Abstract
Several studies have shown that internal tandem duplication (ITD) of FMS-like tyrosine kinase 3 (FLT3) can result in the failure of leukemia treatment and contribute to a poor prognosis. However, the role of the overexpression of FLT3 in leukemia remains to be fully elucidated. By mining public database, the present study first identified that the expression of FLT3 in leukemia was markedly higher, compared with that in other types of tumor and cell lines, indicating that FLT3 is important in leukemia. In leukemia, FLT3 was found to be significantly upregulated in acute myeloid leukemia and acute lymphoblastic leukemia, and a high expression of FLT3 contributed to reduced survival rates. By analyzing Gene Expression Omnibus and The Cancer Genome Atlas data, it was found that genetic alterations and modification of DNA methylation increased the expression of FLT3 in leukemia. FLT3-ITD and FLT3 tyrosine kinase domain point mutations increased the expression of FLT3 in four independent datasets. In addition, the status of FLT3 gene methylation was negatively correlated with the expression of FLT3, and haploinsufficiency of DNA methyltransferase 1 increased the expression of Flt3 in mouse leukemia cells. By analyzing the enrichment of differentially-expressed genes in chemical and genetic perturbation datasets, it was found that genes, which were upregulated in the FLT3 high expression group had myeloid lymphoid leukemia- and nucleophosmin 1-like signatures, indicating that the overexpression of FLT3 may use the same mechanism to promote leukemia. Collectively, the results of the present study showed that the overexpression of FLT3 is a potential risk factor in leukemia.
Keywords: FMS-like tyrosine kinase 3, acute myeloid leukemia, prognostic factor, DNA methylation, differentially-expressed gene
Introduction
The FMS-like tyrosine kinase 3 (FLT3) gene, encoding a membrane-bound receptor tyrosine kinase, is crucial in normal hematopoiesis (1,2). It has been reported that FLT3 has two mutation types in leukemia, the most common form of FLT3 mutation is an internal tandem duplication (ITD) within the juxtamembrane domain, which occurs in 15–35% of patients with acute myeloid leukemia (AML) (3–13) and 5–10% of patients with myelodysplasia (MDS) (14,15). Another mutation type is the missense point mutation on the tyrosine kinase domain (TKD), which occurs in 5–10% of patients with AML, 2–5% of patients with MDS and 1–3% of patients with acute lymphoblastic leukemia (ALL) (9,16,17). FLT3-ITD can promote ligand-independent dimerization, autophosphorylation and constitutive activation of the receptor, which lead to the aberrant activation of multiple signaling pathways, including phosphatidylinositol 3-kinase/AKT, mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and signal transducer and activator of transcription 5 (STAT5) (18–20). FLT3-TKD also promotes constitutive phosphorylation of the receptor and ligand-independent cell growth (16,17,21).
FLT3 mutations are high risk factors in leukemia, and contribute to increased risk of treatment failure and poor prognosis (7,8). Mutations of FLT3-ITD have been reported to confer resistance to multiple tyrosine kinase inhibitors (22). The mutant allelic burden and ITD length are significantly associated with reduced overall survival and disease-free survival rates (23,24). The detection of ITD mutations at diagnosis is now a routine clinical practice to provide guidance for the optimal treatment of patients with AML.
Several previous studies have shown that the expression of FLT3 is activated in acute promyelocytic leukemia (APL) and adult B lymphoblastic leukemia (25,26), and the upregulation of FLT3 is a passive event in Hoxa9/Meis1-induced AML (27) indicating that the overexpression of FLT3 may have a tumor-promotion effect. In addition, the overexpression of FLT3 has been reported to activate the AKT and MAPK pathways, but not the STAT5 pathway (28), which suggests that the overexpression of FLT3 has overlapping downstream pathways with FLT3-ITD.
In the present study, it was found that FLT3 was upregulated in leukemia, and that the high expression of FLT3 indicated a poor prognosis. By analyzing differentially-expressed genes (DEGs), certain leukemic oncogenes were identified, and the high expression of FLT3 was found to have myeloid lymphoid leukemia (MLL)- and nucleophosmin 1-like (NPM1)-like signatures.
Materials and methods
Expression profile analysis
FLT3 expression data were collected from the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), cBioportal (29,30), Oncomine (http://www.oncomine.org), Cancer Cell Line Encyclopedia (CCLE; https://portals.broadinstitute.org/ccle/home) (31) and the Human Protein Atlas (HPA; http://www.proteinatlas.org) (32). The expression levels of FLT3 in various normal human tissues and cells were analyzed using three independent databases, which included the GEO GDS3834 dataset, and the HPA and Genotype-Tissue Expression (GTEx; https://www.gtexportal.org/home/) databases. The FLT3 expression data were directly downloaded from the GEO and HPA databases; the tissue with the highest expression of FLT3 was normalized to 1.
To compare the expression of FLT3 in different types of cancer, data were directly downloaded from cBioportal, Oncomine and CCLE, and cancer cell lines with the same tissue origin were classified as the same group.
The expression levels of FLT3 in different types of leukemia, including AML, B-cell ALL, B-cell childhood ALL, chronic lymphocytic leukemia, chronic myelogenous leukemia, myelodysplastic syndrome, pro-B ALL, and T-cell ALL, were analyzed using Oncomine. The peripheral blood mononuclear cells were considered a normal control.
The GDS4306 dataset was used to evaluate the effect of a DNA methyltransferase 1 (DNMT1) haploinsufficiency on the expression of FLT3. To compare the correlation between FLT3 methylation and expression, data were downloaded from cBioportal to perform linear regression. The FLT3 mutated samples and wild-type samples were analyzed separately.
Overall survival analysis
The GSE12417 GEO dataset, which contains data on survival rates and survival status, was selected to draw the overall survival curve, and the median expression of FLT3 was used as the cut-off, according to a previous report (33), to classify patients into a high expression group and low expression group. The top 50% of patients were classified as the FLT3 high expression group and the lowest 50% patients were defined as the FLT3 low expression group, according to the expression of FLT3 from high to low. The statistical difference between two curves was calculated using a log-rank test.
Analysis of genetic alterations
In order to summarize the genetic alterations of FLT3 in different types of cancer, The Cancer Genome Atlas (TCGA) data were downloaded through cBioportal. A total of 30 types of cancer, including AML, skin cutaneous melanoma, colorectal adenocarcinoma, esophageal carcinoma, lung adenocarcinoma, stomach adenocarcinoma, lung squamous cell cancer, lymphoid neoplasm diffuse large B-cell lymphoma, bladder urothelial carcinoma, uterine corpus endometrial carcinoma, sarcoma, cholangiocarcinoma, prostate adenocarcinoma, breast invasive carcinoma, glioblastoma multiforme, liver hepatocellular carcinoma, ovarian serous cystadenocarcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, head and neck squamous cell carcinoma, kidney renal clear cell carcinoma, uterine carcinosarcoma, brain lower grade glioma, pancreatic adenocarcinoma, pheochromocytoma and paraganglioma, adrenocortical carcinoma, thyroid carcinoma, testicular germ cell cancer, kidney renal papillary cell carcinoma, thymoma, kidney chromophobe and mesothelioma, were selected to analyze FLT3 mutations and copy number alterations.
Screening of DEGs
Three GEO datasets of AML (GSE10358, GSE14468 and GSE34860) were selected to analyze the DEGs between the FLT3 high expression group and FLT3 low expression group. Initially, the raw data in the CEL file were downloaded from the GEO database (34), and the robust multiarray average algorithm in the affy R-3.3.1 package was used to perform background correction, normalization and expression calculation (35–37). The Limma package in R (38) was used to identify the DEGs at the probe level between these two groups. P<0.05 and |log2 fold change (FC)|>0.585 were used as the cut-off criteria. Finally, these probes were annotated into gene names.
Chemical and genetic perturbations enrichment of DEGs
In order to identify the association between the overexpression of FLT3 and other risk factors for leukemia, the enrichment of DEGs in the chemical and genetic perturbations gene set were determined using Gene Set Enrichment Analysis (http://software.broadinstitute.org/gsea) (39,40). Input of the upregulated and downregulated genes was performed on the website separately, and chemical and genetic perturbations was selected to calculate the enrichment. P<0.05 and FDR q-value <0.05 were used as the cut-off criteria.
Statistical analysis
Student's t-test was used to calculate statistically significant differences between quantitative variables. The log-rank test was used to compare the overall survival curve. P<0.05 was considered to indicate a statistically significant difference. GraphPad Prism 5.01 was used for statistical analysis (GraphPad Software, Inc., La Jolla, CA, USA).
Results
FLT3 is upregulated in leukemia and a high expression of FLT3 is a prognostic biomarker
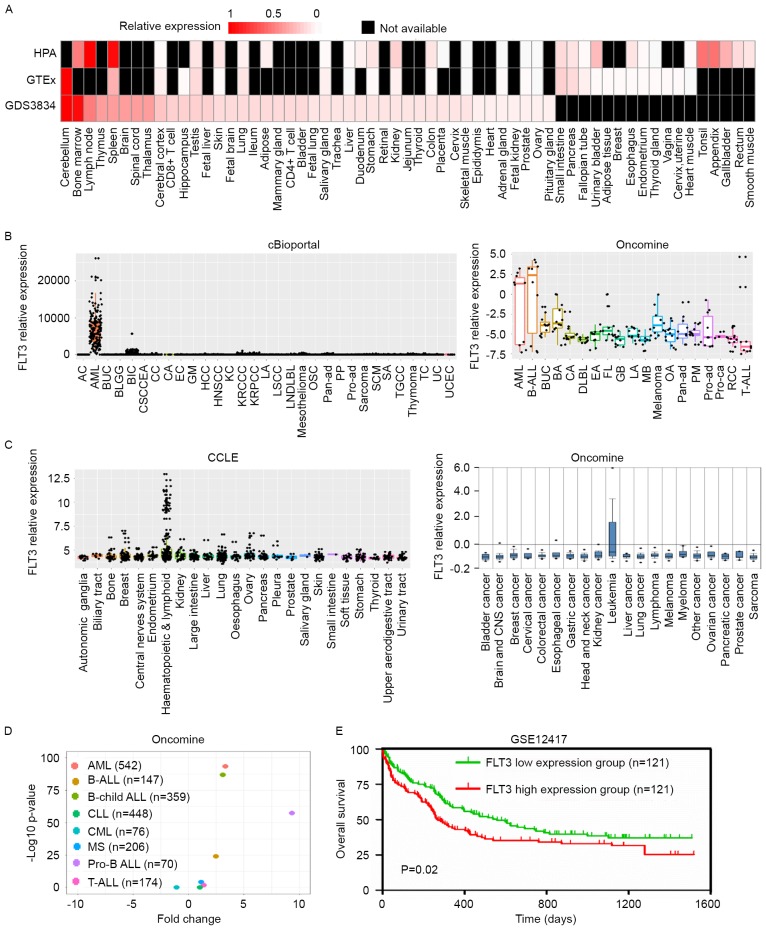
It has been reported that the FLT3-ITD mutation is correlated with prognosis and drug response in leukemia. To investigate the role of the expression of FLT3 in leukemia, the present study firstly evaluated the expression of FLT3 in different types of cancer and normal tissues. In normal tissues, FLT3 was expressed at high levels in bone marrow, lymph nodes, the thymus and spleen in three independent databases (Fig. 1A), which indicated that FLT3 was involved in hematopoiesis. In cancer, the expression of FLT3 was significantly higher in leukemia, compared with other types of tumor (Fig. 1B and C), and FLT3 was specifically upregulated in AML and ALL (Fig. 1D), suggesting that FLT3 contributed to the progression of AML and ALL. In order to analyze the association between the expression of FLT3 and survival rates in AML, the GSE12417 dataset, in which patients with AML exhibited a normal karyotype, was selected for analysis. Kaplan-Meier survival analysis showed that patients with AML with high expression levels of FLT3 (n=121) had reduced survival rates, compared with those with low expression levels of FLT3 (n=121; P<0.05; Fig. 1E). These collective data indicated that FLT3 was overexpressed in leukemia and was a prognostic biomarker.
Figure 1.
Tissue expression profile identifying the overexpression of FLT3 in leukemia. (A) RNA expression of FLT3 in various normal human tissues and cells, analyzed using three independent databases (HPA, GTEx and GDS3834). Red color indicates relative expression of FLT3, black indicates expression data are not available. (B) TCGA and Oncomine databases were used to evaluate expression levels of FLT3 in different types of tumor. (C) Expression of FLT3 in different cancer cell lines was analyzed using CCLE and Oncomine databases. Cell lines of the same tissue origin were classified into the same group. (D) FLT3 was significantly upregulated in several types of leukemia. The X-axis shows the fold change between different types of leukemia and peripheral blood mononuclear cells. The Y-axis shows the log 10 transformed P-value. Different colors of points indicate different types of leukemia. (E) Results of Kaplan-Meier survival analysis showed that patients with AML and a high expression of FLT3 (n=121) had shorter overall survival rates, compared with those with a low expression of FLT3 (n=121). FLT3, FMS-like tyrosine kinase; HPA, Human Protein Atlas; GTEx, Genotype-Tissue Expression; CCLE, Cancer Cell Line Encyclopedia; AC, adrenocortical carcinoma; AML, acute myeloid leukemia; BUC, bladder urothelial carcinoma; BLGG, brain lower grade glioma; BIC, breast invasive carcinoma, CSCCEA, cervical squamous cell carcinoma and endocervical adenocarcinoma; CC, cholangiocarcinoma; CA, colorectal adenocarcinoma; EC esophageal carcinoma; GM, glioblastoma multiforme; HCC hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; KC, kidney chromophobe; KRCCC, kidney renal clear cell carcinoma; KRPCC, kidney renal papillary cell carcinoma; LA, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; LNDLBL, lymphoid neoplasm diffuse large B-cell lymphoma; OSC, ovarian serous cystadenocarcinoma; Pan-ad, pancreatic adenocarcinoma; PP, pheochromocytoma and paraganglioma; Pro-ad, prostate adenocarcinoma; SCM, skin cutaneous melanoma; SA, stomach adenocarcinoma; TGCC, testicular germ cell cancer; TC, thyroid carcinoma; UC, uterine carcinosarcoma; UCEC, uterine corpus endometrial carcinoma; B-ALL, B-cell acute lymphoblastic leukemia; BA, breast adenocarcinoma; DLBL, diffuse large B-cell lymphoma; EA, endometrial adenocarcinoma; FL, follicular lymphoma GB, glioblastoma; MB, medulloblastoma; OA, ovarian adenocarcinoma; PM, pleural mesothelioma; Pro-ca, prostate carcinoma; RCC, renal cell carcinoma.
Expression of FLT3 is regulated by genetic and epigenetic alterations
In order to determine the mechanism increasing the expression of FLT3 in leukemia, the present study evaluated genetic and epigenetic alterations of FLT3 in AML. By mining TCGA data, it was found that FLT3 was significantly mutated in AML, compared with other types of cancer, which was similar to the results obtained on the expression of FLT3. The results revealed ~28% of patients with AML had somatic mutations in FLT3 (Fig. 2A). Therefore, it was hypothesized that FLT3 mutations may increase the expression of FLT3 in AML. To confirm this, the expression of FLT3 was compared between wild-type and mutated groups in four independent databases, (GSE10358, GSE14468, GSE34860 and TCGA). It was found that ITD and TKD mutations significantly increased the expression of FLT3 (Fig. 2B). In addition, methylation data from TCGA was used to compare the association between the expression of FLT3 and methylation status. In AML, the expression of FLT3 was negatively correlated with its methylation (Fig. 2C), indicating that the hypomethylation of FLT3 may be a potential mechanism resulting in the upregulation of FLT3. Of note, mining of the GEO dataset (GDS4306) revealed that the haploinsufficiency of DNMT1 significantly increased the expression of Flt3 in mouse leukemia cells (Fig. 2D), suggesting that DNMT1 modified the methylation of FLT3. Collectively, these data showed that genetic and epigenetic alterations may be potential mechanisms by which the expression of FLT3 is increased in AML.
Figure 2.
Genetic and epigenetic alterations increase the expression of FLT3 in AML. (A) cBioportal database was used to analyze mutations and copy number alterations of FLT3. Genetic alterations of FLT3 in different types of cancer showed the most mutations of FLT3 in leukemia. (B) Four databases (GSE10358, GSE14468, GSE34860 and TCGA) were utilized to examine the effect of FLT3 mutations on the expression of FLT3. (C) cBioportal result showed the expression of FLT3 was negatively correlated with FLT3 methylation in leukemia. (D) Gene Expression Omnibus dataset (GDS4306) showed that DNMT1 haploinsufficiency (haplo) increased expression of Flt3 in mouse leukemia cells. FLT3, FMS-like tyrosine kinase; AML, acute myeloid leukemia; DLBC, diffuse large B-cell; GBM, glioblastoma multiforme; ccRCC, clear cell renal cell carcinoma; UC, uterine carcinosarcoma; PCPG, pheochromocytoma and paraganglioma; ACC, adenoid cystic carcinoma; pRCC, papillary renal cell carcinoma; chRCC, chromophobe renal cell carcinoma; WT, wild-type; ITD, internal tandem duplication; TKD, tyrosine kinase domain. DNMT1, DNA methyltransferase 1; *P<0.05, **P<0.01 and ***P<0.001; ns, not significant.
High expression of FLT3 has MLL- and NPM1-like signatures
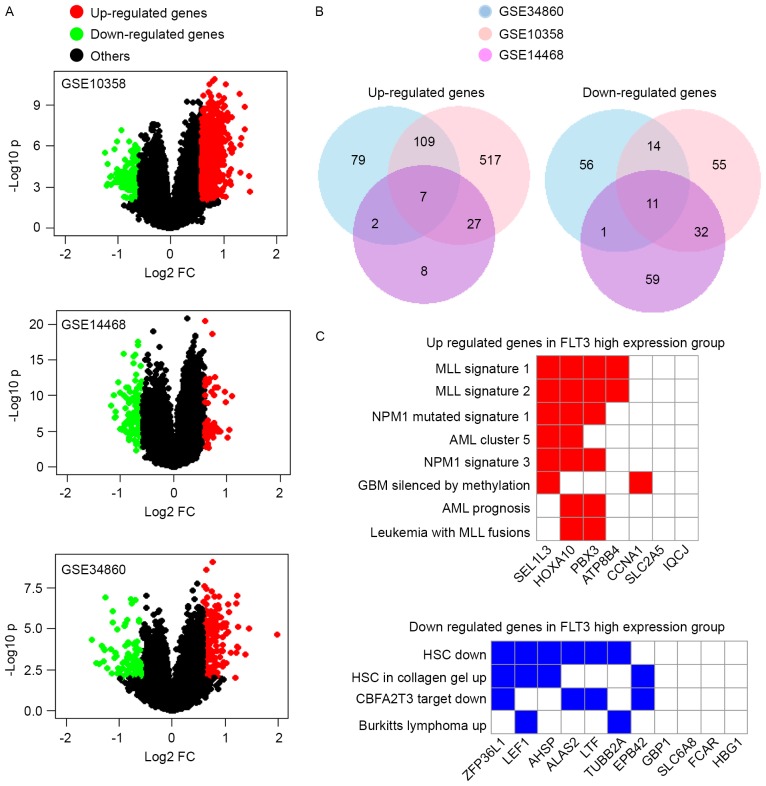
In order to identify the DEGs associated with a high expression of FLT3, three independent AML databases (GSE10358, GSE14468 and GSE34860) were examined (Fig. 3A). The expression of seven genes were significantly upregulated in the FLT3 high expression group, comprising ATP8B4, CCNA1, HOXA10, IQCJ, PBX3, SEL1L3 and SLC2A5. CCNA1, HOXA10, PBX3 and SLC2A5 have been reported to function as oncogenes in leukemia (41–44). A total of 11 genes were downregulated, comprising AHSP, ALAS2, EPB42, FCAR, GBP1, HBG1, LEF1, LTF, SLC6A8, TUBB2A and ZFP36L1, as shown in Fig. 3B. The deletion of RNA-binding protein ZFP36L1 has been reported to lead to perturbed thymic development and T lymphoblastic leukemia (45). Subsequently, the present study analyzed the enrichment of these DEGs in the chemical and genetic perturbations dataset. Of note, the seven overexpressed genes were enriched in MLL and NPM1 signatures, indicating that the majority were also upregulated in MLL- or NPM1-mutated AML (Fig. 3C). The 11 downregulated genes were reduced in hematopoietic stem cells (Fig. 3C). Taken together, these results showed that a high expression of FLT3 was associated with MLL- and NMP1-like signatures.
Figure 3.
DEGs between the FLT3 high expression group and FLT3 low expression group. Three databases (GSE10358, GSE14468 and GSE34860) were used to analyze DEGs. (A) Genes with P<0.05 and FC >1.5 are indicated in red and green colors in the volcano plot. Red indicates genes upregulated in the FLT3 high expression group, and green indicates genes downregulated in the FLT3 high expression group. The X-axis is the log2-transformed fold change, and the Y-axis is the log10-transformed P-value. (B) Pie charts shows number of DEGs in the three databases. A total of seven genes were upregulated in the FLT3 high expression group (left) and 11 genes were downregulated (right). (C) Gene Set Enrichment Analysis was used to assess the association between DEGs and chemical and genetic perturbations. The seven upregulated genes were mainly associated with the MLL signature and NPM1 signature (above). The majority of the downregulated genes were also reduced in HSCs (below). DEGs, differentially-expressed genes; FC, fold change; HCSs, hematopoietic stem cells; MLL, myeloid lymphoid leukemia; NPM1, nucleophosmin 1-like; AML, acute myeloid leukemia; GBM, glioblastoma multiforme.
Discussion
In the present study, it was found that the expression of FLT3 was high in normal hemopoietic tissues (Fig. 1A) and, in leukemia, FLT3 was specifically upregulated in AML and ALL (Fig. 1D), indicating that a high expression of FLT3 may contribute to the progression of leukemia. It has been reported that the overexpression of FLT3 can induce autophosphorylation (46), and activate the AKT and MAPK pathways in AML (47). ITD mutations clustered in the juxtamembrane domain of FLT3 are the most frequent forms in AML, and FLT3-ITD mutations are associated with a poor prognosis (47–49). In the present study, it was also found that a high expression of FLT3 was a prognostic factor for poor prognosis in AML using the GEO database (Fig. 1E).
FLT3-related pathways were activated in AML by ITD mutations or the overexpression of FLT3. Several interacting proteins are also reported to interact with FLT3 to negatively or positively regulate FLT3 pathways. Spleen tyrosine kinase (SYK) and the mucin 1-C-terminal subunit (MUC1-C) oncoprotein are reported to directly bind to and activate FLT3-related pathways (50,51), whereas suppressor of cytokine signaling 2 (SOCS2) and src-like adaptor protein 2 (SLAP2) interact with FLT3 protein to inhibit its signaling (52,53). In addition, the transcription of FLT3 can be regulated in AML. Certain AML-related transcription factors, including CCAAT/enhancer binding protein α and the proto-oncogene MYB, can bind to the FLT3 promoter to activate the transcription of FLT3 (54). In the present study, another two mechanism were found to increase the expression of FLT3 in AML. ITD and TKD mutations, and the methylation of FLT3 increased its expression in AML (Fig. 2B and C). In addition, DNMT1 was identified as is a potential regulator of FLT3 (Fig. 2D).
By analyzing DEGs in the FLT3 high expression group, a total of 18 genes were identified using three independent AML datasets, seven of which were upregulated and 11 of which were downregulated (Fig. 3B). These upregulated genes were enriched in MLL and NPM1 signatures (Fig. 3C), and CCNA1, HOXA10, PBX3 and SLC2A5 were reported to function as oncogenes in leukemia. CCNA1 was overexpressed in ALL, and patients with high levels of CCNA1 exhibit poor event-free survival rates (55). CCNA1 transgenic mice have also been shown to exhibit abnormal myelopoiesis and progressed to overt AML (40). Similarly, the overexpression of HOXA10 can cooperate with active SHP2 to induce AML (41), and HOXA10 was usually fused with the NUP98, collaborating with overexpressed FLT3 receptor tyrosine kinase to induce aggressive AML (56). PBX3 is an important cofactor of HOXA9 in leukemogenesis (57) and the coexpression of PBX3 and MEIS1 (PBX3/MEIS1) can cause AML in vivo (58). SLC2A5 is also overexpressed in AML and can increase the fructose utilization of leukemic cells, with a high expression of SLC2A5 being associated with poor outcomes (44). Among these 11 downregulated genes, ZFP36L1 has a tumor suppressor role in leukemia. ZFP36L1 is an RNA-binding protein and leads to mRNA degradation; deletion of ZFP36L1 in mice can induce T cell ALL (45).
Collectively the findings obtained in the present study showed that FLT3 is overexpressed in leukemia and is prognostic factor for poor prognosis in AML. Patients with a high expression of FLT3 simultaneously express high levels of leukemic oncogenes. Therefore, a high expression of FLT3 is a risk factor in leukemia.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 8130044).
References
- 1.Volpe G, Clarke M, Garcìa P, Walton DS, Vegiopoulos A, Del Pozzo W, O'Neill LP, Frampton J, Dumon S. Regulation of the Flt3 gene in haematopoietic stem and early progenitor cells. PLoS One. 2015;10:e0138257. doi: 10.1371/journal.pone.0138257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebihara Y, Wada M, Ueda T, Xu MJ, Manabe A, Tanaka R, Ito M, Mugishima H, Asano S, Nakahata T, Tsuji K. Reconstitution of human haematopoiesis in non-obese diabetic/severe combined immunodeficient mice by clonal cells expanded from single CD34+CD38- cells expressing Flk2/Flt3. Br J Haematol. 2002;119:525–534. doi: 10.1046/j.1365-2141.2002.03820.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 4.Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 5.Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K, Takeshita A, Saito K, Hasegawa S, Shimodaira S, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho) Leukemia. 1997;11:1447–1452. doi: 10.1038/sj.leu.2400756. [DOI] [PubMed] [Google Scholar]
- 6.Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, Radich JP. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.V97.11.3589. [DOI] [PubMed] [Google Scholar]
- 7.Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, Bernstein ID, Radich JP. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.V97.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.V100.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, Wermke M, Bornhäuser M, Ritter M, Neubauer A, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.V99.12.4326. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Duhier FM, Goodeve AC, Wilson GA, Gari MA, Peake IR, Rees DC, Vandenberghe EA, Winship PR, Reilly JT. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–195. doi: 10.1046/j.1365-2141.2000.02317.x. [DOI] [PubMed] [Google Scholar]
- 11.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.V98.6.1752. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Taki T, Yang HW, Hanada R, Hongo T, Ohnishi H, Kobayashi M, Bessho F, Yanagisawa M, Hayashi Y. Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol. 1999;105:155–162. doi: 10.1111/j.1365-2141.1999.01284.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, Asou N, Kuriyama K, Jinnai I, Shimazaki C, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- 14.Horiike S, Yokota S, Nakao M, Iwai T, Sasai Y, Kaneko H, Taniwaki M, Kashima K, Fujii H, Abe T, Misawa S. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia. 1997;11:1442–1446. doi: 10.1038/sj.leu.2400770. [DOI] [PubMed] [Google Scholar]
- 15.Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, Sonoda Y, Abe T, Kahsima K, Matsuo Y, Naoe T. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.V97.8.2434. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, Naoe T. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 19.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–2563. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 20.Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C, Grüning W, Kratz-Albers K, Serve S, Steur C, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- 21.Spiekermann K, Bagrintseva K, Schoch C, Haferlach T, Hiddemann W, Schnittger S. A new and recurrent activating length mutation in exon 20 of the FLT3 gene in acute myeloid leukemia. Blood. 2002;100:3423–3425. doi: 10.1182/blood-2002-03-0953. [DOI] [PubMed] [Google Scholar]
- 22.Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, Small D. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27:48–55. doi: 10.1038/leu.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Lee GD, Park J, Yoon JH, Kim HJ, Min WS, Kim M. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015;5:e336. doi: 10.1038/bcj.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, Slovak ML, Willman CL, Radich JP. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith M, Griffith OL, Krysiak K, Skidmore ZL, Christopher MJ, Klco JM, Ramu A, Lamprecht TL, Wagner AH, Campbell KM, et al. Comprehensive genomic analysis reveals FLT3 activation and a therapeutic strategy for a patient with relapsed adult B-lymphoblastic leukemia. Exp Hematol. 2016;44:603–613. doi: 10.1016/j.exphem.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilakos K, Viniou NA, Mavrogianni D, Vassilakopoulos TP, Dimopoulou MN, Plata E, Angelopoulou MK, Variami E, Stavrogianni N, Liapi D, et al. FLT3 overexpression in acute promyelocytic leukemia patients without detectable FLT3-ITD or codon 835–836 mutations: A pilot study. Anticancer Res. 2006;26:1201–1207. [PubMed] [Google Scholar]
- 27.Staffas A, Arabanian LS, Wei SY, Jansson A, Ståhlman S, Johansson P, Fogelstrand L, Cammenga J, Kuchenbauer F, Palmqvist L. Upregulation of Flt3 is a passive event in Hoxa9/Meis1-induced acute myeloid leukemia in mice. Oncogene. 2017;36:1516–1524. doi: 10.1038/onc.2016.318. [DOI] [PubMed] [Google Scholar]
- 28.Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W. Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res. 2003;9:2140–2150. [PubMed] [Google Scholar]
- 29.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 33.Wu BH, Chen H, Cai CM, Fang JZ, Wu CC, Huang LY, Wang L, Han ZG. Epigenetic silencing of JMJD5 promotes the proliferation of hepatocellular carcinoma cells by down-regulating the transcription of CDKN1A. Oncotarget. 2016;7:6847–6863. doi: 10.18632/oncotarget.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 36.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–525. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 37.Fujita A, Sato JR, Rodrigues Lde O, Ferreira CE, Sogayar MC. Evaluating different methods of microarray data normalization. BMC Bioinformatics. 2006;7:469. doi: 10.1186/1471-2105-7-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles; Proc Natl Acad Sci USA; 2005; pp. 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 41.Liao C, Wang XY, Wei HQ, Li SQ, Merghoub T, Pandolfi PP, Wolgemuth DJ. Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin A1; Proc Natl Acad Sci USA; 2001; pp. 6853–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Lindsey S, Konieczna I, Bei L, Horvath E, Huang W, Saberwal G, Eklund EA. Constitutively active SHP2 cooperates with HoxA10 overexpression to induce acute myeloid leukemia. J Biol Chem. 2009;284:2549–2567. doi: 10.1074/jbc.M804704200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Cuellar MP, Steger J, Füller E, Hetzner K, Slany RK. Pbx3 and Meis1 cooperate through multiple mechanisms to support Hox-induced murine leukemia. Haematologica. 2015;100:905–913. doi: 10.3324/haematol.2015.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen WL, Wang YY, Zhao A, Xia L, Xie G, Su M, Zhao L, Liu J, Qu C, Wei R, et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell. 2016;30:779–791. doi: 10.1016/j.ccell.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodson DJ, Janas ML, Galloway A, Bell SE, Andrews S, Li CM, Pannell R, Siebel CW, MacDonald HR, De Keersmaecker K, et al. Deletion of the RNA-binding proteins ZFP36L1 and ZFP36L2 leads to perturbed thymic development and T lymphoblastic leukemia. Nat Immunol. 2010;11:717–724. doi: 10.1038/ni1010-969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, Miyawaki S, Kuriyama K, Shimazaki C, Akiyama H, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 47.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 48.Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:89–99. doi: 10.1002/ajh.22246. [DOI] [PubMed] [Google Scholar]
- 49.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puissant A, Fenouille N, Alexe G, Pikman Y, Bassil CF, Mehta S, Du J, Kazi JU, Luciano F, Rönnstrand L, et al. SYK is a critical regulator of FLT3 in acute myeloid leukemia. Cancer Cell. 2014;25:226–242. doi: 10.1016/j.ccr.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Yin L, Stroopinsky D, Rajabi H, Puissant A, Stegmaier K, Avigan D, Kharbanda S, Kufe D, Stone R. MUC1-C oncoprotein promotes FLT3 receptor activation in acute myeloid leukemia cells. Blood. 2014;123:734–742. doi: 10.1182/blood-2013-04-493858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moharram SA, Chougule RA, Su X, Li T, Sun J, Zhao H, Rönnstrand L, Kazi JU. Src-like adaptor protein 2 (SLAP2) binds to and inhibits FLT3 signaling. Oncotarget. 2016;7:57770–57782. doi: 10.18632/oncotarget.10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kazi JU, Rönnstrand L. Suppressor of cytokine signaling 2 (SOCS2) associates with FLT3 and negatively regulates downstream signaling. Mol Oncol. 2013;7:693–703. doi: 10.1016/j.molonc.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volpe G, Walton DS, Del Pozzo W, Garcia P, Dassé E, O'Neill LP, Griffiths M, Frampton J, Dumon S. C/EBPα and MYB regulate FLT3 expression in AML. Leukemia. 2013;27:1487–1496. doi: 10.1038/leu.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holm C, Ora I, Brunhoff C, Anagnostaki L, Landberg G, Persson JL. Cyclin A1 expression and associations with disease characteristics in childhood acute lymphoblastic leukemia. Leuk Res. 2006;30:254–261. doi: 10.1016/j.leukres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Palmqvist L, Argiropoulos B, Pineault N, Abramovich C, Sly LM, Krystal G, Wan A, Humphries RK. The Flt3 receptor tyrosine kinase collaborates with NUP98-HOX fusions in acute myeloid leukemia. Blood. 2006;108:1030–1036. doi: 10.1182/blood-2005-12-007005. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Zhang Z, Li Y, Arnovitz S, Chen P, Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H, et al. PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood. 2013;121:1422–1431. doi: 10.1182/blood-2012-07-442004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Chen P, Su R, Hu C, Li Y, Elkahloun AG, Zuo Z, Gurbuxani S, Arnovitz S, Weng H, et al. PBX3 and MEIS1 cooperate in hematopoietic cells to drive acute myeloid leukemias characterized by a core transcriptome of the MLL-rearranged disease. Cancer Res. 2016;76:619–629. doi: 10.1158/0008-5472.CAN-15-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]