Abstract
Microglial activation and the release of pro-inflammatory cytokines occur during early glaucoma. However, the exact mechanism underlying the initiation of the microglial activation process remains unclear. Thus, the present study investigated the potential role of a purine receptor subtype, the P2X purinoceptor 7 (P2X7) receptor, during microglial activation in the retinal tissues of a rat chronic ocular hypertension (COH) model. This was achieved by cauterizing 3 of the 4 episcleral veins. Microglial activation and caspase-1 upregulation were observed in COH rat retinas by immunohistochemical and western blotting techniques. Intravitreal injection of 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP), a P2X7 receptor agonist, induced microglial activation in normal rat retinal tissues, which was alleviated by pretreatment with the P2X7 receptor antagonist, Brilliant Blue G (BBG). BBG further attenuated caspase-1 increment in COH rat retinal tissues. The data demonstrated that BBG reduced TUNEL-positive retinal ganglion cells in whole-mount retinal tissues with COH and normal retinal tissues following intravitreal injection with BzATP. One may conclude that the P2X7 receptor may be involved in microglial activation in the COH retina and could be considered a target for neuronal protection in glaucoma.
Keywords: chronic ocular hypertension; microglial activation; retinal ganglion cells; 2′,3′-O-(4-benzoylbenzoyl)-ATP; Brilliant Blue G; P2X purinoceptor 7
Introduction
Glaucoma is a disease, which is characterized by progressive visual loss, irreversible optic nerve degeneration and retinal ganglion cell (RGC) apoptosis. Currently there is a lack of effective therapy for glaucoma, due to the irreversible nature of the induction of the RGCs apoptosis. Although higher intraocular pressure (IOP) is often considered as the major cause for this ophthalmopathy, the treatment that targets the reduction of the IOP is not always successful. This occurs due to abnormal IOP and/or IOP-independent mechanisms underlying the induction of apoptosis of RGCs of the patients with glaucoma (1–4).
It has been reported that the immune-inflammatory response is involved in the pathology of glaucoma (5,6). Microglia are a type of glia cells that act as the main form of active immune defense in the CNS and are implicated in the development of various neurodegenerative diseases (7–9). Once stimulated by infectious agents, microglia become activated, leading to hypertrophy and rapid proliferation. Microglia can scavenge damaged neurons, although their cytokine-releasing function causes inhibition of nerve regeneration. The inflammasome, which is an important part of the innate immune system, is a multi-protein complex consisting of the following parts: 1, nucleotide-binding domain and leucine-rich repeat containing receptors (NLR) also called pattern recognition receptors (PRRs); 2, adaptor protein, mainly the apoptosis-associated speck-like protein containing a caspase-recruitment and activation domain (ASC); 3, the effector, mainly caspase proteins. NLR family is classified to four subfamilies, namely the NLRA, NLRB, NLRC and NLRP subfamilies, on the basis of their N-terminal domain configuration (10). NLRP3 inflammasome activation of microglia is caused by different foreign pathogen associated molecular patterns (PAMPs) and/or innate danger associated molecular patterns (DAMPs), and results in the recruitment of procaspase-1 in order to produce activated caspase-1. This leads to the release of proinflammatory cytokines, such as IL-1β and IL-18.
Recent studies have suggested that microglia are present in the early stage of glaucoma. Using DBA/2J mice, which is a type of spontaneous model of glaucomatous neurodegeneration, Bosco et al demonstrated that microglia activation is mainly evident in the retina at 3 months of age. This process persisted between the 5th and the 8th month of age. The authors of this study suggested that microglia activation might contribute to the onset and/or progression of glaucoma (11). Significant microglia activation was noted in the retinal tissues of experimental glaucoma. Microglia activation was positively associated with the optic nerve axon damage when the IOP was lower than the normal levels (12). The results of this study were in accordance with the data reported in a similar study that was conducted in human glaucoma optic nerve head (13). The aforementioned data suggest that regulation of microglia activation is significant for the development of the glaucomatous optic nerve and/or the protection of RGCs. The compounds minocycline and triptolide, which is a bioactive component isolated from Tripterygium wilfordi Hook F, could improve optic nerve integrity and/or RGCs survival via inhibition of microglial activation in DBA/2J mice (14,15). However, the mechanisms underlying microglial activation in glaucoma are not clear. Thus the investigation of the major pathways that are involved in this process is important in order to identify new regulators of microglial activation and targets for RGCs and/or optic nerve protection.
P2X purinoceptor 7 (P2X7) receptor is a ligand-gated ion channel that is a member of the purine receptor family. P2X7 receptor can mediate cytokine release through microglial activation during the development of CNS diseases (16). The elevated levels of extracellular ATP that act via the stimulation of the P2X7 receptor are toxic to RGCs both in vitro and in vivo (17,18), while the inhibition of the P2X7 receptor could protect RGCs from ischemic neurodegeneration in a human retinal tissue model (19). It is not clear whether the same mechanism of action applies for glaucomatous retina and whether an association with microglial activation exists. In order to address this hypothesis, we investigated the role of P2X7 receptor in microglial activation in a COH model. The data indicate that the intravitreal injection of the P2X7 receptor agonist, 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP), to normal rat eyes, induced microglial activation and RGCs apoptosis, which could be attenuated by pretreatment of Brilliant Blue G (BBG), the antagonist of the P2X7 receptor.
Materials and methods
Rat chronic ocular hypertension (COH) model
All experimental procedures described were in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and the Guidelines of the Yangtz University on the Ethical Use of Animals. Care and use of animals were also approved by the ethics committee of First Affiliated Hospital of Yangtze University. All efforts were made to minimize the number of animals used. Male Sprague Dawley rats that were 3 to 4 weeks of age and weighted 80 to 100 g were used for the experiments. The animals were obtained from the SLAC Laboratory Animal Company (Shanghai, China) and were housed at a 12 h light/dark cycle. A rat COH model was established according to previously published studies (20,21). Briefly, rats were anesthetized with a mixture of ketamine (25 mg/kg, im) and xylazine (10 mg/kg, im), and the eyes were locally anesthetized with 0.4% oxybuprocaine hydrochloride drops (Benoxil, Santen Pharmaceutical, Ishikawa, Japan). A total of 3 episcleral veins were carefully separated from the left eye and cauterized under an OPMI VISU 140 microscope (Carl Zeiss, Oberkochen, Germany). In sham-operated control eyes, the surgery was conducted using a similar procedure, although veins were not occluded. Sham-operations were carried out on the eyes of other rats, and not on the contralateral eye of the operated animal. Following surgery, the eyes were flushed with saline and covered with chlorotetracycline eye ointment (Shanghai General Pharmaceutical Co., Ltd, Shanghai, China) in order to avoid bacterial infection. IOP was measured using a handheld digital tonometer (Tonopen XL; Mentor O&O, Norwell, IL, USA) under general and local anesthesia as described above. The average value of five consecutive acceptable measurements with a deviation of less than 5% (<5%) was recorded. All measurements were conducted at 9 o'clock in the morning in order to avoid possible circadian differences. The IOPs of both eyes were measured prior to surgery (baseline), immediately following surgery (day 0), the first day following surgery (G1 day), the third day following surgery (G3 days) and weekly thereafter. The term G was used for the glaucomatous model under COH surgery.
Intravitreal injection
Intravitreal injection was conducted as previously described (21). The pupil was dilated with tropicamide drops. Subsequently, BzATP (300 µM) (B6396-25MG; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and BBG (1 µM) (B0770-5G; Sigma-Aldrich; Merck KGaA) were dispersed in 2 µl of 0.9% saline. Alternatively, saline was injected into the vitreous space via a postlimbus spot using a microinjector (Hamilton Medical AG, Bonaduz, Switzerland), under a stereoscopic microscope (Carl Zeiss). A 30-gauge needle was inserted 2 mm behind the temporal limbus and directed toward the optic nerve. The eyes that received saline only were used as vehicle controls.
Immunohistochemistry
The method has been previously described (20,21). The retinal sections were examined by immunohistochemistry. The rats were anesthetized with ethyl carbamate (1.25 g/kg, ip; Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) and transcardially perfused with 4% paraformaldehyde (PFA; in 0.1 M phosphate buffer, pH 7.4). The eyes were post-fixed in 4% PFA for 2 to 4 h and then dehydrated with graded sucrose solutions at 4°C (4 h in 20% and overnight in 30% solutions). The retinal tissues were vertically sectioned (14 µm; Leica, Wetzlar, Germany), and the sections were mounted on chrome-alum-gelatin-coated slides (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The sections were blocked for 2 h in 6% normal donkey serum, 1% bovine serum, and 0.2% Triton X-100, and subsequently dissolved in PBS at room temperature. The sections were incubated with the following primary antibodies at 4°C for 24 to 48 h: polyclonal goat anti-Iba-1 (ab107159, 1:2,000; Abcam, Cambridge, MA, USA) and/or polyclonal rabbit anti-P2X7 (ARP-004, 1:200; Alomone Labs, Jerusalem, Israel). The binding sites of the primary antibodies were visualized by incubating the sections with cy3/488-conjugated donkey anti-rabbit (711-165-152) and/or anti-goat (705-545-003) IgG (1:400; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at room temperature. The sections were visualized and photographed with a Leica SP2 confocal laser-scanning microscope. To avoid reconstruction stacking artifacts, double labeling was evaluated by sequential scanning on single-layer optical sections at 1.0 µm intervals.
Western blot analysis
Western blot analysis was conducted as previously described with some modifications (20,21). The retinal tissues were homogenized in radio-immunoprecipitation Assay (RIPA) lysis buffer (Pierce; Thermo Fisher Scientific, Inc.) supplemented with protease and phosphatase inhibitor cocktail (Roche Applied Science, Mannheim, Germany). The concentration of the total proteins was measured using a standard bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). The extracted whole protein samples (50 µg) were resolved by a 10% dodecyl sulfate sodium salt-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electroblotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P; EMD Millipore, Billerica, MA, USA) using a Mini-PROTEAN 3 Electrophoresis System and Mini Trans-Blot Electrophoretic Transfer System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following blocking in 5% nonfat milk at room temperature for 1.5 h, the membranes were incubated overnight at 4°C with the following primary antibodies: monoclonal mouse anti-β-actin (A2228, 1:3,000 dilution; Sigma-Aldrich; Merck KGaA) and monoclonal mouse anti-caspase-1 (sc-392736, D-3; 1:500 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Following washing in Tris-buffered saline-Tween-20 (TBST) for three times (5–10 min per time), the blots were incubated with horseradish-peroxidase (HRP)-conjugated donkey anti-mouse secondary antibody (715-035-151, 1:5,000; Jackson ImmunoResearch Laboratories, Inc.) for 2 h at room temperature. The membranes were washed in TBST for three times and finally incubated with chemofluorescent reagent (Pierce; Thermo Fisher Scientific, Inc.). The membranes were exposed to X-ray films in a dark room. The experiments were conducted in triplicate. The protein bands were quantitatively analyzed with the NIH Image Analysis software (Image J, version 1.38x; National Institutes of Health, Bethesda, MD, USA).
Apoptosis assay
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was used to measure apoptosis (20,22) in whole flat-mounted retinal tissues, using a DeadEnd Fluorometric TUNEL System G3250 kit (Promega Corporation, Madison, WI, USA) according to the manufacturer's instructions. TUNEL signals were visualized with a confocal laser scanning microscope using a 20× objective (FluoView 1000; Olympus Corporation, Tokyo, Japan). Retinal tissues were mounted on top of ganglion cell layers (GCL), and serial deep scanning was carried out only in the GCL according to the 4,6-diamino-2-phenyl indole (DAPI) staining results. All TUNEL-positive signals that were merged adequately with DAPI in each retinal tissue in GCL were counted. The inner nuclear layer (INL) exhibited a larger cell body in GCL compared with the outer nuclear layer (ONL).
Data analysis
The data derived from the TUNEL assay and the IOP monitoring were presented as mean ± SEM. For western blotting experiments, the expression levels of caspase-1 were initially normalized to the corresponding pro-caspase-1 levels and subsequently to the β-actin expression levels. The relative expression levels were averaged for all samples. The mean values of the data obtained during different postoperational time periods and BBG treatments, were normalized according to the mean value of the control group. The data were presented as the mean ± SEM. A one-way ANOVA with LSD's post hoc test (for protein analysis and RGCs apoptosis), or t-test (paired data for IOP analysis) were used and P<0.05 was considered to indicate a statistically significant difference.
Results
Microglial activation in COH retinas
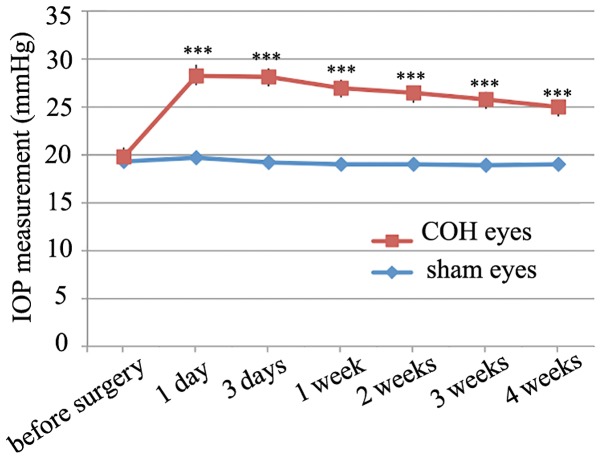
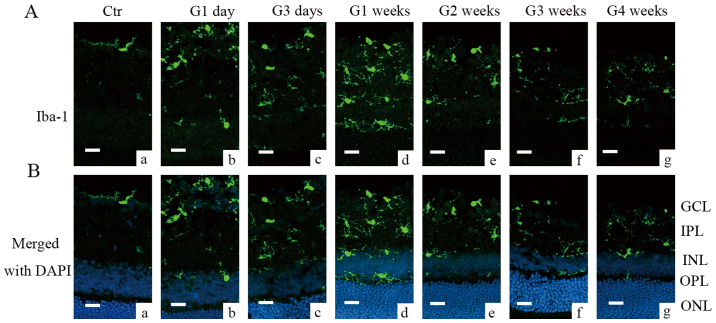
The rat COH model was successfully established and the changes in the IOP of the operated eyes were similar to those reported previously (20,21). As Fig. 1 showed, the average IOP of the operated eyes was higher (25.1±0.7 to 28.2±1.2 mmHg, n=10–74) compared to that noted in the controls (19.0±0.6 to 19.7±0.9 mmHg, n=10–65) and the non-operated eyes (19.8±1.0 mmHg, n=9, all P<0.001). Although microglial activation in rat retinal tissues following induction of IOP by a different COH model has been reported (23,24), the mechanism is unclear. In order to add insight to the time course of the COH retinal microglial activation, we initially measured the change in the Ionized calcium binding adaptor molecule 1 (Iba-1) at different time points following COH surgery by immunohistochemistry. Iba-1 was used to label the majority of the activated microglia cells in the retinal tissues (25). Fig. 2 indicated that the Iba-1 signal was minimal in the control group [Fig. 2A and B (a)]. The Iba-1 signals were enhanced from week G1 to week G4 notably in the inner retina including the GCL and the IPL. The data showed effective labeling of the activated microglia cells with enlarged round cell bodies [Fig. 2A and B (b-g)]. The microglial activation was robust in the retinal tissues of the G3 days, the G1 and G2 weeks. During the G3 and G4 weeks the microglial activation was lessened, although it remained to a higher level compared with the controls.
Figure 1.
IOP measurements in COH eyes compared with sham controls. ***P<0.001 vs. the corresponding sham control eyes at the same time point. IOP, intraocular pressure; COH, chronic ocular hypertension.
Figure 2.
Microglial activation in COH retinas. (A) Images a-g present microglial activation indicated by immunohistochemistry with Iba-1 in control and COH rat retinas. (B) Images a-g are the merged pictures of Iba-1 and DAPI staining in the same slices. Scale bars=20 µm. Ctr, control; G, glaucomatous model; COH, chronic ocular hypertension; G1 day, 1 day following COH surgery; G3 days, 3 days following COH surgery; G1 week, 1 week following COH surgery; G2 weeks, 2 weeks following COH surgery; G3 weeks, 3 weeks following COH surgery; G4 weeks, 4 weeks following COH surgery; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; Iba-1, ionized calcium binding adaptor molecule 1.
Involvement of the P2X7 receptor in COH retinal microglial activation
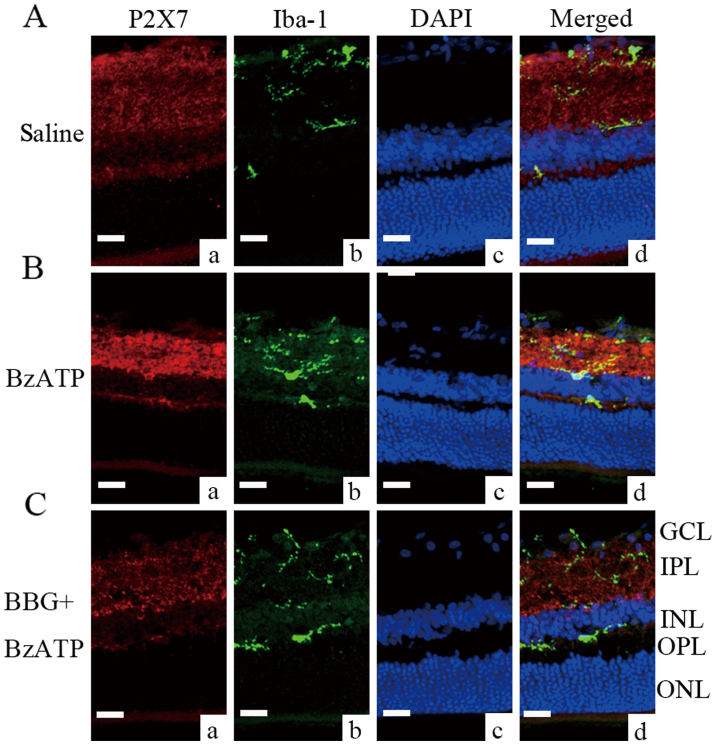
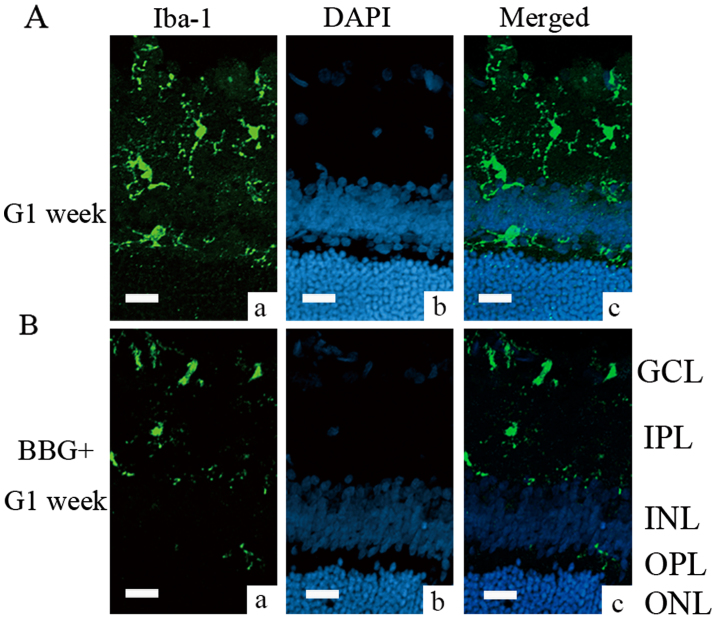
To identify whether the P2X7 receptor is involved in microglial activation, an intravitreal injection of BzATP (300 µM) was conducted. BzATP is a P2X7 receptor agonist. This compound causes an increase in P2X7 expression in the inner plexiform layer [Fig. 3B (a)] and robust microglial activation [Fig. 3B (b)] in normal retinas. The pretreatment with the P2X7 receptor antagonist BBG (1 µM) alleviated the effect of BzATP [Fig. 3C (a and b)]. In addition, the expression of the P2X7 receptor was evident but not limited in enlarged cell bodies of Iba-1-positive microglia [Fig. 3B (b and d)]. Subsequently, the ability of BBG to inhibit microglial activation was tested in COH retinal tissues. The G3 days and the G1 week were selected as optimal time periods for microglial activation compared with the other groups (data not shown for the G3 days period). Microglial activation in retinas that were pretreated with BBG at G1 week was alleviated compared with saline-injected G1 week retinas (Fig. 4). There were smaller cell bodies of microglia and less cellular staining for Iba-1 in the BBG treated group.
Figure 3.
Microglial activation and alleviation by intravitreal injection of BzATP and BBG, respectively in normal retinas. (A) Immunostaining images of (a) the P2X7 receptor, (b) Iba-1 and (c) DAPI staining in saline-injected retinas, and (d) the merged image. (B) Immunostaining images of (a) the P2X7 receptor, (b) Iba-1 and (c) DAPI staining in BzATP-injected retinas, and (d) the merged image. (C) Immunostaining images of (a) the P2X7 receptor, (b) Iba-1 and (c) DAPI staining in BBG plus BzATP-injected retinas, and (d) the merged image. Scale bars=20 µm. BzATP, 2′,3′-O-(4-benzoylbenzoyl)-ATP; BBG, Brilliant Blue G; Iba-1, ionized calcium binding adaptor molecule 1; P2X7, P2X purinoceptor 7.
Figure 4.
Alleviation of microglial activation during week G1 in retinal tissues that were pretreated with BBG. (A) Immunostaining images of (a) Iba-1 and (b) DAPI staining in retinal tissues of G1 week, and (c) the merged image. (B) Immunostaining images of (a) Iba-1 and (b) DAPI staining in retinal tissues that were pretreated with BBG at G1 week, and (c) the merged image. Scale bars=20 µm. BBG, Brilliant Blue G; Iba-1, ionized calcium binding adaptor molecule 1; G, glaucomatous model; G1 week, 1 week following chronic ocular hypertension surgery; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Effect of BBG on caspase-1 expression in COH retinas
Caspase-1, is cleaved from pro-caspase-1 and plays a key role in the processing of pro IL1β to IL1β via the canonical inflammasome pathway, which occurs during inflammation caused by microglial activation in ischemia injury (26–28). The pathological state of ischemia injury includes retinal injury following ischemia. In an acute glaucoma model, caspase-8 was reported to mediate the NRLP1/NLRP3 inflammasome formation, whereas a recent study demonstrated that the main signaling pathway that was involved notably the NF-κB pathway (29,30). In the present study, the association of caspase-1 expression with microglial activation was examined in the COH model. On day 1 (G1 day) the protein levels of caspase-1 were increased to 141.5±13.5% (n=3, P=0.019) compared with the control tissues (Fig. 5). The expression of this protein was further increased to 175.8±14.0% (n=3, P<0.001) and 169.5±14.9% (n=3, P=0.001) compared with the control tissues on the G3 days and at the G1 week, respectively (Fig. 5). Caspase-1 expression was retained at high levels during the G2 (161.5±8.8%, n=3, P=0.002) and G3 weeks (154.8±16.2%, n=3, P=0.004) compared with the control group. Subsequently, at the G4 weeks, the caspase-1 protein levels were decreased to similar levels noted for the control group (115.6±15.0%, n=3, P=0.321). Concomitantly, a single group (G1 week) with apparent microglial activation was selected in order to assess the effects of the P2X7 receptor antagonist BBG on the expression levels of caspase-1. The pretreatment of the tissues with BBG significantly attenuated the increase in the expression of caspase-1 (102.8±12.8% of control, n=3, P=0.001 compared with G1 week, last lane of the immunoblot in Fig. 5A).
Figure 5.
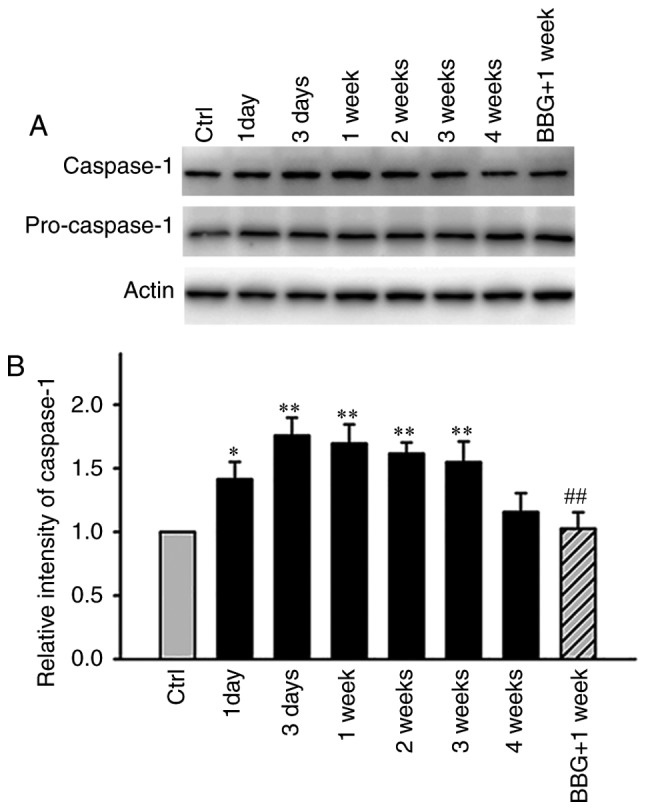
Differences in the protein levels of caspase-1 in COH retinas and BBG-induced effects on caspase-1 expression in G1 week retinas. (A) Representative immunoblots presenting the pro-caspase-1 and caspase-1 levels in the Ctr and COH retinal extracts at different postoperational time periods. The immunoblot of G1 week retinas following BBG pretreatment is displayed in the last lane. (B) Bar chart summarizing the average densitometric quantification of immunoreactive bands of the relative caspase-1 expression, according to pro-caspase-1 among the different groups (n=3/group). *P<0.05 and **P<0.01 vs. Ctr; ##P<0.01 vs. 1W (G1 week). Ctr, control; G, glaucomatous model; COH, chronic ocular hypertension; BBG, Brilliant Blue G; 1D, 1 day following COH surgery; 3D, 3 days following COH surgery; G1 week/1W, 1 week following COH surgery; 2W, 2 weeks following COH surgery; 3W, 3 weeks following COH surgery; 4W, 4 weeks following COH surgery; BBG+1W, BBG pretreatment in G1 week retinas.
Neuron protection by BBG occurs both in COH retinas and normal retinas pre-treated with BzATP
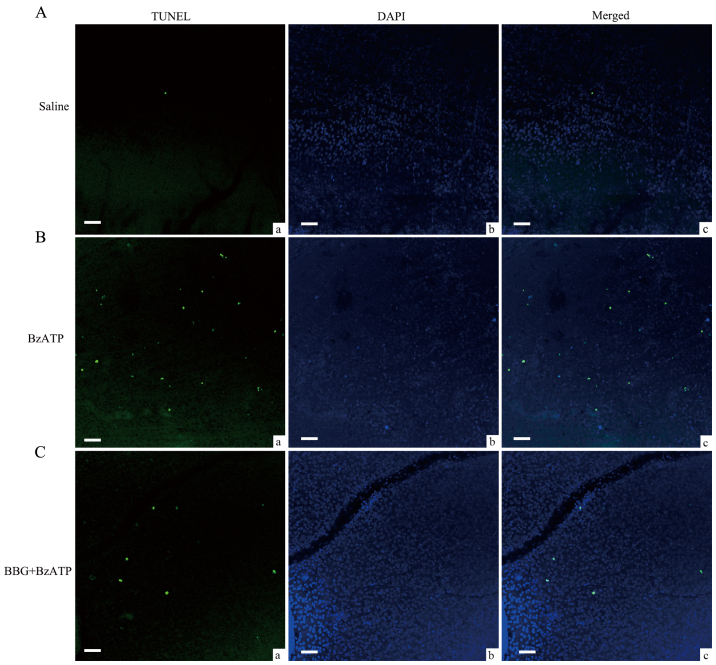
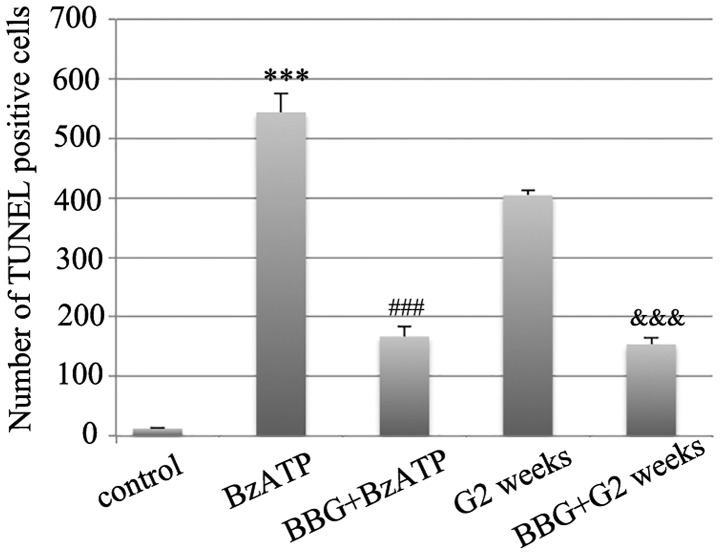
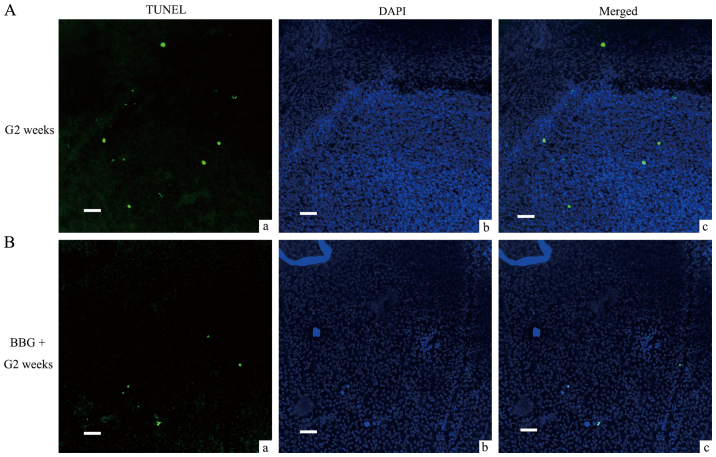
It was suggested that microglial activation was related with RGCs loss and nerve injury (12,24). The aforementioned data suggested that the agonist and the antagonist of the P2X7 receptor could induce and inhibit microglia activation, respectively in normal retinas. Consequently, we further explored whether they would affect the induction of RGCs apoptosis under different conditions. BzATP induced microglial activation in normal retinas and caused abnormal induction of RGCs apoptosis as demonstrated by TUNEL assay (Fig. 6). As summated in Fig. 8, the mean number of TUNEL-positive cells in saline injected retinas was 12.7±1.7, while in BzATP-injected retinas the number was increased to 534.7±31.9, which was considerably higher compared with the saline group (n=6, P<0.001). The pretreatment of the tissues by BBG significantly reduced the number of TUNEL-positive cells (167±17.5, n=6, P<0.001 compared with BzATP group). We further tested the protective function of BBG on the retinal tissues during the G2 weeks period. The G3 days and/or the G1 week time periods were not selected since in the previous study the induction of RGCs apoptosis was not significant until the G2 weeks period (20,21). BBG prevented the induction of RGCs apoptosis in the retinal tissues at the G2 weeks period (Figs. 7 and 8, 154.2±11.3 vs. 404±8.8, n=6, P<0.001), which was consistent with the results reported from the human retinal model of the ischemic neurodegeneration (18).
Figure 6.
Induction of retinal ganglion cell apoptosis following the activation of the P2X7 receptor via an intravitreal injection of BzATP. (A) Representative images of (a) TUNEL staining, (b) DAPI staining and (c) the merged staining image of retinal tissues in the saline group. (B) Representative images of (a) TUNEL staining, (b) DAPI staining and (c) the merged staining image of retinal tissues in the BzATP group. (C) Representative images of (a) TUNEL staining, (b) DAPI staining and (c) the merged staining image of retinal tissues of BBG treatment with BzATP. Scale bars=50 µm. P2X7, P2X purinoceptor 7; BzATP, 2′,3′-O-(4-benzoylbenzoyl)-ATP; BBG, Brilliant Blue G.
Figure 8.
Bar chart depicting the data obtained in the TUNEL assay, showing the average number of TUNEL-positive cells in the whole flat-mount retinal tissues under different conditions. BBG (1 µM, 2 µl) pretreatment was injected 3 days prior to saline and BzATP (300 µM, 2 µl) injections or COH surgery. ***P<0.001 vs. control; ###P<0.001 vs. BzATP; &&&P<0.001 vs. G2 weeks. BzATP, 2′,3′-O-(4-benzoylbenzoyl)-ATP; BBG, Brilliant Blue G; COH, chronic ocular hypertension; G, glaucomatous model; G2 weeks, 2 weeks following COH surgery.
Figure 7.
Alleviation of the induction of retinal ganglion cell apoptosis following pretreatment with BBG in COH retinal tissues. (A) Representative images of (a) TUNEL staining, (b) DAPI staining and (c) the merged staining image of retinal tissues in the G2 weeks group. (B) Representative images of (a) TUNEL staining, (b) DAPI staining and (c) the merged staining image of retinal tissues that were pretreated with BBG during the G2 weeks. Scale bars=50 µm. BzATP, 2′,3′-O-(4-benzoylbenzoyl)-ATP; BBG, Brilliant Blue G; COH, chronic ocular hypertension; G, glaucomatous model; G2 weeks, 2 weeks following COH surgery.
Discussion
Although generalized cascades regarding the induction of RGCs apoptosis and cell death in glaucoma have been well reviewed (2,4), the initial pathways involved in these processes remain poorly defined. RGCs respond to the mechanical force caused by increased IOP with pannexin-mediated ATP release and autostimulation of the P2X7 receptor (31). However, whether the downstream signaling contributes to RGCs injury has not been discovered to date. In the present study, microglial activation was evident in retinal tissues with COH following 1 day of surgery, whereas the P2X7 receptor inhibitor BBG could alleviate the induction of RGCs apoptosis caused by high IOP during inhibition of microglial activation.
The P2X7 receptor has been implicated in CNS-induced inflammation (32,33). Furthermore, the inflammation caused by microglial activation was frequently reported in various neurodegenerative diseases (34,35). Both microglial activation and P2X7 receptor were reported to be involved in neuron injury in tissues of glaucomatous optic nerves and/or retinas (12,13,16,17). In addition, the P2X7 receptor agonist BzATP was shown to cause microglial activation and induction of RGCs apoptosis in normal rat retina, which could also be attenuated by BBG to some extent. Moreover, BBG reduced the elevated expression levels of caspase-1 in COH retinal tissues at the G1 week. The results suggested that P2X7 receptor was involved in the microglial activation via caspase-1 signaling and resulted in the induction of RGCs apoptosis in retinal tissues with COH.
Several inconsistencies were noted with regard to the results of the present study. Specifically, the autostimulation of the P2X7 receptor has been shown to be reduced under IOP, as reported by Lim et al in a recent paper (36). This study demonstrated in vitro and in vivo that cytokine IL-3 release via mechanosensitive autostimulation of the P2X7 receptor alleviated RGCs injury in IOP tissues, which contradicts to our findings. This inconsistency might result from the different IOP models. The study by Lim et al (36) used a model by direct injection of PBS into the anterior chamber in order to cause an instant IOP elevation and animals were sacrificed 24 h following the IOP elevation. Microglial activation in the acute phase of inflammation exhibited beneficial effects by the induction of immune reactions and/or the stimulation of macrophages with regard to the elimination of hazardous factors. The minor microglial activation might serve as a stressor in order to improve the immune function of the body. However, the continuous activation of microglia, as demonstrated in the current COH model and/or the activation of P2X7 with BzATP, could lead to constant cytokine release and/or gliosis which are considered obstacles for tissue regeneration (25).
In the present study, the mechanism by which the P2X7 receptor is involved in the activation of microglia with COH was not fully elucidated, although its large pore formation across the cell membrane facilitated the efflux of K+ and the influx of Ca2+ (37). ATP and K+ are released following apoptosis and necrosis of cells under high IOP and/or ischemia (normal IOP) in glaucoma. During these processes both innate DAMPs can activate the NLRP3 inflammasome (38). Although it has been shown that ATP activates NLRP3 in biovine monocytes in a P2X7-independent fashion (39), the studies that have been conducted in mice peritoneal microglia cells demonstrated that the P2X7 receptor directly interacted with the NLRP3 inflammasome (32). In this study, the NLRP3 protein levels were reduced by ATP stimulation in genetic ATP resistant microglia cells (N13ATPR), which confirmed that the activation of NLRP3 occurs by the P2X7 receptor. The reduction in NLRP3 levels was characterized by reduced P2X7 receptor expression in N13ATPR cells compared with the wild type microglia cell line (N13wt). It should be emphasized that microglia activation in retinal tissues under higher IOP in vivo may influence the expression of the P2X7 receptor and consequently the activation of NLRP3 activation from single stimuli (such as LPS, ROS, BzATP or ATP) applied to in vitro cultured cells. This is mainly due to the contribution of glial cells that regulate K+ homeostasis in retinas especially under higher IOP condition (40).
Acknowledgements
This study was supported by the Natural Science Foundation of Hubei Province (no. 2014CFB213), Health and Birth Control Department of Hubei Province (no. 81000380/H1204) and Jingzhou Science and Technology funding (no. 2014AC47B).
References
- 1.Shiose Y, Kitazawa Y, Tsukahara S, Akamatsu T, Mizokami K, Futa R, Katsushima H, Kosaki H. Epidemiology of glaucoma in Japan-a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35:133–155. [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Crish SD, Calkins DJ. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience. 2011;176:1–11. doi: 10.1016/j.neuroscience.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickells RW. From ocular hypertension to ganglion cell death: A theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287. doi: 10.3129/canjophthalmol.i07-036. [DOI] [PubMed] [Google Scholar]
- 5.Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009;88:825–830. doi: 10.1016/j.exer.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol. 2013;13:23–31. doi: 10.1016/j.coph.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naert G, Rivest S. The role of microglial cell subsets in Alzheimer's disease. Curr Alzheimer Res. 2011;8:151–155. doi: 10.2174/156720511795256035. [DOI] [PubMed] [Google Scholar]
- 8.Wang XJ, Yan ZQ, Lu GQ, Stuart S, Chen SD. Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: Role of microglia. Neurochem Int. 2007;50:39–50. doi: 10.1016/j.neuint.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen S, Imitola J, Ayuso-Sacido A, Wang Y, Starossom SC, Kivisäkk P, Zhu B, Meyer M, Bronson RT, Garcia-Verdugo JM, Khoury SJ. Reversible neural stem cell niche dysfunction in a model of multiple sclerosis. Ann Neurol. 2011;69:878–891. doi: 10.1002/ana.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagenals M, Skeldon A, Saleh M. The inflammasome: In memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519:599–620. doi: 10.1002/cne.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: Spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010;51:6448–6460. doi: 10.1167/iovs.10-5284. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- 14.Bosco A, Inmma DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–1446. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Wu L, Guo X, Wang D, Li Y. Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the DBA/2J mouse model of glaucoma. Ocul Immunol Inflamm. 2013;21:378–389. doi: 10.3109/09273948.2013.806989. [DOI] [PubMed] [Google Scholar]
- 16.Monif M, Burnstock G, Williams DA. Microglia: Proliferation and activation driven by the P2×7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2×7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH. Stimulation of the P2×7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyadurupola N, Sidaway P, Ma N, Rhodes JD, Broadway DC, Sanderson J. P2×7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Invest Ophthalmol Vis Sci. 2013;54:2163–2170. doi: 10.1167/iovs.12-10968. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Miao Y, Wang XH, Wang Z. Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal ganglion cell apoptosis in a rat experimental glaucoma model. Neurobiol Dis. 2011;43:455–464. doi: 10.1016/j.nbd.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Dong LD, Gao F, Wang XH, Miao Y, Wang SY, Wu Y, Li F, Wu J, Cheng XL, Sun XH, et al. GluA2 trafficking is involved in apoptosis of retinal ganglion cells induced by activation of EphB/EphrinB reverse signaling in a rat chronic ocular hypertension model. J Neurosci. 2015;35:5409–5421. doi: 10.1523/JNEUROSCI.4376-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Tay SS, Ng YK. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp Brain Res. 2000;132:476–484. doi: 10.1007/s002210000360. [DOI] [PubMed] [Google Scholar]
- 24.Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962–2968. [PubMed] [Google Scholar]
- 25.Ellis-Behnke RG, Jonas RA, Jonas JB. The microglial system in the eye and brain in response to stimuli in vivo. J Glaucoma. 2013;22(Suppl 5):S32–S35. doi: 10.1097/IJG.0b013e3182934aca. [DOI] [PubMed] [Google Scholar]
- 26.Tsung A, Tohme S, Billiar TR. High-mobility group box-1 in sterile inflammation. J Intern Med. 2014;276:425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu J, Kang N, Wang X. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediators Inflamm. 2014;2014:370530. doi: 10.1155/2014/370530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvoriantchikova G, Hernandez E, Grant J, Santos AR, Yang H, Ivanov D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Invest Ophthalmol Vis Sci. 2011;52:7187–7194. doi: 10.1167/iovs.11-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J, et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma; Proc Natl Acad Sci USA; 2014; pp. 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J Neuroinflammation. 2015;12:137. doi: 10.1186/s12974-015-0360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2×7 receptors. J Physiol. 2012;590:2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschini A, Capece M, Chiozzi P, Falzoni S, Sanz JM, Sarti AC, Bonora M, Pinton P, Di Virgilio F. The P2×7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015;29:2450–2461. doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 33.Murphy N, Cowley TR, Richardson JC, Virley D, Upton N, Walter D, Lynch MA. The neuroprotective effect of a specific P2×7 receptor antagonist derives from its ability to inhibit assembly of the NLRP3 inflammasome in glial cells. Brain Pathol. 2012;22:295–306. doi: 10.1111/j.1750-3639.2011.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizza V, Agresta A, D'Acunto CW, Festa M, Capasso A. Neuroinflammation and ageing: Current theories and an overview of the data. Rev Recent Clin Trials. 2011;6:189–203. doi: 10.2174/157488711796575577. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JC, Lu W, Beckel JM, Mitchell CH. Neuronal release of cytokine IL-3 triggered by mechanosensitive autostimulation of the P2×7 receptor is neuroprotective. Front Cell Neurosci. 2016;10:270. doi: 10.3389/fncel.2016.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2×7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 38.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Hussen J, Düve A, Koy M, Schuberth HJ. Inflammasome activation in bovine monocytes by extracellular ATP does not require the purinergic receptor P2×7. Devel Comp Immunol. 2012;38:312–320. doi: 10.1016/j.dci.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Ji M, Miao Y, Dong LD, Chen J, Mo XF, Jiang SX, Sun XH, Yang XL, Wang ZF. Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model. J Neurosci. 2012;32:12744–12755. doi: 10.1523/JNEUROSCI.1291-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]