Abstract
Skin can be infected by many types of microorganisms, most commonly by gram-positive strains of Staphylococcus and Streptococcus spp. Treatment of Staphylococcus aureus (S. aureus) infections, particularly that of methicillin resistant Staphylococcus aureus (MRSA), is a challenge in clinical practice. Ozone therapy has proven to be one of the strongest antiseptics against the majority of microorganisms involved in skin infections. The purpose of the present study was to evaluate the microbicidal effects of topical ozone therapy on S. aureus and MRSA, and determine the clinical efficacy of ozone therapy on patients with MRSA skin infection. Microbicidal effects of ozonated oil and ozonated water were determined by plating and Kirby Bauer methods. Clinical efficacy and safety of topical ozone were evaluated in two cases with skin MRSA infection. The killing rates of ozonated oil for S. aureus and MRSA were greater when compared with the control oil group. Almost 100% of S. aureus were eliminated by ozonated oil following 5 min. Almost 100% MRSA were eliminated by ozonated oil following 15 min. In addition, 100% S. aureus and 100% MRSA were eliminated by ozonated water in 1 min. The inhibition zone diameters of ozonated oil for S. aureus and MRSA were 17 and 13 mm, respectively, which were significantly larger than the control group. Both cases of skin MRSA infection were completely healed with ozone therapy. In conclusion, ozone therapy is a potential treatment for S. aureus and MRSA skin infection as it has great efficacy, few side effects and low-costs.
Keywords: ozone therapy, Staphylococcus aureus, methicillin resistant Staphylococcus aureus, skin infection
Introduction
Skin and soft tissue bacterial infections occur frequently in the general population. They are characterized by erythema, edema, and/or inflammation (1). and usually begin with an inflammatory process in epidermis, dermis, or subcutaneous tissues, they can spread to other parts of the body, leading to more serious symptoms (2).
Antibiotic therapy is the option of choice for the treatment. However, most of antibiotics could lose their potency over time due to the antimicrobial resistance (3). Microbial resistance to Staphylococcus aureus (S. aureus), especially methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant-MRSA, is a grand challenge in clinical practices globally (4,5).
Ozone is naturally occurring gaseous molecule of triatomic allotrope of oxygen, formed recombination of oxygen atoms and represented as O3 (6,7). The original application of Ozone was to sterilize microorganisms in drinking water (8). Now ozone has been used for treatment of open wounds, Herpes Zoster and Herpes Simplex (9,10), because of its anti-microorganism effectiveness (11). Additionally, ozone has other advantages like such as improving wound healing, enhancing immune, no side effect, no-toxic, environmental friendly and high efficacy (12). A few studies have shown that ozone therapy is efficient in killing many kinds of microorganisms, such as S. aureus, Streptococci spp, Escherichia coli, Enterococcus faecalis, and P. aeruginosa (13,14).
Studies also have shown that ozone therapy can disinfect against S. aureus and MRSA strain in vitro (14,15). In vivo studies have suggested that ozone therapy is safe and exhibits antibacterial effects for the treatment of peritonitis (16,17). Ozone has shown its efficacy on healing MRSA skin infections when combined with other drugs (18,19). However, the effect of ozone therapy alone in the treatment of MRSA skin infections is not to be determined.
This study aims to evaluate the microbicidal effects of topical ozone therapy on S. aureus and MRSA and determine its clinical efficacy on MRSA skin infections.
Materials and methods
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Third Xiangya Hospital, Central South University (Changsha, China) and was carried out in accordance with the approved guidelines. All patients provided written informed consent.
Material and equipment
The bacterial strains used in this study were S. aureus (ATCC 6538) and MRSA (ATCC 43300). Bacterial culture medium was purchased from Zhengzhou An Tu Biological Engineering Co., Ltd. (Zhengzhou, China). Dimethylsulfoxide (DMSO) was from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany. Ozonated oil at the peroxide value of 2,000 to 2,200 mmol-equiv/Kg [provided by Hunan Health Care Technology Co., Ltd. (Hunan, China)] was obtained from the chemical reaction between ozone and camellia oil because of its high composition of unsaturated fatty acids. Ozonated water was created by Ozone Water Generating Instrument (from Hunan Health Care Technology Co., Ltd.) at the Dermatology Department of the Third Xiangya Hospital.
Plating method
The bacterial strains were diluted with PBS to get the concentration reaching at 108 CFU/ml.
For ozonated oil: The test oil suspension was constituted by 400 µl ozonized oil mixes, 50 µl DMSO and 50 µl microorganism suspension. Control oil suspension was constituted by 400 µl basal oil mixes 50 µl DMSO and 50 µl microorganism suspension. Both groups were incubated in 37°C incubator for 1, 5, 10, 15, 20, and 30 min respectively. Then the suspension was plated into Petri dishes and grew at 37°C for 24 h. Then the number of colonies in each agar plate was calculated. The control suspension constituted by 400 µl normal saline mixes 50 µl DMSO and 50 µl microorganism suspension was used as control to calculate the killing rate. Killing Rate (%)=(control colony number-oil colony number)/control colony number).
For ozonated water: 1.0 ml of bacteria liquid was mixed with 4.0 ml of ozonated water or PBS. 0.5 ml mixture was added into 4.5 ml neutralizer (Phosphate buffer solution including sodium thiosulfate) after oscillation for one minute. After that the sample was planted into Petri dishes and cultured in an incubator chamber at 37°C. The number of colonies in each agar plate was calculated after growing for 24 h. Killing Rate (%)=(control colony number-tested colony number)/control colony number).
Kirby bauer method
The bacteria samples prepared above were dropped on sterile cotton swabs. The surface was lightly and uniformly inoculated by cotton swab on agar plate. Then the scrips impregnated with ozonated oil or control oil were pasted into the agar plate, followed by incubation at 37°C for 16–18 h. The inhibitory ring test was performed. If the inhibition zone diameter was bigger than 7 mm, it was considered effective; otherwise, it was not.
Patients and ozone treatment
The Ethnic Committee of Third Xiangya Hospital approved the study, and the informed consents of all the participants were obtained. Two patients with skin MRSA infection were recruited in this study. In addition to skin infections, no other diseases were present in the two patients. The skin lesions were washed or debrided by ozonated water in our therapy room once a day, followed by application of ozonated oil twice per day at home. Antibiotics and other drugs were not administrated during the ozone therapy.
Bacteria culture, drug sensitive test and PCR test of lesions
The lesions of patients were inoculated in the blood plate, then cultured in an incubator chamber with 5% CO2 concentration at 35°C for 12 h. If there was colony formation of microorganism, the microorganism colony was stained by Gram staining. After confirming the gram-positive bacterial by the microscope examination, three bacterial colonies were added into physiological saline and were prepared with 0.5 turbidity ratio in VITEK2 system (VITEK2 gram-positive identification card) by electronic turbidimeter (DensiCHEK Plus, BioMérieux, Durham, NC, USA). Then bacterial species and drug sensitivity results of the samples were detected by Automatic Microorganisms Identification System (VITEK2-compact). The drug sensitivity results were further confirmed by VITEK2 AST-GP67 Test kit according to the manufacturer's protocol. Following the preliminary result of MRSA infection detected by Automatic Microorganisms Identification System, the microorganism colony was further confirmed as MRSA infection by the MRSA Quantitative Standards kit (no. Z-DD-0096-B; Liferiver Bio-Tech, San Diego, CA, USA) according to the manufacturer's protocol in CFX96™Red-Time System.
Statistical analysis
The data were analyzed in SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). t-test was used to assess statistical differences between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Killing rate of ozone on S. aureus and MRSA
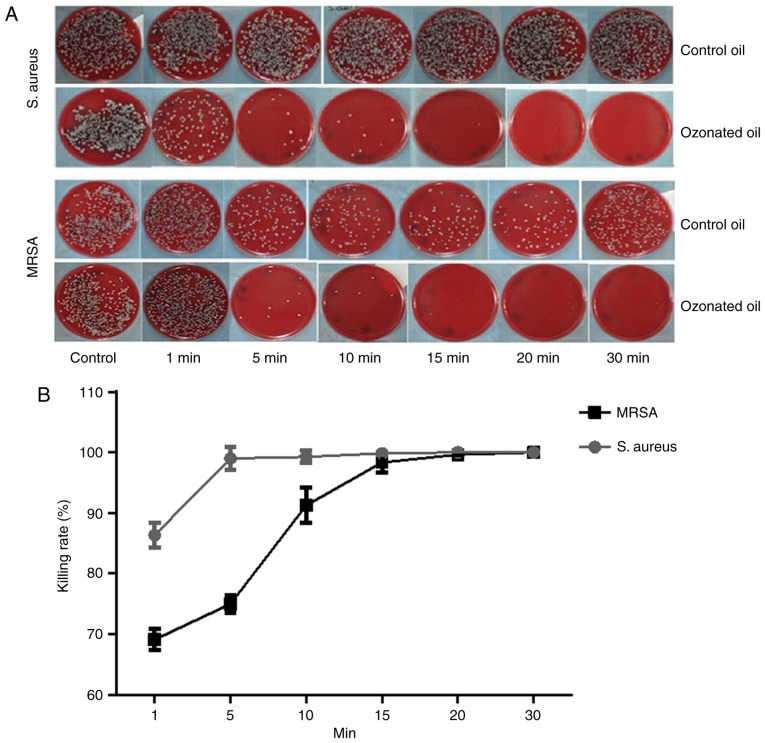
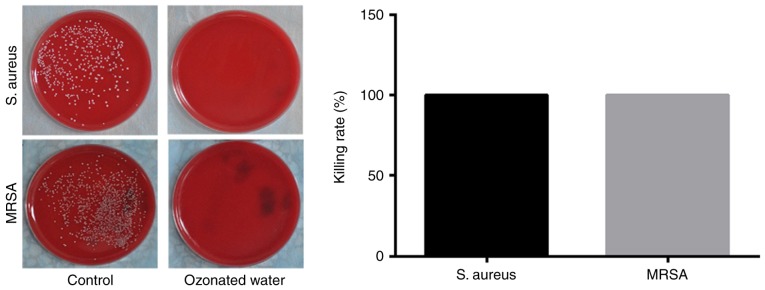
The killing rate of ozonated oil on S. aureus was much higher than the control. Almost 100% S. aureus were killed in 5 min. For MRSA, the killing rate of ozonated oil was also much higher than the control oil. Almost 100% MRSA were killed within 15 min (Fig. 1). The killing rates over time between ozonated oil and control for S. aureus and MRSA were presented in Tables I and II. The ozonated water (1 mg/l) can sterilize 100% S. aureus and 100% MRSA in one minute (Fig. 2).
Figure 1.
Killing rate of ozonated oil for S. aureus and MRSA. (A and B) Almost 100% S. aureus were killed in 5 min by ozonated oil. Almost 100% MRSA were killed within 15 min by ozonated oil. S. aureus, Staphylococcus aureus; MRSA, methicillin resistant Staphylococcus aureus.
Table I.
Killing rate of ozonated oil on S. aureus.
| Killing rate | |||
|---|---|---|---|
| Time (min) | Control oil (%) | Ozonated oil (%) | P-value |
| 1 | 31.80±1.05 | 86.35±2.10 | <0.0001 |
| 5 | 40.81±8.27 | 99.01±1.90 | <0.0001 |
| 10 | 35.91±1.74 | 99.25±1.00 | <0.0001 |
| 15 | 36.04±3.37 | 99.84±0.24 | <0.0001 |
| 20 | 37.26±3.82 | 100±0.00 | <0.0001 |
| 30 | 37.16±1.44 | 100±0.00 | <0.0001 |
S. aureus, staphylococcus aureus.
Table II.
Killing rate of ozonated oil on MRSA.
| Killing rate | |||
|---|---|---|---|
| Time (min) | Control oil (%) | Ozonated oil (%) | P-value |
| 1 | 44.70±0.97 | 69.09±1.73 | 0.0037 |
| 5 | 47.31±1.42 | 74.96±1.44 | 0.0004 |
| 10 | 42.99±5.69 | 91.25±2.91 | 0.0001 |
| 15 | 42.99±5.28 | 98.37±1.71 | <0.0001 |
| 20 | 41.65±6.7 | 99.65±0.09 | <0.0001 |
| 30 | 47.37±7.45 | 100±0.00 | <0.0001 |
MRSA, methicillin resistant staphylococcus aureus.
Figure 2.
Killing rate of ozonated water for S. aureus and MRSA. The ozonated water can sterilize 100% S. aureus and 100% MRSA in one minute. S. aureus, Staphylococcus aureus; MRSA, methicillin resistant Staphylococcus aureus.
Bacterial inhibitory: Inhibition zone diameter
The inhibition zone diameters of ozonated oil for S. aureus and MRSA were 17 and 13 mm respectively, which were significantly much larger than the control (Fig. 3).
Figure 3.
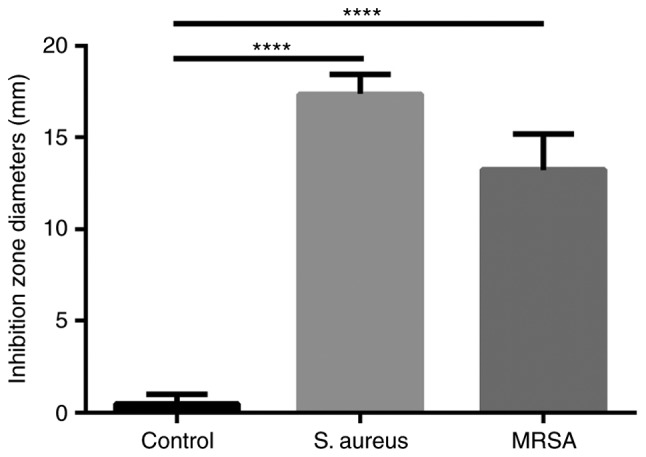
The inhibition zone diameters of ozoned oil for S. aureus and MRSA. The inhibition zone diameters of ozonated oil for S. aureus and MRSA were 17 and 13 mm, respectively. ****P<0.0001. S. aureus, Staphylococcus aureus; MRSA, methicillin resistant Staphylococcus aureus.
Practicing treatment
The first case
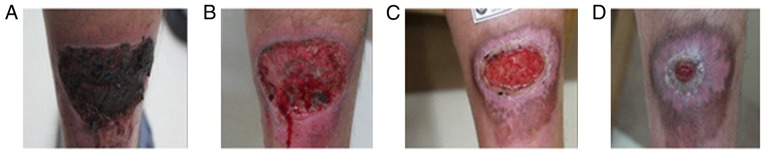
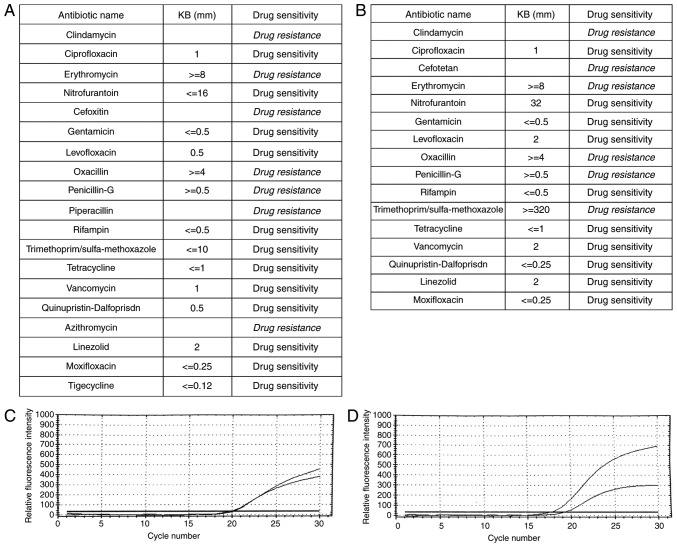
A 21-year-old male patient presented to the Dermatology Department of our hospital complaining of a painful abscess on his left calf muscle for approximately 20 days. Denied any systemic diseases or health issues. Skin examination revealed induration, bleeding and surrounding erythema in the left upper Achilles tendon (Fig. 4A). The overlying skin had become thin and felt fluctuant with spontaneous pus secretion. Previous treatments included systemic antibiotics, cleaning and dressing changes for more than 20 days without any visible effect. Tests for diabetes and immune-suppression syndrome were negative. Bacteria culture, drug sensitive test and PCR test confirmed skin MRSA infection (Fig. 5A and C). The strain was resistant to clindamycin, erythromycin, cefoxitin, oxacillin, penicillin-G, piperacillin, and azithromycin (Fig. 5A). After diagnosis of MRSA infection, the patient voluntarily was put on topical application of ozone therapy. Ozonated water was used to wash the lesion for 10 min immediately, followed by soak the lesion for 20 min after debridement if necessary in our therapy room every day. The application of topical ozonated oil twice per day was carried out at home by the patient. Four days after treatment, the lesion was cleaned to remove necrotic tissue and pus secretion (Fig. 4B). Affected areas were reduced by more than 70% during the first month (Fig. 4C) and was almost healed by the end of second month (Fig. 4D). Bacteria culture test revealed that tissues from the lesion did not develop bacteria one month after the topical ozone treatment.
Figure 4.
Topical application of ozone therapy on left calf muscle MRSA infection. (A) The lesion before ozone therapy. (B) The lesion after removing necrotic tissue and pus secretion. (C) The lesion after topical application of ozone therapy for one month. (D) The lesion after topical application of ozone therapy for two months. MRSA, methicillin resistant Staphylococcus aureus.
Figure 5.
Drug sensitive test and PCR test confirmed skin MRSA infection. (A) The results of drug sensitive test of the first case. (B) The results of drug sensitive test of the second case. (C) The melting curve of PCR test confirmed the MRSA infection of the first case. (D) The melting curve of PCR test confirmed the MRSA infection of the second case. PCR, polymerase chain reaction; MRSA, methicillin resistant Staphylococcus aureus.
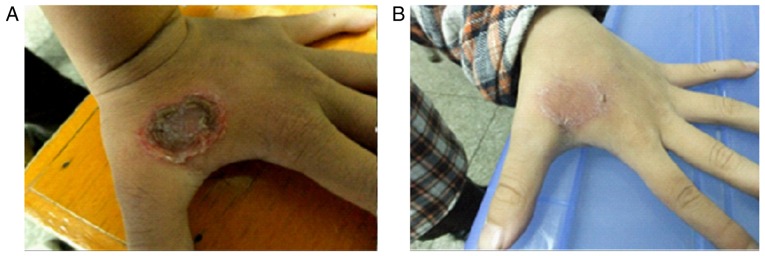
The second case
An 8-year-old male patient presented to our department complaining of a big blister on the dorsal aspect of the left hand. Denied any systemic diseases and any treatment at the moment. On skin examination, a 2×2 cm ulcerated lesion with yellow crusts was seen with surrounding erythema and fluid exudation on the dorsal aspect of the left hand. Laboratory tests confirmed MRSA skin infection, resistant to clindamycin, cefotetan, erythromycin, oxacillin, penicillin-G, and trimethoprim/sulfa-methoxazole (Fig. 5B and D). A complete healing from the lesion was achieved after 12 days of treatment with a combination of ozonated water and ozonated oil using the same remedy as we described in the first case (Fig. 6). Bacteria culture test revealed that scales from the lesion did not develop bacteria 12 days after the topical ozone treatment.
Figure 6.
Topical application of ozone therapy on the left-hand MRSA infection. (A) The lesion before ozone therapy. (B) The lesion after topical application of ozone therapy for 12 days. MRSA, methicillin resistant Staphylococcus aureus.
Discussion
In this study, we demonstrated that ozonated oil and ozonated water have strong in vitro antibacterial effects against S. aureus and MRSA. This is the first study to prove that the topical application of ozone alone can be a powerful treatment option for MRSA skin infections.
Data in this study have shown that ozonated oil can sterilize up to 98% of S. aureus in 5 min and up to 98% of MRSA in 15 min while ozonated water (1 mg/l) can sterilize 100% of S. aureus and 100% of MRSA in one minute. This results indicate that ozone therapy has very powerful anti-microbial properties against gram positive microorganisms, which was confirmed by the bacterial inhibitory experiment of ozonated oil. Our results are consistent with reports regarding bacterial elimination in S. aureus or MRSA by ozone therapy (20–23). Notably ozone can sterilize both gram positive bacteria and gram negative bacteria (24). Ozone is an unstable molecule that rapidly decays to O2 and releases a single oxygen atom. The single oxygen atom reacts with the cell membrane of the bacteria, attacks the cellular components, interrupts the normal cell activity and then destroys bacteria (23,25).
Ozone therapy has been used for infectious diseases such as conjunctivitis and keratitis (26), peritonitis (27), and surgical sepsis (28). In our clinical practice, topical application of ozone therapy is very effective for healing MRSA induced skin ulceration. The two cases got remarkable therapeutic effects after ozone treatment alone. Besides the high efficiency for sterilization, ozone therapy exhibits a potential effect in wound healing. The possible mechanisms include: i) Increasing oxygen levels, glucose and ATP transporter molecules in ischemic tissues; ii) increasing the activity of bone marrow stem cells, so as to promote angiogenesis and tissue regeneration; iii) the upregulation of the expression of antioxidant enzymes in blood; iv) Promoting the neuronal medium rise (29,30); and v) Inducing growth factors (31). Ozone therapy was also reported to reduce pain and swelling (32). Patients in this study also presented significant decrease in pain and swelling.
Diabetic foot ulcers are a challenging clinical problem, characterized by neuropathy, peripheral arterial diseases, foot deformities, and infection (33,34). S. aureus was the most common pathogen identified in Diabetic foot ulcers, representing 46% of culture-positive patients. And 15% were classified as MRSA (35). Ozone application was not only to kill S. aureus or MRSA, but also reported to significantly reduce the lesion area in patient with diabetic foot (31,36,37). Ozone also can improve glycemic control by controlling hyperglycemia and insulin sensitivity and preventing oxidative stress associated with diabetes mellitus and its complications (38). Because of the low-cost, ozone therapy can also reduce the medical bills for patients with diabetes mellitus and its complications.
Ozonated water can keep the bactericidal effect for approximately 30 min, while ozonated oil can maintain its sterilization ability persistently (39–42). This is why ozonated oil was applied to our patients after washing with ozonated water to increase the effective time.
Although ozone therapy can kill microbes, improve wound healing, reduce pain and swelling at minimal cost with almost no side effect, it is contraindicated in several diseases such as Blood Coagulation Failure, Bleeding Organs, Thrombocytopenia, Ozone Alergia, Hemorrhagic or Apoplectic Stroke, Ozone Intolerance (43).
In summary, ozone therapy is potential treatment for S. aureus and MRSA skin infections with great efficacy, low side effects, and low-cost.
Acknowledgements
This study was supported by Natural Science Foundation of Hunan Province (no. 2015JJ6120), Scientific Research Program of Department of Health of Hunan Province (no. B2015-034), National Natural Science Foundation of China (no. 81703101), Development and Reform Commission of Hunan Province [no. (2014) 658], Administration of Traditional Chinese Medicine of HunanProvince (no. 201520), and the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (no. JY201623). Special thanks to Dr Wenbin Tan and Dr Xiaoqi Wang for the review.
Glossary
Abbreviations
- S. aureus
Staphylococcus aureus
- MRSA
methicillin resistant Staphylococcus aureus
- DMSO
Dimethylsulfoxide
References
- 1.Dréno B, Araviiskaia E, Berardesca E, Gontijo G, Sanchez Viera M, Xiang LF, Martin R, Bieber T. Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol. 2016;30:2038–2047. doi: 10.1111/jdv.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz JH, Lopez FA. Skin, soft tissue and systemic bacterial infections following aquatic injuries and exposures. Am J Med Sci. 2015;349:269–275. doi: 10.1097/MAJ.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Sully EK, Geller BL. Antisense antimicrobial therapeutics. Curr Opin Microbiol. 2016;33:47–55. doi: 10.1016/j.mib.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Zhang J, Yu S, Wu Q, Guo W, Huang J, Cai S. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in retail ready-to-eat foods in China. Front Microbiol. 2016;7:816. doi: 10.3389/fmicb.2016.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller WR, Bayer AS, Arias CA. Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb Perspect Med. 2016;6:pii: a026997. doi: 10.1101/cshperspect.a026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene AK, Few BK, Serafini JC. A comparison of ozonation and chlorination for the disinfection of stainless steel surfaces. J Dairy Sci. 1993;76:3617–3620. doi: 10.3168/jds.S0022-0302(93)77702-4. [DOI] [PubMed] [Google Scholar]
- 7.Fiessinger F, Richard Y, Montiel A, Musquere P. Advantages and disadvantages of chemical oxidation and disinfection by ozone and chlorine dioxide. Sci Total Environ. 1981;18:245–261. doi: 10.1016/S0048-9697(81)80062-9. [DOI] [PubMed] [Google Scholar]
- 8.Stalder K, Klosterkötter W. Studies on the reappearing of a bacterial flora in drinking water after ozonization (author's transl) Zentralbl Bakteriol Orig B. 1976;161:474–481. (In German) [PubMed] [Google Scholar]
- 9.Liu J, Zhang P, Tian J, Li L, Li J, Tian JH, Yang K. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev: Cd008474. 2015 doi: 10.1002/14651858.CD008474.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassi P, Sbrascini S, Mattassi R, D'Angelo F, Franchina A. Ozone in the treatment of herpes zoster. Riv Neurobiol. 1982;28:328–333. (In Italian) [PubMed] [Google Scholar]
- 11.Moureu S, Violleau F, Ali Haimoud-Lekhal D, Calmon A. Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem Phys Lipids. 2015;186:79–85. doi: 10.1016/j.chemphyslip.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhong J, Allen K, Rao X, Ying Z, Braunstein Z, Kankanala SR, Xia C, Wang X, Bramble LA, Wagner JG, et al. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal Toxicol. 2016;28:383–392. doi: 10.1080/08958378.2016.1179373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farac RV, Pizzolitto AC, Tanomaru JM, Morgental RD, Lima RK, Bonetti-Filho I. Ex-vivo effect of intracanal medications based on ozone and calcium hydroxide in root canals contaminated with Enterococcus faecalis. Braz Dent J. 2013;24:103–106. doi: 10.1590/0103-6440201301992. [DOI] [PubMed] [Google Scholar]
- 14.Heß S, Gallert C. Sensitivity of antibiotic resistant and antibiotic susceptible Escherichia coli, Enterococcus and Staphylococcus strains against ozone. J Water Health. 2015;13:1020–1028. doi: 10.2166/wh.2015.291. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Hudson JB. Ozone gas is an effective and practical antibacterial agent. Am J Infect Control. 2008;36:559–563. doi: 10.1016/j.ajic.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Gadzhiev ND, Nasirov Mla, Sushkov SV, Klimova EM. Effect of combined and local cytokine- and ozone therapy on the indices of lipid peroxidation, endogenous intoxication and ferroproteins in diffuse peritonitis. Vestn Khir Im I I Grek. 2014;173:38–41. (In Russian) [PubMed] [Google Scholar]
- 17.Kolesova OE, Vasil'ev IT, Volkhovskaia NB, Mumladze RB, Tkachenko SB, Savina GD. Correction of the antioxidative system during ozone therapy in peritonitis. Vestn Ross Akad Med Nauk. 2010:34–39. (In Russian) [PubMed] [Google Scholar]
- 18.Tamai M, Matsushita S, Miyanohara H, Imuta N, Ikeda R, Kawai K, Nishi J, Sakamoto A, Shigihara T, Kanekura T. Antimicrobial effect of an ultrasonic levitation washer disinfector with silver electrolysis and ozone oxidation on methicillin-resistant Staphylococcus aureus. J Dermatol. 2013;40:1020–1026. doi: 10.1111/1346-8138.12327. [DOI] [PubMed] [Google Scholar]
- 19.Solovăstru LG, Stîncanu A, De Ascentii A, Capparé G, Mattana P, Vâţă D. Randomized, controlled study of innovative spray formulation containing ozonated oil and α-bisabolol in the topical treatment of chronic venous leg ulcers. Adv Skin Wound Care. 2015;28:406–409. doi: 10.1097/01.ASW.0000470155.29821.ed. [DOI] [PubMed] [Google Scholar]
- 20.Gulmen S, Kurtoglu T, Meteoglu I, Kaya S, Okutan H. Ozone therapy as an adjunct to vancomycin enhances bacterial elimination in methicillin resistant Staphylococcus aureus mediastinitis. J Surg Res. 2013;185:64–69. doi: 10.1016/j.jss.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 21.Al-Saadi H, Potapova I, Rochford ET, Moriarty TF, Messmer P. Ozonated saline shows activity against planktonic and biofilm growing Staphylococcus aureus in vitro: A potential irrigant for infected wounds. Int Wound J. 2016;13:936–942. doi: 10.1111/iwj.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilczyńska-Borawska M, Leszczyńska K, Nowosielski C, Stokowska W. Ozone in dentistry: Microbiological effects of gas action depending on the method and the time of application using the ozonytron device. Experimental study. Ann Acad Med Stetin. 2011;57:99–103. [PubMed] [Google Scholar]
- 23.Yamayoshi T, Tatsumi N. Microbicidal effects of ozone solution on methicillin-resistant Staphylococcus aureus. Drugs Exp Clin Res. 1993;19:59–64. [PubMed] [Google Scholar]
- 24.Almaz ME, Sönmez IŞ. Ozone therapy in the management and prevention of caries. J Formos Med Assoc. 2015;114:3–11. doi: 10.1016/j.jfma.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Komanapalli IR, Lau BH. Inactivation of bacteriophage lambda, Escherichia coli, and Candida albicans by ozone. Appl Microbiol Biotechnol. 1998;49:766–769. doi: 10.1007/s002530051244. [DOI] [PubMed] [Google Scholar]
- 26.Gierek-Lapińska A, Antoszewski Z, Myga B, Skowron J. Preliminary report on using therapeutic ozone in infectious conjunctivitis and keratitis and in corneal degeneration. Klin Oczna. 1992;94:137–138. (In Polish) [PubMed] [Google Scholar]
- 27.Erginel B, Erginel T, Aksoy B, Dokucu AI. Effect of Ozone Therapy (OT) on healing of colonic anastomosis in a rat model of peritonitis. Balkan Med J. 2014;31:249–253. doi: 10.5152/balkanmedj.2014.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkhisenko IuA, Glukhov AA. Use of ozone therapy and hydro-pressure technologies in complex intensive therapy of surgical sepsis. Khirurgiia (Mosk) 2001:55–58. (In Russian) [PubMed] [Google Scholar]
- 29.Verrazzo G, Coppola L, Luongo C, Sammartino A, Giunta R, Grassia A, Ragone R, Tirelli A. Hyperbaric oxygen, oxygen-ozone therapy, and rheologic parameters of blood in patients with peripheral occlusive arterial disease. Undersea Hyperb Med. 1995;22:17–22. [PubMed] [Google Scholar]
- 30.Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Guan M, Xie C, Luo X, Zhang Q, Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475. doi: 10.1155/2014/273475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazancioglu HO, Kurklu E, Ezirganli S. Effects of ozone therapy on pain, swelling, and trismus following third molar surgery. Int J Oral Maxillofac Surg. 2014;43:644–648. doi: 10.1016/j.ijom.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr. 2017;11:149–156. doi: 10.1016/j.dsx.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Kateel R, Adhikari P, Augustine AJ, Ullal S. Topical honey for the treatment of diabetic foot ulcer: A systematic review. Complement Ther Clin Pract. 2016;24:130–133. doi: 10.1016/j.ctcp.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Reveles KR, Duhon BM, Moore RJ, Hand EO, Howell CK. Epidemiology of methicillin-resistant Staphylococcus aureus diabetic foot infections in a large academic hospital: Implications for antimicrobial stewardship. PLoS One. 2016;11:e0161658. doi: 10.1371/journal.pone.0161658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazin IK. Pathophysiological aspects of endotoxicosis complicated with purulent infection of the foot and correction of endotoxicosis with conventional treatment and with application of ozonized physiological solution in patients suffering from diabetes mellitus. Patol Fiziol Eksp Ter. 2008:23–25. (In Russian) [PubMed] [Google Scholar]
- 37.Martínez-Sánchez G, Al-Dalain SM, Menéndez S, Re L, Giuliani A, Candelario-Jalil E, Alvarez H, Fernández-Montequín JI, León OS. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523:151–161. doi: 10.1016/j.ejphar.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Al-Dalain SM, Martínez G, Candelario-Jalil E, Menéndez S, Re L, Giuliani A, León OS. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharmacol Res. 2001;44:391–396. doi: 10.1006/phrs.2001.0867. [DOI] [PubMed] [Google Scholar]
- 39.Bialoszewski D, Pietruczuk-Padzik A, Kalicinska A, Bocian E, Czajkowska M, Bukowska B, Tyski S. Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Med Sci Monit. 2011;17:BR339–BR344. doi: 10.12659/MSM.882044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke FJ. Ozone and caries: A review of the literature. Dent Update. 2012;39:271–272, 275–278. doi: 10.12968/denu.2012.39.4.271. [DOI] [PubMed] [Google Scholar]
- 41.Valacchi G, Zanardi I, Lim Y, Belmonte G, Miracco C, Sticozzi C, Bocci V, Travagli V. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int J Pharm. 2013;458:65–73. doi: 10.1016/j.ijpharm.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Pai SA, Gagangras SA, Kulkarni SS, Majumdar AS. Potential of ozonated sesame oil to augment wound healing in rats. Indian J Pharm Sci. 2014;76:87–92. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang YB, Xiang YP, Huang JH, Gao L, Chen M, Kathy W, Li M, Chen J, Yang S, Lu J. Combined ozone hydrotherapy for atopic dermatitis: Evaluation of efficacy and detection of interleukin-4 and nerve growth factor levels in peripheral blood from patients before and after treatment. Chin J Dermatol. 2016;49:736–738. (In Chinese) [Google Scholar]