Abstract
West Nile virus (WNV) and Rift Valley fever virus (RVFV) are two emerging arboviruses transmitted by Culex pipiens species that includes two biotypes: pipiens and molestus. In Lebanon, human cases caused by WNV and RVFV have never been reported. However, the introduction of these viruses in the country is likely to occur through the migratory birds and animal trades. In this study, we evaluated the ability of Cx. pipiens, a predominant mosquito species in urban and rural regions in Lebanon, to transmit WNV and RVFV. Culex egg rafts were collected in the West Bekaa district, east of Lebanon and adult females of Cx. pipiens were experimentally infected with WNV and RVFV Clone 13 strain at titers of 1.6×108 and 1.33×107 plaque forming units (PFU)/mL, respectively. We estimated viral infection, dissemination and transmission at 3, 7, 14 and 19 days post infection (dpi). Results showed that infection was higher for WNV than for RVFV from 3 dpi to 19 dpi. Viral dissemination and transmission started from 3 dpi for WNV; and only from 19 dpi for RVFV. Moreover, Cx. pipiens were able to excrete in saliva a higher number of viral particles of WNV (1028 ± 405 PFU/saliva at 19 dpi) than RVFV (42 PFU/saliva at 19 dpi). Cx. pipiens from Lebanon are efficient experimental vectors of WNV and to a lower extent, RVFV. These findings should stimulate local authorities to establish an active entomological surveillance in addition to animal surveys for both viruses in the country.
Author summary
West Nile virus (WNV) and Rift Valley fever virus (RVFV) are two emerging mosquito-borne arboviruses mainly transmitted by Culex mosquitoes. WNV considered one of the most important causative agent of viral encephalitis has a wide distribution in many tropical and temperate countries including the Middle East. RVFV is mainly distributed in Sub-Saharan Africa but epizootics were also reported in Egypt, Saudi Arabia and Yemen. The mosquito vector belongs to the Culex pipiens species which includes two biotypes: pipiens and molestus. Both biotypes are the most widely distributed mosquitoes in Lebanon. Using experimental infections of mosquitoes, our study showed that Cx. pipiens populations collected in West Bekaa were susceptible to infection by these two viruses and ensured efficient transmission of WNV and to a lesser extent, RVFV. Our findings may help to prepare a control strategy more adapted to these mosquito vectors.
Introduction
West Nile virus (WNV) and Rift Valley fever virus (RVFV) are two important emerging mosquito-borne zoonotic agents transmitted by Culex pipiens, a complex of sibling species that includes Cx. pipiens s.s., Cx. quinquefasciatus and possibly Cx. australicus [1, 2]. Cx. pipiens s.s. includes two biotypes or subspecies: Cx. pipiens pipiens and Cx. pipiens molestus [1, 3]. The first biotype is primarily a bird-feeding mosquito present in temperate areas while Cx. pipiens biotype molestus feeds on mammals (mainly human) and thrives in sewers in temperate and sub-tropical regions [3, 4]. Because morphological identification of these biotypes is not possible, they can only be distinguished using molecular techniques [3–5].
WNV is a member of the Flavivirus genus (Flaviviridae family) and was first isolated in Uganda in 1937 [6]. Usually, only 20% of infected individuals develop symptoms and less than 1% of infected people develop serious and potentially fatal neurological illnesses such as meningitis and encephalitis. Birds are considered the main reservoir of the virus and Cx. pipiens is recognized as one of the primary enzootic vector [7]. WNV infections have been reported in many tropical and temperate countries in Africa, Europe, Asia and America. The Middle East and North Africa (MENA) region has been long considered as a WNV-endemic area [8, 9]. Locally acquired cases have been recently reported in Israel [10], Greece [11], Turkey [12], and Italy [13]. In addition, evidence of WNV circulation has been reported in Jordan [14] and Egypt [15]. The introduction of WNV into the United States in 1999, which constitutes a turning point in WNV epidemiology, is thought to have originated from Israel following introduction from Africa [16, 17].
RVFV belongs to Phlebovirus genus (Bunyaviridae family). It was first identified in Kenya in 1931 [18]. This virus usually affects livestock and causes abortion. The main enzootic vectors belong to the Aedes genus [19]. However, several Culex species, including Cx. pipiens are considered secondary vectors and contribute to the transmission of RVFV to humans [19]. Infected people can be asymptomatic or develop a mild febrile disease. In less than 10% of cases, people may develop more severe symptoms such as encephalitis and hemorrhagic fever. RVFV was responsible for numerous outbreaks among animals and humans in Sub-Saharan Africa [20] up to Mauritania [21] but also in Egypt [22]. In the Middle East, epizootics were reported in Saudi Arabia and Yemen [23].
In Lebanon, Cx. pipiens is a predominant mosquito species besides another vector of arboviruses, Aedes albopictus [24, 25]. Cx. pipiens colonizes urban and rural habitats whereas Ae. albopictus is mostly present in the densely populated coastal fringe. Local Ae. albopictus are competent to transmit Chikungunya virus and to a lesser extent, Dengue virus [26]. The vector competence of local populations of Cx. pipiens to transmit WNV and RVFV has never been evaluated. Diseases caused by these two viruses have never been reported in Lebanon. Nevertheless, a serological study conducted in a main hospital in the capital city of Beirut, confirmed the presence of neutralizing WNV antibodies in blood donors [27]. In fact, Lebanon is situated in a WNV-endemic area and located on the flyways of migratory birds with potential introduction of the virus into the country. Moreover, Lebanon is geographically close to Yemen and Saudi Arabia, regions where RVFV had circulated actively. Intensive livestock trade between Lebanon and these countries increases the risk of RVFV introduction.
Here, we assess the vector competence of local populations of Cx. pipiens towards WNV and RVFV. We estimate viral infection, dissemination and transmission at different days after experimental infections.
Materials and methods
Mosquito collections
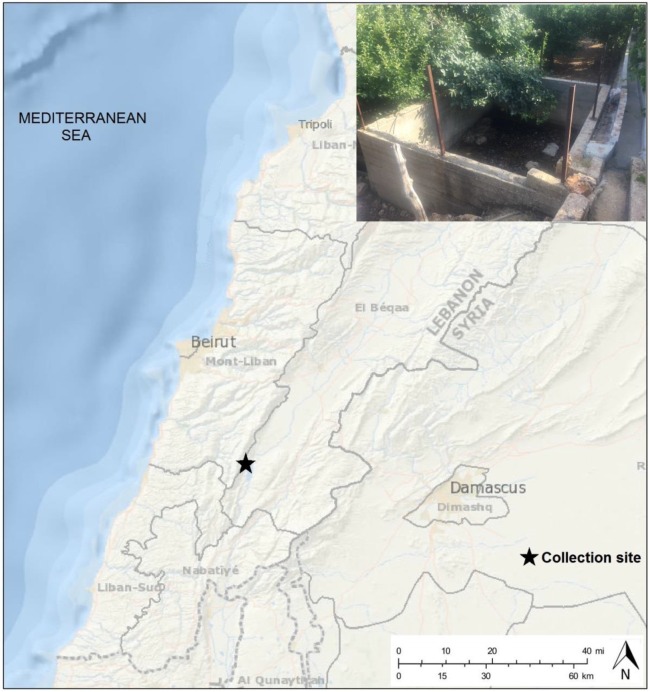
Culex egg rafts were sampled in June 2015 in Bab Mareh, in the West Bekaa district, a sub-humid, agricultural area in east of Lebanon with large stagnant water systems (Fig 1). Egg rafts were collected on the water surface in an artificial basin and placed individually in a tube containing 30 mL of water collected from the breeding site.
Fig 1. Map showing the geographic location of Lebanon East of the Mediterranean basin.
This map indicates the site of mosquito egg collections in West Bekaa region. The map was modified using PowerPoint from http://landsatlook.usgs.gov/ (Credit: U.S. Geological Survey Department of the Interior/USGS—U.S. Geological Survey). Photo: breeding site (N. Haddad).
Mosquito preparation for vector competence study
Collected egg rafts were shipped to the Laboratory of Arboviruses and Insect Vectors (AIV) at the Institut Pasteur, Paris. They were reared until the adult stage. Adults emerging from each raft were morphologically identified and only Cx. pipiens species were retained for this study.
Viruses and blood meal preparation
Two viruses were used in this study: WNV lineage 1 strain isolated from a horse in Camargue (France) in 2000 [9] and an avirulent RVFV strain Clone 13 isolated from a human case in Bangui (Central African Republic) in 1974 [28]. After passages on Vero (E6) cells (ATCC cell lines), both viruses were produced on C6/36 mosquito cells. Viral stocks were stored at -80°C until use. The infectious blood meal was composed of a viral suspension (1:3) diluted in washed rabbit erythrocytes (New Zealand White rabbit, Charles River) collected at the day of mosquitoes infection. A phagostimulant (ATP) was added at a final concentration of 5 mM. Virus titer in the blood meal was 1.6×108 plaque forming units (PFU)/mL for WNV and 1.33×107 PFU/mL for RVFV.
Artificial feeding of female mosquitoes
The susceptibility of Lebanese Cx. pipiens mosquitoes to WNV and RVFV was tested on F0 and F1 generation respectively. Ten-to-twelve day-old female Cx. pipiens mosquitoes were left to starve for 48 h in Biosafety Level 3 (BSL3) insectary at 28±1°C with 80% relative humidity and a 16h:8h photoperiod. Females were then allowed to feed for one hour through a chicken skin membrane (obtained from a commercially purchased chicken) covering the base of a capsule of the feeding system (Hemotek) containing the blood-virus mixture maintained at 37°C. Fully engorged females were sorted, then transferred in cardboard containers and maintained with 10% sucrose at 28±1°C until examination.
Saliva collection
Around 20 female mosquitoes were tested at 3, 7, 14 and 19 days post-infection (dpi). For each mosquito, saliva was collected using the forced salivation technique [29]. Briefly, mosquitoes were chilled, their legs and wings removed and the proboscis was inserted into 20 μL tip filled with 5 μL of Fetal Bovine Serum (FBS). After 45 min, medium containing the saliva was expelled into 0.2 mL tube containing 45 μL of Dulbecco’s MEM (DMEM) medium. Collected saliva and the remaining mosquito bodies were conserved at -80°C for further analysis.
Viral detection in mosquitoes
In order to assess the ability of both viruses to invade and cross the midgut barrier, the infection rate (IR) and the dissemination efficiency (DE) were determined. IR reflects the proportion of female mosquitoes with infected bodies (thorax and abdomen including the midgut) among tested specimens while DE is the proportion of female mosquitoes with infected head (detection of the virus having succeeded to reach the mosquito general cavity) among tested ones. Thus, heads and bodies were separated and ground each in 300 μL DMEM supplemented with 3% FBS. After centrifugation, the supernatant of each homogenate was conserved at -80°C. Then, 20 μL of each sample were diluted in 180 μL DMEM supplemented with 2% FBS and distributed in serial dilutions from 10−1 to 10−3 in duplicates on Vero cell monolayers (3.105 cells/well) in 96-well plates. After incubation at 37°C for 6 days, inoculum was removed and the cells were fixed and stained using a crystal violet solution (0.2% in 10% formaldehyde and 20% ethanol). After washing, the presence or absence of cytopathic effect was noted.
The capacity of the WNV and RVFV to cross the salivary glands barrier was evaluated by determining the transmission efficiency (TE) which corresponds to the proportion of female mosquitoes that secrete infectious saliva among tested specimens. The number of infectious particles within collected saliva samples was estimated on Vero cell culture and expressed as PFU/saliva. Briefly, 20 μL of each saliva were diluted in 280 μL DMEM 2% FBS. The total volume was inoculated on a monolayer of Vero cells (8.105cells/well) in six-well plates. Cells were incubated at 37°C for 6 days under an overlay consisting of DMEM, 2% FBS, 1% antibiotic-antimycotic mix and 1% agarose. The lytic plaques were counted after staining with a crystal violet solution.
Statistical analysis
Proportions (IR, DE and TE) were compared using Fisher’s exact test and sample distributions (number of viral particles) with the Kruskal-Wallis test. Statistical analyses were conducted using the Stata software (StataCorp LP, Texas, and USA). P-values<0.05 were considered significant.
Results
Artificial feeding
Collected egg rafts were hatched in laboratory conditions and provided 480 adult females of Culex pipiens (F0 generation); of those only 174 (36.25%) had successfully fed on a WNV-infected blood. A batch of 600 F1 female mosquitoes was used for the RVFV infection assay. Of those, only 91 (15.16%) had successfully fed on infected blood.
Viral infection
Infection rate (IR) for each virus was estimated by determining the number of infected bodies (abdomen and thorax) among all engorged female mosquitoes examined at each dpi (3, 7, 14 and 19) (Fig 2A). For WNV, IRs were very high (94.7–100%) from 3 to 19 dpi. For RVFV, IRs were much lower: at 3 dpi, the IR was 44.0% and increased gradually to reach 65.0% at 14 dpi and 64.3 at 19 dpi.
Fig 2.
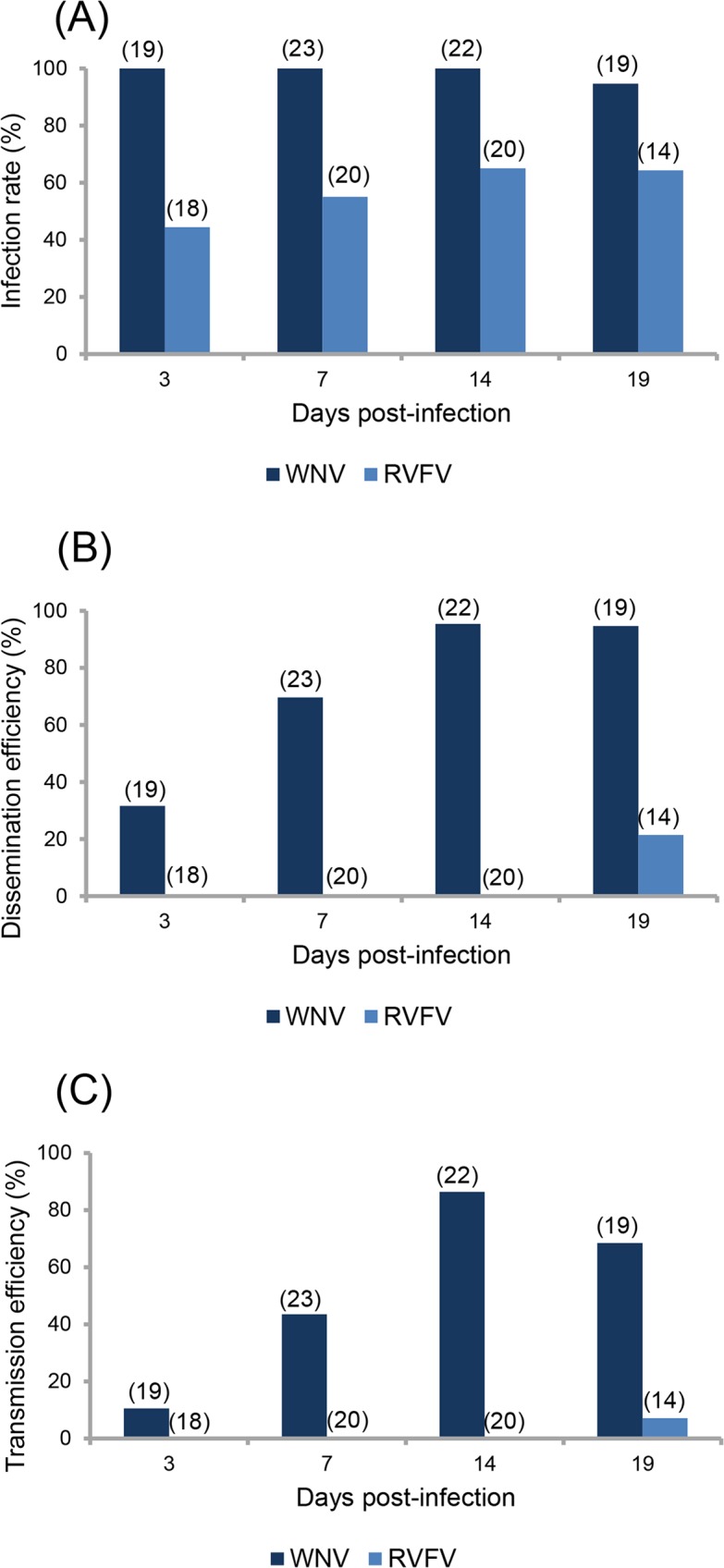
Infection (A), Dissemination (B) and Transmission (C) of Culex pipiens at different days after ingestion of an infectious blood meal containing WNV or RVFV. Culex pipiens F0/F1 mosquitoes were orally challenged with WNV at a titer of 1.6×108 PFU/mL and RVFV at titer of 1.33×107 PFU/mL using an artificial feeding system (Hemotek). At 3, 7, 14, and 19 dpi, homogenates of mosquito bodies and heads, and saliva collected from females were tested for the presence of virus on Vero cells to estimate infection rate, dissemination efficiency, and transmission efficiency, respectively. In brackets, the number of tested mosquitoes.
Viral dissemination
The detection of viral particles in mosquito heads allowed estimating the ability of the virus to disseminate from the midgut to internal organs. For WNV, dissemination efficiency (DE) increased from 31.6% (3 dpi) to 94.7% (19 dpi) (Fig 2B). For RVFV, virus was only detected at 19 dpi with a DE of 21.4% (Fig 2B).
Viral transmission
The ability of mosquitoes to transmit the virus was measured by detecting viral particles in saliva expectorated by mosquitoes. With WNV, transmission efficiencies were much higher than with RVFV (Fig 2C). TE increased gradually from 10.5% at 3 dpi to 68.4% at 19 dpi (Fisher’s exact test: p < 10−4). To note, TE decreased slightly but not significantly from 86.4% at 14 dpi to 68.4% at 19 dpi (Fisher’s exact test: p = 0.17). For RVFV, viral particles were only detected in saliva at 19 dpi with a TE of 7.1% (Fig 2C).
Intensity of transmission
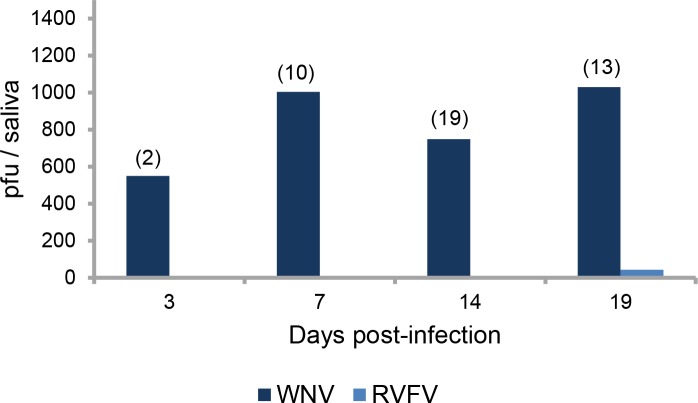
Mosquitoes were able to deliver an average of 550 (±450) PFU/saliva at 3 dpi with WNV, which increased to reach 1004 (±442) PFU/saliva at 7 dpi (Fig 3). Despite a decrease at 14 dpi, the viral load remained high at 19 dpi with 1028 (±405) PFU/saliva. For RVFV, only one female had infectious particles in saliva at 19 dpi with a viral load of 42 PFU (Fig 3).
Fig 3. Mean titer of infectious viral particles secreted in saliva of Culex pipiens at different days after ingestion of an infectious blood meal containing WNV or RVFV.
The number of infectious particles in saliva was estimated by inoculation of collected saliva on Vero cells and expressed as pfu/saliva. In brackets, the number of tested mosquitoes.
Discussion
Culex pipiens is the most widely distributed mosquito species in Lebanon and is suspected to transmit WNV and RVFV in several countries [7, 19]. Using experimental infections, we showed that Cx. pipiens populations collected from West Bekaa, Lebanon were susceptible to infection by these two viruses and ensured efficient transmission of WNV and to a lesser extent, RVFV.
Cx. pipiens was capable to ensure viral infection, dissemination and transmission starting from 3 dpi. Most mosquitoes exposed to the infectious blood-meal were infected as IRs reached 100% at the 4 dpi examined (3, 7, 14 and 19 dpi). Dissemination and transmission were slightly lower suggesting that not all infected mosquitoes were able to transmit WNV. Mosquitoes delivered more than 500 viral particles in saliva from 3 dpi. On the other side, infections with RVFV present different patterns: lower IR, DE and TE. Only 21% of mosquitoes were able to ensure viral dissemination at 19 dpi and 7% were able to transmit at 19 dpi. This suggests a significant role of the midgut and the salivary glands as respective barriers to the release of viruses into the body cavity and their excretion in saliva [30]. In this manner, Cx. pipiens was less susceptible to RVFV than to WNV.
Overall, Cx. pipiens can transmit experimentally both viruses but the time interval between the ingestion of the viremic blood-meal and the ability to transmit the virus termed the extrinsic incubation period (EIP) was 3 days for WNV. For RVFV, we only found one mosquito able to transmit the virus 19 days after ingestion. It is likely that more females would have been able to transmit the virus if more mosquitoes were able to feed on RVFV-infected blood. Cx. pipiens from Tunisia showed similar EIP of 3 days with WNV and a much shorter EIP of 3 days with RVFV [31] underlining the significant role of mosquito genotype in specific interactions between mosquito and virus genotypes; these interactions promoting adaptation of viral lineages to specific mosquito vector genotypes influence the outcome of transmission [32]. In addition, when viral dose increases in blood meals, transmission efficiency also increases suggesting that hosts presenting a high viremia may infect more mosquitoes [33]. Animals susceptible to RVFV can develop very high viremia, higher than 1010.1 MIPLD50 (mouse intraperitoneal 50% lethal dose/mL) in lambs [34]. Then, at higher titers of blood meal, RVFV may infect more mosquitoes.
In conclusion, the predominant Cx. pipiens mosquito in Lebanon is susceptible to both viruses, WNV and RVFV. As Lebanon is located in a region where WNV and RVFV can be potentially introduced (respectively through migratory birds and animal trades), local health authorities should establish an active surveillance to detect any new human cases in addition to reinforce the entomological surveillance allowing an early viral detection in field-collected mosquitoes.
Acknowledgments
We are grateful Anubis Vega-Rua for technical advice.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the Institut Pasteur and the French Government's Investissement d'Avenir program, Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases" (grant n°ANR-10-LABX-62-IBEID). RZ was supported by the Institut Pasteur during her 6-month stay in Paris and by the Doctoral School for Science and Technology at the Lebanese University for her PhD program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harbach RE (2012) Culex pipiens: species versus species complex taxonomic history and perspective. J Am Mosq Control Assoc 28(4 Suppl):10–23. doi: 10.2987/8756-971X-28.4.10 [DOI] [PubMed] [Google Scholar]
- 2.Turell MJ (2012) Members of the Culex pipiens complex as vectors of viruses. J Am Mosq Control Assoc 28(4 Suppl):123–126. doi: 10.2987/8756-971X-28.4.123 [DOI] [PubMed] [Google Scholar]
- 3.Fonseca DM, et al. (2004) Emerging vectors in the Culex pipiens complex. Science 303(5663):1535–1538. doi: 10.1126/science.1094247 [DOI] [PubMed] [Google Scholar]
- 4.Bahnck CM & Fonseca DM (2006) Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg 75(2):251–255. [PubMed] [Google Scholar]
- 5.Price DC & Fonseca DM (2015) Genetic divergence between populations of feral and domestic forms of a mosquito disease vector assessed by transcriptomics. PeerJ 3:e807 doi: 10.7717/peerj.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithburn KC HT, Burke AW, Paul JH. (1940) A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am J Trop Med Hyg. s1-20(4):471–492. [Google Scholar]
- 7.Andreadis TG (2012) The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J Am Mosq Control Assoc 28(4 Suppl):137–151. doi: 10.2987/8756-971X-28.4s.137 [DOI] [PubMed] [Google Scholar]
- 8.Marka A, et al. (2013) West Nile virus state of the art report of MALWEST Project. Int J Environ Res Public Health 10(12):6534–6610. doi: 10.3390/ijerph10126534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murgue B, Murri S, Triki H, Deubel V, & Zeller HG (2001) West Nile in the Mediterranean basin: 1950–2000. Ann N Y Acad Sci 951:117–126. [DOI] [PubMed] [Google Scholar]
- 10.Paz S & Semenza JC (2013) Environmental drivers of West Nile fever epidemiology in Europe and Western Asia—a review. Int J Environ Res Public Health 10(8):3543–3562. doi: 10.3390/ijerph10083543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa A (2017) Emerging arboviral human diseases in Southern Europe. J Med Virol 89(8):1315–1322. doi: 10.1002/jmv.24803 [DOI] [PubMed] [Google Scholar]
- 12.Failloux AB, et al. (2017) Surveillance of Arthropod-Borne Viruses and Their Vectors in the Mediterranean and Black Sea Regions Within the MediLabSecure Network. Current tropical medicine reports 4(1):27–39. doi: 10.1007/s40475-017-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calistri P, et al. (2010) Epidemiology of west nile in europe and in the mediterranean basin. The open virology journal 4:29–37. doi: 10.2174/1874357901004020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batieha A, et al. (2000) Seroprevalence of West Nile, Rift Valley, and sandfly arboviruses in Hashimiah, Jordan. Emerg Infect Dis 6(4):358–362. doi: 10.3201/eid0604.000405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soliman A, et al. (2010) Studies on West Nile virus infection in Egypt. Journal of infection and public health 3(2):54–59. doi: 10.1016/j.jiph.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Giladi M, et al. (2001) West Nile encephalitis in Israel, 1999: the New York connection. Emerg Infect Dis 7(4):659–661. doi: 10.3201/eid0704.010410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, et al. (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286(5448):2333–2337. [DOI] [PubMed] [Google Scholar]
- 18.Daubney R, Hudson J. R. and Garnham P. C. (1931) Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. J. Pathol. 34:545–579. [Google Scholar]
- 19.Tantely LM, Boyer S, & Fontenille D (2015) A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am J Trop Med Hyg 92(4):722–729. doi: 10.4269/ajtmh.14-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himeidan YE, Kweka EJ, Mahgoub MM, El Rayah el A, & Ouma JO (2014) Recent outbreaks of rift valley Fever in East Africa and the middle East. Front Public Health 2:169 doi: 10.3389/fpubh.2014.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Mamy AB, et al. (2011) Unexpected Rift Valley fever outbreak, northern Mauritania. Emerg Infect Dis 17(10):1894–1896. doi: 10.3201/eid1710.110397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meegan JM (1979) The Rift Valley fever epizootic in Egypt 1977–78. 1. Description of the epizzotic and virological studies. Trans R Soc Trop Med Hyg 73(6):618–623. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad K (2000) More deaths from Rift Valley fever in Saudi Arabia and Yemen. Lancet (London, England) 356(9239):1422. [DOI] [PubMed] [Google Scholar]
- 24.Haddad N, Harbach RE, Chamat S, & Bouharoun-Tayoun H (2007) Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc 23(2):226–228. doi: 10.2987/8756-971X(2007)23[226:POAAIL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 25.Knio KM, Markarian N, Kassis A, & Nuwayri-Salti N (2005) A two-year survey on mosquitoes of Lebanon. Parasite 12(3):229–235. doi: 10.1051/parasite/2005123229 [DOI] [PubMed] [Google Scholar]
- 26.Haddad N, et al. (2012) Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC infectious diseases 12:300 doi: 10.1186/1471-2334-12-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallian P, de Micco P, & Ghorra P (2010) Seroprevalence of West Nile virus in blood donors at Hotel Dieu de France, Beirut, Lebanon. Transfusion 50(5):1156–1158. [DOI] [PubMed] [Google Scholar]
- 28.Muller R, et al. (1995) Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg 53(4):405–411. [DOI] [PubMed] [Google Scholar]
- 29.Dubrulle M, Mousson L, Moutailler S, Vazeille M, & Failloux AB (2009) Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 4(6):e5895 doi: 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer LD & Ebel GD (2003) Dynamics of flavivirus infection in mosquitoes. Adv Virus Res 60:187–232. [DOI] [PubMed] [Google Scholar]
- 31.Amraoui F, et al. (2012) Culex pipiens, an experimental efficient vector of West Nile and Rift Valley fever viruses in the Maghreb region. PLoS One 7(5):e36757 doi: 10.1371/journal.pone.0036757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fansiri T, et al. (2013) Genetic mapping of specific interactions between Aedes aegypti mosquitoes and dengue viruses. PLoS Genet 9(8):e1003621 doi: 10.1371/journal.pgen.1003621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin E, Moutailler S, Madec Y, & Failloux AB (2010) Differential responses of the mosquito Aedes albopictus from the Indian Ocean region to two chikungunya isolates. BMC Ecol 10:8 doi: 10.1186/1472-6785-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Easterday BC, Mc GM, Rooney JR, & Murphy LC (1962) The pathogenesis of Rift Valley fever in lambs. Am J Vet Res 23:470–479. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.