Abstract
Purpose
To evaluate the expression of 19 angiogenic biomarkers in the aqueous humor before and after intravitreal bevacizumab injection (IVB) in eyes with neovascular age-related macular degeneration (AMD).
Design
Prospective, noncomparative, interventional case series.
Participants
Twenty-three eyes of 23 treatment-naïve patients with choroidal neovascularization (CNV) secondary to neovascular AMD.
Methods
Eyes were diagnosed with CNV secondary to neovascular AMD and were treated with 3 monthly IVBs. Aqueous humor samples were obtained by anterior chamber paracentesis at baseline and immediately before each intravitreal bevacizumab injection.
Main Outcome Measures
Aqueous humor levels of 19 angiogenic biomarkers (angiopoietin 2, bone morphogenetic protein 9 [BMP-9], epidermal growth factor [EGF], endoglin, endothelin 1, fibroblast growth factor [FGF]-1 and FGF-2, follistatin, granulocyte colony-stimulating factor [GCSF], heparin-binding EGF-like growth factor [HB-EGF], hepatocyte growth factor [HGF], interleukin 8, leptin, placental growth factor [PLGF], vascular endothelial growth factor [VEGF]-A, VEGF-C, VEGF-D, and tissue inhibitor of metalloproteinases [TIMP]-1 and TIMP-2) were measured. Best-corrected visual acuity (BCVA), spectral-domain OCT parameters, and intraocular pressure also were evaluated.
Results
Baseline aqueous VEGF-A expression was elevated in all study eyes before treatment initiation. A statistically significant decrease of VEGF-A was observed at the 1- and 2-month follow-ups. A statistically significant increased concentration was observed in 7 biomarkers: VEGF-C, angiopoietin 2, endothelin 1, follistatin, HB-EGF, HGF, and interleukin 8. The other 11 study biomarker levels (VEGF-D, BMP-9, EGF, endoglin, FGF-1, FGF-2, GCSF, leptin, PLGF, TIMP-1, and TIMP-2) did not show any significant difference during follow-up. The BCVA statistically improved significantly at 2 months. Spectral-domain OCT parameters improved significantly at all follow-ups. Mean intraocular pressure values were not statistically different during the study period.
Conclusions
Despite a decrease in VEGF-A, the aqueous levels of VEGF-C, angiopoietin 2, endothelin 1, follistatin, HB-EGF, HGF, and interleukin 8 increased significantly after intravitreal injection of bevacizumab. These upregulated angiogenic biomarkers may represent new therapeutic targets in exudative AMD.
Angiogenesis is a fundamental physiologic process characterized by the development of new vessels from preexisting blood vessels and involves the stimulation of angiogenic growth factor receptors on vascular endothelial cells, proteolytic breakdown of the endothelial cell basal membrane, and endothelial cell proliferation and migration.1 The vasculogenesis process is controlled by a dynamic balance between positive angiogenesis regulator factors (vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF], interleukin 8, transforming growth factors α and β, connective tissue growth factor, fibroblast growth factor [FGF], and angiopoietin) and negative angiogenesis regulator factors (pigment epithelium–derived factor, endostatin, and vasostatin). Angiogenesis occurs in the setting of imbalance of such factors that leads to overactivity of the proangiogenic cytokines.2
Neovascular age-related macular degeneration (AMD) is the main cause of severe visual loss among individuals older than 55 years in developed countries, and inhibition of angiogenesis is pivotal to the prevention and treatment of choroidal neovascularization (CNV) associated with neovascular AMD.3 Vascular endothelial growth factor is a potent cytokine modulator of angiogenesis, promotes the growth of both retinal and choroidal new vessels, and is considered critical for CNV development in neovascular AMD eyes.4 The establishment of VEGF as an important regulator of angiogenesis in AMD revolutionized the field by stimulating the development of anti-VEGF agents that inhibit angiogenesis. Bevacizumab, ranibizumab, and aflibercept are commercially available immunoglobulin antibodies that bind and inhibit the biological activity of VEGF isoforms, and their intravitreal use has been associated with visual acuity improvement in neovascular AMD.5
Despite the success of anti-VEGF therapy, an incomplete response to anti-VEGF agents is observed in many neovascular AMD patients. Therefore, other biomarkers may be implicated in the pathogenesis of CNV resulting from AMD. The purpose of this study was to measure the expression of 19 vasogenic biomarkers, including VEGF, in the aqueous humor after 2 monthly intravitreal bevacizumab injections (IVBs) in treatment-naïve eyes with neovascular AMD. Additionally, mean vasogenic biomarkers levels, best-corrected visual acuity (BCVA), and spectral-domain (SD) OCT parameters were compared. We hypothesized that alternative biomarker pathways may maintain angiogenic stimulus in eyes with neovascular AMD despite anti-VEGF blockage with bevacizumab intravitreal injection.
Methods
In this prospective study, the aqueous levels of 19 angiogenic biomarkers were measured in eyes with neovascular AMD treated with IVB at the Retina Department of Public Service Hospital of São Paulo (IAMSPE), São Paulo, Brazil. The institutional review board of the Federal University of São Paulo (reference number, 215195) and the Public Service Hospital of São Paulo (reference number, 0115/10) approved the off-label use of bevacizumab and collection of aqueous humor in the current study. All patients provided informed consent before treatment.
Participants
Aqueous humor samples were obtained before each IVB from 23 eyes of 23 consecutive patients (10 men, 13 women; mean age±standard deviation, 76.4±9.4 years) with active CNV resulting from neovascular AMD. All study patients underwent 3 monthly IVBs (1.25 mg/0.05 ml; Avastin; Genentech, South San Francisco, CA). Active CNV resulting from AMD was confirmed by fluorescein angiography and SD OCT. None of the study participants had been treated previously for neovascular AMD.
Ophthalmologic Examination
Patients underwent a comprehensive ophthalmologic examination (at baseline and 1 and 2 months after IVB) that included BCVA, biomicroscopic examination, and intraocular pressure measurement. Color fundus photography and fluorescein angiography were performed with a Topcon TRC-50IA fundus camera (Tokyo Optical Co. Ltd, Tokyo, Japan).
Three OCT parameters (central retinal thickness [CRT], macular volume [MV], and macular thickness [MT]) were measured using the SD OCT Cirrus 4000 (Carl Zeiss Meditec, Dublin, CA) at the baseline, 1-month, and 2-month follow-up examinations. Central retinal thickness, MV, and MT were calculated after acquiring a sequence of 128 horizontal sections recorded in the high-resolution mode (27 000 A-scans per second). Macular cube 512 × 128 and 5-line raster scans were performed.
Sample Collection
Aqueous humor samples (volume, 0.1 ml) were obtained from the anterior chamber using a 30-gauge needle paracentesis before each IVB (at baseline and 1 and 2 months after IVB, but before the third injection). Topical anesthesia was induced before the injection with instillation of tetracaine 1% eye drops. Povidon–eiodine was applied to the eyelid margins, and a lid speculum was inserted after application of a sterile drape. The IVBs were performed via the pars plana using a 30-gauge needle. Undiluted aqueous humor (0.1 ml) was collected in sterile tubes and stored immediately at −80° C until analysis.
Measurement of Biomarkers
The concentrations of 19 vasogenic biomarkers (angiopoietin 2, bone morphogenetic protein 9 [BMP-9], epidermal growth factor [EGF], endoglin, endothelin 1, FGF-1, FGF-2, follistatin, granulocyte colony-stimulating factor [GCSF], heparin-binding EGF-like growth factor [HB-EGF], HGF, interleukin 8, leptin, placental growth factor [PLGF], VEGF-A, VEGF-C, VEGF-D, and tissue inhibitor of metalloproteinases [TIMP] 1 and TIMP-2) were measured in the aqueous humor using an enzyme-linked immunometric assay (Luminex Merk Millipore; Merck KGaA, Darmstadt, Germany).
The assay manufacturer reported minimum detectable doses of 0.50, 0.080, 0.13, 0.74, 0.56, 3.17, 7.61, 0.15, 2.46, 0.13, 4.37, 0.085, 14.37, 0.068, 0.81, 0.77, 0.37, 7.64, and 20.66 pg/ml for angiopoietin 2, BMP-9, EGF, endoglin, endothelin 1, FGF-1, FGF-2, follistatin, GCSF, HB-EGF, HGF, interleukin 8, leptin, PLGF, VEGF-A, VEGF-C, VEGF-D, TIMP-1, and TIMP-2, respectively. The minimum detectable dose was confirmed by adding 2 standard deviations to the mean optical density value of standard replicates and calculating the corresponding concentration. Standard curves for each biomarker level were generated using the reference standard supplied with the kit.
Statistical Analysis
Data were analyzed using commercially available R software (R Development Core Team, 2016; https://www.r-project.org/), version 3.2.2. Linear mixed models with a random intercept for subject were used to compare the mean vasogenic biomarkers levels, BCVA, and SD OCT measurements at each time point (baseline and 1-month and 2-month follow-ups), as illustrated in Figure 1 and Table 1. We treated time as a categorical variable, and thus estimated means for the outcomes separately at each time point. P values less than 0.05 were considered statistically significant.
Figure 1.
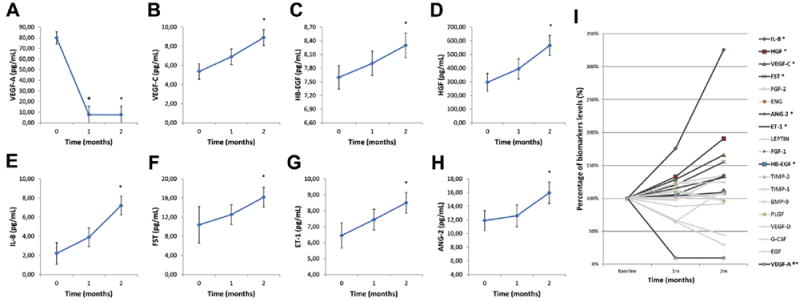
A–H, Graphs showing angiogenesis-related biomarker aqueous humor levels (in picograms per milliliter) in patients with neovascular age-related macular degeneration (AMD) before (0, 1, and 2 months) each bevacizumab intravitreal injection. A, Vascular endothelial growth factor (VEGF)-A concentration was reduced statistically significantly at the first and second months when compared with baseline. B–H, Seven of 19 studied biomarkers showed a statistically significant increase in their levels at the second month when compared with baseline. I, Percentage variation of studied angiogenesis-related biomarker aqueous humor levels in patients with neovascular AMD before (0, 1, and 2 months) each bevacizumab intravitreal injection. Baseline (month 0) levels were considered as 100%. Biomarkers listed in the legend at the right of panel I were ordered according to final concentration. Dark gray lines correspond to statistically significant biomarkers (VEGF-A was reduced significantly at the first and second months, whereas interleukin 8 [IL-8], hepatocyte growth factor [HGF], VEGF-C, follistatin [FST], angiopoietin 2 [ANG-2], endothelin 1 [ET-1], and heparin-binding epidermal growth factor–like growth factor [HB-EGF] concentrations increased significantly at the second month when compared with baseline), whereas light gray lines correspond to those without statistical significance (VEGF-D, bone morphogenetic protein 9 [BMP-9], epidermal growth factor [EGF], endoglin [ENG], fibroblast growth factor [FGF]-1, FGF-2, granulocyte colony-stimulating factor [GCSF], leptin, placental growth factor [PLGF], tissue inhibitor of metalloproteinases [TIMP]-1, and TIMP-2). P < 0.05 when baseline aqueous humor values were compared with 1-month (Φ) and 2-month (*) values.
Table 1.
Intraocular Pressure, Visual Acuity, and Spectral-Domain OCT Retinal Thickness Parameters
| Feature | Baseline | 1 Month | 2 Months |
|---|---|---|---|
| IOP | 17.17±0.56 | 17.83±0.57 | 17.83±0.57 |
| BCVA | 0.12±0.04 | 0.15±0.03 | 0.22±0.03* |
| CRT | 376.00±20.63 | 273.78±21.28* | 255.17±21.28* |
| MV | 10.77±0.33 | 10.12±0.28† | 9.69±.28* |
| MT | 303.39±9.58 | 280.26±9.72† | 275.17±9.73* |
BCVA = best-corrected visual acuity (decimal values); CRT = central retina thickness; IOP = intraocular pressure; MT = macular thickness; MV = macular volume.
Values are mean±standard error.
P < 0.01 compared with baseline.
P < 0.05 compared with baseline.
Pathway Analysis
A proteine–protein interaction network associated with the 8 statistically significant angiogenic biomarkers (VEGF-A, VEGF-C, angiopoietin 2, endothelin 1, follistatin, HB-EGF, HGF, and interleukin 8) was created using Qiagen’s Ingenuity Pathway Analysis (Qiagen, Redwood City, CA). Ingenuity Pathway Analysis is a commercial software package that makes use of computational algorithms and statistical tests to identify the most highly connected and significant protein interactions. Figure 2 was adapted from Ingenuity Pathway Analysis results.
Figure 2.
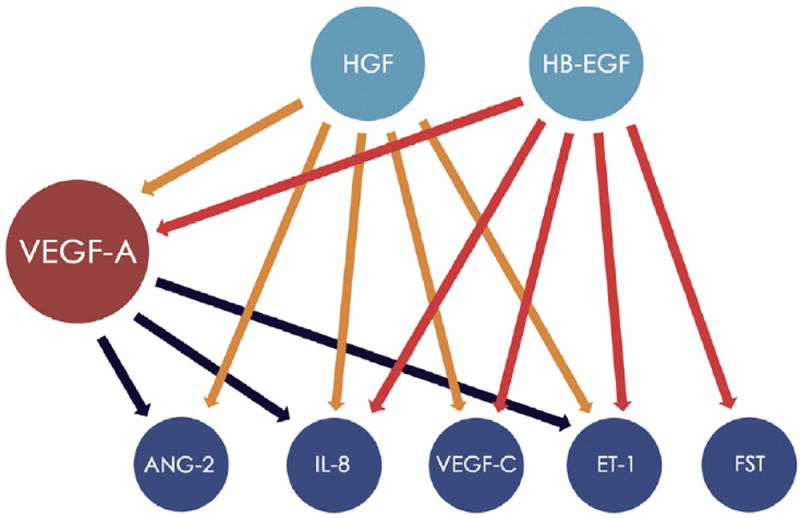
Diagram showing the protein–protein interaction network of statistically significant biomarkers investigated in the present study. Each circle represents a significant pathway, and each arrow symbolizes a pathway crosstalk between proteins. Orange and red arrows represent, respectively, the influence of hepatocyte growth factor (HGF) and heparin-binding epidermal growth factor–like growth factor (HB-EGF; shown as light blue circles) on 5 angiogenic factors (dark blue circles) and on the vascular endothelial growth factor (VEGF)-A pathway (red circle). It is important to highlight that HGF does not present significant connection with follistatin (FST), nor does epidermal growth factor (EGF) with angiopoietin 2 (ANG-2). Black arrows represent the influence of VEGF-A on 3 protein pathways (ANG-2, interleukin 8 [IL-8], and endothelin 1 [ET-1]). The VEGF-A blockage represents the upregulation of the 7 represented biomarkers directly (HGF and EGF) and indirectly (ANG-2, IL-8, VEGF-C, ET-1, and FST). The network was adapted from Qiagen’s Ingenuity Pathway Analysis (Qiagen, Redwood City, CA), generated in October 2016.
Results
In total, 69 anterior chamber biopsies were collected from 23 eyes, and there were no complications, such as uveitis, lens opacification, or endophthalmitis. At baseline, before the first intravitreal injection of bevacizumab, the VEGF-A concentration was increased in all patients, and a significant decrease in its levels was observed at the 1- and 2-month follow-ups (Fig 1A). The mean±standard deviation aqueous concentration of VEGF-A was 79.91±48.17 pg/ml at baseline and was reduced significantly to 7.66±3.52 pg/ml at 1 month and 7.62±3.42 pg/ml at 2 months after injection (P < 0.0001; Fig 1A).
Interestingly, 7 vasogenic biomarkers (VEGF-C, angiopoietin 2, endothelin 1, follistatin, HB-EGF, HGF, and interleukin 8) showed a statistically significant increase after intravitreal injection of bevacizumab (Fig 1B–H). Compared with baseline, the percentage of VEGF-A levels reduced to 9.59% at 1 month and to 9.54% at 2 months after injection. As previously described, statistically significant concentrations of other biomarkers (interleukin 8, HGF, VEGF-C, follistatin, angiopoietin 2, endothelin 1, and HB-EGF) were observed only at 2 months of follow-up. At this follow-up point, their percentages increased to 325.74% (interleukin 8), 190.76% (HGF), 165.93% (VEGF-C), 155.50% (follistatin), 134.10% (angiopoietin 2), 131.76% (endothelin 1), and 109.24% (HB-EGF) when compared with baseline. The percentage variation of the levels of all the biomarkers is shown in Figure 1I.
We also determined a protein–protein interaction network that allowed the comparison between our findings and those of the current literature. Ingenuity Pathway Analysis results suggested that HGF and HB-EGF are important regulatory proteins of VEGF-A,6 interleukin 8,7 VEGF-C,7 and endothelin 1.8 Also, HGF influences angiopoietin 2,9 because HB-EGF influences the follistatin pathway.10 Thus, VEGF-A blockage with bevacizumab may lead to a counterbalance mechanism that enhances HGF and HB-EGF pathways.11 Consequently, angiopoietin 2,12 interleukin 8,13 VEGF-C,14 endothelin 1,15 and follistatin16 were upregulated and angiogenesis stimuli persist through an alternative route to the VEGF-A17 (Fig 2).
The mean BCVA±standard deviation of study patients improved at all follow-up periods. However, it was statistically significant only at 2 months of follow-up. In SD OCT measurements, decreases in CRT, MV, and MT were observed 1 and 2 months after the IVB. In comparison with baseline, CRT, MV, and MT showed the largest reduction at 2 months (P < 0.01, linear mixed models). Intraocular pressure was not altered significantly during the study period (Table 1). There was no case of worsening visual acuity. However, despite improvement on OCT and of VEGF-A levels, the BCVA of 7 patients did not change. This finding was not associated with any statistically significant biomarker (data not shown).
Discussion
Approximately 30 years ago, angiogenesis was first proposed to have a key role in the survival of cancer cells and in local tumor growth.18 In this setting, the development of new blood vessels is critical for affording appropriate oxygen and nutrient amounts to the growing tumor tissue. Such blood vessel formation within the neoplastic mass is controlled by the production of various growth factors and growth inhibitors.19 Similar to tumor growth, the formation of new blood vessels in neovascular AMD is related to pathologic circumstances, such as ischemia, hypoxia, or inflammation, that may influence the balance in favor of proangiogenic factors and the formation of new vessels (i.e., CNV). Consequently, inhibition of angiogenesis by blockage of proangiogenic factors has proven essential for the prevention and treatment of neovascular AMD.20
Among several growth factors associated with angiogenesis, VEGF is considered the most critical regulator of ocular angiogenesis, and increased production of VEGF is thought to be the main driver of CNV development in patients with neovascular AMD.21 Intraocular injections of anti-VEGF agents provide benefit by reducing CNV and considerably improving visual acuity in most AMD patients.4 However, in patients with recalcitrant CNV and decreased visual acuity, despite aggressive treatment with anti-VEGF agents, we believe other vascular mediators contribute to CNV. Therefore, alternative angiogenic factors and pathways have been proposed to explain the failure of anti-VEGF therapy in AMD patients.22
Currently, there is no consensus regarding the optimal model to measure biomarkers in the eye, and several studies have shown that vitreous levels of secreted cytokines have been used as a substitute for retinal levels. For example, levels of VEGF in the retina and choroid are supposed to correspond to VEGF expression in the vitreous humor.23 Nonetheless, vitreous biopsies are risky and may cause adverse effects such as vitreous hemorrhage, endophthalmitis, lens opacity, and retinal detachment. Conversely, samples of aqueous humor are safer and easier to collect, and equivalent levels of VEGF have been reported in both aqueous and vitreous humors.24
In this study, a broad spectrum of related angiogenic and inflammatory markers was measured in the aqueous humor at baseline and before monthly intravitreal injections of bevacizumab in eyes with neovascular AMD. As expected, the decrease of VEGF-A concentration in the aqueous humor was observed after IVB in all study eyes. Concurrently, with the reduction of VEGF-A expression, there was a statistically significant increase in the expression of 7 vasogenic biomarkers (VEGF-C, angiopoietin 2, endothelin 1, follistatin, HB-EGF, HGF, and interleukin 8). The other 11 vasogenic biomarkers studied (VEGF-D, BMP-9, EGF, endoglin, FGF-1, FGF-2, GCSF, leptin, PLGF, TIMP-1, and TIMP-2) did not show statistically significant differences in their levels after treatment with bevacizumab. Although percentage variation does not allow us to infer which of the factors may have greater significance in maintaining the proangiogenic stimuli, it may be helpful to drive future research. As shown in Figure 1I, interleukin 8, HGF, VEGF-C, and follistatin showed the highest increase (more than 50% from baseline) among the 7 statistically significant biomarkers. Therefore, investigating their angiogenic stimulus strength may allow researchers to direct their efforts toward what may be considered the most important angiogenesis-related alternative targets.
The increased aqueous levels of VEGF-C, HGF, angiopoietin 2, endothelin 1, HB-EGF, follistatin, and interleukin 8 after anti-VEGF intravitreal injections observed in the present study may be a consequence of VEGF-A blockage resulting in ischemic injury and upregulation of other angiogenic factors. Vascular endothelial growth factor C belongs to the growth factors related to platelet-derived growth factor, and strong VEGF-C staining in most pigment-containing cells in all CNV specimens has been described.25 Hepatocyte growth factor contributes to blood vessel formation, and several studies have suggested an important role for HGF in choroidal and retinal angiogenesis and its association with proliferative diseases.26 Angiopoietin 2 is upregulated by hypoxia and has been detected in surgically removed human CNV membranes.27 Single and combined inhibition of angiopoietin 2 and VEGF-A are showing promising results in the management of ocular angiogenesis.28 Endothelin 1 is considered a potent endogenous vasoconstrictor in small vessels, has an important regulatory role for retinal blood flow, and also may induce angiogenesis.15 Heparin-binding EGF-like growth factor is a potent stimulator of cell proliferation and migration, promoting angiogenesis in processes such as wound healing and recovery from injury.29 Follistatin is a regulator of angiogenin, an important protein involved in inflammatory processes, and some studies have reported high levels of angiogenin in the eyes of patients with AMD.30 Interleukin 8 is a proinflammatory cytokine related to ocular angiogenesis and to increased vascular permeability.31 In an investigation of biomarkers related to cancer resistance to bevacizumab, Hayashi et al32 detected higher serum concentrations of interleukin 8, angiopoietin 2, and VEGF-C in nonresponders compared with responders to bevacizumab.
Clinical outcomes also were evaluated, and our results demonstrated that the improvement of BCVA was statistically significant at the 2-month follow-up. The study SD OCT parameters (CRT, MV, and MT) improved significantly at all follow-up periods when compared with baseline. These findings corroborate previous studies that demonstrated a reduction in SD OCT parameters before BCVA improvement in neovascular AMD eyes treated with intravitreal injections of anti-VEGF.33
Neovascular AMD is a heterogeneous disease with multiple genetic and environmental risks, and it is unlikely that only one signaling pathway is driving the pathologic behavior of all CNV lesions. Although the anti-VEGF treatment of neovascular AMD has a favorable effect on disease progression, the anti-VEGF treatment alone seems to be insufficient to treat all neovascular lesions. One explanation may be that there are other important angiogenic factors involved in AMD that are not addressed by the anti-VEGF agents. Also, it is possible that a downregulation of VEGF may lead to an upregulation of other angiogenic pathways. Our data suggest that several other angiogenic growth factors may act independently or in combination with VEGF-A, and suppression or downregulation of these biomarkers may provide a new therapeutic approach for neovascular AMD. We hypothesize that, as a compensatory mechanism, the upregulation of VEGF-C, HGF, angiopoietin 2, endothelin 1, HB-EGF, follistatin, and interleukin 8 may sustain the neovascularization stimuli in neovascular AMD and may be responsible for the persistent and recurrent CNV after anti-VEGF therapy, similar to previous reports in cancer disease.34 The current study had some limitations, for instance, its small sample size and the short follow-up period. Furthermore, the neovascular disease activity was not correlated with the aqueous biomarker measurements. A correlation between VEGF expression and that of other biomarkers with morphologic changes of the retina, such as fluid and blood on OCT, should be assessed better in a future study. Exosomal proteins also are promising biomarkers of ocular neovascularization35; however, they were not evaluated in the present study. Our research provides evidence that suggests the participation of VEGF-C, HGF, angiopoietin 2, endothelin 1, HB-EGF, follistatin, and interleukin 8 in alternative pathways and supports the need for future, more complex study designs with larger samples to clarify the importance of these biomarkers in maintaining the angiogenic stimulus despite VEGF-A blockage. In addition, studies with different angiogenic factor inhibitors, such as aflibercept, which binds to PLGF and VEGF-B, will help to understand better the pathophysiologic features of wet AMD and the impact of combined treatment in the management of CNV.
Our findings suggest a correlative relationship between VEGF and 7 other biomarkers in the eye with regard to its expression, which may have therapeutic significance. There are several alternative antiangiogenic ocular drugs under development. Fovista (E10030; Ophthotech, New York, NY),36 CrossMAb (RG7716; Roche, Basel, Switzerland),28 Conbercept (KH902; Chengdu Kanghong Biotech Co, Ltd, Sichuan, China),37 Pazopanib (GW786034; GlaxoSmithKline, Research Triangle Park, NC),38 AdPEDF (GenVec, Inc, Gaithersburg, MD),39 and many others are showing interesting outcomes. In the future, we likely will have a combination of angiogenesis inhibitors, or a more effective one, that concurrently addresses various vasogenic mediators and pathways. Therefore, the dysregulation of these angiogenic biomarker levels may be potential targets for CNV secondary to AMD.
Acknowledgments
Supported by the National Institutes of Health, Bethesda, Maryland (grant nos.: K08EY020530, R01EY024665, R01EY025225, R01EY024698, and R21AG050437 [V.B.M.]).
Abbreviations and Acronyms
- AMD
age-related macular degeneration
- BCVA
best-corrected visual acuity
- BMP-9
bone morphogenetic protein 9
- CNV
choroidal neovascularization
- CRT
central retinal thickness
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GCSF
granulocyte colony-stimulating factor
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- HGF
hepatocyte growth factor
- IVB
intravitreal bevacizumab injection
- MT
macular thickness
- MV
macular volume
- PLGF
placental growth factor
- SD
spectral-domain
- TIMP
tissue inhibitor of metalloproteinases
- VEGF
vascular endothelial growth factor
Footnotes
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author Contributions:
Conception and design: Cabral, Polido, Oshima, Serracarbassa, Regatieri, Belfort Jr.
Analysis and interpretation: Cabral, Lima, Mello, Duong, Regatieri, Mahajan, Belfort Jr.
Data collection: Cabral, Polido, Correa
Overall responsibility: Cabral, Lima, Mello, Mahajan, Belfort Jr.
Human Subjects: This study includes human subject/tissues. Study protocol was approved by IRB. Informed consent was obtained from all human subjects. All tenets of the Declaration of Helsinki were followed.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 3.Group EDPR. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Dong G, Lee TL, Yeh NT, et al. Metastatic squamous cell carcinoma cells that overexpress c-Met exhibit enhanced angiogenesis factor expression, scattering and metastasis in response to hepatocyte growth factor. Oncogene. 2004;23:6199–6208. doi: 10.1038/sj.onc.1207851. [DOI] [PubMed] [Google Scholar]
- 7.Enholm B, Paavonen K, Ristimaki A, et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh HL, Lin CC, Chan HJ, et al. c-Src-dependent EGF receptor transactivation contributes to ET-1-induced COX-2 expression in brain microvascular endothelial cells. J Neuroinflam. 2012;9:152. doi: 10.1186/1742-2094-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Yang N, Park JW, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–3412. [PubMed] [Google Scholar]
- 10.Wankell M, Kaesler S, Zhang YQ, et al. The activin binding proteins follistatin and follistatin-related protein are differentially regulated in vitro and during cutaneous wound repair. J Endocrinol. 2001;171:385–395. doi: 10.1677/joe.0.1710385. [DOI] [PubMed] [Google Scholar]
- 11.Cascone T, Herynk MH, Xu L, et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 2011;121:1313–1328. doi: 10.1172/JCI42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labussiere M, Cheneau C, Prahst C, et al. Angiopoietin-2 may be involved in the resistance to bevacizumab in recurrent glioblastoma. Cancer Invest. 2016;34:39–44. doi: 10.3109/07357907.2015.1088948. [DOI] [PubMed] [Google Scholar]
- 13.Jeon S, Lee WK. Effect of intravitreal triamcinolone in diabetic macular edema unresponsive to intravitreal bevacizumab. Retina. 2014;34:1606–1611. doi: 10.1097/IAE.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Xie K, Ding G, et al. Tumor resistance to anti-VEGF therapy through up-regulation of VEGF-C expression. Cancer Lett. 2014;346:45–52. doi: 10.1016/j.canlet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol. 2016;310:R230–R234. doi: 10.1152/ajpregu.00373.2015. [DOI] [PubMed] [Google Scholar]
- 16.Gokmen-Polar Y, Goswami CP, Toroni RA, et al. Gene expression analysis reveals distinct pathways of resistance to bevacizumab in xenograft models of human ER-positive breast cancer. J Cancer. 2014;5:633–645. doi: 10.7150/jca.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano S, Pages G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie. 2013;95:1110–1119. doi: 10.1016/j.biochi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 19.Klagsbrun M, D’Amore PA. Regulators of angiogenesis. Ann Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- 20.Kvanta A. Ocular angiogenesis: the role of growth factors. Acta Ophthalmol Scand. 2006;84:282–288. doi: 10.1111/j.1600-0420.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 21.Bressler SB. Introduction: understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116:S1–S7. doi: 10.1016/j.ophtha.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira Dias JR, Rodrigues EB, Maia M, et al. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95:1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 23.Wells JA, Murthy R, Chibber R, et al. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996;80:363–366. doi: 10.1136/bjo.80.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 25.Otani A, Takagi H, Oh H, et al. Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc Res. 2002;64:162–169. doi: 10.1006/mvre.2002.2407. [DOI] [PubMed] [Google Scholar]
- 26.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Park DY, Park DY, et al. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Invest Ophthalmol Vis Sci. 2014;55:2191–2199. doi: 10.1167/iovs.14-13897. [DOI] [PubMed] [Google Scholar]
- 28.Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8:1265–1288. doi: 10.15252/emmm.201505889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollborn M, Iandiev I, Seifert M, et al. Expression of HB-EGF by retinal pigment epithelial cells in vitreoretinal proliferative disease. Curr Eye Res. 2006;31:863–874. doi: 10.1080/02713680600888807. [DOI] [PubMed] [Google Scholar]
- 30.Agawa T, Usui Y, Wakabayashi Y, et al. Profile of intraocular immune mediators in patients with age-related macular degeneration and the effect of intravitreal bevacizumab injection. Retina. 2014;34:1811–1818. doi: 10.1097/IAE.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 31.Hautamaki A, Kivioja J, Vavuli S, et al. Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. 2013;33:1815–1827. doi: 10.1097/IAE.0b013e318285cf92. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi H, Arao T, Matsumoto K, et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget. 2014;5:2588–2595. doi: 10.18632/oncotarget.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 34.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang G-Y, Bang JY, Choi AJ, et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J Proteome Res. 2014;13:581–595. doi: 10.1021/pr400751k. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe GJ, Eliott D, Wells JA, et al. A phase 1 study of intravitreous E10030 in combination with ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2016;123:78–85. doi: 10.1016/j.ophtha.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TT, Guymer R. Conbercept (KH-902) for the treatment of neovascular age-related macular degeneration. Expert Rev Clin Pharmacol. 2015;8:541–548. doi: 10.1586/17512433.2015.1075879. [DOI] [PubMed] [Google Scholar]
- 38.Csaky KG, Dugel PU, Pierce AJ, et al. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age-related macular degeneration. Ophthalmology. 2015;122:579–588. doi: 10.1016/j.ophtha.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 39.Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Human Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]


