Abstract
Background
Transcatheter aortic valve implantation (TAVI) is a well-established procedure; however, atrioventricular block requiring permanent pacemaker implantation (PPI) is a common complication.
Objectives
To determine the incidence, predictors and clinical outcomes of PPI after TAVI, focusing on how PPI affects left ventricular ejection fraction (LVEF) after TAVI.
Methods
The Brazilian Multicenter TAVI Registry included 819 patients submitted to TAVI due to severe aortic stenosis from 22 centers from January/2008 to January/2015. After exclusions, the predictors of PPI were assessed in 670 patients by use of multivariate regression. Analysis of the ROC curve was used to measure the ability of the predictors; p < 0.05 was the significance level adopted.
Results
Within 30 days from TAVI, 135 patients (20.1%) required PPI. Those patients were older (82.5 vs. 81.1 years; p = 0.047) and mainly of the male sex (59.3% vs 45%; p = 0.003). Hospital length of stay was longer in patients submitted to PPI (mean = 15.7 ± 25.7 vs. 11.8 ± 22.9 days; p < 0.001), but PPI affected neither all-cause death (26.7% vs. 25.6%; p = 0.80) nor cardiovascular death (14.1% vs. 14.8%; p = 0.84). By use of multivariate analysis, the previous presence of right bundle-branch block (RBBB) (OR, 6.19; 3.56-10.75; p ≤ 0.001), the use of CoreValve® prosthesis (OR, 3.16; 1.74-5.72; p ≤ 0.001) and baseline transaortic gradient > 50 mm Hg (OR, 1.86; 1.08-3.2; p = 0.025) were predictors of PPI. The estimated risk of PPI ranged from 4%, when none of those predictors was present, to 63%, in the presence of all of them. The model showed good ability to predict the need for PPI: 0.69 (95%CI: 0.64 - 0.74) in the ROC curve. The substudy of 287 echocardiograms during the 1-year follow-up showed worse LVEF course in patients submitted to PPI (p = 0.01).
Conclusion
BRD prévio, gradiente aórtico médio > 50 mmHg e CoreValve® são preditores independentes de implante de MPD pós-TAVI. Ocorreu implante de MPD em aproximadamente 20% dos casos de TAVI, o que prolongou a internação hospitalar, mas não afetou a mortalidade. O implante de MPD afetou negativamente a FEVE pós-TAVI.
Keywords: Aortic Valve Stenosis; Atroventricular Block; Transcatheter Aortic Valve Replacement / complications; Pacemaker, Artificial; Stroke Volume
Introduction
Transcatheter aortic valve implantation (TAVI) is an alternative to conventional surgery for patients with severe aortic stenosis at high surgical risk.1-3 For more than one decade, that technology has proved to increase the quality of life and survival of patients, rapidly becoming a solid treatment option. Atrioventricular block (AVB) and the need for permanent pacemaker implantation (PPI) are complications commonly reported after surgical or percutaneous aortic valve replacement. The PPI rate after surgical aortic valve replacement has been recently reported as 5.8%,4 while that after TAVI ranges from 8% to 33.7%,4,5 according to the largest studies and meta-analyses. Previous publications of data from the Brazilian Multicenter TAVI Registry have reported an incidence of TAVI-related PPI around 25% in the first 30 days.6
The risk factors for the need for PPI remain inaccurate, being related to the characteristics of the patient (previous conduction system disease: right bundle-branch block - RBBB) and of the procedure, in which the intervention causes direct mechanical trauma, inflammation due to prosthesis positioning and balloon dilation,4,7 or even related to the device itself (self-expandable, balloon-expandable, tissue penetration). By analyzing data from the Brazilian Multicenter TAVI Registry, this study aimed at determining the incidence, predictors and clinical outcomes of PPI after TAVI, focusing on how PPI affects left ventricular ejection fraction (LVEF) after TAVI.
Methods
Study population
From January/2008 to January/2015, 819 patients submitted to TAVI with significant aortic valve stenosis, aortic valve area < 1 cm2 and mean transaortic gradient ≥ 40 mm Hg were included. After excluding those who died during the procedure, those who already had PPI and implantable cardioverter defibrillator, those who received an Inovare® prosthesis, and those with unavailable or incomplete information about AVB prior to the intervention, 670 patients were left for analysis. The choice of the prosthesis was at the discretion of the operating physician. The indication for PPI was based on the institutional protocols of each participating hospital. The registry was approved by the Ethics Committee of all participating centers, and written informed consent was provided by all patients. Data were electronically monitored for identification and correction of inconsistent information. Local verification of the documents was randomly performed in 20% of all procedures.
Evolution of LVEF
This study assessed the evolution of LVEF in a subgroup of 287 patients, whose echocardiographic data were available before the procedure and 1 year after that. In that subanalysis, clinical data related to the procedure and echocardiographic outcomes were compared between patients who underwent PPI within the first 30 days after TAVI and patients who did not. The outcome assessed was LVEF variation in 1 year, calculated according to the Simpson’s method.
Statistical analysis
Atrioventricular block with subsequent PPI was attributed to TAVI when occurring within 30 days from that procedure. The patients were divided into two groups: "Group PPI", formed by patients who underwent PPI, and "Group non-PPI", formed by those who did not. Only two types of bioprostheses were included in the analysis: CoreValve® (Medtronic Inc.; Minneapolis, MN, USA) and SapienXT® (Edwards Lifesciences; Irvine, CA, USA). Categorical variables were presented as frequencies, being compared by using the chi-square or Fisher exact test. Continuous variables were presented as mean and standard deviation, being compared by using non-paired Student t test. The Kolmogorov-Smirnov test was used to assess if the quantitative variables had a normal distribution, and that supposition was confirmed.
Logistic regression was used to assess factors potentially associated with the need for PPI, with variables included in the model with level of significance ≤ 0.10. Multivariate regression analysis was performed adjusted for age, sex, pre- and post-dilation, heart rate before the procedure and presence of RBBB, and other types of intraventricular conduction disorders or the degree of AVB. Differences were statistically significant when p < 0.05. The ROC curves were analyzed to determine the ability of the risk factors to predict PPI. Outcomes within 30 days and 1 year were assessed with Kaplan-Meier curves and compared between the groups with the log-rank test. Predictors of LVEF change over time were analyzed with the use of a univariate and multivariate linear regression model. Statistical analysis was performed with the IBM-SPSS for Windows software, version 20.0.
Results
From January/2008 to January/2015, data from 819 patients submitted to TAVI at 22 hospitals in Brazil were collected. Of those, 149 patients were excluded from the analysis due to: previous PPI or cardioverter defibrillator implantation (n = 86); incomplete or unavailable data about AVB prior to the intervention (n = 36); death during the procedure (n = 25); or Inovare®prosthesis implantation (Braile Biomedica; São José do Rio Preto, SP, Brazil; n = 20). Therefore, the study population was comprised of 670 patients as follows: Group PPI, formed by 135 patients (20.1%), and Group non-PPI, formed by 535 patients.
Table 1 lists the pre-procedure demographic and baseline clinical characteristics of the study population. Group PPI patients were slightly older (mean age, 82.5 ± 6.6 years vs. 81.1 ± 7,4 years; p = 0.047) and predominantly of the male sex (59.3% vs. 45%; p = 0.003). The risk scores (EuroScore I and Society of Thoracic Surgeons Score - STS) were similar between the groups. The presence of some degree of AVB on baseline electrocardiogram (ECG) increased the risk for need for PPI. It is worth noting that of the 135 patients requiring PPI, 36 (27.3%) had RBBB or RBBB associated with anterior hemiblock (AHB). That characteristic significantly predicted PPI after TAVI when compared to other conduction disorders (p ≤ 0.001).
Table 1.
Pre-procedure demographic and clinical data of the population submitted to TAVI and its effect on permanent pacemaker implantation (PPI)
| PPI (n = 135) | Non-PPI (n = 535) | p value | |
|---|---|---|---|
| Age (years) | 82.5 ± 6.6 | 81.1 ± 7.4 | 0.047 |
| Male sex | 59.3% (80) | 45.0% (241) | 0.003 |
| Systemic arterial hypertension | 70.4% (95) | 76.1% (407) | 0.172 |
| Dyslipidemia | 48.9% (66) | 48.6% (260) | 0.952 |
| Diabetes mellitus | 34.8% (47) | 31.6% (169) | 0.474 |
| Chronic kidney disease | 71.1% (96) | 76.8% (411) | 0.167 |
| Previous myocardial infarction | 13.3% (18) | 14.4% (77) | 0.753 |
| Previous TIA/stroke | 9.6% (13) | 8.0% (35) | 0.550 |
| Previous PCI | 31.9% (43) | 34.0% (182) | 0.634 |
| CABG | 23.0% (31) | 16.3% (87) | 0.068 |
| Peripheral vascular disease | 13.3% (26) | 15.9% (85) | 0.346 |
| Porcelain aorta | 6.7% (9) | 7.3% (39) | 0.802 |
| Pulmonary hypertension | 17.8% (24) | 21.3% (114) | 0.365 |
| COPD | 22.2% (30) | 18.3% (98) | 0.302 |
| Previous valvuloplasty | 7.4% (10) | 6.5% (35) | 0.720 |
| Previous valve replacement | 1.5% (2) | 4.5% (24) | 0.106 |
| Angina | 29.6% (40) | 22.1% (118) | 0.064 |
| Syncope | 25.9% (35) | 22.4% (120) | 0.389 |
| I or II | 20.7% (28) | 18.3% (98) | |
| III or IV | 79.3% (107) | 81.7% (437) | |
| EuroScore I | 20.2 ± 15.3 | 20.1 ± 14.4 | 0.972 |
| STS score | 11.1 ± 8.4 | 10.2 ± 7.9 | 0.252 |
| Creatinine clearance | 49.3 ± 21.5 | 49.2 ± 22.1 | 0.951 |
| Heart rhythm | 0.834 | ||
| Sinus | 85.8% (115) | 86.5% (462) | |
| Atrial fibrillation/flutter | 14.2% (19) | 13.5% (72) | |
| Atrioventricular block | 0.045* | ||
| 1st degree | 21.5 % (29) | 14.0% (75) | |
| 2nd degree - Mobitz I | 0.7% (1) | 0% (0) | |
| 2nd degree - Mobitz II | 0% (0) | 0.2% (1) | |
| Conduction disorder | < 0,001 | ||
| RBBB or RBBB+AHB | 27.3% (36) | 6.6% (35) | |
| LBBB | 11.4% (15) | 14.8% (78) | |
| AHB or none | 61.4% (81) | 78.6% (414) |
TIA: transient ischemic attack; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; RBBB: right bundle branch block; LBBB: left bundle-branch block; AHB: anterior hemiblock.
Likelihood ratio; Student t test for continuous variables; chi-square test for categorical variables.
Table 2 shows the pre-TAVI echocardiographic data. Group PPI patients had slightly higher mean aortic gradient (52.8 ± 16.0 mmHg vs. 49.5 ± 15.9 mmHg; p = 0.037) and thicker interventricular septum (12.7 ± 2.2 mmHg vs. 12.1 ± 2.2 mmHg; p = 0.013). There was no significant difference between the groups regarding pre-procedure LVEF (60.7% ± 12.1% in Group PPI vs. 59.0% ± 15.1% in Group non-PPI; p = 0.15).
Table 2.
Baseline echocardiographic findings in patients with and without PPI after TAVI
| PPI (n = 135) | Non-PPI (n = 535) | p value | |
|---|---|---|---|
| Aortic valve area (cm2) | 0.65 ± 0.17 | 0.67 ± 0.20 | 0.427 |
| Aortic valve ring (mm) | 23.3 ± 3.1 | 22.9 ± 3.0 | 0.189 |
| LVEF (%) | 60.7 ± 12.1 | 59.0 ± 15.1 | 0.149 |
| Peak gradient (mm Hg) | 86.5 ± 26.2 | 81.5 ± 24.7 | 0.043 |
| Mean gradient (mm Hg) | 52.8 ± 16.0 | 49.5 ± 15.9 | 0.037 |
| LV diastolic diameter (mm) | 50.5 ± 9.0 | 50.6 ± 9.4 | 0.952 |
| Septal thickness (mm) | 12.7 ± 2.2 | 12.1 ± 2.2 | 0.013 |
| LV posterior wall thickness (mm) | 11.9 ± 2.4 | 11.6 ± 1.9 | 0.229 |
| Aortic regurgitation | 85.5% (112) | 86.5% (453) | 0.011* |
| Mild | 76.3% (100) | 71.8% (376) | |
| Moderate + Severe | 9.2% (12) | 14.7% (77) | |
| Mitral regurgitation | 88.6% (117) | 88.2% (463) | 0.826* |
| Mild | 72.7% (96) | 69.9% (365) | |
| Moderate + Severe | 15.9% (21) | 18.8% (98) |
PPI: permanent pacemaker implantation; LVEF: left ventricular ejection fraction; LV: left ventricular.
Likelihood ratio; Student t test for continuous variables.
Regarding the type of prosthesis, the need for PPI was more frequent in patients receiving the CoreValve® prosthesis as compared to those receiving the Sapien® device (23.9% vs. 9.3%, respectively; p ≤ 0.001). The other characteristics related to the procedure had no impact on the need for PPI (Table 3).
Table 3.
Characteristics of the procedure in patients with and without PPI after TAVI
| PPI (n = 135) | Non-PPI (n = 535) | p value | |
|---|---|---|---|
| Anesthesia | 0.769 | ||
| Sedation | 8.9% (12) | 9.7% (52) | |
| General | 91.1% (123) | 90.3% (483) | |
| Vascular access | 0.537 | ||
| Transfemoral or iliac | 97.0% (131) | 95.9% (513) | |
| Others | 3.0% (4) | 4.1% (12) | |
| Successful device implantation | 88.9% (120) | 89.2% (417) | 0.928 |
| Poor overlapping | 3.7% (5) | 4.5% (24) | 0.690 |
| Prosthesis migration or embolization | 3.0% (4) | 2.6% (14) | 0.824* |
| Need for a second prosthesis | 3.7% (5) | 4.1% (22) | 0.829 |
| Transesophageal echocardiography | 75.6% (102) | 82.2% (440) | 0.077 |
| Pre-dilation | 54.1% (73) | 48.2% (258) | 0.224 |
| Bioprosthesis type | < 0,001 | ||
| CoreValve | 88.1% (119) | 70.8% (379) | |
| SapienXT | 11.9% (16) | 29.2% (156) | |
| Post-dilation | 40.7% (55) | 37.0% (198) | 0.424 |
Likelihood ratio; Student t test for continuous variables; chi-square test for categorical variables.
Predictors of PPI
The multivariate analysis (Table 4), describing the independent risk factors for PPI within 30 days after TAVI, confirmed RBBB alone or in association with AHB as a strong risk factor (OR 6.19; 95%CI: 3.56-10.76; p < 0.001), as well as the CoreValve® device (OR 3.16; 95%CI: 1.74-5.72; p < 0.001). In addition, mean transaortic gradient (OR 1.86; 95%CI: 1.08-3.20; p = 0.025), the innovative finding of this study, was an independent predictor of the need for PPI. Table 5 shows the likelihood of the need for PPI estimated by multiple logistic regression combining the independent predictors of PPI within 30 days after TAVI. To build the model, the mean transaortic gradient value was analyzed as a categorical variable, using the cutoff point of 50.05 mmHg, determined based on the mean of the total population of the registry.
Table 4.
Independent predictors of the need for PPI after TAVI
| Variable | OR (95%CI) | p value |
|---|---|---|
| Conduction disorder | ||
| RBBB or RBBB+AHB | 6.19 (3.56-10.76) | < 0.001 |
| Bioprosthesis type | ||
| CoreValve | 3.16 (1.74-5.72) | < 0.001 |
| Mean gradient | ||
| ≥ 50 mm Hg | 1.86 (1.08-3.20) | 0.025 |
RBBB: right bundle-branch block; AHB: anterior hemiblock; the mean transaortic gradient was the mean found in the population: 50.05 mm Hg. Multiple logistic regression.
Table 5.
Likelihood of PPI within the first 30 days after TAVI according to 3 independent variables on multivariate analysis
| Conduction disorder | Bioprosthesis type | Mean gradient | PPI likelihood (%) within 30 days | |||
|---|---|---|---|---|---|---|
| AHB or LBBB | RBBB or RBBB+AHB | CoreValve | SapienXT | < 50 | ≥ 50 | |
| X | X | X | 4.4 | |||
| X | X | X | 8.0 | |||
| X | X | X | 12.8 | |||
| X | X | X | 21.5 | |||
| X | X | X | 22.4 | |||
| X | X | X | 34.9 | |||
| X | X | X | 47.6 | |||
| X | X | X | 62.9 | |||
PPI: permanent pacemaker implantation; AHB: anterior hemiblock; LBBB: left bundle-branch block; RBBB: right bundle-branch block.
Impact of PPI on hospitalization, clinical outcomes and LVEF
The hospital length of stay in the Group PPI was significantly prolonged (mean = 15.7 ± 25.7 days - Group PPI vs. 11.8 ± 22.9 days - Group non-PPI; p < 0.001). No difference was observed between the groups regarding all-cause mortality (26.7% vs. 25.6% for groups PPI and non-PPI, respectively; p = 0.80) and cardiovascular mortality (14.1% vs. 14.8% for groups PPI and non-PPI, respectively; p = 0.84) during hospitalization.
In the substudy of 287 patients with echocardiograms before the procedure and 1 year after that, 74 patients received PPI. The groups did not differ regarding baseline LVEF (Group PPI: 60.7% ± 12.1% vs. Group non-PPI: 59.0% ± 15.1%; p = 0.15), but differed significantly regarding the 1-year follow-up after TAVI (mean variation: -2.27% ± 13.46 for Group PPI vs. 3.28% ± 11.99 for Group non-PPI; p = 0.01). Baseline LVEF and need for PPI within 30 days after TAVI were the only independent predictors of LVEF worsening over time (estimated coefficient -0.51; 95%CI: -0.62 to -0.40; p < 0.001; and -4.92; 95%CI: -8.14 to -1.69; p = 0.003, R2= 0.35, respectively; Table 6). That negative association of PPI with LVEF had no impact on the NYHA functional class (p = 0.35 on multivariate analysis).
Table 6.
Univariate and multivariate predictors of changes in left ventricular ejection fraction over time (12 month follow-up)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| Clinical variables | ||||
| Age | -0.043 (-0.259 to 0.173) | 0.699 | ||
| Sex | 0.179 (-2.89 to 3.252) | 0.909 | ||
| Hypertension | -3.673 (-6.938 to -0.408) | 0.318 | -0.667 (-3.548 to 2.214) | 0.650 |
| Diabetes mellitus | -1.753 (-5.187 to 1.681) | 0.318 | ||
| eGFR < 60 mL/min | 1.475 (-2.253 to 5.203) | 0.439 | ||
| Paroxysmal/chronic atrial fibrillation | 1.937 (-2.828 to 6.702) | 0.426 | ||
| Coronary artery disease | 0.274 (-2.801 to 3.349) | 0.861 | ||
| Echocardiography | -0.511 (-0.619 to -0.403) | |||
| LVEF | -0.466 (-0.554 to -0.378) | <0.001 | 0.033 (-0.061 to 0.127) | <0.001 |
| Mean gradient (≥ 50.05 mm Hg) | -0.143 (-0.24 to -0.043) | 0.006 | 0.491 | |
| Aortic valve area | -0.216 (-8.227 to 7.795) | 0.958 | -0.131 (-0.286 to 0.024) | |
| LV diastolic diameter | 0.166 (-0.001 to 0.333) | 0.053 | 0.098 | |
| Variables of the procedure | ||||
| Moderate or greater AR | -0.085 (-4.595 to 4.425) | 0.971 | -4.917 (-8.141 to -1.693) | |
| Within 30 days from PPI | -5.55 (-9.221 to -1.879) | 0.003 | 0.003 | |
| CoreValve | -0.708 (-4.577 to 3.161) | 0.720 | ||
| Pre-dilation | -2.516 (-5.648 to 0.616) | 0.117 | 1.652 (-1.772 to 5.076) | |
| HF (III or IV) | 5.578 (1.676 to 9.480) | 0.005 | 0.345 | |
AR: aortic regurgitation; CI: confidence interval; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; LV: left ventricular; PPI: permanent pacemaker implantation; HF: heart failure. Linear regression; multivariate model R2 = 0.347.
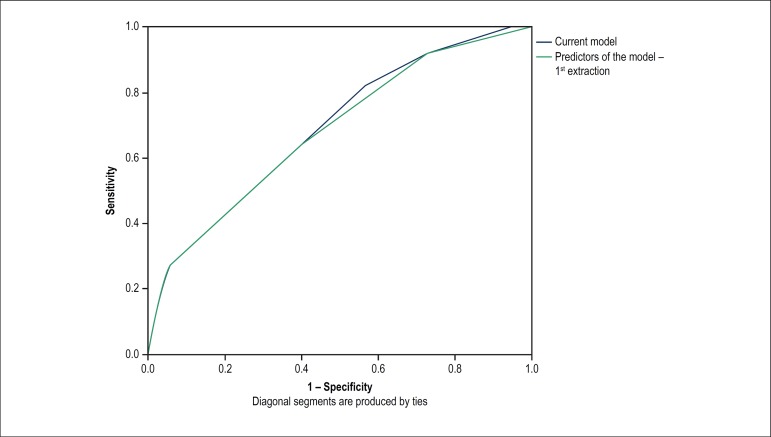
The area under the ROC curve for the model of predictors (Figure 1) showed good competence to predict the need for PPI: 0.69 (95%CI: 0.64 - 0.74).
Figure 1.
ROC curve comparing the performance of the predictors previously published by the Brazilian Multicenter TAVI Registry and the new ones
Discussion
Transcatheter aortic valve implantation has been established not only as an effective treatment for patients for whom conventional surgery is not an option, but also as an alternative to patients at high8 and, more recently, moderate risk. The need for PPI due to total AVB is a frequent complication of TAVI. Under other clinical circumstances, PPI has been associated with left ventricular systolic function impairment, possibly secondary to the negative impact of PPI on LVEF due to the dyssynchrony inflicted by the artificial electromechanical activation on left ventricular performance.9 The major findings of this study are the description of the predictors of need for post-TAVI PPI in the Brazilian population and the description of the unfavorable effect of PPI on LVEF by the end of the first year after the implantation.
The native aortic valve apparatus lies very close to the AV node and His bundle, therefore, TAVI might harm the infra-Hisian conduction system, probably due to direct pressure and compression, hemorrhage/hematoma, ischemia or inflammation of the His bundle and compact AV node during the prosthesis positioning or expansion.4,7,10-12 Thus, heart block can occur early after TAVI. The Valve Academic Research Consortium (VARC) has highlighted the risk of AVB requiring PPI as one of the most relevant complications associated with TAVI.10,13-17 However, TAVI has been shown to improve the left ventricular systolic function,18 but patients requiring PPI might fail to recover as expected due to the right ventricular stimulus, unfavorable to left ventricular systolic performance.4,9,18-21
In this study population, considering a pre-TAVI LVEF similar in both groups and adjusting for clinical, echocardiographic and procedural variables, the patients submitted to post-TAVI PPI showed a significantly reduced LVEF by the end of the first year. In fact, PPI within the first 30 days after TAVI and baseline LVEF were the only factors that significantly worsened left ventricular performance (approximately 6%) in that period. Such data are in accordance with previously published reports.19,21 However, that is not a consensus and has been recently challenged by the findings of other studies,4,20 showing that the issue requires further consideration. However, from the clinical perspective, in our substudy, the negative association of PPI with LVEF had no impact on the NYHA functional class of heart failure. This can be partially explained by the fact that the baseline LVEF was normal in most of the population, because of the small deterioration of LVEF observed in most patients and because of the positive hemodynamic effects related to aortic stenosis repair.
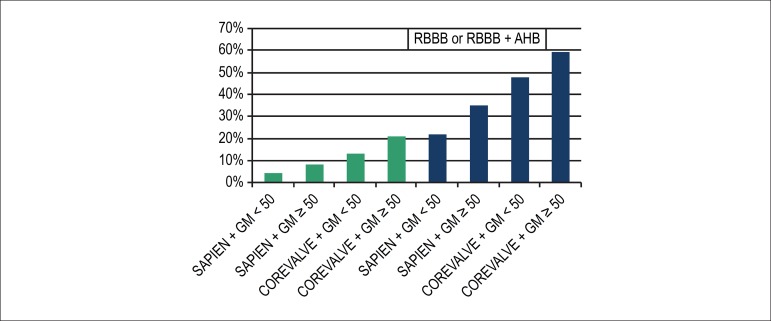
The major findings of the analysis of the risk factors for the need for PPI after TAVI were: 1. One PPI for every five TAVI performed (20.1%); 2. Previous RBBB (isolated or associated with AHB), mean transaortic gradient and use of CoreValve® bioprosthesis were independent predictors of PPI; and 3. The likelihood of PPI after TAVI ranges from 4.4%, when none of those risk factors are present, to 62.9%, in the presence of those three.
The proportion of patients from the Brazilian Multicenter TAVI Registry requiring PPI after TAVI is in accordance with data from European countries (16.3% in the UK TAVI Registry,22 and 13% in the Belgian National Registry23). However, that is approximately half of the 33.7% incidence observed in the German TAVI Registry.24,25 In a recent meta-analysis,26 comprising more than 11000 patients, 17% of them required PPI after TAVI. In another systematic review27 with more than 2000 patients from European and North American retrospective studies, the incidence of PPI after TAVI was 14.2% (ranging from 0 to 34%; median of 9.7%).
The indication for PPI and its time of performance are frequently individualized according to the center and/or the operating physician’s preference. The current European Society of Cardiology guidelines28 on cardiac pacing and cardiac resynchronization therapy recommend, regarding AVB after TAVI, PPI be performed before completing the observation period of 7 days only if the escape rhythm is considered low or unstable (class of recommendation I, level of evidence C).
The finding that PPI prolongs the hospital length of stay is no surprise, being in accordance with previous studies.4,21,29,30 Although this study does not assess costs, the need for PPI is intuitively associated with an increased use of hospital resources and might have resulted in a considerable increase in the general costs of TAVI. In addition, PPI requires an additional surgical procedure that is not risk-free. However, in accordance with previous publications,21 our data show that PPI influences neither global mortality nor cardiovascular mortality.
The reported predictors of PPI after TAVI have shown some variability and heterogeneity between the publications, 4,6,18,20,21,26,29-33 indicating that the mechanism associated with AVB could be multifactorial. Being a factor related to the patient, the conduction disorders have been consistently reported in the literature, but with different importance. While the predictive role of RBBB has been accepted, the meaning of developing left bundle-branch block (LBBB), a common disorder after TAVI, is still uncertain.1,34,35 Likewise, the influence of age and the differences related to sex remain controversial. Some anatomical and echocardiographic characteristics, such as septal wall dimensions, non-coronary cusp thickness, porcelain aorta, aortic subvalvular calcification, valvular ring diameter, have been reported. This analysis of the Brazilian Multicenter TAVI Registry failed to show an association of those characteristics with the need for PPI. However, we found a new independent predictor associated with the likelihood of PPI after TAVI, the mean transaortic gradient. We interpreted that as representing the greater severity of the valvular apparatus calcification. There is neither a study nor a registry investigating directly the effects of that echocardiographic parameter or its influence as a predictor of the need for PPI. Therefore, that finding might have a speculative importance, requiring further investigation.
Regarding the aspects related to the device, there are differences in composition and design, delivery mechanism and tissue penetration ability. In this study, the need for PPI among patients receiving the SapienXT® device (Edwards Lifesciences; Irvine, CA, USA) is very close to that reported in the literature4,28(5.9% - 6.5%). In addition, the PPI rates related to CoreValve® implantation (Medtronic Inc.; Minneapolis, MN, USA) are known to be substantially greater and in accordance with recent publications4,26 (24.5% - 25.8%).
Finally, our data are in accordance with those of most studies and registries, in which previous RBBB (isolated or associated with AHB) and the CoreValve®prosthesis type are almost unanimously accepted as independent predictors of the risk for requiring PPI after TAVI.18,20,21,26,31,33,34,36
Study limitations
This is an analysis from a non-randomized registry, of voluntary participation, which has inherent restrictions, associated with the limitations of retrospective data analysis, issues related to the uniformity of patient selection process and outcome description. This registry represents neither all centers nor the total number of TAVI performed in Brazil. Furthermore, it does not include all devices available for TAVI in the Brazilian market, contemplating only two types of bioprostheses internationally implanted. The PPI was performed at the discretion of the participating centers and the registry had no information on that procedure, and the following aspects could not be assessed: stimulation site, QRS duration, and AVB reversibility potential (up to 50% in some publications1,27,37-40). Finally, the echocardiographic data before the procedure and 1 year after it were available in approximately half of the population (287 patients). The LVEF was reported by each participating center, which can add more variability to the findings.
Conclusion
Permanent pacemaker implantation is the most frequent post-TAVI complication, and its consequences extend beyond the surgical procedure inherent in implantation. In this analysis of the Brazilian Multicenter TAVI Registry, the need for PPI after TAVI is a relatively frequent finding (incidence of 20.1%), and PPI can have adverse effects, such as worse LVEF recovery. In addition, the need for PPI prolonged the post-procedure hospital length of stay, but was not associated with global mortality, cardiovascular death or heart failure functional class worsening. In accordance with previous reports, RBBB (isolated or associated with AHB) and the use of CoreValve®prosthesis were important predictors of the need for PPI after TAVI. In addition, this study identified pre-procedure mean transaortic gradient as a new risk factor. A simple model of predictors (Figure 2) was elaborated to estimate the absolute risk of PPI after TAVI in the Brazilian population. These risk factors can be used to identify individuals at high risk for PPI, which can be a useful tool for resource planning.
Figure 2.
Risk model: likelihood of permanent pacemaker implantation within 30 days after TAVI based on predictors of the Brazilian Multicenter TAVI Registry. RBBB: right bundle-branch block; AHB: anterior hemiblock; the mean transaortic gradient was the mean found in the population: 50.05 mm Hg
Footnotes
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the Residency Conclusion Paper in Hemodynamics by Cláudio Monteiro, from Hospital São Lucas da PUC/RS.
Author contributions
Conception and design of the research: Monteiro C, Caramori PRA, De Brito Junior FS; Acquisition of data: Monteiro C, Caramori PRA, Carvalho LAF, Siqueira DAA, Thiago LEKS, Perin M, Lima VC, Guérios E, De Brito Junior FS; Analysis and interpretation of the data: Monteiro C, Ferrari ADL, Caramori PRA; Writing of the manuscript: Monteiro C; Critical revision of the manuscript for intellectual content: Monteiro C, Ferrari ADL, Caramori PRA, Carvalho LAF, Siqueira DAA, Thiago LEKS, Perin M, Lima VC, Guérios E, De Brito Junior FS.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Muñoz-García AJ, Muñoz-García E, Alonso-Briales JH, Hernandez-Garcia JM. Trastornos de la conducción auriculoventricular tras el implante valvular aórtico transcatéter. Rev Esp Cardiol Suppl. 2015;15(C):44–48. [Google Scholar]
- 2.Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. 2009;53(20):1829–1836. doi: 10.116/j.jacc.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 4.Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement. Pt AJACC Cardiovasc Interv. 2015;8(1):60–69. doi: 10.1016/j.jcin.2014.07.0022. [DOI] [PubMed] [Google Scholar]
- 5.Mollmann H, Kim W-K, Kempfert J, Walther T, Hamm C. Complications of transcatheter aortic valve implantation (TAVI): how to avoid and treat them. Heart. 2015;101(11):900–908. doi: 10.1136/heartjnl-2013-304708. [DOI] [PubMed] [Google Scholar]
- 6.Gensas CS, Caixeta A, Siqueira D, Carvalho LA, Sarmento-Leite R, Mangione JA, et al. Predictors of permanent pacemaker requirement after transcatheter aortic valve implantation: insights from a Brazilian Registry. Int J Cardiol. 2014;175(2):248–252. doi: 10.1016/j.ijcard.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Rubin JM, Avanzas P, del Valle R, Renilla A, Rios E, Calvo D, et al. Atrioventricular conduction disturbance characterization in transcatheter aortic valve implantation with the CoreValve prosthesis. Circ Cardiovasc Interv. 2011;4(3):280–286. doi: 10.1161/CIRCINTERVENTIONS.111.961649. [DOI] [PubMed] [Google Scholar]
- 8.Reinöhl J, Kaier K, Reinecke H, Schmoor C, Frankenstein L, Vach W, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373(25):2438–2447. doi: 10.1056/NEJMoa1500893. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari AD, Borges AP, Albuquerque LC, Sussenbach CP, Rosa PR, Piantá RM, et al. Cardiac pacing induced cardiomyopathy: mith or reality sustained by evidence? Rev Bras Cir Cardiovasc. 2014;29(3):402–413. doi: 10.5935/1678-9741.20140104. http://dx.doi.org/10.5935/1678-9741.20140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda T, Hawley RL, Edwards JE. Lesions of conduction tissue complicating aortic valvular replacement. Chest. 1976;69(5):605–614. doi: 10.1378/chest.69.5.605. [DOI] [PubMed] [Google Scholar]
- 11.Moreno R, Dobarro D, Lopez de Sa E, Prieto M, Morales C, Calvo Orbe L, et al. Cause of complete atrioventricular block after percutaneous aortic valve implantation: insights from a necropsy study. Circulation. 2009;120(5):e29–e30. doi: 10.1161/CIRCULATIONAHA.109.849281. [DOI] [PubMed] [Google Scholar]
- 12.Sinhal A, Altwegg L, Pasupati S, Humphries KH, Allard M, Martin P, et al. Atrio- ventricular block after transcatheter balloon expandable aortic valve implantation. JACC Cardiovasc Interv. 2008;1(3):305–309. doi: 10.1016/j.jcin.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Ghadimi K, Patel PA, Gutsche JT, Sophocles A, Anwaruddin S, Szeto WY, et al. Perioperative conduction disturbances after transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2013;27(6):1414–1420. doi: 10.1053/j.jvca.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J. 2016 Mar 07;37(10):803–810. doi: 10.1093/eurheartj/ehv574. [DOI] [PubMed] [Google Scholar]
- 15.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart. 2011;32(2):205–217. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Valve Academic Research Consortium-2 Updated standardized endpoint definitions for transcatheter aortic valve implantation the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145(1):6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Neragi-Miandoab S, Michler RE. A review of most relevant complications of transcatheter aortic valve implantation. ISRN Cardiol. 2013 May 12;2013:956252–956252. doi: 10.1155/2013/956252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordana F, D'Ascenzo F, Nijhoff F, Moretti C, D'Amico M, Biondi Zoccai G. Meta-analysis of predictors of all-cause mortality after transcatheter aortic valve implantation. Am J Cardiol. 2014;114(9):1447–1455. doi: 10.1016/j.amjcard.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 19.Dizon JM, Nazif TM, Hess PL, Biviano A, Garan H, Douglas PS, et al. PARTNER Publications Office Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart. 2015;101(20):1665–1671. doi: 10.1136/heartjnl-2015-307666. [DOI] [PubMed] [Google Scholar]
- 20.Weber M, Bruggemann E, Schueler R, Momcilovic D, Sinning JM, Ghanem A, et al. Impact of left ventricular conduction defect with or without need for permanent right ventricular pacing on functional and clinical recovery after TAVR. Clin Res Cardiol. 2015;104(11):964–974. doi: 10.1007/s00392-015-0865-9. [DOI] [PubMed] [Google Scholar]
- 21.Urena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A, Dager AE, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129(11):1233–1243. doi: 10.1161/CIRCULATIONAHA.113.005479. [DOI] [PubMed] [Google Scholar]
- 22.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58(20):2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, et al. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12(5):762–767. doi: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- 24.Ledwoch J, Franke J, Gerckens U, Kuck KH, Linke A, Nickenig G, et al. German Transcatheter Aortic Valve Interventions Registry Investigators Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: analysis from the German Transcatheter Aortic Valve Interventions Registry. Catheter Cardiovasc Interv. 2013;82(4):E569–E577. doi: 10.1002/ccd.24915. [DOI] [PubMed] [Google Scholar]
- 25.Hamm CW, Mollmann H, Holzhey D, Beckmann A, Veit C, Figulla HR, et al. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2013;35(24):1588–1598. doi: 10.1093/eurheartj/eht381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siontis GCM, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64(2):129–140. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Bates MG, Matthews IG, Fazal IA, Turley AJ. Postoperative permanente pacemaker implantation in patients undergoing trans-catheter aortic valve implantation: what is the incidence and are there any predicting factors? Interact Cardiovasc Thorac Surg. 2011;12(2):243–253. doi: 10.1510/icvts.2010.256578. [DOI] [PubMed] [Google Scholar]
- 28.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2013;34(29):2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 29.Buellesfeld L, Stortecky S, Heg D, Hausen S, Mueller R, Wenaweser P, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60(6):493–501. doi: 10.1016/j.jacc.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 30.De Carlo M, Giannini C, Bedogni F, Klugmann S, Brambilla N, De Marco E, et al. Safety of a conservative strategy of permanent pacemaker implantation after trasncatheter aortic CoreValve implantation. Am Heart J. 2012;163(3):492–499. doi: 10.1016/j.ahj.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Erkapic D, De Rosa S, Kelava A, Lehmann R, Fichtlscherer S, Hohnloser SH. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol. 2012;23(4):391–397. doi: 10.1111/j.1540-8167.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira E, Ferreira N, Caeiro D, Primo J, Adão L, Oliveira M, et al. Transcatheter aortic valve implantation and requirements of pacing over time. Pacing Clin Electrophysiol. 2013;36(5):559–569. doi: 10.1111/pace.12104. [DOI] [PubMed] [Google Scholar]
- 33.Boerlage-Van Dijk K, Kooiman KM, Yong ZY, Wiegerinck EM, Damman P, Bouma JB, et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37(11):1520–1529. doi: 10.1111/pace.12460. [DOI] [PubMed] [Google Scholar]
- 34.Egger F, Nürnberg M, Rohla M, Weiss TW, Unger G, Smetana P, et al. High-degree atrioventricular block in patients with preexisting bundle branch block or bundle branch block occurring during transcatheter aortic valve implantation. Heart Rhythm. 2014;11(12):2176–2182. doi: 10.1016/j.hrthm.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Van Der Boon RM, Houthuizen P, Nuis RJ, Van Mieghem NM, Prinzen F, De Jaegere PP. Clinical implications of conduction abnormalities and arrhythmias after transcatheter aortic valve implantation topical collection on valvular heart disease. Curr Cardiol Rep. 2014;16(1):429–429. doi: 10.1007/s11886-013-0429-4. [DOI] [PubMed] [Google Scholar]
- 36.Hoyt MJ, Hathaway J, Palmer R, Beach M. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2015;29(5):1162–1166. doi: 10.1053/j.jvca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Rivard L, Schram G, Asgar A, Khairy P, Andrade JG, Bonan R, et al. Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm. 2015;12(2):321–329. doi: 10.1016/j.hrthm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Ramazzina C, Knecht S, Jeger R, Kaiser C, Schaer B, Osswald S, et al. Pacemaker implantation and need for ventricular pacing during follow-up after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37(12):1592–1601. doi: 10.1111/pace.12505. [DOI] [PubMed] [Google Scholar]
- 39.Holmes DR, MacK MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Nuis RJ, Van Mieghem NM, Schultz CJ, Tzikas A, Van der Boon RM, Maugenest AM, et al. Timing and potential mechanisms of new conduction abnormalities during the implantation of the Medtronic CoreValve System in patients with aortic stenosis. Eur Heart J. 2011;32(16):2067–2074. doi: 10.1093/eurheartj/ehr110. [DOI] [PubMed] [Google Scholar]