Summary
Cyclic nucleotide-gated (CNG) channels are essential for vision and olfaction. They belong to the voltage-gated ion channel superfamily but their activities are controlled by intracellular cyclic nucleotides instead of transmembrane voltage. Here we report a 3.5 Å-resolution single-particle electron cryomicroscopy structure of a CNG channel from C. elegans in the cGMP-bound open state. The channel has an unusual voltage-sensor-like domain (VSLD), accounting for its deficient voltage dependence. A C-terminal linker connecting S6 and the cyclic nucleotide-binding domain interacts directly with both the VSLD and pore domain, forming a gating ring that couples conformational changes triggered by cyclic nucleotide binding to the gate. The selectivity filter is lined by the carboxylate side chains of a functionally important glutamate and three rings of backbone carbonyls. This structure provides a new framework for understanding mechanisms of ion permeation, gating and channelopathy of CNG channels and cyclic nucleotide modulation of related channels.
Vertebrate vision and olfaction signal transduction depends critically on cyclic nucleotide-gated (CNG) channels1–3. In photoreceptors, light activation of the photopigments decreases intracellular cyclic guanosine monophosphate (cGMP) concentration and closes CNG channels, resulting in membrane hyperpolarization4–5. In olfactory sensory neurons, odorant activation of olfactory receptors increases intracellular cyclic adenosine monophosphate (cAMP) concentration and opens CNG channels, leading to membrane depolarization6. CNG channels also play an important role in chemosensation in invertebrates1. Moreover, CNG channels are expressed in the central nervous system where they regulate neuronal and glial functions1,3. Mutations in CNG channel genes have been associated with debilitating visual disorders such as retinitis and achromatopsia1,3.
CNG channels are members of the voltage-gated ion channel (VGIC) superfamily that includes voltage-gated potassium (Kv), sodium (Nav) and calcium (Cav) channels and the transient receptor potential (TRP) channels1–3,7–8. Like Kv and TRP channels, CNG channels are composed of four subunits, each containing six transmembrane (TM) segments (S1-S6) and a pore-loop (P-loop) between S5 and S61–3. Vertebrates have four CNGA and two CNGB subunits. VGICs possess two structural and functional modules: a voltage-sensor domain (VSD) or voltage-sensor-like domain (VSLD) consisting of S1-S4 and the S4-S5 linker, and a pore domain consisting of S5, P-loop and S6. Although possessing a VSLD, CNG channels are not gated by TM voltage1–4,6,8, a property critical for proper phototransduction and olfactory transduction. Instead, CNG channels are gated by intracellular cAMP or cGMP. These ligands bind to a cyclic nucleotide-binding domain (CNBD) in the cytoplasmic C-terminus1–3,7–8. A ~80-amino acid linker, called the C-linker, connects the CNBD to S6 and is crucial for CNG channel gating9–14. Rich knowledge has been gained from extensive functional investigation of CNG channels1–3, but why CNG channels are insensitive to membrane voltage and how cyclic nucleotide binding opens the channel remains largely unclear.
Structures of major classes of VGICs have been obtained15–20, but no high resolution structure of a full-length CNG channel or domain has been reported. In this study we determined, by using single particle electron cryo-microscopy (cryo‐EM), a 3.5 Å‐resolution cGMP-bound open-state structure of a full-length eukaryotic CNG channel formed by TAX-4, a CNGA subunit from C. elegans. TAX-4 forms functional homomeric channels in heterologous expression systems14,21–22 (Extended Data Fig. 1), and its amino acid sequence shares 60% identity with that of bovine and human CNGA1 in the core region encompassing S1 through the CNBD (Extended Data Fig. 2). Our structure is the first high-resolution full-length structure of CNG channels and related hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels and reveals features distinct from other VGICs.
Overall architecture
Full-length TAX-4 was heterologously expressed in High Five inset cells. Purified TAX-4 protein bound with cGMP and amphipol was used for single particle cryo-EM analysis (Extended Data Figs 3–5). A cryo-EM density map of the cGMP-bound TAX-4 tetramer was obtained at an overall resolution of 3.5 Å with C4 symmetry imposed (Fig. 1a and Extended Data Figs 3–5). The local resolution varies from 3.0 Å in some transmembrane and cytoplasmic regions to <5.0 Å in the peripheries of the 3D reconstruction (Extended Data Fig. 4e–i). The electron densities were of good quality for most functional regions (Extended Data Fig. 6), and we were able to model 514 of the 733 amino acids of TAX-4 in the final structure. Residues M1 to I105 and N621 to K733, which constitute most of the N-terminus and the distal C-terminus beyond the CNBD, respectively, were not modeled because their densities were weak or missing. The regions with the best resolutions include the pore helix, the selectivity filter, S5, S6 and the C-linker.
Figure 1. Architecture of TAX-4.
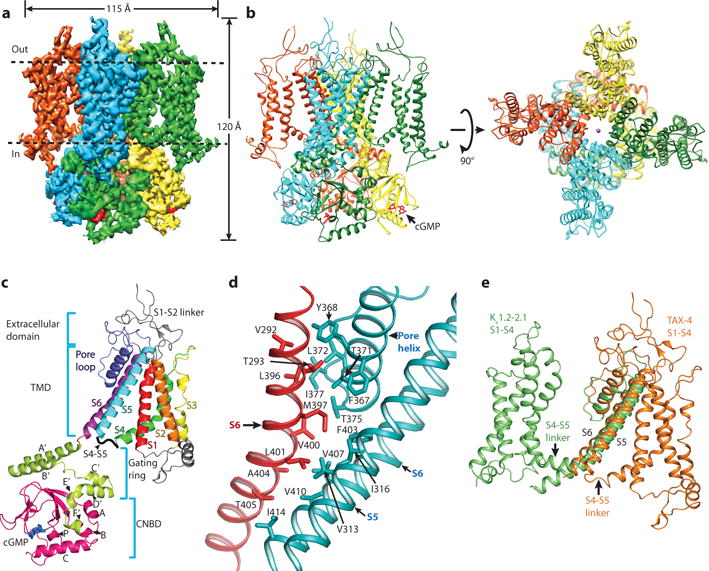
a, Cryo-EM density map of TAX-4. Dashed lines mark the membrane boundaries. b, TAX-4 structure, viewed parallel to the membrane (left) and from the extracellular side (right). c, Structure of a TAX-4 protomer, viewed parallel to the membrane. Different regions (S1-S6, pore-loop, C-linker and CNBD) are illustrated in different colors. d, Intersubunit interface in the TAX-4 TMD. e, Superposition of the TMDs of TAX-4 and the Kv1.2–2.1 chimera (PDB code: 2R9R), aligned by S5 and S6.
TAX-4 forms a four-fold symmetric tetramer with a central ion-conducting pore (Fig. 1a, b). Each protomer can be divided into four structural layers along the fourfold axis: the extracellular domain, the transmembrane domain (TMD), the gating ring, and the CNBD (Fig. 1c). The gating ring is formed by helices A′B′C′D′ of the C-linker, which contains two additional helices E′ and F′, and is strategically positioned between the TMD and the CNBD (Fig. 1c).
Two interaction interfaces contribute to TAX-4 tetramerization. One interface is in the TMD, between the pore domain of one subunit and S6 of a neighboring subunit (Fig. 1d). This interface involves predominantly hydrophobic interactions (Fig. 1d). Another interface is in the gating ring, where helices A′B′C′D′ of two neighboring subunits form extensive intersubunit interactions (see below).
A common defining feature of VGICs and other VSLD-containing channels is that, despite their divergent amino acid sequences, the VSD (or VSLD) of one subunit associates with the pore domain of a neighboring subunit in a domain-swapped configuration (Extended data Fig. 7a–c). Strikingly, the VSLD of TAX-4 interacts only with the pore domain of the same subunit in a non-domain swapped configuration (Fig. 1b, e and Extended Data Fig. 7b, c). These interactions include hydrophobic and hydrogen bonding interactions between the extracellular ends of S1 and S5 and the pore helix, and between the intracellular ends of S4 and S5 (Extended Data Fig. 7d). The importance of the non-domain swapped architecture in CNG channel function awaits elucidation.
An unusual voltage-sensor-like domain
VGICs open and close in response to membrane potential changes by moving the S4 voltage sensor and, via the S4-S5 linker, the S6 helix16,23. Although CNG channels have a VSLD, they are not gated by membrane voltage1–4,6,8 (Extended Data Fig. 1b, c). The TAX-4 channel structure reveals two striking differences between its VSLD and the canonical VGIC VSD that evidently explain CNG channels’ lack of voltage-dependent gating.
First, a typical S4, exemplified by that of Kv channels, adopts a continuous helical structure with regularly spaced arginine or lysine residues throughout, and tilts at a 65° angle from the plane of the membrane16,23 (Fig. 2a, c). The TAX-4 S4, however, breaks up into three sub-segments, which we name (from external to internal side) S4a (loop), S4b (310 helix), and S4c (α-helix). These sub-segments have different secondary structures and orientations in the membrane: S4c tilts at a shallow 25° angle from the membrane, but S4b bends abruptly such that the remainder of S4 stands at a much steeper 72° angle. Eight positive charges are scattered in all three sub-segments, of which five arginines (R280, R283, R286, R289 and R296) are conserved in mammalian CNGA1 subunits (Fig. 2a). Among the positive charges, four (K288, R289, R291 and R296) are located in S4c and thus sense only a fraction of the transmembrane voltage field. Moreover, S4c is likely restrained owing to its interaction with the C-linker (see later). Three of the positive charges (R280, R283 and 286) are located in S4b. They, along with R289, are engaged in interactions with a string of negative charges in S2, including D208, D211, D218 (Fig. 2d). These negative charges are conserved in all human CNGA and CNGB subunits (Extended Data Fig. 8a). These interactions may serve to restrict the movement of S4b and also stabilize S2. These physico-chemical features of S4 likely render S4 nonresponsive to membrane potential changes, consistent with the lack of voltage-dependent gating currents in CNG channels24.
Figure 2. An unusual voltage-sensor-like domain.

a, Amino acid sequence alignment of S4 and neighboring regions of the indicated channel subunits. Positions of positive charges in TAX-4 and Kv1.2–2.1 chimera are marked. b, c, Comparison of S4 of TAX-4 and the Kv1.2–2.1 chimera, viewed parallel to the membrane and after a 25-degree clockwise rotation from Fig. 1e. Positions R0-R6 in c correspond to R287, Q290, R293, R296, R299, K302 and R305, respectively. d, Ion-pair interactions involving the indicated TAX-4 S4 positive charges.
Second, a typical S4-S5 linker in VGICs is a 10-12-amino acid α-helix running parallel to the plane of the membrane (Fig. 2c) that interacts with S6, an interaction crucial for voltage-dependent gating16,23. The TAX-4 S4-S5 linker is only five amino acids long (Fig. 2a) and does not interact with S6 due to the non-domain swapped arrangement between the VSLD and pore domain (Fig. 1e). Instead, it forms a short loop such that S4, S5 and the S4-S5 linker adopt a helix-turn-helix motif (Fig. 1C and 2b). As described below, this motif interacts with the C-linker, suggesting that the VSLD is actually involved in ligand gating of CNG channels.
Ion conduction in an open channel
The ion-conduction pathway traverses all four layers, with a total length of ~120 Å (Fig. 3a). The wide outer pore tapers sharply toward a narrow and electronegative selectivity filter (Fig. 3a, b and Extended Data Fig. 9a). The cavity and inner pore, formed by S6, widen from 9.3 Å below the selectivity filter to 21.5 Å at the cytoplasmic end of S6. The wide-splayed S6 indicates that the channel is open.
Figure 3. The ion conduction pathway.

a, The solvent-accessible pathway generated with the HOLE program, shown in the center. b, Pore-size profile generated with the HOLE program of different sections of the solvent-accessible pathway. The origin of the pore axis is set at the cytoplasmic end of S6. c, Close-up view of the selectivity filter. Only two diagonally opposed subunits are shown. The cryo-EM density map and the modeled amino acids are shown in mesh and sticks, respectively. Distances between the atoms are measured as the center-to-center distance of two diagonally opposed atoms. Purple spheres mark presumed ion binding sites. d, Protein packing around the selectivity filter. Amino acids in and immediately adjacent to the selectivity filter are colored in red.
The selectivity filter is formed by four continuous conserved amino acids –T376, I377, G378 and E379, and is lined by the carboxylate of E379, the backbone carbonyl oxygens of T376, I377 and G378, and the hydroxyl side chain of T376 (Fig. 3c). The narrowest constriction of the selectivity filter and the entire ion-conduction pathway is at the E379 side chains and is 4.7 Å in diameter. The rest of the selectivity filter is substantially wider (7.0 Å to 10.1 Å) (Fig. 3c), indicating that the selectivity filter is in an open conformation. Previous studies examining state-dependent block and cysteine modification of the CNG channel pore suggest that the selectivity filter acts as the activation gate25–27. Whether the selectivity filter indeed narrows to form a gate in the absence of cyclic nucleotide binding awaits structural elucidation.
A continuous elongated density is observed in the center of the selectivity filter (Fig. 3c). This density likely represents bound ions rather than noise since no density peaks are present elsewhere along the fourfold axis. We tentatively assign three ion binding sites to this density and speculate that they are occupied by Na+ (since no Ca2+ was added in the protein sample). Multiple ion-binding sites formed by a combination of acidic side chains and backbone carbonyl oxygens are a common characteristic of Ca2+-conducting channels (Extended Data Fig. 9b).
The structural hallmarks of the TAX-4 channel selectivity filter account for the observed ion permeation properties of CNG channels. An electronegative but relatively wide selectivity filter would allow ions with one or more hydration waters to pass, consistent with the poor monovalent cation selectivity of CNG channels1–4,6–8,28. Mutagenesis studies show that the glutamate occupying the analogous position of E379 plays a critical role in pH-dependent ion permeation and divalent cation (Ca2+ and Mg2+) block29–31. E379 is well positioned to be protonated by external protons and to attract and directly interact with external permeant and blocking cations. The close proximity of E379 side chains is consistent with a previous finding in a vertebrate CNG channel that a single protonation site is made by two glutamates31. On the other hand, the luminal projection of the E379 side chain is in contrast with the side chain orientation of the analogous glutamate in NaK2CNG-E, an engineered chimeric NaK channel containing four amino acids (ETPP) of the CNG channel selectivity filter sequence32–34 (Extended Data Fig. 9c). In NaK2CNG-E, the glutamate side chain points toward the protein interior and is engaged in dynamic protein packing around the selectivity filter32–34.
In contrast to the elaborate hydrogen bonding network surrounding the selectivity filter of K+ channels35, the selectivity filter of TAX-4 is stabilized primarily by hydrophobic interactions (Fig. 3d). The ‘greasy’ hydrophobic interactions likely impart flexibility to the selectivity filter, allowing it to change its conformation during both ion conduction and ligand gating.
The cyclic nucleotide-binding domain and C-linker
Structures of isolated C-linker/CNBD regions of HCN channels have been reported36–41. Our study reveals for the first time the structure of this region in a full-length channel context. The overall structure of the cGMP-bound CNBD of TAX-4 is similar to the ligand-bound structures of the CNBD of other cyclic-nucleotide binding proteins, including HCN channels38. A conserved fold consisting of four α helices (designated A, P, B and C) and eight β sheets that form a β-roll is preserved in the TAX-4 CNBD (Fig. 4a). cGMP binds in a conserved pocket formed by helix C and the β-roll (Fig. 4a), and engages in the same interactions observed in other cGMP-bound CNBD structures36,42 (Fig. 4b). Superposition of the cGMP-bound TAX-4 and HCN2 CNBD36 structures shows a high degree of overlap in the β sheets (rmsd=0.69) (Fig. 4c).
Figure 4. The cyclic nucleotide binding domain.

a, Structure of the cGMP-bound CNBD of TAX-4. b, Amino acids and interactions involved in the binding of cGMP, shown in a ball-and-stick model. c, Comparison of the structures of the cGMP-bound CNBD of TAX-4 and HCN2 (PDB code: 1Q3E), aligned by the β strands.
The structure of the TAX-4 C-linker is similar to that of the HCN2 C-linker36. The C-linker is divided into six α helices, designated A′-F′ (Fig. 5a). Helix A′ is connected to S6 via a mere 2-amino acid-linker. This direct connection is crucial for cGMP activation of TAX-4 (see later). Helices A′B′ lay at a 25° angle to the plane of the membrane and are sandwiched between the TMD and the rest of the C-terminus (Fig. 1c and Extended Data Fig. 10a). The topside and downside of A′B′ are both engaged in intersubunit interactions (Fig. 5b, c). The downside of one subunit associates with helices C′D′ of the clockwise (viewing from top down) neighboring subunit (Fig. 5b and Extended Data Fig. 10a). The intersubunit interactions (Fig 5b) are similar but, importantly, not identical to those found in the HCN2 C-linker36. Through these interactions, the C-linker/CNBD tetramerizes, which likely contributes to the assembly of the full-length channel.
Figure 5. The C-linker.

a, Superposition of the structures of the cGMP-bound C-linker/CNBD regions of TAX-4 and HCN2 (PDB code: 1Q3E), aligned by the β strands. b, Interactions between the C-linkers of two adjacent subunits. c, Interactions between the C-linker of one subunit and S4/S4-S5 linker/S5 of an adjacent subunit. For clarity, an ion-pair interaction between R300 in the S4-S5 linker and D119 in S1 is not illustrated. d, Comparison of the liganded structure of the TAX-4 C-linker/CNBD and the unliganded structure of the HCN2 C-linker/CNBD (PDB code: 2MPF). The structures are aligned by the β strands. Each arrow marks the positions of the same atom in the two structures.
The topside of helices A′B′ interacts with the VSLD and pore domain of the same clockwise neighboring subunit (Fig. 5c). R421, Q425 and D429 in helix A′ form an intertwining network of salt bridges and hydrogen bonds with E295 and E298 in S4 and R308 in S5. D453 in helix B′ and R296 in S4 form another ion pair interaction. In addition, K432 in helix A′ forms a hydrogen bond with the backbone carbonyl oxygen of S301 in the S4-S5 linker, which, together with an ion-pair interaction between R300 in the S4-S5 linker and D119 in S1, stabilizes this 5-residue linker. These A′B′-TMD interactions are likely critical for maintaining S6 in an open state. Through these interactions, helices A′B′ directly transmits conformational changes in the CNBD to the TMD and ultimately to the selectivity filter.
Comparison of the structures of the cGMP-bound TAX-4 C-linker/CNBD and an unbound HCN2 C-linker/CNBD37 reveals marked conformational changes in both the CNBD and the C-linker in the unbound state (Fig. 5d). First, helix C moves downward, away from the β-roll, by 7.2 to 9.6 Å. Second, helices A and B, which interact with helix F′, move away from helix F′, dragging the latter downward. Consequently, helix E′ is pulled downward by 3.8 to 4.6 Å (Fig. 5d). Because helix D′ is unresolved and helices A′B′C′ are not included in the apo HCN2 C-linker/CNBD structure37, it is unclear how these helices change their structures. But superposition of the bound and a hypothetical unbound structures of TAX-4 suggests that because of the downward movement of helices E′F′, helix D′ must adopt a different conformation in the unbound state to avoid clash with helix A (Extended Data Fig. 10 c–e).
Model for cyclic nucleotide gating
Based on our structure we propose a model for cyclic nucleotide gating of CNG channels (Fig. 6a). Several experimental observations are taken into account in constructing this model. First, helix C undergoes large conformational changes upon ligand binding/unbinding37,41,43–45. Second, isolated unliganded C-linker/CNBD of HCN channels can exist as monomers and dimmers in solution13,36,40. Third, the inner pore formed by S6 narrows in the closed state but does not fully close25–27,46. Fourth, the selectivity filter forms the activation gate26–27. Our model starts with the cGMP-bound, open-state structure (Fig. 6a, I): (1) As cGMP unbinds, helix C moves downward. (2). Helices E′F′ in the C-linker move downward, resulting in conformational changes in helices C′D′. (3) Interactions between C′D′ and A′B′ are weakened, causing a weakening of the interactions between A′B′ and the TMD (Fig. 6a, II); or alternatively, C′D′ and A′B′ dissociate, causing A′B′ to dissociate from the TMD (Fig. 6a, III). In either case, the end result is a conformational change of S6. In essence, helices A′B′C′D′ constitute a gating ring13,36 that changes its conformation and its interaction with the TMD upon cyclic nucleotide binding and unbinding, and these conformational changes are transmitted directly to S6. (4) S6 movements perturb the hydrophobic interactions between S6, the pore helix and the selectivity filter (depicted in Fig. 1d and 3d) and thereby close the selectivity filter.
Figure 6. Model for cyclic nucleotide gating.

a, Schematic of the TAX-4 channel in the cGMP-bound and unbound states. In the liganded state (I), helices A′B′C′D′ of the C-linker form a compact, firmly bound gating ring, which is highlighted in color. A′B′ (green) and C′D′ (blue) of two neighboring subunits associate tightly, and A′B′ interact strongly with S4/S4-S5 linker/S5. These interactions, schematized in red, cause S6 to splay wide and the selectivity filter gate to open. In the unliganded state, the gating ring loosens or dissociates as the interactions between A′B′ and C′D′ and between A′B′ and S4/S4-S5 linker/S5 are either greatly weakened (II) or virtually abolished (III), causing S6 to constrict and the selectivity filter gate to close. For clarity, only two subunits are shown, but in (I) and (III), helices A′B′C′D′ of all four subunits are included to depict the gating ring. b, Whole-cell currents recorded at ‐100 mV from HEK 293T cells expressing WT TAX-4 and the indicated mutant channels, without or with 100 μM cGMP in the recording pipette. Number of measurements is indicated for each condition. Error bars represent S.E.M.
According to this model, alterations of the interactions between helices A′B′ and C′D′ or between helices A′B′ and the TMD would affect the allosteric coupling between the gating ring and the TMD and hence cyclic nucleotide gating. The different affinities and efficacies of cyclic nucleotide activation of different CNG channels can thus be partly accounted for by the amino acid sequence divergence in these interaction interfaces (Extended Data Fig. 8a).
We functionally tested two aspects of this model. First, we disrupted the interactions between the gating ring and the TMD by collectively mutating to alanine R421, Q425, D429, K432 and D453 in helices A′B′ (Fig. 5c). The mutant channel, named TAX-4_5A, did not respond to a saturated concentration (100 μM) of intracellular cGMP (Fig. 6b and Extended Data Fig. 1g). Second, we disrupted the coupling between the gating ring and S6 by inserting one to three glycines between M417 and S418, which connect helix A′ and S6. All three mutant channels (named TAX-4_1G, TAX-4_2G and TAX-4_3G), failed to produce currents in the presence of 100 μM intracellular cGMP (Fig. 6b and Extended Data Fig. 1i–j). All mutant channels were robustly expressed on the plasma membrane (Extended Data Fig. 1k), suggesting that the mutations disrupted channel gating. These results are consistent with the predictions of the model.
Concluding remarks
The TAX-4 channel structure reveals several novel features: a non-domain swapped architecture, a segmented and restrained S4, a short S4-S5 linker, and direct interactions between the C-linker and both the pore domain and VSLD. The non-domain swapped architecture has been recently suggested by a structural and biochemical study of the TRPV6 channel47 and observed in a cryo-EM structure of the Eag1 channel48. The amino acid sequence homology among CNG, TRPV6 and Eag1 channels is low, and the pore domain/VSD (or VSLD) interface is different among them, suggesting that different structural elements underlie the non-domain swapped architecture. A key element might be the length and/or secondary structure of the S4-S5 linker.
The structural features revealed by the TAX-4 structure are in good agreement with CNG channel properties elucidated in functional studies and provide new insights into the allosteric mechanisms of how cyclic nucleotide binding opens the channel. The physico-chemical characteristics of the selectivity filter and its surroundings are consistent not only with nonselective permeation of monovalent cations and block by extracellular Ca2+ and Mg2+, but also with the proposition that the selectivity filter is the activation gate. The structure also provides a blueprint for understanding and investigating the functional effects of hereditary disease-causing single amino acid missense mutations, many of which are distributed in regions critical for cyclic nucleotide gating (Extended Data Fig. 8b).
METHODS
Molecular biology
The full-length TAX-4 gene was cloned from a C. elegans cDNA library by PCR. For protein expression, the DNA fragment encoding the entire TAX-4 was cloned into a modified pFastBac1 vector with BamHI and NotI restriction sites. The endogenous BamHI site in the TAX-4 DNA sequence was removed by mutagenesis without altering the protein sequence. A maltose binding protein (MBP) tag was added before the N-terminus of TAX-4, and a linker sequence of NNNNNNENLYFQGGGGS, which contains the tobacco etch virus (TEV) protease recognition sequence (underlined) and flanking sequences, was inserted between the MBP tag and TAX-4. For electrophysiology experiments, the full-length WT TAX-4 gene was cloned into the pcDNA3.1(-) vector using EcoRI and HindIII restriction sites, producing a construct is named TAX-4_WT. For surface expression experiments, a construct named GFP-TAX-4_WT-HA was created in two steps: first, the full-length WT TAX-4 gene was cloned into a modified pEGFP_C1 vector using BamHI and NotI restriction sites, generating a construct called GFP-TAX-4_WT; second, a sequence of GGGYPYDVPDYAGGG, which contains the hemagglutinin (HA) tag (underlined) and flanking sequences, was inserted between G162 and T163 in GFP-TAX-4_WT. The resulting GFP-TAX-4_WT-HA channel thus contains a GFP tag on its N-terminus and an HA tag in the extracellular linker between S1 and S2. All site-specific mutants were subsequently generated in TAX-4_WT and GFP-TAX-4_WT-HA by PCR-based overlapping extension mutagenesis.
Protein purification
The baculovirus of TAX-4 was generated with Sf9 cells using the standard Bac-to-Bac method. High Five insect cells were infected with the TAX-4 virus. 48 hours after infection, cells were harvested by centrifugation at 4 °C, and suspended in a buffer containing 50 mM HEPES-NaOH (pH 7.4), 500 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol and 50 μM cGMP (buffer A) in the presence of a protease inhibitor cocktail (Roche). After cell disruption by sonication, cell debris was removed by centrifugation at 3,200 g for 10 minutes at 4 °C and cell membrane was pelleted from the supernatant by ultracentrifugation at 150,000 g for 1 hour at 4 °C. Membrane was suspended in buffer A containing the above protease inhibitor cocktail and homogenized with a glass dounce homogenizer. TAX-4 protein was extracted with 1% Lauryl Maltose Neopentyl Glycol (LMNG, Anatrace) for 1 hour at 4 °C. The solubilized membrane was clarified by ultracentrifugation at 150,000 g for 30 minutes and incubated with amylose resin (NEB) for 2 hours at 4 °C with gentle agitation. Subsequently, the resin was collected by low speed spin at 800 g, transferred into a gravity column, and washed with buffer A containing 0.5 mM LMNG and 0.1 mg/ml soybean lipids (Avanti polar lipids). 20 mM maltose in the wash buffer was used to elute the MBP-tagged TAX-4 protein. The eluted protein was then mixed with amphipol A8-35 (Anatrace) at 1:6 (w/w) ratio and incubated overnight at 4 °C with gentle agitation. Detergent was removed by incubation with Bio-Beads SM-2 (Bio-Rad) for 8 hours. After removal of Bio-Beads, TEV protease was added to the protein sample and incubated at 4 °C overnight to cleave the MBP tag. TAX-4 protein was concentrated and further purified on a superose 6 column in a buffer containing 20 mM HEPES-NaOH (pH7.4), 150 mM NaCl, 2 mM β-mercaptoethanol and 50 μM cGMP. Fractions containing the protein were examined by negative staining EM, and then pooled and concentrated. Proteins were concentrated by ultrafiltration using the Amicon™ Centrifugal Filter Units (EMD Millipore), and their concentrations were measured by using the Bradford Protein Assay kit (Bio-Rad).
Cryo-EM sample preparation and data acquisition
cGMP was added to purified TAX-4 protein (0.6 mg/ml) to a final concentration of 2 mM. 4 μl of this protein sample was loaded onto glow-discharged Quantifoil R1.2/1.3 holey carbon grid, and blotted for 4.0 s under 100% humidity and 8 °C using FEI Vitrobot (FEI Company). After waiting for 3 s (double-sided, blot force 1), the grid was immediately plunged into liquid ethane cooled by liquid-nitrogen. Micrographs were acquired by a Titan Krios microscope operated at 300 kV, equipped with a K2 Summit direct electron detector (Gatan Company) working at super-resolution counting mode. UCSFImage449 was used for data collection following standard FEI low-dose procedure. Nominal magnification of 22,500x was used for imaging the samples, corresponding to a final pixel size of 0.66 Å on image. The defocus ranges from −1.7 μm to −2.7 μm. Each micrograph was dose-fractionated to 32 frames under a dose rate of 8.2 counts/physical pixel/second, with a total exposure time of 8 s and a frame exposure time of 0.25 s, which results in a total dose of ~50 e−/Å2.
Image processing
All micrographs were first 2x binned, generating a pixel size of 1.32 Å. Motion correction was performed using the MotionCorr program50. The resulting integrated micrographs and stacks of motion-corrected frames were used for further processing. The contrast-transfer function parameters of the micrographs were estimated using the CTFFIND3 program51. Initially, ~19,000 particles were semi-automatically picked with the EMAN boxer swarm tool52 from a subset of 70 micrographs. Subsequent image processing was performed using Relion 1.353. Five class averages representing common features of the picked particles were selected from the 2D classification of the subset, and used as templates for automatic particle picking from the full dataset of 2,215 micrographs. The picked particles were screened by several rounds of 2D classification, and a total of 231,328 particles were finally selected and subjected to a cascade of 3D classification and refinement. The full set of 2,215 micrographs was divided into 3 subsets with 579, 897, and 739 micrographs, respectively, and each subset was classified into 3 or 4 classes in the 3D classification procedure (Extended Data Fig. 3e). Particle heterogeneity was observed among the resulting 3D classes, which presented two typical conformations - a near-perfect tetramer and a defective tetramer with a missing/flexible domain. To isolate the particles belonging to different 3D conformations, two 3D reconstructions corresponding to the two tetramer conformations were selected from the 3D classification of one subset (class 2# and 4# from the subset with 579 micrographs), and used as initial references (after low-pass filtered to 60 Å resolution) for the next two rounds of supervised 3D classification. In each round, the particles were classified into 2 classes, and only the particles classified into the class of the near-perfect tetramer were used for the next round of classification. Finally, 99,934 particles corresponding to the near-perfect tetramer were gathered and subjected to final 3D refinement with C4 symmetry imposed. The final 3D reconstruction produced a resolution of 3.7 Å. A ‘particle polishing’ subroutine in Relion was subsequently carried out and yielded a 3D reconstruction at a 3.5 Å resolution. All resolutions are estimated using the gold-standard FSC=0.143 criterion. ResMap54 was used to calculate the local resolution map.
Model building
The 3.5 Å EM map was used for de novo model building in COOT55. The S6-C-linker region was easily identified in the map because of the good quality of its density, and was used as the starting point of model building. Atomic model for S2, S3, S4, S5, S6 and the P-loop was built manually, using bulky residues Trp, Tyr and Phe as guide posts for sequence assignment. Predicted transmembrane segments were also used to help guide sequence assignment. The selectivity filter motif TIGE confirmed the correct sequence register in the pore loop. A polyalanine model was first built into the density of S1; subsequent sequence assignment was achieved by identification of residues with large side chains. The density of residues 1–105 was missing in the map. The density of the S1-S2 loop was poor but mostly continuous. Although the density of some side chains was missing, atomic model was built for this region with the exception of amino acids 162–166. Atomic model of the C-terminus was also built manually. The homologous structure of the C-linker/CNBD region of HCN2 (PDB code: 1Q3E) was docked into the C-terminal density and was used as a reference for model building. The cGMP molecule from the HCN2 structure fit well to the density; thus, it was put in the model without change. Residues 621–733 were not visible in the density map. There was weak density under the C-linker/CNBD region. This density could represent the coiled coil region, but it was too weak for model building. During model building, the fitting of every modeled residue was optimized by using the real-space refinement tool in COOT. Once an atomic model was built for one subunit, it was rotated and fitted into the densities of the other three subunits. The models for all four subunits were then merged. Three Na+ ions were added to the model to fit the density in the center of the selectivity filter.
Reciprocal-space refinement of the entire model was carried out using Phenix56. A restraints file was generated by ReadySet and manually modified for the cGMP molecule. The final masked maps were put into an artificial unit cell with P1 symmetry. Structure factors were calculated using Phenix, and were then used for maximum likelihood refinement in reciprocal space. Coordinates and individual B-factors were refined with secondary structure restraints and strict NCS constraints.
Model validation
A cross-validation method previously described57 was used. In brief, atoms in the final model were randomly shifted by up to 0.2 Å, and the new model was then refined against one of the two half maps generated during the final 3D reconstruction. FSC values were calculated between the map generated from the resulting model and the two half maps, as well as the averaged map of two half maps (Extended Data Fig. 5b).
Electrophysiology
Human embryonic kidney (HEK) 293T cells were grown in DMEM (HyClone) plus 10% newborn calf serum (Gibco) and penicillin (100 U/ml)/streptomycin (0.1 mg/ml) (Biological Industries). HEK 293T cells were transiently transfected with WT or mutant TAX-4 and enhanced GFP plasmids together using LipoD293™ (SignaGen Laboratories) and used in 48 hours.
All experiments were performed at room temperature (~22 °C). Pipettes were fabricated from borosilicate glass (World Precision Instruments) using a micropipette puller (P-1000, Sutter Instrument), and were fire-polished to resistances of 1~2 MW for inside-out patch recording and ~3 MW for whole-cell recording. Whole-cell currents were elicited by 20-ms voltage steps from −100 to +150 mV with 10-mV increments, with a holding potential of 0 mV. For inside-out patch recording, currents were elicited by 100-ms voltage steps from −150 to +150 mV with 10-mV increments, with a holding potential of 0 mV. Currents were amplified by Axopatch 200B and digitized by Digidata 1440A (Molecular Devices). Currents were low-pass filtered at 2 kHz and sampled at 10 kHz. pCLAMP 10 software (Molecular Devices) was used for data acquisition and analysis. Both intracellular and extracellular solutions contained (in mM) 140 NaCl, 5 KCl, 1 mM EGTA and 10 HEPES (pH 7.4 adjusted with NaOH).
Immunofluorescence and imaging
HEK 293T cells were plated on poly-D-lysine-coated (Sigma) glass coverslips in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), and kept in an incubator at 37 °C in a 5% CO2 humid atmosphere. Transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. 2.5 μg of GFP-TAX-4_WT-HA or corresponding mutants cDNAs in 200 μl Opti-MEM (Invitrogen) were used to transfect each 12 mm (diameter) coverslip.
Immunofluorescence staining was performed 36 h post-transfection, at which time GFP’s expression was seen to peak. The culture medium was removed, and the transfected cells were briefly rinsed with PBS, and immediately fixed with PBS supplemented with 2% paraformaldehyde-4% sucrose, for 15 min at room temperature. Cells were then washed in PBS, and subsequently blocked in a non-permeabilizing (detergent-free) blocking buffer, which consisted of 1% fish gelatin (Sigma) and 2% goat serum (Sigma) in PBS, for 4 h at room temperature. To visualize surface HA tags, the fixed cells were incubated with a primary antibody against HA (mouse monoclonal anti-HA, BioLegend) at 4°C overnight (diluted in blocking buffer, 1:160), then washed with PBS four times. A goat anti-mouse secondary antibody conjugated with the Alexa594 fluorophore (Life Technologies) was added to the coverslips for 1.5 h at room temperature (in blocking buffer, 1:700). The stained coverslips were finally washed 5 times with PBS before imaging.
Imaging was performed using a spinning disc confocal microscope, built on an inverted Nikon Eclipse TE2000-S microscope. Optics consisted of a spinning disc scanner unit (CSU10, Yokogawa) and a CCD high resolution digital ImageM camera (Hamamatsu). Images were acquired using a 60x color-corrected objective lens (Nikon). The GFP (green) laser line used 491 nm and 520 nm as excitation and emission wavelengths, respectively. The Alexa594 (red) laser optical path consisted of a 561 nm excitation source and a 591 nm emission filter. Laser sources and equipment were from Spectral. Optical filters (Chroma) were sputter-coated, to minimize chromatic aberration.
Focal plane thickness (z-axis) was 200 nm in all conditions. Confocal images for each fluorophore (GFP and HA) were always acquired as sequential scans (every 200 nm), which eliminated any optical bleed-through for the fluorophores used. Images were acquired and analyzed with Volocity 6.3 (PerkinElmer).
Extended Data
Extended Data Figure 1. Functional characterization of WT and mutant TAX-4 channels.

a, Family of macroscopic currents recorded in an inside-out patch from a HEK 293T cell expressing WT TAX-4 channels. Currents were elicited by 10 μM cGMP, in response to voltage steps from −150 mV to +150 mV in 10-mV increments from a holding potential of 0 mV. b, Current-voltage (I-V) relationship from the same patch as in a. Currents were recorded first in the control bath solution (bath), then in the presence of 10μM cGMP, and then again in the control bath solution (washout). c, Averaged I‐V relationship of macroscopic currents from seven independent inside-out patch recordings from HEK 293T cells expressing WT TAX-4 channels. Currents were obtained as in a and b.For each patch, currents at different voltages were normalized by the absolute current value at -150 mV. In this and subsequent figures, error bars represent S.E.M. and are smaller than the symbols at some voltages. Notice the lack of voltage-dependent gating. d, Dose-response relationship of cGMP activation of WT TAX-4 channels at –100 mV. Currents were recorded in inside-out patches obtained from HEK 293T cell expressing WT TAX-4 channels. Data points represent mean ± S.E.M. of the indicated number of measurements. Solid curve represents fit to the Hill equation in the form of I(X)=Xn/(Xn+EC50n), where I(X) is the normalized current, X the cGMP concentration, n the Hill coefficient, and EC50 the cGMP concentration producing half maximal current. The fit yields an EC50 of 0.16 μM and n of 1.7, which are very close to the values of 0.34 μM and 1.6 reported previously. e, Family of currents recorded in the whole-cell configuration from a HEK 293T cell expressing WT TAX-4 channels. Currents were elicited by 100 μM cGMP added in the recording pipette, in response to voltage steps from −100 mV to +150 mV in 10-mV increments from a holding potential of 0 mV. f-j, Averaged I-V relationships of whole-cell currents recorded from the indicated number of HEK 293T cell expressing WT TAX-4 channels and the indicated mutant TAX-4 channels. The recording pipette contained either no cGMP or 100 μM cGMP. The non-liner I-V relationship exhibited by cGMP-activated WT TAX-4 channels (f) is likely caused by block by an endogenous cytoplasmic molecule. In TAX-4_5A, R421, Q425, D429, K432 and D453 in helices A′B′ are mutated to alanine. In TAX-4_1G, TAX-4_2G and TAX-4_3G, one, two or three glycine residues are inserted between M417 and S418. k, Surface expression of WT and mutant TAX-4 channels in HEK 293T cells. The channels contain a GFP tag on its N-terminus and an HA tag in the extracellular linker between S1 and S2, except for GFP-TAX-4_WT, which does not contain the HA tag. Red fluorescence represents surface expression. Similar observations were made in >10 cells for each channel type (including GFP-TAX-4_1G-HA and GFP-TAX-4_2G-HA) in blind experiments.
Extended Data Figure 2. Amino acid sequence alignment of TAX-4, human CNGA1, bovine CNGA1, human HCN2 and the Kv1.2–2.1 chimera.

Sequence alignment begins at D115 of TAX-4 and ends at L677. Green and yellow highlight identical and similar amino acids, respectively. Secondary structures are marked for TAX-4. The TAX-4 channel selectivity filter is boxed in blue. Amino acids in red in TAX-4 participate in intersubunit interactions between helices A′B′ and C′D′. Red triangles mark the amino acids involved in cGMP binding in TAX-4 and HCN2. Red dots mark the amino acids involved in forming the charge transfer center in the Kv1.2–2.1 chimera. Positions of S4 positive charges are boxed in red in the Kv1.2–2.1 chimera.
Extended Data Figure 3. Single-particle cryo-EM analysis of TAX-4.

a, A representative motion-corrected micrograph of TAX-4 recorded using the K2 Summit camera. Typical particles are marked with yellow boxes. b, Fourier power spectrum of the micrograph shown in a with the Thon ring extending to 3Ǻ. c, Gallery of 2D class averages. d, Three enlarged views of representative 2D classes. e, Work flow of two-reference 3D classification. Structures produced by the final refinement with or without C4 imposed are given. Particle number is given below each reconstruction.
Extended Data Figure 4. 3D reconstruction and refinement of TAX-4.

a, Two isosurface levels (low is grey and high is blue) of the final density map filtered to 5 Ǻ. The density contributed by amphipol is visible at the low isosurface level. b, Selected z-slice views of the unfiltered map in a at the corresponding layers indicated by the arrows. c, FSC curve of the final 3D reconstruction with C4 imposed (black) marked with a resolution of 3.5 Å corresponding to the FSC=0.143 cut-off criterion. FSC curve of the final 3D reconstruction without C4 imposed (red) marked with a resolution of 4.5 Å corresponding to the FSC=0.143 cut-off criterion. The FSC curve between the final reconstruction and the map calculated from the atom model (blue) shows a resolution of 3.8 Å according to the FSC=0.5 cut-off criterion. d, Euler angle distribution of all particles used in the final map reconstruction. Each orientation is represented by a cylinder, for which both the height and color (from blue to red) are proportional to the number of particles for that specific direction. e-i, Color-coded final 3D reconstruction of TAX-4 showing local resolutions. The tetramer (e) and four different orientations (f-i) of the protomer are shown, viewed parallel to the membrane. The orientation in f is the same as that in Fig. 1c.
Extended Data Figure 5. Validation of the TAX-4 model.

a, 3D reconstruction and model refinement statistics. b, FSC curves for cross-validation of the final model. Blue: model versus the summed map. Red: model versus half 1 map (called ‘work’, used for model refinement). Black: model versus half 2 map (called ‘free’, not used for model refinement).
Extended Data Figure 6. Cryo-EM density maps and atomic models of selected key TAX-4 regions.

Maps were low-pass filtered to 3.5 Å and amplified by a temperature factor of -160 Å2, and were contoured at 3.0σ.
Extended Data Figure 7. Unique arrangement of the TAX-4 pore domain and voltage-sensor-like domain.
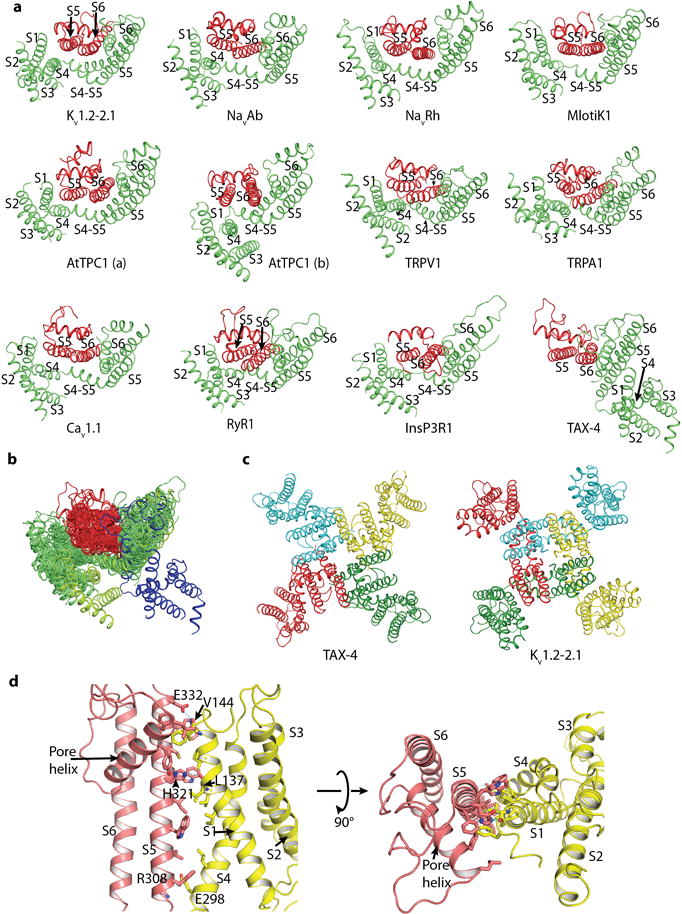
a, Comparison of the arrangement of the pore domain (S5/P-loop/S6) and voltage-sensor domain (VSD, i.e., S1-S4/S4-S5 linker) or voltage-sensor-like domain (VSLD) of TAX-4 and the selected channels. The pore domain and VSD or VSLD of one subunit (for AtTPC1 and Cav1.1, one homologous repeat) is shown in green, and the pore domain of an adjacent subunit (or homologous repeat) is shown in red. In all comparison channels, the pore domain of the red subunit (or repeat) cross-interacts with the VSD or VSLD of the green subunit (or repeat). In TAX-4, however, the pore domain and VSLD of the green subunit form intrasubunit interactions. AtTPC1 is made up of two identical two-pore subunits. Each subunit contains two homologous 6-TM repeats, each of which has its own pore domain and VSD. AtTPC1(a) and AtTPC1(b) represent the two types of pore domain/VSD interactions present in AtTPC1 (ref). Cav1.1 contains 4 homologous 6-TM repeats, each of which has its own pore domain and VSD. Only one representative pore domain/VSD interaction is shown, with green and red representing repeat III and IV, respectively. The PDB codes of the comparison channels are: Kv1.2–2.1, 2R9R; NavAb, 3RVY; NavRh, 4DXW; Mlotik1, 3BEH; AtTPC1, 5E1J; TRPV1, 3J5P; TRPA1, 3J9P; Cav1.1, 3JBR; RyR1, 3J8H; InsP3R, 3JAV. b, Superposition of all structures in a, aligned by S5 and S6 of the green subunit. For contrast, green is changed to indigo for TAX-4. c, Comparison of the tertiary and quaternary structures of TAX-4 and the Kv1.2–2.1 chimera, viewed from the extracellular side of the membrane. d, Interactions between the pore domain and VSLD of TAX-4, viewed parallel to the membrane (left) and from the extracellular side of the membrane (right).
Extended Data Figure 8. Structural and functional annotation of TAX-4.

a, Amino acid sequence alignment of TAX-4 with human CNG channel subunits and TAX-2, a CNGB subunit in C. elegans. Secondary structures and selected functionally important amino acids are annotated for TAX-4. The selectivity filter is boxed in blue, and S4 positive charges are colored in blue. Residues boxed in purple are involved in ion-pair interactions between S4 and S2-S3. Residues boxed in red in S6 and the pore helix interact with residues in or immediately adjacent to the selectivity filter. Residues boxed in green participate in intersubunit interactions between helices A′B′ and C′D′. Residues boxed in orange are engaged in interactions between helices A′B′ of one subunit and S4/S4-S5 linker/S5 of an adjacent subunit. Positions of single-amino acid missense mutations that cause retinitis pigmentosa are highlighted in red in hCNGA1 and orange in hCNGB1, and those cause achromatopsia are highlighted in cyan in hCNGA3 and green in hCNGB3. The disease-causing mutations are listed and color-coded on both sides of the sequences. TAX-2 does not form functional homomeric channels but associates with TAX-4 to form functional heteromeric channels, both in heterologous expression systems and native cells. b, c, Mapping the disease-causing mutations listed in a on the TAX-4 protomer structure, shown in the same orientation here as in Fig. 1c.
Extended Data Figure 9. The TAX-4 channel selectivity filter.

a, Electrostatic surface representation of TAX-4, viewed from the extracellular side of the membrane, showing a highly electronegative external entrance to the selectivity filter in the center. b, Comparison of the selectivity filter of TAX-4 and the selected Ca2+-conducting channels. For clarity, only two diagonally opposed subunits are shown. Cav1.1(a) and Cav1.1(b) represent repeats I/III and II/IV of Cav1.1, respectively. Notice the reoccurring utilization of a combination of negative side chains and backbone carbonyls to line the selectivity filter. The PDB codes of the comparison channels are: Cav1.1, 3JBR; RyR1, 3J8H; TRPV1, 3J5P; TRPA1, 3J9P. c, Superposition of the selectivity filter of TAX-4 and NaK2CNG-E (PDB code: 3K0G). Only two diagonally opposed subunits are shown. Purple spheres mark presumed ion binding sites in TAX-4.
Extended Data Figure 10. The TAX-4 channel gating ring.

a, The gating ring, formed by helices A′B′C′D′ of the C-linker, is depicted in cylinder form and shown in the TAX-4 structure, viewed parallel to the membrane. b, The gating ring shown in isolation, viewed from the extracellular side of the membrane. c, A composite figure demonstrating that helix D′ of the gating ring must change its conformation in the unliganded state. The figure was generated in two steps: (1) The liganded structure of the TAX-4 C-linker/CNBD and the unliganded structure of the HCN2 C-linker/CNBD (PDB code: 2MPF) are aligned by the β strands of the CNBD, and only the indicated α helices are shown here for comparison. (2) Helices A, B, C and E′ of TAX-4 are aligned with those of HCN2, producing a hypothetical unliganded TAX-4 structure. The resulting helix D′, however, clashes with helix A, as shown in e. d, Space-filling model of the interface between helices A and D′ in the liganded TAX-4 structure, showing a snugly fit between these helices. e, Space-filling model of the interface between helices A and D′ in the hypothetical unliganded TAX-4 structure generated in c, showing clash between these helices, indicating that helix D′ must adopt a different conformation.
Acknowledgments
We thank Yushu Chen and Martin Chalfie for providing the C. elegans cDNA library, the China National Center for Protein Sciences (Beijing) for providing facility support, and Jianmin Cui, Steven Siegelbaum, Ming Zhou and Yang lab members for reading and commenting on the manuscript. This work was supported by grants to J.Y. from the National Key Basic Research Program of China (2014CB910301), the National Institutes of Health (R01GM085234 and RO1NS053494), the National Natural Science Foundation of China (31370821), the Top Talents Program of Yunnan Province (2011HA012), and the High-level Overseas Talents of Yunnan Province; to X.L. from the China Youth 1000-Talent Program of the State Council of China, Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, and the National Natural Science Foundation of China (31570730); and to S.W. from the Key Research Program of the Chinese Academy of Sciences (KJZD-EW-L03), the National Natural Science Foundation of China (81302865), West Light Foundation of the Chinese Academy of Sciences, Yunnan Applied Basic Research Projects (2013FB074), and Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Footnotes
Online Content Any additional Methods, Extended Data display items and Source Data are available in the online version of the paper; references unique to these sections appear only in the online paper.
Author Contributions M.L and J.Y. conceived the project. M.L., X.Z., S.W., I.M., X.L. and J. Y. designed experiments, analyzed results, and wrote the manuscript. M.L. performed all molecular biological and biochemical experiments and built the atomic model. X.Z. and X.L. performed all cryo-EM experiments, including data acquisition and processing, and checked the model. S.W., Y.G., D.S. and H. L. carried out electrophysiology experiments. Ioannis Michailidis performed confocal imaging experiments. All authors contributed to manuscript discussion, preparation and editing.
Author Information 3D cryo-EM density maps of TAX-4 without and with low-pass filter and amplitude modification have been deposited in the Electron Microscopy Data Bank under the accession number EMD-6656. The coordinates of the atomic model of TAX-4 have been deposited in the Protein Data Bank under the accession number 5H3O. Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
The authors declare no competing financial interests.
Data availability The authors declare that the data supporting the findings of this study are available within the paper. 3D cryo-EM density maps of TAX-4 without and with low-pass filter and amplitude modification have been deposited in the Electron Microscopy Data Bank under the accession number EMD-6656. The coordinates of the atomic model of TAX-4 have been deposited in the Protein Data Bank under the accession number 5H3O.
References
- 1.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 2.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 3.Varnum MD, Dai G. Cyclic nucleotide-gated channels. In: Zheng J, Trudeau MC, editors. The Hankbook of Ion Channels. CRC Press; Boca Raton: 2015. pp. 361–382. [Google Scholar]
- 4.Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- 5.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaupp UB, et al. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- 8.Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 9.Gordon SE, Zagotta WN. A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron. 1995;14:177–183. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 10.Gordon SE, Zagotta WN. Localization of regions affecting an allosteric transition in cyclic nucleotide-activated channels. Neuron. 1995;14:857–864. doi: 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 11.Brown RL, Snow SD, Haley TL. Movement of gating machinery during the activation of rod cyclic nucleotide-gated channels. Biophys J. 1998;75:825–833. doi: 10.1016/s0006-3495(98)77571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong X, Zucker H, Hofmann F, Biel M. Three amino acids in the C-linker are major determinants of gating in cyclic nucleotide-gated channels. EMBO J. 1998;17:353–362. doi: 10.1093/emboj/17.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Olivier NB, Yao H, Young EC, Siegelbaum SA. A conserved tripeptide in CNG and HCN channels regulates ligand gating by controlling C-terminal oligomerization. Neuron. 2004;44:823–834. doi: 10.1016/j.neuron.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti P, Young EC, Siegelbaum SA. C-Linker of cyclic nucleotide-gated channels controls coupling of ligand binding to channel gating. J Gen Physiol. 1999;113:17–34. doi: 10.1085/jgp.113.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 16.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 17.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, et al. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350:aad2395. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 19.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu H, et al. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 1999;821:160–168. doi: 10.1016/s0006-8993(99)01111-7. [DOI] [PubMed] [Google Scholar]
- 23.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 24.Arcangeletti M, Marchesi A, Mazzolini M, Torre V. Multiple mechanisms underlying rectification in retinal cyclic nucleotide-gated (CNGA1) channels. Physiol Rep. 2013;1:e00148. doi: 10.1002/phy2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn GE, Zagotta WN. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 2001;30:689–698. doi: 10.1016/s0896-6273(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 26.Contreras JE, Holmgren M. Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide-gated channels: implications for the location of the gate. J Gen Physiol. 2006;127:481–494. doi: 10.1085/jgp.200509440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras JE, Srikumar D, Holmgren M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2008;105:3310–3314. doi: 10.1073/pnas.0709809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulding EH, Tibbs GR, Liu D, Siegelbaum SA. Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature. 1993;364:61–64. doi: 10.1038/364061a0. [DOI] [PubMed] [Google Scholar]
- 29.Eismann E, Muller F, Heinemann SH, Kaupp UB. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci U S A. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Root MJ, MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993;11:459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- 31.Morrill JA, MacKinnon R. Isolation of a single carboxyl-carboxylate proton binding site in the pore of a cyclic nucleotide-gated channel. J Gen Physiol. 1999;114:71–83. doi: 10.1085/jgp.114.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derebe MG, et al. Tuning the ion selectivity of tetrameric cation channels by changing the number of ion binding sites. Proc Natl Acad Sci U S A. 2011;108:598–602. doi: 10.1073/pnas.1013636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derebe MG, Zeng W, Li Y, Alam A, Jiang Y. Structural studies of ion permeation and Ca2+ blockage of a bacterial channel mimicking the cyclic nucleotide-gated channel pore. Proc Natl Acad Sci U S A. 2011;108:592–597. doi: 10.1073/pnas.1013643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napolitano LM, et al. A structural, functional, and computational analysis suggests pore flexibility as the base for the poor selectivity of CNG channels. Proc Natl Acad Sci U S A. 2015;112:E3619–3628. doi: 10.1073/pnas.1503334112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 36.Zagotta WN, et al. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–205. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- 37.Saponaro A, et al. Structural basis for the mutual antagonism of cAMP and TRIP8b in regulating HCN channel function. Proc Natl Acad Sci U S A. 2014;111:14577–14582. doi: 10.1073/pnas.1410389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schunke S, Stoldt M. Structural snapshot of cyclic nucleotide binding domains from cyclic nucleotide-sensitive ion channels. Biol Chem. 2013;394:1439–1451. doi: 10.1515/hsz-2013-0228. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Vysotskaya ZV, Liu Q, Zhou L. Structural basis for the cAMP-dependent gating in the human HCN4 channel. J Biol Chem. 2010;285:37082–37091. doi: 10.1074/jbc.M110.152033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lolicato M, et al. Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2011;286:44811–44820. doi: 10.1074/jbc.M111.297606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taraska JW, Puljung MC, Olivier NB, Flynn GE, Zagotta WN. Mapping the structure and conformational movements of proteins with transition metal ion FRET. Nat Methods. 2009;6:532–537. doi: 10.1038/nmeth.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altieri SL, et al. Structural and energetic analysis of activation by a cyclic nucleotide binding domain. J Mol Biol. 2008;381:655–669. doi: 10.1016/j.jmb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puljung MC, Zagotta WN. A secondary structural transition in the C-helix promotes gating of cyclic nucleotide-regulated ion channels. J Biol Chem. 2013;288:12944–12956. doi: 10.1074/jbc.M113.464123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puljung MC, DeBerg HA, Zagotta WN, Stoll S. Double electron-electron resonance reveals cAMP-induced conformational change in HCN channels. Proc Natl Acad Sci U S A. 2014;111:9816–9821. doi: 10.1073/pnas.1405371111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou L, Siegelbaum SA. Gating of HCN channels by cyclic nucleotides: residue contacts that underlie ligand binding, selectivity, and efficacy. Structure. 2007;15:655–670. doi: 10.1016/j.str.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair AV, Nguyen CH, Mazzolini M. Conformational rearrangements in the S6 domain and C-linker during gating in CNGA1 channels. Eur Biophys J. 2009;38:993–1002. doi: 10.1007/s00249-009-0491-4. [DOI] [PubMed] [Google Scholar]
- 47.Saotome K, Singh AK, Yelshanskaya MV, Sobolevsky AI. Crystal structure of the epithelial calcium channel TRPV6. Nature. 2016;534:506–511. doi: 10.1038/nature17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whicher JR, MacKinnon R. Structure of the voltage-gated K(+) channel Eag1 reveals an alternative voltage sensing mechanism. Science. 2016;353:664–669. doi: 10.1126/science.aaf8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Zheng S, Agard DA, Cheng Y. Asynchronous data acquisition and on-the-fly analysis of dose fractionated cryoEM images by UCSFImage. J Struct Biol. 2015;192:174–178. doi: 10.1016/j.jsb.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142:334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 52.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 53.Scheres SH. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amunts A, et al. Structure of the yeast mitochondrial large ribosomal subunit. Science. 2014;343:1485–1489. doi: 10.1126/science.1249410. [DOI] [PMC free article] [PubMed] [Google Scholar]


