Abstract
Chronic pain occurs in ~85–90% of chronic pancreatitis (CP) patients. However, as the pathogenesis of CP pain remains to be fully understood, the current therapies for CP pain remain inadequate. Emerging evidence has suggested that the epigenetic modulations of genes are involved in chronic pain. In the present study, intrapancreatic trinitrobenzene sulfonic acid infusions were used to establish a CP model in rats. Mechanical allodynia was measured with von Frey filaments. Immunofluorescent staining analysis was used to observe the expression changes of histone deacetylase 2 (HDAC2) and µ-opioid receptor (MOR), and intrathecal administration of the selective HDAC2 inhibitor AR-42 was used to assess the underlying mechanisms. The expression levels of c-Jun N-terminal kinase (JNK) in the thoracic spinal cord were detected by western blotting, and the mRNA expression levels of interleukin (IL)1-β, IL-6 and tumor necrosis factor (TNF)-α were detected by reverse transcription-quantitative polymerase chain reaction. The results demonstrated that HDAC2 expression was upregulated during the course of CP induction, while MOR activity in the thoracic spinal dorsal horn was significantly suppressed. Intrathecal infusion of AR-42 significantly attenuated CP-induced mechanical allodynia, with rescued MOR activity. Additionally, HDAC2 facilitated the release of inflammatory cytokines, including IL-1β, IL-6 and TNF-α. These results suggested that the underlying mechanisms of HDAC2 regulating MOR activity under CP induction may occur via promoting the release of inflammatory cytokines, thus activating the JNK signaling pathway. The present study suggested that the epigenetic-regulated disturbance of MOR is dependent on the endogenous analgesia system in CP, which may a provide novel therapeutic strategy for treating pain in CP.
Keywords: chronic pancreatitis, histone deacetylase, epigenetic, µ-opioid receptor, allodynia
Introduction
Chronic pancreatitis (CP) is a painful inflammatory disease, characterized by progressive destruction of the pancreatic gland and severe abdominal pain (1). Approximately 85–90% CP patients suffer from abdominal pain (2). Pain in CP has been associated with malnutrition, narcotic addiction, and physical and emotional disability, which leads to major socioeconomic problems (3). However, the currently available therapies for CP pain remain inadequate and the underlying mechanisms remain to be completely elucidated. Previous reports have demonstrated that CP-induced pain exhibits numerous characteristics similar to neuropathic pain, especially the alterations located in the central nervous system (CNS) (4). Epigenetic modulations of gene expression have been indicated to be involved in the development of chronic pain (5). Epigenetic alterations are required for long-lasting neuronal plasticity that is essential for the development of chronic pain state modifications (6,7). Epigenetic modifications regulate the compaction of chromatin and include a variety of facets; significant epigenetic control is achieved via histone acetylation. Histone acetylation modification is dynamic and reversible, and is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) (8). Typically, HATs acetylate the histones to produce an open chromatin conformation, thus favoring gene expression, while HDACs deacetylate the DNA and result in a closed chromatin conformation and ultimately gene repression (9). Studies on HDACs inhibitors have demonstrated obvious analgesic effect on nociceptive responses of rodents, either delivered systemically or intrathecally (10–13). However, little is known on how this mechanism operates and which target genes are involved (14). Furthermore, whether selective interruption of HDAC activity on CP could alleviate allodynia remains to be investigated.
In the nervous system, activation of µ-opiod receptor (MOR) leads to neuronal inhibition and causes an endogenous analgesia effect (15). The expression level of MOR in the mouse brain correlates with alterations in histone modifications (16). However, limited studies have analyzed the epigenetic processes that contribute to gene repression and activation in pain states. Furthermore, a study reported that MOR is mainly negatively regulated by the c-Jun NH2-terminal kinase (JNK) signaling pathway (17). Our previous study demonstrated that HDAC2 activity was significantly upregulated in the thoracic spinal cord, based on a rat CP model induced by intrapancreatic infusion of trinitrobenzene sulfonic acid (TNBS) (18), indicating that epigenetic regulation mechanisms are involved in chronic pain induced by CP.
The present study aimed to investigate whether upregulation of HDAC2 affects MOR expression, and thus has an impact on CP allodynia. It was hypothesized that the elevated HDAC2 expression suppressed MOR activation via JNK signaling pathways, and aggravated CP pain. To test this hypothesis, AR-42 was used as a selective HDAC2 inhibitor (19), and the underlying mechanisms of CP pain were investigated.
Materials and methods
Animals
The present study was approved by the Animal Use and Care Committee for Research and Education of the Fourth Military Medical University (Xi'an, China), following the ethical guidelines on investigating experimental pain in conscious animals. 54 young adult male Sprague-Dawley rats (age, 10–12 weeks; weight, 180–220 g) were purchased from the Laboratory Animals Center (Fourth Military Medical University, Xi'an, China) and caged in a temperature-controlled environment at 22–25°C and 55±5% relative humidity with a 12-h light/dark cycle. Free access to water and food was available until 12 h before pancreatitis induction. Minimum animals were used for consistent effects.
Induction of pancreatitis and pain behavioral test
All rats were randomly divided into three main groups: TNBS (n=30), sham (n=18) and controls (n=6). Rats in the TNBS and sham group were further divided for drug injection: TNBS-HDAC inhibitor (i)/saline and sham-HDACi/saline groups (n=6/group). In order to study the time course of HDAC2 and MOR changes, 6 rats in the TNBS group were sacrificed at 1, 3 and 5 weeks each following TNBS infusion, in addition to 6 rats in the control and 6 rats in the sham group. All rats were sacrificed following anesthetization with pentobarbital (cat. no. 1507,002; Sigma-Aldrich; Merck KGaA; Darmstadt, Germany; 60 mg/kg, intraperitoneal injection) for further experiments 5 weeks after surgery. The protocol has been published in our previous study (18) with certain improvement for minimizing surgical injury. All the procedures in sham group were identical to the TNBS group, except the same volume of saline infusion. Mechanical allodynia was measured with von Frey filaments (Stoelting Co., Wood Dale, IL, USA) and performed fully randomized and blinded. The protocol was performed according to our previous study (18).
Intrathecal operation and drug administration
Intrathecal drug administration procedure was performed as previous described (18). The HDAC2-selective inhibitor AR-42 (AdooQ Bioscience, Irvine, CA, Canada) was used for the testing (19). The treatment group received an intrathecal injection of AR-42 (30 nmol) at each time point (0, 1, 3 and 5 weeks), while the same volume of saline was administered to the control group. Drug administration was performed under anesthetizing with isoflurane using a Vaporizer (Harvard Apparatus, Holliston, MA, USA), through an intrathecal catheter controlled by a micro-injection pump.
Immunofluorescent staining analysis
Rats were perfused through the ascending aorta with 100 ml 0.9% saline after deeply anesthetizing with pentobarbital (60 mg/kg, intraperitoneally), following administration of 500 ml 0.1 M phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde and 2% picric acid. The thoracic spinal segments T10-T12 were harvested and post-fixed with the same fixative for 4 h, then cryosectioned at 4°C for 24 h in 0.1 M PB containing 30% sucrose. Samples were transversely cut (25-µm thick) in a cryostat, then washed in PBS (0.01 M, pH 7.3) for 10 min three times. Sections were then blocked with 2% goat serum (EMD Millipore, Billerica, MA, USA) in 0.01 M PBS containing 0.3% Triton X-100 for 1 h at room temperature. These sections were incubated at 4°C overnight with the following primary antibodies: rabbit anti-HDAC2 (cat. no. sc-437285; 1:1,000; Santa-Cruz Biotechnology, Inc., Dallas, TX, USA) guinea pig anti-MOR (cat. no. AB5509; 1:200; EMD Millipore), mouse anti-neuronal-specific nuclear protein (NeuN; cat. no. MAB377; 1:500; EMD Millipore), mouse anti-glial fibrillary acidic protein (GFAP; cat. no. NE1015; 1:500; EMD Millipore) and mouse anti-integrin α-M (CD11b) antibody (OX-42; cat. no. CBL1512; 1:500; EMD Millipore). The sections were then washed three times with PBS (10 min each) and then incubated for 2 h at room temperature with the corresponding secondary antibody: Alexa 488-conjugated donkey anti-rabbit IgG (cat. no. R37118; 1:800; Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Alexa 594-conjugated donkey anti-guinea pig IgG (cat. no. A-11076; 1:200; Molecular Probes; Thermo Fisher Scientific, Inc.). Images were obtained under a confocal laser microscope (FV1000; Olympus Corporation, Tokyo, Japan), and digital pictures were captured with the Fluoview 1000 imaging system (Olympus Corporation). A total of 12 nonadjacent sections were randomly selected for scanning. The z-separation was 4.6 µm under a ×20 objective magnification, and was 1.0 µm under a ×60 objective magnification. For semiquantification, the fluorescent brightness value of immunoreactivities was detected on the same areas of the thoracic spinal dorsal horn using software (Fluoview; version 1.5.0.14; Olympus Corporation, Tokyo, Japan) under an Olympus IX-70 confocal microscope (Olympus Corporation).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Rat thoracic spinal cord samples were harvested as described above. RNA extraction was performed using TRIzol (Gibco; Thermo Fisher Scientific, Inc.), and oligo(dT) primer and SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) were used in cDNA RT. The mRNA expression levels of the pro-inflammatory cytokines interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α were assessed by RT-qPCR. The protocol was performed according to a previous report (18): 3 min at 9°C, followed by 45 cycles of 10 sec at 95°C for denaturation, and 45 sec at 60°C for annealing and extension. All experiments were repeated twice, and PCR reactions were triplicate in each test. Target cDNA quantities were evaluated from the quantitation cycle number (Cq) (20) using Sequence Detection System software (version 2.4.1; Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer sequences are provided in Table I. GAPDH served as an endogenous internal standard control.
Table I.
Primers sequence for the rat genes characterized in this experiment.
| Gene | Sequence |
|---|---|
| TNF-α | F: 5′-TGATCGGTCCCAACAAGGA-3′ |
| R: 5′-TGCTTGGTGGTTTGCTACGA-3′ | |
| IL-1β | F: 5′-TGCTGATGTACCAGTTGGGG-3′ |
| R: 5′-CTCCATGAGCTTTGTACAAG-3′ | |
| IL-6 | F: 5′-GCCCTTCAGGAACAGCTATG-3′ |
| R: 5′-CAGAATTGCCATTGCACAAC-3 | |
| GAPDH | F: 5′-CCCCCAATGTATCCGTTGTG-3′ |
| R: 5′-TAGCCCAGGATGCCCTTTAGT-3′ |
F, forward; R, reverse; TNF-α, tumor necrosis factor-α; IL, interleukin.
Western blotting
Rats were sacrificed and T10-T12 spinal cord sections were rapidly harvested and frozen on dry ice. Samples were quickly micro-dissected and homogenized using a hand-held pestle with ice-cold SDS sample lysis buffer (Roche Diagnostics, Basel, Switzerland; 10 ml/mg tissue), containing a cocktail of proteinase inhibitors. The homogenates were centrifuged at 10,000 × g for 10 min at 4°C. Subsequent to the protein concentration being measured using a bicinchoninic acid protein assay kit (cat. no. 23225; Pierce; Thermo Fisher Scientific, Inc.), the homegenates were heated at 100°C for 5 min. Proteins (50 µg) were separated using SDS-PAGE on a 10% gel with standard Laemmli solutions (Bio-Rad Laboratories, Hercules, CA, USA) and subsequently transferred onto a polyvinylidene difluoride membrane (Immobilon-P, EMD Millipore). The membranes were washed with Tris-buffered saline with 0.02% Tween (TBST) and blocked with 5% non-fat dry milk for 1 h at room temperature, and incubated overnight with gentle agitation with rabbit anti-JNK (cat. no. sc-7345; 1:1,000; Santa-Cruz Biotechnology, Inc.) and anti-β-actin (cat. no. A0483; 1:1,000; Sigma-Aldrich; Merck KGaA) primary antibodies. Subsequently, membranes were incubated overnight at 4°C with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (cat. no. RPN4301; 1:10,000; GE Healthcare, Chicago, IL, USA). Between each of these steps, the membranes were rinsed with TBST. All reactions were detected through the enhanced chemiluminescence (ECL) detection method (GE Healthcare). The densities of protein blots were analyzed using Labworks Software (version 4.5; UVP, Inc., Upland CA, USA) and normalized against the values of β-actin.
Statistical analysis
After the images were captured, the optical density of the same areas of the superficial dorsal horn (lamina I and II) of the five spinal sections were calculated. All data are presented as mean ± standard error and were analyzed by one-way analysis of variance. Multiple comparisons between the groups were performed using the Student-Newman-Keuls method. All statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulated HDAC2 expression is associated with suppressed MOR activity in the thoracic spinal dorsal horn after CP induction by TNBS
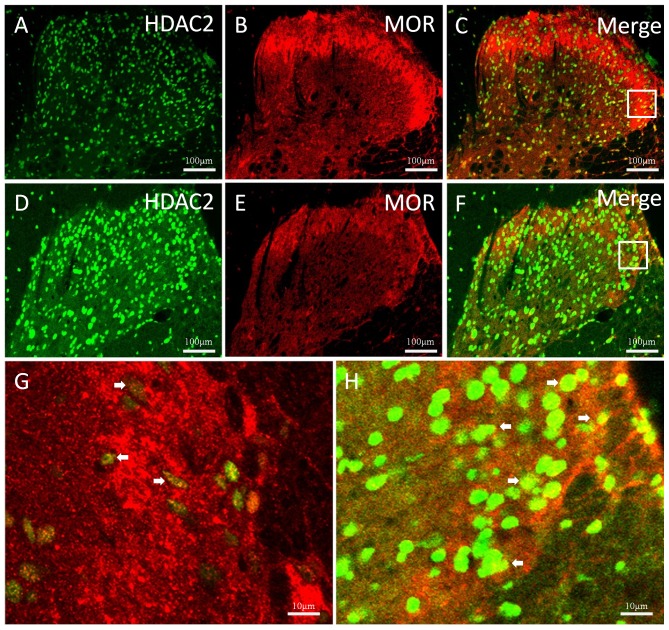
The rats were sacrificed at different time points to investigate the expression changes of HDAC2 and MOR. Double immunofluorescent staining was performed to reveal the association between HDAC2 and MOR in the thoracic spinal cord. Colocalization of HDAC2 and MOR were observed both in the control (Fig. 1A-C) and TNBS-treated (Fig. 1D-H) groups. In the TNBS-treated group, HDAC2 expression levels were significantly elevated and persistent from 1 to 5 weeks. Suppressed MOR activity was simultaneously observed and maintained at a low level. Immunostaining density statistical analysis was used to confirm these changes (P<0.05 vs. control group; Fig. 2).
Figure 1.
Immunofluorescent double staining for HDAC2 and MOR in the thoracic spinal dorsal horn of CP-induced rats. Immunoreactivities of (A) HDAC2 (green), (B) MOR (red) and (C) merge in the control group. Immunoreactivities of (D) HDAC2, (E) MOR and (F) merge in the CP group. (G) and (H) represent magnified images of the rectangles indicated in the above panels. White arrows indicate areas of merged overlap of HDAC2 and MOR. HDAC2, histone deacetylase 2; MOR, µ-opiod receptor; CP, chronic pancreatitis.
Figure 2.
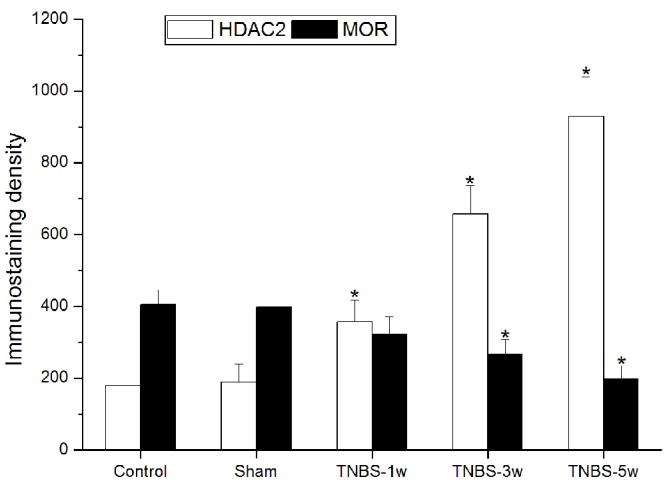
Immunostaining density of HDAC2 and MOR. Data are expressed as the mean ± standard error. *P<0.05 vs. control. HDAC2, histone deacetylase 2; MOR, µ-opiod receptor; TNBS, trinitrobenzene sulfonic acid; w, week.
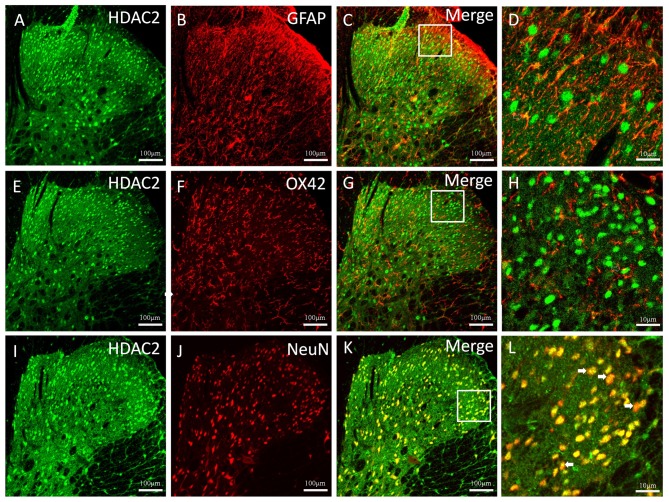
The cellular localization of HDAC2 in the thoracic spinal dorsal horn 5 weeks after CP induction was observed (Fig. 3). Double immunofluorescent staining of HDAC2 with the astrocytic specific marker GFAP (Fig. 3A-C), the microglial marker OX42 (Fig. 3E-G) and the neuronal marker NeuN (Fig. 3I-K) was conducted. The results revealed a clear colocalization between HDAC2 and NeuN (Fig. 3 K and L); however, HDAC2 rarely colocalized with GFAP or OX42 (Fig. 3D and H).
Figure 3.
Double immunostaining of the cellular location of HDAC2. (A) HDAC2 and (B) GFAP immunoreactivity, and (C) their merge; (D) represents a magnified image of the rectangles indicated in (C). (E) HDAC2 and (F) OX42 immunoreactivity, and (G) their merge; (H) represents a magnified image of the rectangles indicated in (G). (I) HDAC2 and (J) NeuN immunoreactivity, and (K) their merge; (L) represents a magnified image of the rectangles indicated in (K). White arrows indicate areas of merged overlap NeuN and HDAC2. GFAP, glial fibrillary acidic protein; NeuN, neuronal-specific nuclear protein; OX42, integrin α-M; HDAC2, histone deacetylase 2.
Intrathecal infusion of HDACi significantly attenuates CP-induced mechanical allodynia
To assess whether upregulated HDAC2 contributes to CP-induced pain, AR-42 was used to selectively suppress HDAC2, and thus investigate the nociceptive behavioral consequences and cellular and molecular alterations. The results demonstrated that AR-42 significantly attenuated mechanical allodynia (P<0.05 vs. TNBS; Fig. 4A). However, AR-42 exerted no influence on the response frequency (RF) of sham controlled rats (P<0.05 vs. sham; Fig. 4A). Different stimulations on RFs were tested, and it was observed that RFs of rats were significantly reduced after AR-42 treatment compared with non-treated CP model rats (Fig. 4B). Furthermore, rats treated with only AR-42 did not exhibit differences in RF compared with sham rats. Therefore, AR-42 may attenuate CP-induced chronic pain without interfering with the basic pain behavior.
Figure 4.
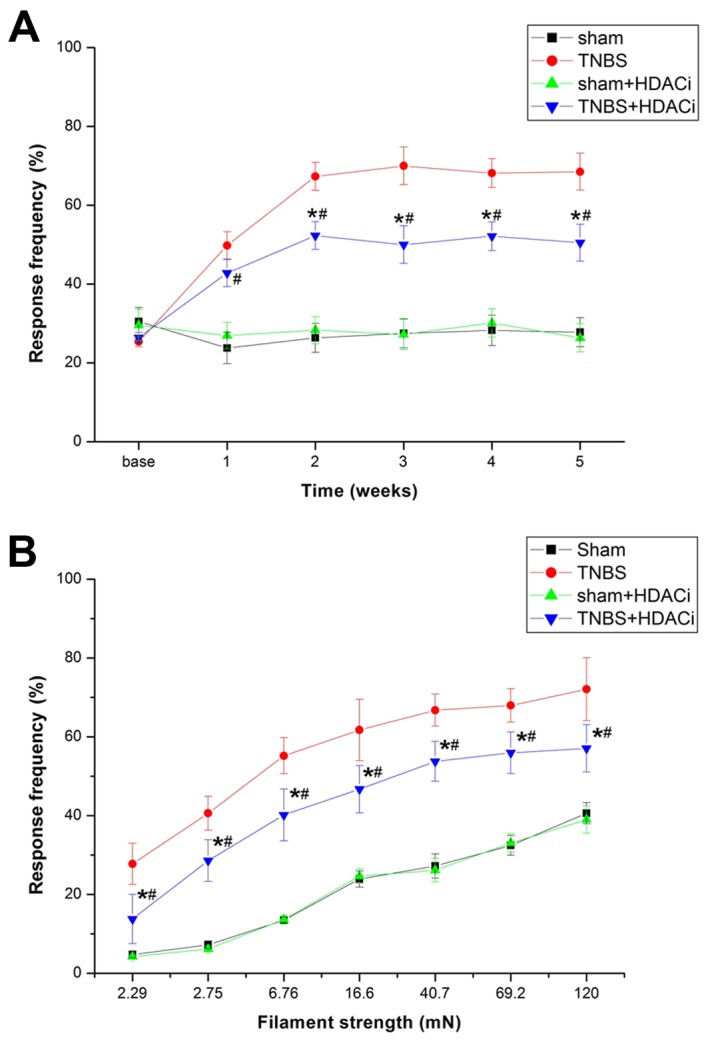
Effects of the HDAC inhibitor AR-42 administration on mechanical allodynia induced by TNBS. Response frequency in relation to (A) time and (B) filament strength. Data are presented as the mean ± standard error (n=6/group). *P<0.05 vs. TNBS group, #P<0.05 vs. sham and sham+HDACi groups. HDAC, histone deacetylase; i, inhibitor; TNBS, trinitrobenzene sulfonic acid.
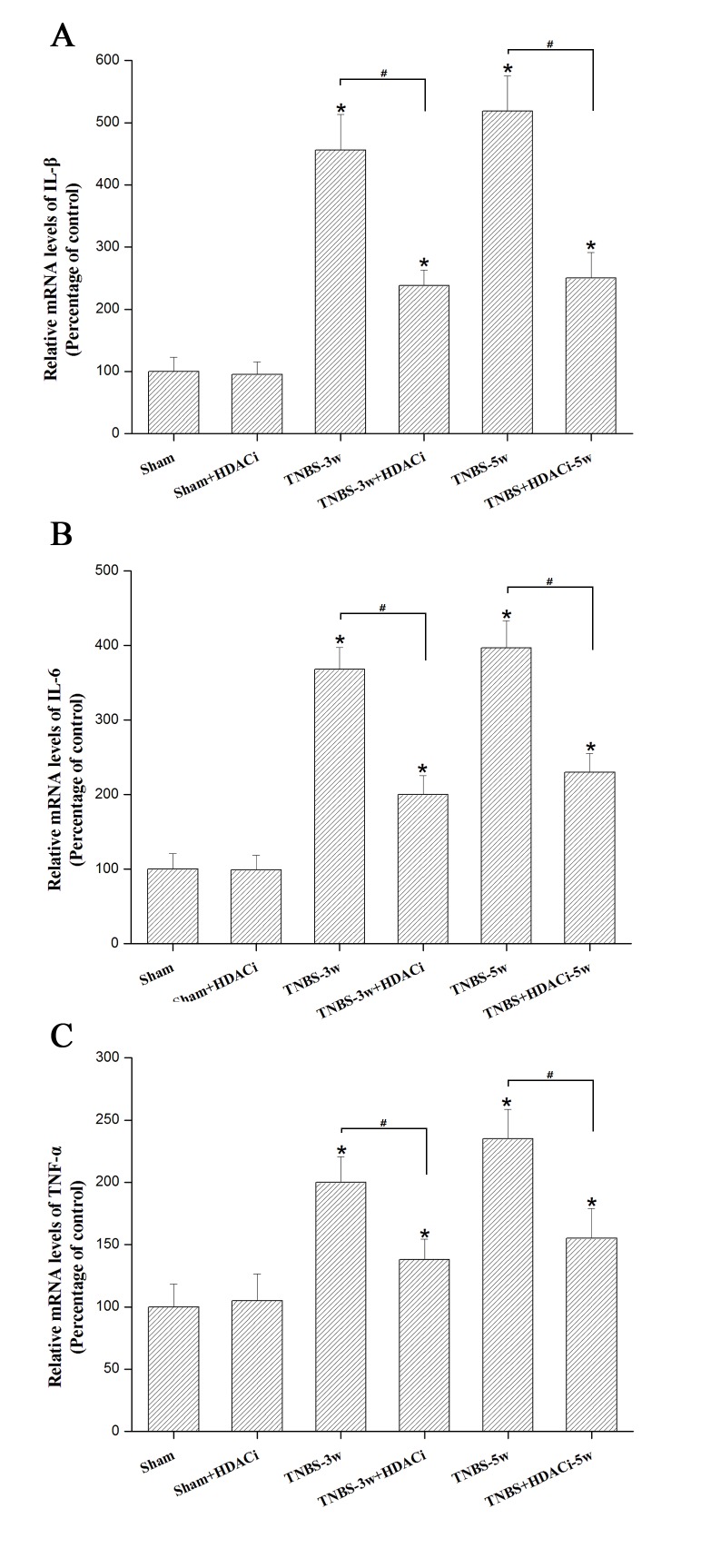
HDAC2 facilitates the release of the pro-inflammatory cytokines IL1-β, IL-6 and TNF-α
Our previous study demonstrated that pro-inflammatory cytokines such as IL-1β IL-6, and TNF-α in the spinal dorsal horn increase the status of CP (18). Using RT-qPCR, it was observed that the mRNA expression levels of these cytokines were significantly reduced by AR-42 at 3 and 5 weeks in CP rats (P<0.05 vs. TNBS control; Fig. 5), though this effect was not completely reversed (P<0.05 vs. sham; Fig. 5). In addition, AR-42 administration did not significantly affect the sham group (P<0.05 vs. sham control; Fig. 5). These data indicated that HDAC2 may promote the release of pro-inflammatory mediators under chronic CP allodynia.
Figure 5.
Pro-inflammatory cytokine levels in the spinal dorsal horn following AR-42 treatment. mRNA expression levels of the inflammatory mediators (A) IL-1β, (B) IL-6 and (C) TNF-α. GAPDH served as an internal reference gene control. Data are presented as the mean ± standard error. *P<0.05 vs. sham and sham+HDACi groups, #P<0.05. IL, interleukin; TNF-α, tumor necrosis factor-α; TNBS, trinitrobenzene sulfonic acid; w, weeks; HDAC, histone deacetylase; i, inhibitor.
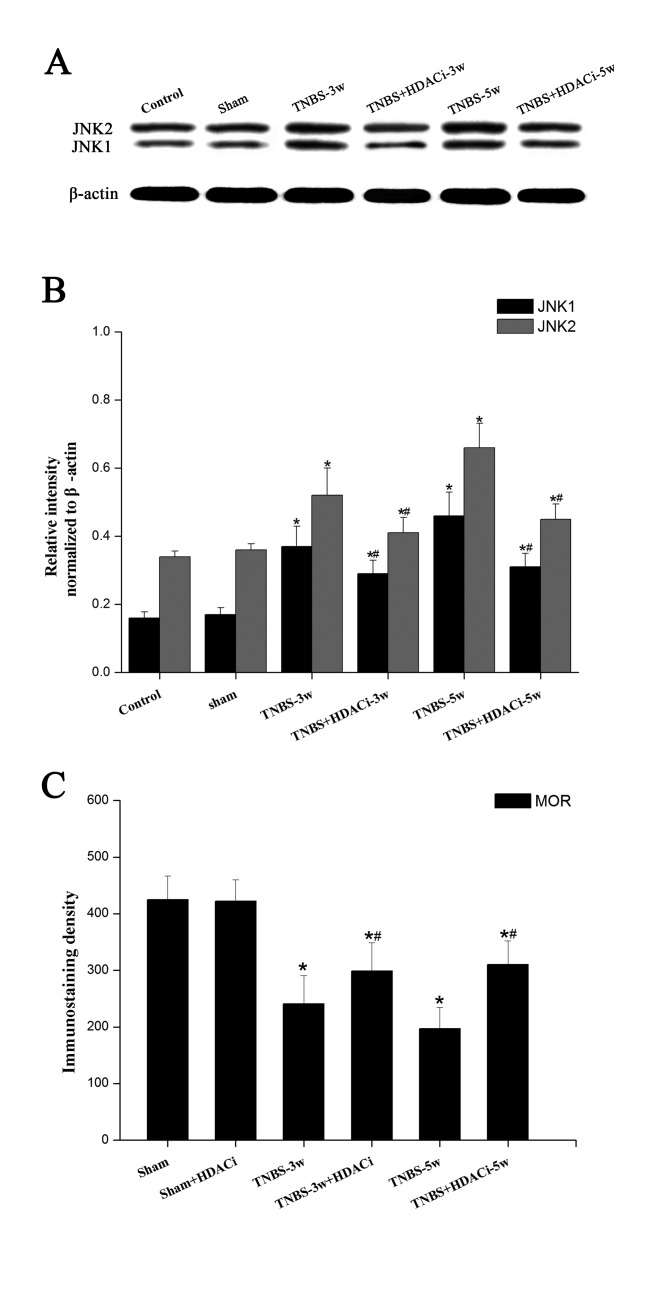
Effects of AR-42 on JNK signaling pathways in CP allodynia
As MOR expression is mainly negatively regulated by the JNK signaling pathway (17), the present study selected to further detected the expression levels of this intracellular kinase. Western blotting data indicated that JNK has two subtypes, JNK1 and JNK2, and that the protein expression levels of both were significantly increased after TNBS infusion (P<0.05 vs. control; Fig. 6A and B). However, HDACi AR-42 administration reversed the overexpression of JNK1/2 in CP rats (P<0.05, vs. TNBS; Fig. 6A-B), although the expression level remained higher than the control and sham groups. As presented in Fig. 6C, following a TNBS-induced reduction in MOR immunostaining density, HDACi AR-42 significantly rescued MOR activity in CP rats. No significant differences were observed between the control and sham group. In addition, no significant differences were observed on basic MOR expression levels following AR-42 treatment (Fig. 6C). These results suggested that HDAC2 may suppress MOR activity via regulating JNK signaling pathways.
Figure 6.
Effects of AR-42 on JNK signaling pathways in chronic pancreatitis. (A) Representative western blot images and (B) quantification of protein expression levels of JNK1/2 in the spinal dorsal horn. (C) Quantification of immunostaining density of MOR following AR-42 treatment. Data are presented as the mean ± standard error. *P<0.05 vs. control; #P<0.05 vs. group untreated with AR-42 at the same time point. MOR, µ-pioid receptor; JNK, c-Jun N-terminal kinase; TNBS, trinitrobenzene sulfonic acid; w, weeks; HDAC, histone deacetylase; i, inhibitor.
Discussion
The present study demonstrated that HDAC2 was upregulated and MOR activity was reduced in the spinal cord after CP induction, and HDAC2 was primarily upregulated in neurons rather than in glial cells in the spinal dorsal horn. As MOR is mainly expressed in neurons (16), it was hypothesized that the contrary expression between HDAC2 and MOR during CP might be involved in CP-induced allodynia. In the present study, it was demonstrated that HDAC2 increased in the spinal dorsal horn after CP induction. This was also confirmed by immunostaining analysis. Furthermore, the molecular mechanisms underlying the inflammatory process in the CNS following CP induction were investigated. Intrathecal injection of AR-42 significantly attenuated CP-induced mechanical allodynia and pro-inflammatory cytokine expression levels, following suppressed JNK expression and rescued MOR activity. Though these effects were not completely reversed, these results somewhat elucidated the underlying mechanisms supporting that HDAC2 serves important roles in suppressing MOR activity, thus inducing endogenous analgesia that is disrupted after CP induction.
In chronic pain, studies on HDACs and their inhibitors are only just beginning to emerge (21). HDACi have attracted increasing interest in recent years due to their potential usage in enhancing neuroplasticity and synaptic activity (22), and reducing neuronal damage (23,24) and neuroinflammation (25). Previous studies have demonstrated that HDAC inhibitors can alleviate inflammatory pain and attenuate the development of hypersensitivity in models of neuropathic pain. Thus, HDACi could be a valid alternative to traditional therapeutic agents by eliminating the side effect of pain (9). However, a clear demonstration that selective interference with HDAC activity can affect CP-induced chronic pain remains lacking. After the HDAC2 selective inhibitor AR-42 was intrathecally administrated, mechanical allodynia was significantly in TNBS treated rats, though not completely. In addition, it was confirmed that AR-42 could attenuate CP-induced chronic pain without interfering with basic pain behavior. This suggested the potential role of HDAC2 in CP chronic pain induction.
It has been reported that the overexpression of pro-inflammatory cytokines during CP induction, such as TNF-, IL-1β and IL-6 may promote the allodynia of CP rats (18,26), and these mediators may induce the activation of JNK and extracellular signal-regulated kinase (ERK) signaling pathways, which serve important roles in the maintenance of chronic pain. IL-1β is the most important inflammatory mediator that causes pancreatic islet dysfunction and destruction, which may promote activation of JNK (27–29); previous studies have also demonstrated positive effect of TNF-α and IL-6 on JNK activation (30–32). MOR expression is mainly regulated by signaling pathways such as JNK (17). However, ERK exhibits no significantly effect on MOR expression (33,34). Further investigation into the JNK signaling pathway may help reveal the underlying mechanisms involved in CP pain induction. The results of the present study demonstrated that the mRNA expression levels of these cytokines were significantly reduced by AR-42 at 3 and 5 weeks after CP induction, indicating that HDAC2 may promote the release of pro-inflammatory mediators, maintaining CP allodynia. Though this effect was not completely reversed by AR-42, no significant differences were induced by AR-42 in the sham group. These data are consistent with previous studies that HDACi possesses promising anti-inflammatory activities (35,36).
Western blot analysis demonstrated that JNK expression levels were upregulated after CP induction, and that inhibition of HDAC2 with AR-42 could significantly reverse JNK1/2 overexpression. Nevertheless, MOR activity was significantly rescued by AR-42 treatment. Furthermore, AR-42 did not effect on basic MOR activity. Additionally, the cellular localization of HDAC2 was observed in the thoracic spinal dorsal horn, which primarily localized to the cell bodies of neurons and rarely on glial cells, indicating that neuroglia may not directly express HDAC2. These results indicated that HDAC2 may be important in regulating neuroactivity, and is involved in MOR deactivation, causing central sensitization and persistent pain. The underlying mechanisms may facilitate the release of inflammatory cytokines, thus activating JNK signaling pathways, and finally suppressing MOR activity.
In conclusion, pain induced by CP can be as limiting as the disease itself (37), and requires medical treatment; however, treatment options are limited. Previous studies on humans and animal experimental models have implicated several mechanisms involved in pain, including neuroimmune alterations (38,39). To the best of our knowledge, the present study investigated epigenetic regulation in CP-induced allodynia for the first time, and observed increased HDAC2 activity in the spinal cord and behavioral allodynia after TNBS treatment. Therefore, HDAC2 may be facilitating the release of inflammatory cytokines, activating the JNK signaling pathway, and suppressing MOR activity, causing sensitization and pain induction. These results may facilitate the development of novel therapeutics for patients suffering from CP pain. Further studies are required to establish drugs with maximum efficacy and fewer side effects.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81400906, 81370928, 81620108008, 31100861 and 2012YQ0302609) and the China Postdoctoral Science Foundation (grant no. 2012M521864).
References
- 1.Keefe MD, Wang H, De La OJP, Khan A, Firpo MA, Murtaugh LC. β-catenin is selectively required for the expansion and regeneration of mature pancreatic acinar cells in mice. Dis Model Mech. 2012;5:503–514. doi: 10.1242/dmm.007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 3.Olesen SS, Juel J, Graversen C, Kolesnikov Y, Wilder-Smith OH, Drewes AM. Pharmacological pain management in chronic pancreatitis. World J Gastroenterol. 2013;19:7292–7301. doi: 10.3748/wjg.v19.i42.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes AM, Krarup AL, Detlefsen S, Malmstrøm ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: The role of neuropathic pain mechanisms. Gut. 2008;57:1616–1627. doi: 10.1136/gut.2007.146621. [DOI] [PubMed] [Google Scholar]
- 5.Feng W, Teng R, Zhao Y, Gao J, Chu H. Epigenetic modulation of Wnt signaling contributes to neuropathic pain in rats. Mol Med Rep. 2015;12:4727–4733. doi: 10.3892/mmr.2015.3972. [DOI] [PubMed] [Google Scholar]
- 6.Gáranton SM. Targeting epigenetic mechanisms for pain relief. Curr Opin Pharmacol. 2012;12:35–41. doi: 10.1016/j.coph.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Lessans S, Dorsey SG. The role for epigenetic modifications in pain and analgesia response. Nurs Res Pract 2013. 2013:961493. doi: 10.1155/2013/961493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert RE, Huang Q, Thai K, Advani SL, Lee K, Yuen DA, Connelly KA, Advani A. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: Potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int. 2011;79:1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 9.Capasso KE, Manners MT, Quershi RA, et al. Effect of histone deacetylase inhibitor JNJ-26481585 in pain. J Mol Neurosci. 2015;55:570–578. doi: 10.1007/s12031-014-0391-7. [DOI] [PubMed] [Google Scholar]
- 10.Bai G, Wei D, Zou S, Ren K, Dubner R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain. 2010;6:51. doi: 10.1186/1744-8069-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiechio S, Copani A, Zammataro M, Battaglia G, Gereau RW, IV, Nicoletti F. Transcriptional regulation of type-2 metabotropic glutamate receptors: An epigenetic path to novel treatments for chronic pain. Trends Pharmacol Sci. 2010;31:153–160. doi: 10.1016/j.tips.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, IV, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison C. Analgesia: Unravelling epigenetic mechanisms of chronic pain. Nat Rev Drug Discov. 2011;10:900. doi: 10.1038/nrd3606. [DOI] [PubMed] [Google Scholar]
- 15.Jamil MF, Subki MF, Lan TM, Majid MI, Adenan MI. The effect of mitragynine on cAMP formation and mRNA expression of mu-opioid receptors mediated by chronic morphine treatment in SK-N-SH neuroblastoma cell. J Ethnopharmacol. 2013;148:135–143. doi: 10.1016/j.jep.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 16.Hwang CK, Kim CS, Kim DK, Law PY, Wei LN, Loh HH. Up-regulation of the mu-opioid receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Mol Pharmacol. 2010;78:58–68. doi: 10.1124/mol.110.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagley Y, Hwang CK, Lin HY, Kam AF, Law PY, Loh HH, Wei LN. Inhibition of c-Jun NH2-terminal kinase stimulates mu opioid receptor expression via p38 MAPK-mediated nuclear NF-κB activation in neuronal and non-neuronal cells. Biochim Biophys Acta. 2013;1833:1476–1488. doi: 10.1016/j.bbamcr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian NS, Liao YH, Feng QX, Tang Y, Dou KF, Tao KS. Spinal toll like receptor 3 is involved in chronic pancreatitis-induced mechanical allodynia of rat. Mol Pain. 2011;7:15. doi: 10.1186/1744-8069-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravillah D, Mohammed A, Qian L, Brewer M, Zhang Y, Biddick L, Steele VE, Rao CV. Chemopreventive effects of an HDAC2-selective inhibitor on rat colon carcinogenesis and APCmin/+ mouse intestinal tumorigenesis. J Pharmacol Exp Ther. 2014;348:59–68. doi: 10.1124/jpet.113.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Denk F, McMahon SB. Chronic pain: Emerging evidence for the involvement of epigenetics. Neuron. 2012;73:435–444. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggarty SJ, Tsai LH. Probing the role of HDACs and mechanisms of chromatin-mediated neuroplasticity. Neurobiol Learn Mem. 2011;96:41–52. doi: 10.1016/j.nlm.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz KC, Casaccia P. HDAC inhibitors and neurodegeneration: At the edge between protection and damage. Pharmacol Res. 2010;62:11–17. doi: 10.1016/j.phrs.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashioka S, Klegeris A, McGeer PL. The histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates human astrocyte neurotoxicity induced by interferon-γ. J Neuroinflammation. 2012;9:113. doi: 10.1186/1742-2094-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh HS, Choi S, Khattar P, Choi N, Lee SC. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J Neuroimmune Pharmacol. 2010;5:521–532. doi: 10.1007/s11481-010-9192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu PY, Lu CL, Wang CC, Lee IH, Hsieh JC, Chen CC, Lee HF, Lin HC, Chang FY, Lee SD. Spinal microglia initiate and maintain hyperalgesia in a rat model of chronic pancreatitis. Gastroenterology. 2012;142:165–173.e2. doi: 10.1053/j.gastro.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, Xie Z, Jones WP, Wei XT, Liu Z, Wang D, Kulp SK, Wang J, Coss CC, Chen CS, et al. Preclinical pharmacokinetics study of R- and S-enantiomers of the histone deacetylase inhibitor, AR-42 (NSC 731438), in rodents. AAPS J. 2016;18:737–745. doi: 10.1208/s12248-016-9876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Wang D, Zhuang Z, Jin K, Zheng L, Yang Q, Guo K. Hypericin-mediated photodynamic therapy induces apoptosis in K562 human leukemia cells through JNK pathway modulation. Mol Med Rep. 2015;12:6475–6482. doi: 10.3892/mmr.2015.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann JL, Weil BR, Abarbanell AM, Wang Y, Poynter JA, Manukyan MC, Meldrum DR. IL-6 and TGF-α costimulate mesenchymal stem cell vascular endothelial growth factor production by ERK-, JNK-, and PI3K-mediated mechanisms. Shock. 2011;35:512–516. doi: 10.1097/SHK.0b013e31820b2fb9. [DOI] [PubMed] [Google Scholar]
- 31.Lu ZY, Chen WC, Li YH, Li L, Zhang H, Pang Y, Xiao ZF, Xiao HW, Xiao Y. TNF-α enhances vascular cell adhesion molecule-1 expression in human bone marrow mesenchymal stem cells via the NF-κB, ERK and JNK signaling pathways. Mol Med Rep. 2016;14:643–648. doi: 10.3892/mmr.2016.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang R, Zhang W, Song YM. Levels of leptin and IL-6 in lungs and blood are associated with the severity of chronic obstructive pulmonary disease in patients and rat models. Mol Med Rep. 2013;7:1470–1476. doi: 10.3892/mmr.2013.1377. [DOI] [PubMed] [Google Scholar]
- 33.Kim DK, Hwang CK, Wagley Y, Law PY, Wei LN, Loh HH. p38 mitogen-activated protein kinase and PI3-kinase are involved in up-regulation of mu opioid receptor transcription induced by cycloheximide. J Neurochem. 2011;116:1077–1087. doi: 10.1111/j.1471-4159.2010.07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian JM, Wu N, Su RB, Li J. Phosphatidylethanolamine-binding protein is not involved in µ-opioid receptor-mediated regulation of extracellular signal-regulated kinase. Mol Med Rep. 2015;11:3368–3374. doi: 10.3892/mmr.2015.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ZY, Schluesener HJ. HDAC inhibitor MS-275 attenuates the inflammatory reaction in rat experimental autoimmune prostatitis. Prostate. 2012;72:90–99. doi: 10.1002/pros.21410. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, West EJ, Van KC, Gurkoff GG, Zhou J, Zhang XM, Kozikowski AP, Lyeth BG. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Suvannasankha A, Crean CD, White VL, Chen CS, Farag SS. The novel histone deacetylase inhibitor, AR-42, inhibits gp130/Stat3 pathway and induces apoptosis and cell cycle arrest in multiple myeloma cells. Int J Cancer. 2011;129:204–213. doi: 10.1002/ijc.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas DM, Alinari L, West DA, Davis ME, Edwards RB, Johnson AJ, Blum KA, Hofmeister CC, Freitas MA, Parthun MR, et al. The novel deacetylase inhibitor AR-42 demonstrates pre-clinical activity in B-cell malignancies in vitro and in vivo. PLoS One. 2010;5:e10941. doi: 10.1371/journal.pone.0010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji RR. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm Pharmacol Ther. 2015;35:81–86. doi: 10.1016/j.pupt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]