Abstract
Purpose
No previous clinical trial has been conducted for patients with neuroblastoma associated opsoclonus myoclonus ataxia syndrome (OMA), and current treatment is based on case reports. To evaluate the OMA response to prednisone and risk-adapted chemotherapy and determine if the addition of intravenous gammaglobulin (IVIG) further improves response, the Children’s Oncology Group designed a randomized therapeutic trial.
Patient and Methods
Eligible subjects were randomized to receive twelve cycles of IVIG (IVIG+) or no IVIG (NO-IVIG) in addition to prednisone and neuroblastoma risk-adapted chemotherapy. All low-risk patients were treated with cyclophosphamide. The severity of OMA symptoms was evaluated at 2, 6, and 12 months using a scale developed by Mitchell and Pike and baseline versus best response scores were compared. A single patient who did not undergo neurologic assessment was excluded from OMA response analysis. This study is registered with Clinical Trials.gov (identifier NCT00033293).
Results
Of the 53 patients enrolled in the study, 62% (33/53) were female. There were 44 low-risk, 7 intermediate-risk, and 2 high-risk neuroblastoma patients. Twenty-six subjects were randomized to receive IVIG+ and 27 were randomized to NO-IVIG. The neuroblastoma 3-year event-free survival (95% confidence interval (CI)) was 94.1% (87.3%, 100%) and overall survival was 98.0% (94.1%, 100%). Significantly higher rates of OMA response were observed in patients randomized to IVIG+ compared to NO-IVIG [21/26=80.8% for IVIG+; 11/27=40.7% for NO-IVIG (odds ratio=6.1; 95% CI: (1.5, 25.9), p=0.0029)]. For the majority of patients, the IVIG+ OMA regimen combined with cytoxan or other risk-based chemotherapy was well tolerated, although there was one toxic death in a high-risk subject.
Conclusion
This is the only randomized prospective therapeutic clinical trial in children with neuroblastoma-associated OMA. The addition of IVIG to prednisone and risk-adapted chemotherapy significantly improves OMA response rate. IVIG+ constitutes a back-bone upon which to build additional therapy.
Introduction
Opsoclonus myoclonus ataxia syndrome (OMA), also known as dancing eyes and dancing feet syndrome or Kingsbourne syndrome,(1) is a rare neurologic disorder that affects 2–3% of the > 650 children diagnosed with neuroblastoma every year in North America.(2) Symptoms include conjugate rapid eye movements; spontaneous muscle jerking which can affect the trunk, face and extremities; ataxia; personality changes including irritability and behavior disorders; and developmental regression. OMA also occurs in children and adults without the diagnosis of neuroblastoma and may be triggered by intercurrent infection, but in many subjects the triggering event is never identified.(3) The proportion of children with neuroblastoma-associated OMA varies according to the cohort analyzed.(1) In a retrospective study of patients treated at two large pediatric oncology programs and two large neurology centers in France, 22 (64%) of 34 children with OMA had associated neuroblastoma.(4) The majority of children with neuroblastoma-associated OMA have low-risk neuroblastoma and are cured of their neuroblastoma with surgery alone or surgery with moderate-dose chemotherapy.(3–6) However, the neurological sequelae of OMA are often severe and lifelong.(5;6)
Although the cause of OMA remains unknown, there is significant evidence that the disorder results from an autoimmune process. Serum autoantibodies against neuronal tissues have been identified in some patients with neuroblastoma-associated OMA.(7;8) Several groups have documented the presence of B-cells in the cerebrospinal fluid, increased B-cell activating factor in serum and cerebrospinal fluid, and other B-cell related cytokines and increased tumor infiltrating lymphocytes, both B- and T-cells.(9–12) However, the most compelling evidence for the autoimmune nature of this disorder is the clinical response to corticosteroids, intravenous gamma globuilin, rituximab, and/or other immunosuppressive therapy reported in single cases or small retrospective series. (1;13;14) Further, a retrospective analysis of 29 children with neuroblastoma and OMA from the Pediatric Oncology Group (POG) indicated that the immune suppression associated with chemotherapy may also be beneficial to patients with neuroblastoma-associated OMA.(6) All ten children in this series who received chemotherapy as part of their neuroblastoma treatment had resolution of their acute OMA symptoms and six had no long-term neurologic sequelae.
Because of the rarity of this condition, no previous prospective clnical trials have been conducted, and published retrospective series include only small numbers of patients. Thus, the expected OMA response rate to corticosteroids alone or combination immunosuppressive regimens is not known. Based on the promising responses to chemotherapy reported in the retrospective analysis of POG patients,(6) we hypothesized that immunosuppressive therapy with prednisone plus risk-adpated chemotherapy (with cyclophosphamide for low-risk patients) would alleviate the acute neurologic symptoms of OMA and also improve the long-term neurologic outcome. We further hypothesized that the addition of IVIG, an immune modulatory agent, would augment the neurologic recovery in these patients.(1) To test these hypotheses, the Children’s Oncology Group (COG) conducted a prospective randomized phase III clinical trial (ANBL00P3) for children with neuroblastoma-associated OMA, with a primary endpoint of OMA response.
Patients and Methods
Study Design
This trial was approved by the COG and made available to the more than 200 COG institutions. Ninety-two of these institutions opened the trial for enrolment. The study design is a randomized open label clinical trial. This is a standard approach for children with malignancies when the treatment is intravenous and it is impractical across a large cooperative group like COG and unethical to blind the investigators and expose children to an intravenous placebo arm.
Participants
Subjects ≤8 years of age with biopsy-proven, newly diagnosed neuroblastoma or ganglioneuroblastoma associated with OMA were eligible for the study. Any one of the neurologic components of OMA was sufficient for eligibility (Table 1). Patients with neuroblastoma diagnosed within 6 months of the development of OMA and patients with OMA diagnosed within six months of the diagnosis of neuroblastoma were eligible. Patients could not have received prior chemotherapy. Patients who received treatment with prednisone or adrenocorticotropic hormone (ACTH) for ≤14 days were eligible. Patients had to be registered on study no later than 4 weeks after they were deemed to be eligible for the study. Other requirements were that patients had to be free of any organ dysfunction or disorder that would preclude the use of corticosteroid therapy, cyclophosphamide or risk-adapted chemotherapy, and IVIG. Staging of disease was according to the International Neuroblastoma Staging System (INSS).(15) Tumor histopathology, MYCN status, and DNA index were determined in central COG laboratories. The COG Tracking Center assigned neuroblastoma risk-group classification, as described previously.(15) Before the start of therapy, institutional review board approval at participating sites was obtained. Written informed consent was obtained before enrollment onto ANBL00P3 and the neuroblastoma biology study, ANBL00B1.
Table 1.
Opsoclonus Myoclonus Syndrome severity scale, by Drs. Wendy G. Mitchell and Michael G. Pike (5)
|
Stance: 0: standing and sitting balance normal for age 1: mildly unstable standing for age, slightly wide-base 2: unable to stand without support but can sit without support 3: unable to sit without using hands to prop or other support |
|
Gait: 0: walking normal for age 1: mild wide-based gait for age but able to walk indoors and outdoors independently 2: walks only or predominantly with support from person or equipment 3: unable to walk even with support from person or equipment |
|
Arm and hand function: 0: normal for age 1: mild infrequent tremor or jerkiness without functional impairment 2: fine motor function (e.g. pincer grip of small objects, pencil use) persistently impaired for age but less precise manipulative tasks (e.g. playing with larger toys, feeding, dressing) normal or almost normal 3: major difficulty with all age-appropriate manipulative tasks |
|
Opsoclonus: 0: none 1: rare or only when elicited by change in fixation 2: frequent; interfering frequently with fixation and/or tracking 3: persistent; interfering continuously with fixation and tracking |
|
Mood/behavior 0: normal 1: mild increase in irritability but consolable and/or mild sleep disturbance but easily settled 2: irritability and sleep disturbance, interfering substantially with child and family life 3: persistent severe distress |
|
For all children aged 17 months or less, the
following abilities were considered when making the determination of the OMA
ratings: Able to hold head consistently erect when trunk vertical? Able to reach and grasp objects with each hand? Able to roll back to front and front to back? Able to finger-feed self? |
Randomization and masking
Subjects were randomized at the COG statistical office and institutions informed of the unmasked randomization assignment. Randomization was stratified according to the neuroblastoma clinical stage.
Procedures
Treatment
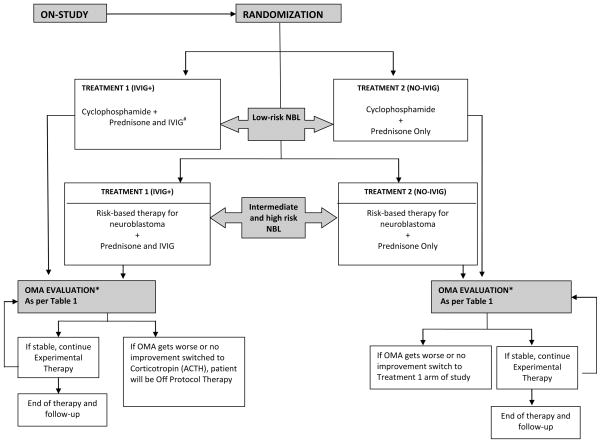
All patients with low-risk neuroblastoma received prednisone and cyclophosphamide and were randomly assigned to also receive: i) IVIG (IVIG+); or ii) no IVIG (NO-IVIG). Patients with intermediate- or high-risk disease were treated with prednisone and corresponding risk-adapted therapy regimens,(16;17) although three intermediate-risk patients received prednisone and cyclophosphamide. The intermediate and high-risk patients were also randomized to receive IVIG or no additional therapy (Figure 1). For low-risk patients, treatment cycles were repeated every 28 days. Prednisone was given at a dose of 2 mg/kg per day divided twice a day for a minimum of two cycles if a complete response was achieved at the two month evaluation. A slow taper of the steroid dose could then be started and titrated to the clinical response. Prednisone could be continued until achieving a response for as long as 18 months, with a slow taper to maintain maximal clinical response. Subjects on NO-IVIG regimen that failed to respond or progressed any time after completing the two month evaluation were allowed to cross-over to the IVIG+ regimen but considered treatment non-responders upon crossing over (see statistical section). Subjects on the IVIG+ who failed to respond or progressed any time after the two month evaluation were allowed to switch to ACTH therapy instead of prednisone but were considered treatment non-responders upon switching (see statistical section). Patients received IV cyclophosphamide at a dose of 25 mg/kg for children ≤20 kg or 750 mg/m2 for children >20 kg on day 0 of each cycle for 6 cycles. Patients randomized to receive IVIG received 1 gm/kg on day 0 and 1 of cycle one; day 0 of cycles 2 to 6; and then on day 0 of cycles 8, 10 and 12. Based on the review of the literature available at the time of study design, which consisted of case reports and small series, one year of therapy was frequently reported. We, thus, empirically chose one year of therapy for this trial. The protocol did not specify an immunization administration policy because it is a well-accepted practice in the pediatric oncology community to withhold immunization in this patient population.
Figure 1.
Study Design Schema for COG Study ANBL00P3
* OMA evaluation time points include: at Dx, monthly for 6 months, then every other month until 1 year, 18 months, 2 years and yearly thereafter two months, six months and one year.
Outcomes
OMA Response Evaluation
The OMA symptoms were evaluated using the previously published neurologic severity scale of Mitchell and Pike (Table 1).(5) Using this system we evaluated stance, gait, arm and hand function, opsoclonus, and mood/behavior at enrollment. Each symptom was scored as 0 (no symptoms); 1 (mild symptoms); 2 (moderate symptoms); or 3 (severe symptoms). The patient’s neurologic symptoms were evaluated and scored at two months, six months, and one year after enrollment for assessment of the primary endpoint of OMA response (Supplemental Table 1). Supplemental Table 2 provides the average and distribution of the total and subscale OMA severity scores at two, six and 12 months. Response was defined as the best score of the three evaluations points. For the secondary endpoint of OMA progression-free survival (OMA-PFS), subjects were followed at their local institutions and the status of OMA neurologic symptoms listed in Table 1 were reported annually for up to ten years. For each symptom, the OMA response was classified as complete response (CR), partial response (PR), no response (NR), or progressive/worsening of OMA (PD). CR was defined as improvement from baseline OMA to normal at two-months, six-months, or one-year without progression of neurologic symptoms; a PR was defined as improvement from baseline OMA at two-months, six-months, or one-year without progression; NR was defined as no change in neurologic symptoms from baseline at two-months, six-months, and one-year; and PD was defined as sustained worsening of neurologic symptoms from baseline at two-months, six months, or one-year in any symptom. For the purposes of the statistical efficacy rule, a patient was classified as an overall OMA “responder” if the combination of the symptom-specific responses were either a CR or a PR (Supplemental Table 1). Subjects were otherwise classified as an overall OMA “non-responder”. All patients who crossed over from NO-IVIG to IVIG+, or switched to ACTH at any time, were classified as overall OMA non-responders.
Neuroblastoma Response Evaluation
Eligible intermediate- and high-risk patients were evaluated for neuroblastoma response using criteria specified in the intermediate- or high-risk treatment protocols. Evaluation of tumor response for low-risk patients treated with cyclophosphamide was conducted according to the guidelines of the COG low-risk neuroblastoma protocol P9641.(18)
Secondary Outcomes
One of the secondary outcomes predefined in our trial protocol was to better define neuroblastoma outcome in children with OMA, and these data are included in this report. Additional secondary outcomes were: 1) to determine if IVIG improved the functional outcome of OMA; 2) to determine the long-term neuro-psychological prognosis of children with OMA; and 3) to better define the epidemiology and pathogenesis of OMA by analyzing serum and cerebrospinal fluid samples for specific anti-neuronal antibodies and evaluating brain magnetic resonance imaging. These outcomes are currently being analyzed and will be included in future reports.
Statistical Analysis
The randomization was stratified by risk group (low, intermediate, high) according to the COG Risk Classification System.(15;19) Analyses were conducted as intent-to-treat, except for a secondary analysis of the primary objective to assess the OMA response within evaluable patients, defined as those who completed the baseline evaluation and at least one other evaluation of OMA response at 2, 6, or 12 months. An interim futility monitoring rule was used to determine that a treatment arm met a minimum required response rate; arm(s) not meeting the minimum were eliminated (see Supplemental Statistical Methods). If both arms met the minimum response rate, a test of proportions was used to identify the arm with the superior OMA response rate, with p<0.2 considered statistically significant. Due to the rarity of this disease, and because it was deemed unlikely that the addition of IVIG would result in a lower reponse rate, the risk of a higher than usual type 1 error was considered acceptable. With a planned sample size of 26 evaluable patients per arm (52 total), the test of proportions had 90% power to detect a 28% difference and 80% power to detect a 23% difference in the proportion of OMA responders for NO-IVIG versus IVIG+.
For event-free survival (EFS), time to event was calculated from study enrollment until the first occurrence of neuroblastoma relapse or progression, secondary malignancy, or death from any cause; patients without an event were censored at the date of last contact. For overall survival (OS), time to event was calculated from study enrollment until death from any cause; living patients were censored at the date of last contact. The Data Monitoring Committee for the protocol recommended that we add OMA-PFS as a secondary analysis of the results. For OMA-PFS, time to event was calculated from study enrollment until time of OMA progression, OMA non-response (see OMA response evaluation above), crossover to IVIG, or switch to ACTH; otherwise a patient was censored at the date last known to be OMA progression-free. Survival curves were generated using the methods of Kaplan and Meier,(20) with standard errors per Peto et al.(21) EFS and OS rates are presented as survival estimates (95% confidence interval (CI)). Within the subset of patients who crossed over to IVIG or switched to ACTH, OMA responses after the crossover or switch were descriptively summarized
Role of the Funding Source
This study is supported by the Chair’s Grant U10 CA-98543 and CA-180886 and Statistical and Data Center Grant U10 CA-98413 and CA-180899 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD. The sponsoring institution does not have a role in study design, data collection, analysis or interpretation or role in writing this report. The corresponding author and statistician co-authors had full access to the data and all authors participated in the designs, analysis, interpretation of the data and the writing of the manuscript. This study is registered with Clinical Trials.gov (identifier NCT00033293).
Results
Patients
A total of 53 eligible patients, from the 92 institutions that opened the trial, were enrolled after the study was activated on May 15, 2004. The study closed to accrual on February 4, 2013. All 53 patients were included in the intent-to-treat analysis. However, one patient was not evaluable for the OMA response analysis because the patient was not seen in the required follow-up visit for neurologic assessment. This patient refused therapy post-randomization but prior to start of therapy; therefore, 52 patients were evaluable for toxicity [graded per Common Terminology Criteria for Adverse Events (CTCAE) version 4.0] and for the secondary analysis of OMA response. The median age at diagnosis was 18.9 (IQR: 16.6, 24.0) months (range 2.9–49.2 months). Thirty-three patients were female and 20 were male with a female:male ratio of 1.7:1.0 (Table 2). Forty-four patients had low-risk neuroblastoma, seven had intermediate-risk neuroblastoma, and two were diagnosed with MYCN-amplified, high-risk neuroblastoma according to the COG Risk Classification System.(15;19) Sixty-four percent of patients (n=34) had favorable histology according to the International Pathology Classification System (INPC) criteria.(22)
Table 2.
Characteristics of 53 patients with OMA on COG study ANBL00P3
| IVIG + risk-based chemotherapy (IVIG+) (n=26) | Risk-based chemotherapy(NO- IVIG)(n=27) | Overall (n=53) | |
|---|---|---|---|
|
| |||
| Median Age at NB diagnosis [IQR] (years) | 1.6 [1.3, 2.2] | 1.4 [1.4, 1.9] | 1.6 [1.4, 2.0] |
|
| |||
| Sex | |||
| Male | 8 | 12 | 20 |
| Female | 18 | 15 | 33 |
|
| |||
| Race | |||
| White | 19 | 17 | 36 |
| African American | 5 | 6 | 11 |
| Asian | 1 | 0 | 1 |
| Unknown or Other | 1 | 4 | 5 |
|
| |||
| Ethnicity | |||
| Hispanic | 5 | 7 | 12 |
| Non-Hispanic | 20 | 20 | 40 |
| Unknown | 1 | 0 | 1 |
|
| |||
| Neuroblastoma risk factors | |||
|
| |||
| Low risk | 23 | 21 | 44 |
| Stage 1 | 16 | 16 | 32 |
| Stage 2A | 2 | 1 | 3 |
| Stage 2B | 5 | 4 | 9 |
|
| |||
| Intermediate risk | 3 | 4 | 7 |
| Stage 2A | 1 | 1 | 2 |
| Stage 2B | 1 | 0 | 1 |
| Stage 3 | 1 | 3 | 4 |
| Stage 4 | 0 | 0 | 0 |
|
| |||
| High risk | 0 | 2 | 2 |
| Stage 3 | 0 | 1 | 1 |
| Stage 4 | 0 | 1 | 1 |
|
| |||
| Histology | |||
| Favorable | 16 | 18 | 34 |
| Unfavorable | 8 | 8 | 16 |
| Unknown | 2 | 1 | 3 |
|
| |||
| MYCN status | |||
| Non-Amplified | 24 | 24 | 48 |
| Amplified | 0 | 2 | 2 |
| Unknown | 2 | 1 | 3 |
|
| |||
| Age at NB diagnosis | |||
| <18 months | 10 | 15 | 25 |
| ≥18 months | 16 | 12 | 28 |
|
| |||
| Ploidy | |||
| Hyperdiploid | 18 | 18 | 36 |
| Diploid | 6 | 7 | 16 |
| Unknown | 2 | 2 | 4 |
Stage: The patients enrolled on this study were all staged using the International Neuroblastoma Staging System (INSS), which is a surgical pathological staging system. All patients with INSS stage 1 disease have undergone complete resection of their primary unilateral localized tumor by definition. Patients with stage 2 disease have some residual tumor tissue (up to 10%) following surgical resection, while those with stages 3 and 4 disease generally undergo a biopsy only at diagnosis and have residual disease. Thus, the extent of surgical resection is captured by INSS staging.
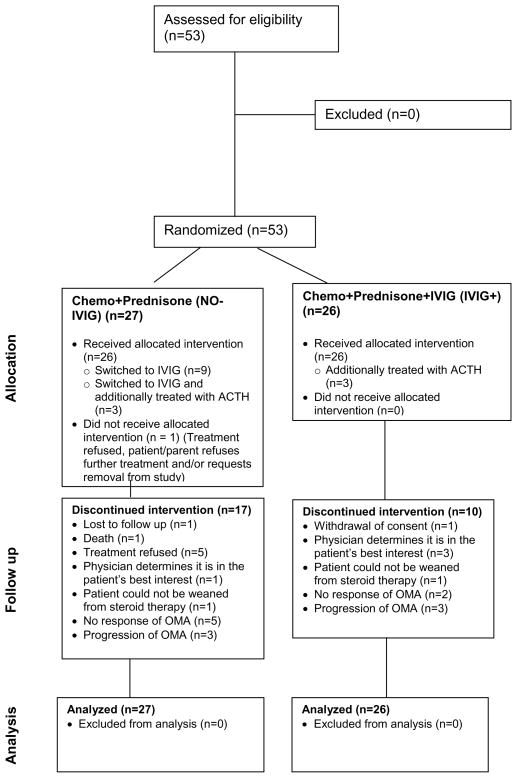
Twenty-six subjects were randomized to IVIG+ and 27 to NO-IVIG. Four of the seven subjects with intermediate-risk neuroblastoma and both high-risk patients were treated with risk-based multi-agent chemotherapy. Thus, a total of 47 (44 low risk and 3 intermediate risk) patients were treated with cyclophosphamide and prednisone with or without IVIG (Figure 2).
Figure 2.
CONSORT Diagram for COG Study ANBL00P3
OMA Response
Applying the protocol futility interim monitoring rule to test the lack of efficacy, neither treatment arm was eliminated for insufficient OMA response rate (see Supplemental Statistical Methods). The OMA response rate for IVIG+ (21/26=80.8%) was statistically significantly higher than for NO-IVIG (11/27=40.7%) (odds ratio=6.1; 95% CI: (1.5, 25.9), p=0.0029; Table 3). In a secondary analysis of 52 patients who received therapy and completed at least one follow-up assessment for OMA response, the OMA response rate remained statistically significantly higher for the IVIG+ cohort compared to the NO-IVIG cohort (p=0.0044).
Table 3.
Overall OMA response rate, by randomized treatment arm
| Randomized to IVIG+ (n=26) | Randomized to NO-IVIG (n=27) | Total (n=53) | P value | |
|---|---|---|---|---|
| OMA Responders | 21 (81%) | 11 (41%) | 32 (60%) | 0.0029* |
| OMA Non-responders | 5 (19%) | 16 (59%) | 21 (40%) | |
| Reasons for OMA non- response: | ||||
| Crossed over from NO- IVIG to IVIG+ only | N/A | 9 (33%) | 9 (17%) | |
| Crossed over from NO- IVIG to IVIG+ then switched to ACTH | N/A | 3 (11%) | 3 (6%) | |
| Switched to ACTH only | 3 (12%) | 0 (0%) | 3 (6%) | |
| Stable disease | 2 (8%) | 4 (15%) | 6 (11%) | |
| OMA Events | 12 (46%) | 19 (70%) | 31 (58%) |
In a secondary analysis of 52 patients who received therapy and completed at least one follow-up assessment of OMA response, p=0.0044.
N/A-not applicable
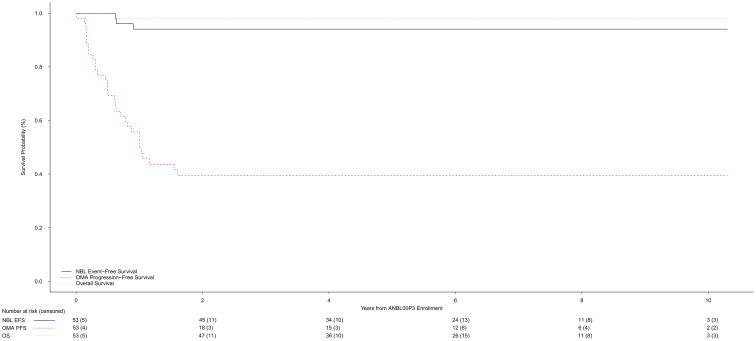
Twelve patients randomized to NO-IVIG crossed over and received IVIG+ at a median time of 143.5 (IQR: 92, 251; range 1–372) days (Table 3). These patients were classified as non-responders. OMA symptoms did not improve and prednisone was discontinued in three patients randomized to IVIG+ and three patients randomized to NO-IVIG. All six patients were treated with ACTH and classified as non-responders. The 3-year OMA-PFS was 39.5% (25.3%, 53.7%) for the overall cohort (Figure 3a). The 27 patients randomized to IVIG+ had a higher OMA-PFS compared to the 26 patients treated with NO-IVIG [53.9% (34.0%, 73.7%) versus 24.2% (5.7%, 42.6%) respectively] (Figure 3b). The median follow-up time for patients who were OMA progression-free was 6.1 (IQR: 3.6, 8.7; range: 1 day, 10.3) years [6.2 (IQR: 4.2, 6.7; range: 1.6, 9.8) years for IVIG+, 4.6 (IQR: 1.0, 9.5; range: 1 day, 10.3) years for NO-IVIG].
Figure 3.
a. Neuroblastoma event-free, OMA progression-free, and overall survival of the overall patient cohort (n=53)
b. OMA progression-free survival for IVIG+ (n=26) versus NO-IVIG (n=27)
Toxicity
The combination of risk-based chemotherapy with or without IVIG was generally well tolerated Five of the low-risk patients developed fever, and one of these patients was admitted to the hospital. A total of twenty-eight subjects reported a Grade 3 or higher toxicity. Toxicity for patients with intermediate- and high-risk neuroblastoma who received multi-agent cytotoxic chemotherapy consisted predominantly of the expected hematological toxicities (chemotherapy induced neutropenia and thrombocytopenia). One patient with high-risk neuroblastoma developed overwhelming adenovirus infection after high-dose chemotherapy followed by autologous stem cell reconstitution and died of the infection. Table 4 summarizes the toxicities that were reported by institutions for each of the patients enrolled on this study. Because the patients randomized to the IVIG+ arm also received corticosteroids and chemotherapy it is not possible to determine if any of the toxicities were due specifically to the IVIG. It is likely that the reported nervous toxicties were related to the patients’ underlying OMA syndrome rather than the treatment received.
Table 4.
Episodes of Grade 3, 4, or 5 Toxicity (CTCAE; version 4.0)
| IVIG+ (N=38)* Number of episodes (%) |
NO-IVIG (N=25) Number of episodes (%) |
|
|---|---|---|
|
| ||
| Hematopoietic | ||
| Anemia | 4 (10·5%) | 3 (12·0%) |
| Leukopenia | 1 (2·6%) | 1 (4·0%) |
| Neutropenia | 5 (13·2%) | 4 (16·0%) |
| Thrombocytopenia | 2 (5·3%) | 2 (8·0%) |
| Febrile neutropenia | 1 (2·6%) | 1 (4·0%) |
| Other | 1 (2·6%) | 1 (4·0%) |
|
| ||
| Gastrointestinal | ||
| ALT increased | 1 (2·6%) | |
| AST increased | 1 (2·6%) | |
| Intra-abdominal hemorrhage | 1 (4·0%) | |
| Mucositis | 1 (4·0%) | |
| Nausea | 1 (4·0%) | |
| Vomiting | 4 (10·5%) | 1 (4·0%) |
| Colitis | 1 (4·0%) | |
| Diarrhea | 1 (2·6%) | |
|
| ||
| Infectious diseses | ||
| Bladder infection | 2 (5·3%) | 1 (4·0%) |
| Fever | 1 (2·6%) | |
| Catheter related | 3 (7·9%) | 2 (8·0%) |
| Enterocolitis | 1 (2·6%) | 1 (4·0%) |
| Lung infection | 1 (4·0%) | |
| Other | 6 (15·8%) | 5 (20·0%) |
|
| ||
| Nervous system | ||
| Irritability | 1 (2·6%) | 1 (4·0%) |
| Nystagmus | 2 (5·3%) | 3 (12·0%) |
| Ataxia | 1 (2·6%) | 3 (12·0%) |
| Agitation | 7 (18·4%) | 7 (28·0%) |
| Personality changes | 1 (2·6%) | |
|
| ||
| Cardiovascular | ||
| Hypertension | 2 (5·3%) | 2 (8·0%) |
|
| ||
| Metabolism and Nutrition | ||
| Anorexia | 1 (2·6%) | |
| Weight gain | 1 (2·6%) | 1 (4·0%) |
| Dehydration | 1 (2·6%) | 1 (4·0%) |
| Hyperglycemia | 2 (5·3%) | 2 (8·0%) |
| Hypoalbuminemia | 1 (2·6%) | |
| Hypoglycemia | 1 (2·6%) | |
| Hypokalemia | 2 (5·3%) | 2 (8·0%) |
| Hyponatremia | 1 (4·0%) | |
| Hypophosphatemia | 1 (2·6%) | 2 (8·0%) |
|
| ||
| Respiratory | ||
| Hypoxemia | 1 (4·0%) | |
The IVIG+ arm also contains patients that crossed over from the NO-IVIG arm
Neuroblastoma Survival
The neuroblastoma 3-year EFS and OS for the entire cohort were 94.1% (87.3%, 100%) and 98.0% (94.1%, 100%), respectively (n=53, Figure 3a). The median follow-up time for patients without a neuroblastoma event was 6.0 (IQR: 3.8, 6.7; range: 1 day, 10.3) years [6.1 (IQR: 4.1, 6.7; range: 1.6, 9.8) years for IVIG+, 5.0 (IQR: 3.2, 7.5; range: 1 day, 10.3) years for NO-IVIG]. For low-risk patients, the 3-year EFS and OS were 97.6% (92.9%, 100%) and 100%, respectively. The median follow-up time for low-risk patients without an event was 5.9 (IQR: 3.8, 6.7; range: 1 day, 10.3) years [6.0 (IQR: 4.1, 6.7; range: 1.6, 9.8) years for IVIG+, 5.6 (IQR: 3.2, 6.6; range: 1 day, 10.3) years for NO-IVIG]. Of the seven intermediate-risk patients, six are alive without evidence of neuroblastoma disease progression. One intermediate-risk patient developed neuroblastoma disease progression associated with OMA relapse, but remains alive. One of the 2 high-risk patients is alive without evidence of neuroblastoma disease progression.
Discussion
The acute neurologic symptoms associated with OMA are often severe, and long-term neurological deficits are common.(5;6) To date, treatment regimens have been based on case reports and small retrospective studies. In an effort to improve neurologic recovery in this patient cohort, we designed and conducted the first prospective, randomized clinical trial for children with neuroblastoma-associated OMA. This neurologic disorder appears to be caused by an autoimmune process, and corticosteroids have been widely used to treat OMA.(1) Prednisone was, therefore, included in both treatment arms in this clinical trial. Based on promising results of a retrospective analysis of neuroblastoma patients with OMA that suggested chemotherapy may enhance the resolution of both acute and long-term neurology sequelae (6) all patients in this study received chemotherapy. Low-risk patients received cyclophosphamide at doses frequently used for immunosuppression. Three intermediate-risk patients were also treated with cyclophosphamide, while the other 4 intermediate-risk patients and the 2 high-risk patients received risk-adpated treatment regimens.(15) Because chemotherapy was included in both treatment arms, it is not possible to assess the clinical impact of chemotherapy on OMA response.
IVIG is also known to modulate the immune response in other autoimmune diseases, and case reports have indicated clinical response to this agent in patients with neuroblastoma-associated OMA.(23;24) To test if the addition of IVIG to prednisone and chemotherapy improved neurologic recovery, this study randomized patients to receive IVIG (IVIG+) or no IVIG (NO-IVIG). We found that patients randomized to IVIG+ had a statistically significantly higher rate of resolution of OMA symptoms compared to those who were randomized to NO-IVIG (p=0.0029). This study was not conducted in a blinded fashion due to ethical and practical concerns regarding intravenous infusion of placebo in pediatric patients. However, because pediatric oncologists routinely enroll patients on unblinded randomized cooperative group trials and remain in equipoise regarding the treatment arms, the vast majority of patients are treated as randomized.
In contrast to the slight predominance of males observed in most neuroblastoma cohorts,(25) 62% (33/53) of the neuroblastoma patients with OMA in our study were female. Although female preponderance is a finding that is common in autoimmune disorders, this particular characteristic of neuroblastoma associated OMA patients has not been recognized previously. Our review of the published literature confirmed a preponderance of female cases in other OMA reports (3–5;26–28).
The majority of children with OMA and neuroblastoma have been reported to have low-risk disease,(1) and our study confirms the association between OMA and favorable biologic features. Forty-four (83%) of the 53 patients enrolled on the study had low-risk disease and seven had intermediate-risk. Only two patients were classified as high-risk. Reflecting the high proportion of low-risk tumors, survival of this cohort was excellent, with 3-year EFS and OS rates of 94.1% (87.3%, 100%) and 98.0% (94.1%, 100%), respectively. The single patient enrolled on this study who died had high-risk neuroblastoma and suffered an infectious complication following myeloablative therapy. Interestingly, the immunoglobulin fraction of serum from OMA patients has been shown to suppress growth of neuroblastoma cell lines,(29) suggesting there may be a biologic rationale for the low numbers of high-risk patients with OMA. However, it is well recognized that the majority of patients with OMA and neuroblastoma have significant neurologic morbidities, and many experience life-long neurologic deficits.(5;6) Additional follow-up is needed to assess the incidence of long-term neurologic sequelae in our cohort, which may include developmental delay and learning disabilities.
OMA symptoms may be exacerbated by concurrent infection. We did not detect any difference in the incidence of infections rising to Grade 3 toxicity or greater infections in either treatment group. Because we did not collect data on lower toxicity grade infections, we are not able to determine if viral infections impacted OMA response rate.
Mitchell and co-workers, in their recent long-term follow-up report of OMA, showed a correlation of outcome and intensity of therapy consistent with the hypothesis that more intensive immunosuppression may be associated with improved long-term neurologic outcome.(30) Other studies have also indicated that a more aggressive treatment approach may lead to improved neurologic outcome for patients with OMA.(5;13) Our study demonstrates the addition of IVIG to prednisone and risk-adapted chemotherapy improves OMA response, indicating that IVIG+ will serve as an effective treatment back-bone to test future strategies. We conducted the trial within the COG which consists of over 200 institutions of varying sizes in North America, Australia and New Zealand. Most, if not all, children diagnosed with neuroblastoma are referred to a COG institution in North America. Although only 92 COG institutions elected to open the trial, and only a subset of these 92 institutions enrolled at least one eligible subject, this study was successfully conducted in small institutions as well as medium-sized hospitals and tertiary centers. These results suggest that the care and complex clinical evaluations required to treat this population of patients can be successfully applied in different hospital settings and are generalizable. At the Advances in Neuroblastoma Research meeting in Genoa, Italy 2004, a task force recommended that three of four of the following criteria be present to diagnose neuroblastoma with OMA: 1) opsoclonus, 2) myoclonus/ataxia, 3) behavioral changes and/or sleep disturbances, and 4) neuroblastoma. Although we did not apply the Genoa criteria in our study, only one of our patients (randomized to NO-IVIG) had less than 3 of the 4 Genoa criteria.(1)
Further follow-up is needed to determine if this more intensive treatment approach improves the long-term neurologic outcome of patients with neuroblastoma-associated OMA. Long-term follow up to assess neuro-developmental and learning problems of this study population is important, and there is broad agreement that the establishment of a registry would be extremely valuable. Unfortunately, the funding required to build this resource has not yet been identified. Long-term follow-up of this cohort and studies to better define the epidemiology and pathogenesis of OMA, including analysis of antineuronal antibodies in serum and cerebrospinal fluid and evaluation of magnetic resonance images of the cerebellum are secondary objectives of this study and data collection is still in progress. The results of these objectives will be the subject of separate reports.
Supplementary Material
Research in Context.
Evidence before this study
We searched the Pubmed database using the terms opsoclonus, myoclonus, ataxia, and neuroblastoma to review prior treatment of patients with neuroblastoma and OMA. There was no date restriction used to search the database, and we did not restrict the language. Our search revealed case reports, small series, and retropsecitve studes that described neurologic response with immunosuppressive therapy in patients with neuroblastoma and OMA. These publications provide compelling evidence that OMA associated with neuroblastoma is an immune-mediated paraneoplastic syndrome. No previous prospective randomized clinical trial for this population has been reported.
Added value of this study
This is the first and, to date, only randomized clinical trial for patients with OMA and neuroblastoma ever conducted. The results of this study demonstrate treatment effect for both arms of therapy, although significantly superior OMA response was seen with the IVIG+ regimen. As the only randomized trial with the largest number of OMA patients ever reported, the results define, for the first time, OMA response to two treatment regimens.
Limitations of the study
Because of the rarity of OMA, our trial has limited number of subjects and we had to accept an 80% power in our analysis. The results of our study need to be interpreted with caution in light of the small numbers of subjects but also taking into account the rarity of the disorder. In our trial, we could not evaluate the role of chemotherapy since both arms of the study contain chemotherapy with the majority of the subjects receiving cyclophosphamide.
Implications of all available evidence
The OMS response observed with the IVIG+ regimen can be used as a historical control to investigate if treatment strategies that include new immunosuppressant agents will lead to improved outcome. Currently there is a single arm European study that is evaluating the use of rituximab in combination with dexamethasone and cyclophosphamide in the therapy of OMA.
Acknowledgments
This work was supported by the Chair’s Grant U10 CA-98543 and CA-180886 and Statistical and Data Center Grant U10 CA-98413 and CA-180899 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
The authors would like to thank Dina Willis and Patrick McGrady for their assistance with data collection and analyses in the COG Statistics and Data Center at the University of Florida in Gainesville, Florida.
The authors would like to dedicate this publication to the memory of Dr. Jessica A. Panzer, a coauthor and basic science investigator for OMA, who unfortunately died between the time of writing this manuscript and publication.
Footnotes
Declaration of Interests:
All the authors equally contributed to the preparation of this manuscript. The authors have no conflicts of interest to declear.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matthay KK, Blaes F, Hero B, Plantaz D, de AP, Mitchell WG, et al. Opsoclonus myoclonus syndrome in neuroblastoma a report from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa, Italy, 2004. Cancer Lett. 2005;228(1–2):275–282. doi: 10.1016/j.canlet.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute; Bethesda, MD: 2017. [Google Scholar]
- 3.Pang KK, de SC, Lang B, Pike MG. A prospective study of the presentation and management of dancing eye syndrome/opsoclonus-myoclonus syndrome in the United Kingdom. Eur J Paediatr Neurol. 2010;14(2):156–161. doi: 10.1016/j.ejpn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Krug P, Schleiermacher G, Michon J, Valteau-Couanet D, Brisse H, Peuchmaur M, et al. Opsoclonus-myoclonus in children associated or not with neuroblastoma. Eur J Paediatr Neurol. 2010;14(5):400–409. doi: 10.1016/j.ejpn.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.De Grandis E, Parodi S, Conte M, Angelini P, Battaglia F, Gandolfo C, et al. Long-term follow-up of neuroblastoma-associated opsoclonus-myoclonus-ataxia syndrome. Neuropediatrics. 2009;40(3):103–111. doi: 10.1055/s-0029-1237723. [DOI] [PubMed] [Google Scholar]
- 6.Russo C, Cohn SL, Petruzzi MJ, de Alarcon PA. Long-term neurologic outcome in children with opsoclonus-myoclonus associated with neuroblastoma: a report from the Pediatric Oncology Group. Med Pediatr Oncol. 1997;28(4):284–288. doi: 10.1002/(sici)1096-911x(199704)28:4<284::aid-mpo7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Blaes F, Fuhlhuber V, Korfei M, Tschernatsch M, Behnisch W, Rostasy K, et al. Surface-binding autoantibodies to cerebellar neurons in opsoclonus syndrome. Ann Neurol. 2005;58(2):313–317. doi: 10.1002/ana.20539. [DOI] [PubMed] [Google Scholar]
- 8.Blaes F, Pike MG, Lang B. Autoantibodies in childhood opsoclonus-myoclonus syndrome. J Neuroimmunol. 2008;201–202:221–226. doi: 10.1016/j.jneuroim.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Moticka EJ, Franz DN, et al. B- and T-cell markers in opsoclonus-myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology. 2004;62(9):1526–1532. doi: 10.1212/wnl.62.9.1526. [DOI] [PubMed] [Google Scholar]
- 10.Pranzatelli MR, Tate ED, McGee NR, Travelstead AL, Colliver JA, Ness JM, et al. BAFF/APRIL system in pediatric OMS: relation to severity, neuroinflammation, and immunotherapy. J Neuroinflammation. 2013;10:10. doi: 10.1186/1742-2094-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NK, Dalmau J. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol. 2003;53(3):347–353. doi: 10.1002/ana.10462. [DOI] [PubMed] [Google Scholar]
- 12.Raffaghello L, Conte M, De GE, Pistoia V. Immunological mechanisms in opsoclonus-myoclonus associated neuroblastoma. Eur J Paediatr Neurol. 2009;13(3):219–223. doi: 10.1016/j.ejpn.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Pranzatelli MR, Tate ED, Travelstead AL, Barbosa J, Bergamini RA, Civitello L, et al. Rituximab (anti-CD20) adjunctive therapy for opsoclonus-myoclonus syndrome. J Pediatr Hematol Oncol. 2006;28(9):585–593. doi: 10.1097/01.mph.0000212991.64435.f0. [DOI] [PubMed] [Google Scholar]
- 14.Pranzatelli MR, Tate ED, Shenoy S, Travelstead AL. Ofatumumab for a rituximab-allergic child with chronic-relapsing paraneoplastic opsoclonus-myoclonus. Pediatr Blood Cancer. 2012;58(6):988–991. doi: 10.1002/pbc.23187. [DOI] [PubMed] [Google Scholar]
- 15.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33(27):3008–3017. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363(14):1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreissman SG, Rackoff W, Lee M, Breitfeld PP. High dose cyclophosphamide with carboplatin: a tolerable regimen suitable for dose intensification in children with solid tumors. J Pediatr Hematol Oncol. 1997;19(4):309–312. doi: 10.1097/00043426-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Strother DR, London WB, Schmidt ML, Brodeur GM, Shimada H, Thorner P, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. J Clin Oncol. 2012;30(15):1842–1848. doi: 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86(2):349–363. [PubMed] [Google Scholar]
- 23.Petruzzi MJ, de Alarcon PA. Neuroblastoma-associated opsoclonus-myoclonus treated with intravenously administered immune globulin G. J Pediatr. 1995;127(2):328–329. doi: 10.1016/s0022-3476(95)70322-5. [DOI] [PubMed] [Google Scholar]
- 24.Borgna-Pignatti C, Balter R, Marradi P, Colamaria V. Treatment with intravenously administered immunoglobulins of the neuroblastoma-associated opsoclonus-myoclonus. J Pediatr. 1996;129(1):179–180. doi: 10.1016/s0022-3476(96)70225-1. [DOI] [PubMed] [Google Scholar]
- 25.Davis S, Rogers MA, Pendergrass TW. The incidence and epidemiologic characteristics of neuroblastoma in the United States. Am J Epidemiol. 1987;126(6):1063–1074. doi: 10.1093/oxfordjournals.aje.a114745. [DOI] [PubMed] [Google Scholar]
- 26.Catsman-Berrevoets CE, Aarsen FK, van Hemsbergen ML, van Noesel MM, Hakvoort-Cammel FG, van den Heuvel-Eibrink MM. Improvement of neurological status and quality of life in children with opsoclonus myoclonus syndrome at long-term follow-up. Pediatr Blood Cancer. 2009;53(6):1048–1053. doi: 10.1002/pbc.22226. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa S, Matsushige T, Kajimoto M, Inoue H, Momonaka H, Oka M, et al. A nationwide survey of opsoclonus-myoclonus syndrome in Japanese children. Brain Dev. 2014 doi: 10.1016/j.braindev.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Singhi P, Sahu JK, Sarkar J, Bansal D. Clinical profile and outcome of children with opsoclonus-myoclonus syndrome. J Child Neurol. 2014;29(1):58–61. doi: 10.1177/0883073812471433. [DOI] [PubMed] [Google Scholar]
- 29.Korfei M, Fuhlhuber V, Schmidt-Woll T, Kaps M, Preissner KT, Blaes F. Functional characterisation of autoantibodies from patients with pediatric opsoclonus-myoclonus-syndrome. J Neuroimmunol. 2005;170(1–2):150–157. doi: 10.1016/j.jneuroim.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell WG, Wooten AA, O’Neil SH, Rodriguez JG, Cruz RE, Wittern R. Effect of Increased Immunosuppression on Developmental Outcome of Opsoclonus Myoclonus Syndrome (OMS) J Child Neurol. 2014;30(8):976–982. doi: 10.1177/0883073814549581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.