Abstract
Stereotactic body radiotherapy (SBRT) for early-stage non–small-cell lung cancer use is evolving, with implementation in a rising geriatric oncologic population. Our study investigated the use of SBRT for non–small-cell lung cancer in 58 consecutive patients ≥ 80 years old at 4 academic centers. SBRT was well-tolerated with expected toxicity rates, excellent disease-specific outcomes, and patients with higher performance status deriving the greatest benefit.
Background
Stereotactic body radiotherapy (SBRT) is the standard of care for medically inoperable early-stage non– small-cell lung cancer. Despite the limited number of octogenarians and nonagenarians on trials of SBRT, its use is increasingly being offered in these patients, given the aging cancer population, medical fragility, or patient preference. Our purpose was to investigate the efficacy, safety, and survival of patients ≥80 years old treated with definitive lung SBRT.
Methods
Patients who underwent SBRT were reviewed from 2009 to 2015 at 4 academic centers. Patients diagnosed at ≥80 years old were included. Kaplan-Meier and multivariate logistic regression and Cox proportional hazard regression analyses were performed. Recursive partitioning analysis was done to determine a subgroup of patients most likely to benefit from therapy.
Results
A total of 58 patients were included, with a median age of 84.9 years (range, 80.1-95.2 years), a median follow-up time of 19.9 months (range, 6.9-64.9 months), a median fraction size of 10.0 Gy (range, 7.0-20.0 Gy), and a median number of fractions of 5.0 (range, 3.0-8.0 fractions). On multivariate analysis, higher Karnofsky performance status (KPS) was associated with higher local recurrence-free survival (hazard ratio [HR], 0.92; P < .01), regional recurrence-free survival (HR, 0.94; P < .01), and overall survival (HR, 0.91; P < .01). On recursive partitioning analysis, patients with KPS ≥75 had improved 3-year cancer-specific and overall survival (99.4% and 91.9%, respectively) compared with patients with KPS < 75 (47.8% and 23.6%, respectively; P < .01).
Conclusion
Definitive lung SBRT for early-stage non–small-cell lung cancer was efficacious and safe in patients ≥80 years old. Patients with a KPS of ≥75 derived the most benefit from therapy.
Keywords: Geriatric oncologic, Nonagenarians, Octogenarians, Performance status, Radiation pneumonitis
Introduction
Stereotactic body radiotherapy (SBRT) is the standard of care for medically inoperable early-stage non–small-cell lung cancer (NSCLC), with local control rates of approximately 90%.1–8 The adoption of lung SBRT has been swift, with its use now being investigated in patients who are surgical candidates9,10 as well as those who would otherwise choose no other therapy.11 With an aging oncologic population,12 the treatment of octogenarians and nonagenarians with definitive lung SBRT will become more common, either owing to medical fragility or patient preference. There is limited literature exploring the efficacy and safety of definitive lung SBRT in this population, with small numbers of these patients on prospective trials and concerns about the utility of this treatment in patients who may die of competing risk factors.1,2,13
The objectives of this study were to evaluate the efficacy of definitive lung SBRT in patients ≥ 80 years old at the time of treatment in a high-volume multicenter academic practice. Additionally, given safety concerns in a potentially more medically fragile group, we sought to explore the toxicity of lung SBRT in this population and examine patient, tumor, and treatment factors associated with treatment-related toxicity.
Methods and Materials
Patient Selection
Under an institutional review board-approved protocol, the records of patients from 2009 to 2015 treated with definitive lung SBRT at Emory University Hospital, Emory University Hospital Midtown, Grady Memorial Hospital, and Emory St. Joseph’s Hospital were reviewed. We excluded patients who started treatment before 80 years of age. Staging was based on the seventh edition of the American Joint Committee on Cancer guidelines.14 Treatment simulation, planning, and delivery was done per modern clinical trial standards.15 All patients were followed with periodic physical exams and surveillance computed tomography (CT) imaging.
Statistical Analysis
All statistics were computed using SAS software version 9.4 (Cary, NC). Patient characteristics examined included gender, age, history of prior cancer, history of prior lung cancer, history of prior thoracic radiation, active smoking status, smoking pack-years, Karnofsky performance status16 (KPS) at time of initial consult (score, 0-100), and use of an angiotensin-converting-enzyme inhibitor (ACE-I) at time of initial consult. ACE-I usage was investigated owing to the evolving literature of its association with radiation pneumonitis (RP).17,18 Tumor characteristics examined included histology (adenocarcinoma, squamous cell carcinoma, and not biopsied), T stage (T1a/T1b, T2a/T2b, and T3), tumor size, and tumor location. Treatment characteristics examined included method of mediastinal staging (positron emission tomography [PET]-CT with or without pathologic sampling), radiation dose per fraction, number of fractions delivered, and treatment delivery modality (intensity-modulated radiotherapy or volumetric arc therapy). Of note, age, KPS at the time of consult, smoking pack-years, tumor size, number of radiation fractions delivered, and radiation dose per fraction were treated as continuous variables. Local recurrence-free survival (LRFS) was defined as time from last treatment date to recurrence at treated site, death, or last follow-up. Regional recurrence-free survival (RRFS) was defined as time from last treatment date to recurrence in the same lobe of treatment, ipsilateral hilum, mediastinum, death, or last follow-up. Metastatic recurrence-free survival (MRFS) was defined as time from last treatment date to recurrence in the contralateral lung, any distant site of disease, death, or last follow-up. Death was included in these endpoints given the low event rate of tumor failures. Cancer-specific survival (CSS) was defined as time to death from causes other than lung cancer or last follow-up. Overall survival (OS) was defined as time to death or time to last follow-up from diagnosis. RP was defined using Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 grade 2 or higher. Survival estimates for endpoints of local control (absence of failure at the treated site), regional control (absence of failure at the lobe of treatment, ipsilateral hilum, or mediastinum), distant control (absence of failure at contralateral lung or distant sites), CSS, and OS were estimated using the Kaplan-Meier product limit method, and survival distributions were compared using the log-rank test.19 Univariate association (UVA) of each variable with LRFS, RRFS, MRFS, CSS, and OS were assessed using Cox proportional hazard models and log rank tests. UVA logistic regression was assessed for RP. Multivariable (MVA) logistic regression and proportional hazard models were generated, using a backward selection with an alpha removal of 0.2. Recursive partitioning analysis was done to determine the relationship between KPS at time of consult to CSS and OS and generate 2 stratified classes.20,21 Significance was assessed at the 0.05 level. Graphics were created using R version 2.15.1.
Results
Patient Characteristics
After review of 266 patient charts, 58 patients met the study inclusion criteria, with the rest excluded for being treated before the age of 80 years. The median follow-up time was 19.9 months (range, 6.9-64.9 months), median age at time of treatment was 84.9 years (range, 80.1-95.2 years), median KPS at time of consult was 75 (range, 50-100), and median tumor size was 2.05 cm (range, 0.8-6.3 cm). All patients were staged prior to therapy with a PET-CT scan. The other patient, tumor, and treatment characteristics of our study population are summarized in Table 1.
Table 1.
Summary of the Patient, Tumor, and Treatment Characteristics of Our Study Population
| Variable | N (%) = 58 |
|---|---|
| Patient characteristics | |
| Gender | |
| Female | 28 (48.3) |
| Male | 30 (51.7) |
| History of prior cancer | |
| No | 28 (48.3) |
| Yes | 30 (51.7) |
| History of prior lung cancer | |
| No | 37 (63.8) |
| Yes | 21 (36.2) |
| History of prior thoracic radiation | |
| No | 47 (81.0) |
| Yes | 11 (19.0) |
| ACE-I usage | |
| No | 32 (55.2) |
| Yes | 26 (44.8) |
| Active smoker | |
| No | 52 (89.7) |
| Yes | 6 (10.3) |
| Smoking pack-years | |
| Median (range) | 40.0 (0.0–130.0) |
| Tumor characteristics | |
| Histology | |
| Adenocarcinoma | 23 (39.7) |
| Squamous cell carcinoma | 17 (29.3) |
| Not biopsied | 18 (31.0) |
| T stage | |
| T1a/T1b | 39 (67.2) |
| T2a/T2b | 12 (20.7) |
| T3 | 7 (12.1) |
| Tumor location | |
| Left lower lobe | 11 (19.0) |
| Left upper lobe | 8 (13.8) |
| Right lower lobe | 14 (24.1) |
| Right middle lobe | 2 (3.4) |
| Right upper lobe | 23 (39.7) |
| Treatment characteristics | |
| Method of mediastinal staging | |
| PET-CT alone | 37 (63.8) |
| PET-CT and pathologic sampling | 21 (36.2) |
| Radiation dose per fraction in Gy | |
| Median (range) | 10.0 (7.0–20.0) |
| Number of radiation fractions delivered | |
| Median (range) | 5.0 (3.0–8.0) |
| Treatment modality | |
| IMRT | 14 (24.1) |
| VMAT | 44 (75.9) |
Abbreviations: ACE-I = angiotensin-converting enzyme inhibitor; CT = computed tomography; IMRT = intensity modulated radiotherapy; KPS = Karnofsky performance status; PET = positron emission tomography; VMAT = volumetric arc therapy.
Local Recurrence
There were 6 tumor recurrences at the treated site (10.4%), with median time to recurrence of 17.7 months (range, 5.0-62.4 months). On UVA, having prior lung cancer (hazard ratio [HR], 2.46; 95% confidence interval [CI], 1.06-5.72) and having any prior cancer (HR, 4.09; 95% CI, 1.50-11.13) were associated with shorter LRFS whereas higher KPS at time of consult (HR, 0.92; 95% CI, 0.89-0.96) was associated with longer LRFS (See Supplemental Table 1 in the online version). On MVA, T1a or T1b tumors (HR, 0.20; 95% CI, 0.04-0.90), and higher KPS at time of consult (HR, 0.92; 95% CI, 0.89-0.96) were associated with longer LRFS, whereas adenocarcinomas (HR, 6.36; 95% CI, 1.57-25.76) were associated with shorter LRFS (Table 2).
Table 2.
Multivariablea Analysis of Patient, Tumor, and Treatment Characteristics and Their Association With Local Recurrence-free Survival
| Covariate | Level | Hazard Ratio (95% CI) | HR P Value |
|---|---|---|---|
| T stage | T1a or T1b | 0.20 (0.04–0.90) | .04 |
| T2a or T2b | 1.28 (0.23-6.97) | .78 | |
| T3 or T4 | – | – | |
| Tumor histology | Adenocarcinoma | 6.36 (1.57–25.76) | .01 |
| Not biopsied | 1.02 (0.22–4.72) | .98 | |
| Squamous cell carcinoma | – | – | |
| History of prior lung cancer | Yes | 2.38 (0.68–8.32) | .17 |
| No | – | – | |
| History of prior cancer | Yes | 2.93 (0.99–8.69) | .06 |
| No | – | – | |
| KPS at the time of consult | 0.92 (0.89–0.96) | <.01 |
Bold P-values indicate statistical significance.
Abbreviations: CI = confidence interval; HR = hazard ratio; KPS = Karnofsky performance status.
Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: age, tumor location, prior lung radiotherapy, gender, radiation fractions and dose per fraction, tumor size, treatment modality.
Regional Recurrence
There were 12 regional tumor recurrences (20.7%), with median time to recurrence of 14.8 months (range, 2.2-64.2 months). On UVA, having prior cancer (HR, 3.08; 95% CI, 1.28-7.42), history of prior lung cancer (HR, 2.58; 95% CI, 1.15-5.77), and right middle lobe tumor location (HR, 5.49; 95% CI, 1.09-27.65) was associated with shorter RRFS, whereas higher KPS at time of consult (HR, 0.93; 95% CI, 0.90-0.96) was associated with longer RRFS (See Supplemental Table 2 in the online version). On MVA, only higher KPS at time of consult (HR, 0.94; 95% CI, 0.90-0.97) was associated with longer RRFS (See Supplemental Table 3 in the online version).
Metastatic Recurrence
There were 6 metastatic disease recurrences (10.4%), with median time to recurrence of 18.1 months (range, 2.2-64.2 months). On UVA, history of prior cancer (HR, 3.56; 95% CI, 1.28-9.89) and history of prior lung cancer (HR, 3.75; 95% CI, 1.47-9.57) was associated with shorter MRFS, whereas higher KPS at the time of consult (HR, 0.91; 95% CI, 0.87-0.95) was associated with longer MRFS (See Supplemental Table 4 in the online version). On MVA, only higher KPS at time of consult (HR, 0.91; 95% CI, 0.87-0.95) was associated with longer MRFS.
CSS
There were 9 deaths attributable to lung cancer (15.5%). On UVA, a history of prior cancer (HR, 4.20; 95% CI, 1.05-16.84) was associated with lower CSS, whereas higher KPS at time of consult (HR, 0.94; 95% CI, 0.89-0.99) was associated with higher CSS (See Supplemental Table 5 in the online version). On MVA, history of prior lung cancer (HR, 7.75; 95% CI, 1.61-37.20) and older age (HR, 1.19; 95% CI, 1.01-1.42) was associated with lower CSS, whereas not being an active smoker (HR, 0.14; 95% CI, 0.02-0.87) was associated with higher CSS (Table 3). Two- and 3-year rates of CSS were 81.6% (95% CI, 64.5%-91.0%) and 72.6% (95% CI, 52.1%-85.4%), respectively.
Table 3.
Multivariablea Analysis of Patient, Tumor, and Treatment Characteristics and Their Association With Cancer-specific Survival
| Covariate | Level | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| History of prior lung cancer | Yes | 7.75 (1.61–37.20) | .01 |
| No | – | – | |
| Active smoker | No | 0.14 (0.02-0.87) | .04 |
| Yes | – | – | |
| Age | 1.19 (1.01–1.42) | .04 |
Bold P-values indicate statistical significance.
Abbreviation: CI = confidence interval.
Backward selection with an alpha level of removal of 0.2 was used. The following variables were removed from the model: Karnofsky performance status at time of initial consult, tumor size, prior lung radiation.
OS
There were 20 deaths at the time of analysis. On UVA, history of prior cancer (HR, 3.30; 95% CI, 1.19-9.11) and history of prior lung cancer (HR, 3.00; 95% CI, 1.22-7.36) were associated with lower OS, whereas higher KPS at time of consult (HR, 0.91; 95% CI, 0.88-0.95) was associated with higher OS (See Supplemental Table 6 in the online version). On MVA, only higher KPS at time of consult (HR, 0.91; 95% CI, 0.88-0.95) was associated with higher OS. Two- and 3-year rates of OS were 69.3% (95% CI, 53.6%-80.6%) and 56.4% (95% CI, 37.6%-71.6%), respectively. Figure 1 summarizes disease outcomes and survival endpoints for our series.
Figure 1.
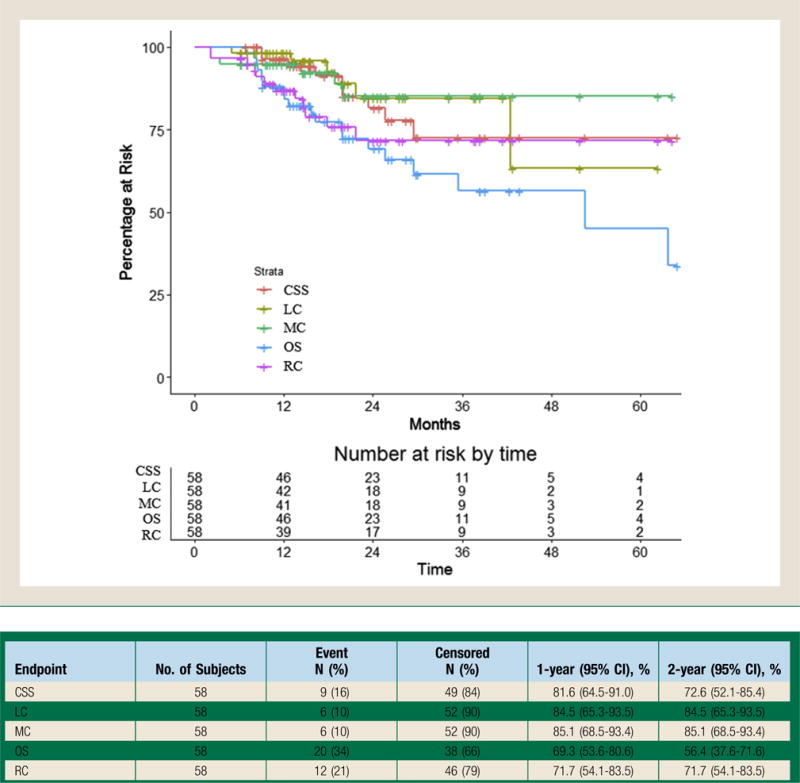
Local Control (LC), Regional Control (RC), Metastatic Control (MC), Cancer-specific Survival (CSS), and Overall Survival (OS) Among the Entire Cohort of Patients 80 Years and Older Treated With Definitive Lung Stereotactic Body Radiotherapy. LC, RC, MC, CSS, and OS Among All 58 Patients in the Study. LC Was Defined as Tumor Control at Treated Site. RC Was Defined as Tumor Control in the Same Lobe of Treatment, Ipsilateral Hilum, or Mediastinum. MC Was Defined as Disease Control in the Contralateral Lung or Free From Any Distant Site of Disease. CSS Was Defined as Survival in the Absence of Any Active Cancer at Any Site, With Other Deaths Censored. OS Was Defined as Time to Death or Time to Last Follow up From Diagnosis. The 2- and 3-year Endpoints With 95% Confidence Intervals (CI) Are Listed Below:
Effect of KPS on CSS and OS
Given the association of KPS with all outcomes, we performed recursive partitioning analysis to determine a cutoff for CSS and OS significance. A KPS of 75 was the cutoff for 2 distinct groups of patients. Patients with KPS ≥75 had significantly improved CSS and OS (Figure 2). For patients with KPS < 75, the 2- and 3-year CSS rates were 67.2% (95% CI, 40.0%-84.2%) and 47.8% (95% CI, 19.1%-71.9%), respectively, whereas for patients with KPS ≥75, the 2- and 3-year CSS rates were both 96.2% (95% CI, 75.7%-99.4%). For patients with KPS < 75, the 2- and 3-year OS rates were 49.9% (95% CI, 29.0%-67.6%) and 23.6% (95% CI, 5.3%-49.2%), respectively, whereas for patients with KPS ≥75, the 2- and 3-year OS rates were both 91.1% (95% CI, 68.4%-97.7%).
Figure 2.
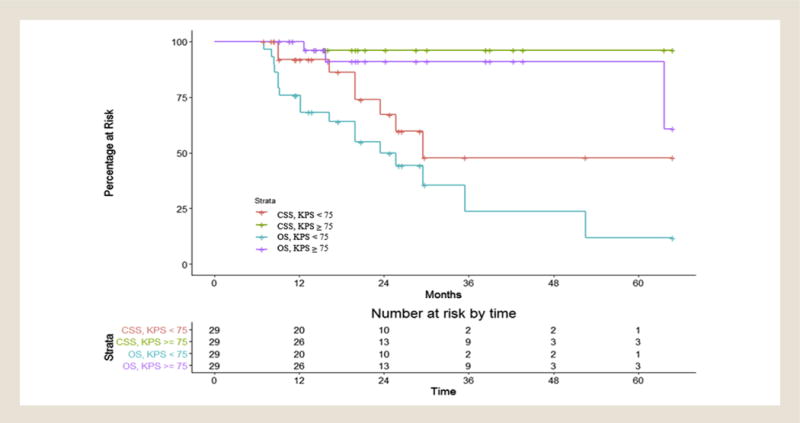
Cancer-specific Survival (CSS) and Overall Survival (OS) Among Patients Stratified by Karnofsky Performance Status (KPS) breakpoint of 75 on Recursive Partitioning Analysis. CSS and OS Were Analyzed Among the Entire Cohort of 58 Patients as Stratified by the KPS Breakpoint of 75 as Determined by Recursive Partitioning Analysis (RPA). CSS Was Defined as Net Survival Measuring Cancer Survival in the Absence of Other Causes of Death. OS Was Defined as Time to Death or Time to Last Follow-up From Diagnosis. The Determined RPA KPS of 75 Was also the Median KPS of the Entire Cohort, as Evidenced by the Equal Distribution of Patients in the Number at Risk Section at the Bottom of the Figure. For Patients With KPS < 75, the 2- and 3-year CSS Rates Were 67.2% (95% CI, 40.0%-84.2%) and 47.8% (95% CI, 19.1%-71.9%), Respectively, Whereas for Patients With KPS ≥ 75, the 2- and 3-year CSS Rates Were Both 96.2% (95% CI, 75.7%-99.4%). For Patients With KPS < 75, the 2- and 3-year OS Rates Were 49.9% (95% CI, 29.0%-67.6%) and 23.6% (95% CI, 5.3%-49.2%), Respectively, Whereas for Patients With KPS ≥ 75, the 2- and 3-year OS Rates Were Both 91.1% (95% CI, 68.4%-97.7%)
RP and Other Treatment-related Toxicity
Overall, 20 patients (34.5%) developed grade ≥2 RP, with only 2 patients (3.5%) developing grade 3 RP. The median time to RP development was 5.2 months (range, 1.7-21.1 months). On logistic regression, UVA of variables associated with RP, T1 tumors (odds ratio [OR], 0.04; 95% CI, 0.01-0.41) were associated with lower rates of RP, whereas larger tumors (OR, 1.58; 95% CI, 1.01-2.48) and not being on an ACE-I (OR, 3.71; 95% CI, 1.12-12.27) were associated with higher rates of RP (See Supplemental Table 7 in the online version). On MVA, T1 tumors (vs. T3) (OR, 0.03; 95% CI, 0.01-0.37) were associated with lower rates of RP, whereas not being on an ACE-I (OR, 5.83; 95% CI, 1.29-26.32) was associated with higher rates of RP (Table 4).
Table 4.
Multivariablea Analysis of Patient, Tumor, and Treatment Characteristics and Their Association With Developing Radiation Pneumonitis
| Covariate | Level | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| T stage | T1a or T1b | 0.03 (0.01–0.37) | .01 |
| T2a or T2b | 0.07 (0.01–1.06) | .06 | |
| T3 | – | – | |
| ACE-I usage | No | 5.83 (1.29–26.36) | .02 |
| Yes | – | – |
Bold P-values indicate statistical significance.
Abbreviations: ACE-I = angiotensin converting enzyme inhibitor; CI = confidence interval.
Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: age, radiation dose/fraction, number of radiation fractions, tumor size, gender, and smoking pack-years.
There were a total of 7 other non-RP treatment-related toxicities (12.1%). Specifically, there were 3 grade 1 chest wall pain (mild pain) toxicities, 1 grade 2 chest wall pain (moderate pain limiting an instrumental activity of daily living) toxicity, 2 grade 3 chest wall pain (severe pain limiting self-care) toxicities, and 1 grade 3 esophagitis (severely altered swallowing requiring PEG tube placement) toxicity. There were no grade 4 or 5 toxicities among the entire cohort at last follow-up. All patients completed their entire prescribed course of radiation.
Discussion
The present study specifically addresses octogenarians and nonagenarians managed with definitive lung SBRT for early stage NSCLC in a multi-center setting. We demonstrated that treatment is efficacious, with overall tumor outcomes in line with other series reporting outcomes after definitive lung SBRT.1–3,5,7,9,10,22,23 We also demonstrated that KPS at the time of consult was not only associated with OS outcomes but also disease-specific outcomes. Using recursive partitioning analysis, we showed that patients with KPS ≥75 have significantly improved CSS and OS.
Importantly, this study also examines prior lung cancer and prior thoracic radiation as patient factors, a common clinical scenario in patients ≥80 years old with early-stage lung cancer. In terms of treatment tolerance and toxicity, we found treatment was well-tolerated in this elderly cohort, with expected rates of RP and other treatment-related toxicities.2,3,24,25 To our knowledge, this is the largest study examining definitive lung SBRT in octogenarians and nonagenarians in the United States with full disease and treatment toxicity information.
Patients ≥80 years old have not been well-represented on the landmark randomized trials establishing the effectiveness of lung SBRT. In the phase II multi-center study (RTOG 0236) by Timmerman et al,1,2 they examined biopsy-proven T1-2N0M0 NSCLC in medically inoperable patients treated with SBRT to a total dose of 54 Gy in 3 fractions. Of the 55 evaluable patients, they found a 3-year primary tumor control rate of 97.6%, 3-year lobar control rate of 90.6%, and 3-year OS rate of 55.8%. The median age of patients on this trial was 72 years, with 86% of patients having a Zubrod performance status of 0 to 1. Despite the age difference in our series (median age, 84.9 years), we observed similar tumor control outcomes, with 10.4% local failures, 20.7% regional failures, and a 3-year OS rate of 56.4%, suggesting that lung SBRT is similarly efficacious in octogenarians. In the recently reported prospective trial JCOG0403 of biopsy-proven T1N0M0 NSCLC, patients were treated to 48 Gy delivered over 4 fractions.10 They accrued 100 inoperable patients and 64 operable patients and showed a 3-year OS rate of 59.9% in inoperable patients and 76.5% in operable patients. Whereas the median age of 78 years in this study was higher than RTOG 0236, only 28% and 31% of the inoperable and operable patients, respectively, were over 80 years old. Ongoing randomized trials in lung SBRT including RTOG 0813 and RTOG 0618 do not have a distinct age cutoff, but it is difficult to report the number of patients ≥80 years old at this time, as recently reported results of RTOG 0813 in abstract form comment that “patients were elderly” without additional information.26
There are few studies examining lung SBRT in elderly patients. In a study by Hayashi et al, they examined outcomes of 81 patients treated with lung SBRT.25 They dichotomized patients at 85 years, with 20 patients being placed in a “very elderly” cohort of ≥85 years old. They showed that 3-year local control rates were worse in the “very elderly” cohort compared with others (83.1% vs. 93.8%, respectively) as was 3-year OS rates (40.7% vs. 75.0%, respectively). These results differ from our series, with 3-year OS rate of 56.4% (95% CI, 37.6%-71.6%). This difference is intriguing as no patient in the “very elderly” group in their series had a Zubrod performance status score of > 1, only 20% had tumors larger than T1, and all were treated with effective SBRT doses. In fact, our series represents a more high-risk cohort, with 32.8% having tumors larger than T1, with similarly good performance status (median, KPS 75). It is likely that the difference in outcomes could be attributed to either the 5-year difference in age (85 years old vs. 80 years old) or from the small number of patients in their “very elderly” cohort. These differences highlight one of the challenges with oncologic management: determine a subgroup of patients who are likely to experience a survival benefit despite competing risk factors, which generally would be much greater in an aging population. In an effort to answer this, we performed a recursive partitioning analysis using KPS to determine a threshold for improved outcomes. On this analysis, we found that patients with KPS ≥75 had improved CSS and OS (Figure 2). This finding will hopefully provide guidance for selecting a group of patients most likely to benefit from therapy. Other retrospective literature examining lung SBRT in older patients includes a database analysis examining 3147 patients ≥70 years old where 96 of the 258 patients treated with SBRT were ≥80 years old.27 The authors found that SBRT had improved OS versus observation (HR, 0.64). In a recent series by Mancini et al, examining 251 patients treated with lung SBRT, they dichotomized patients at age 75. There were 126 patients ≥75 years old, with a median age of 82.1 years22 defining their “elderly cohort.” They found no differences in 3-year local control among elderly and non-elderly cohorts (84.2% vs. 86.4%, respectively) or 3-year OS (47.5% vs. 41.0%, respectively). They did observe that distant disease control was improved in the elderly cohort, a finding also seen in other series.23,28 We similarly found a low rate of distant recurrence (10.4%), with previous lung cancer history (HR, 3.75; 95% CI, 1.47-9.57) associated with increased rates. This finding is hypothesized to be related to less aggressive management of equivocal findings on follow up and more conservative restaging.22,23
One of the most significant concerns with treatment of octogenarians and nonagenarians is treatment-related toxicity. In a retrospective series from Japan examining 109 patients ≥80 years old with T1-2N0M0 NSCLC treated with lung SBRT there was a reported early grade 3+ RP rate of 4.6%.29 The reported low rates of late toxicity, with grade 2 symptomatic rib fracture in 2 patients and grade 2 transient chest wall pain in 7 patients. In the previously mentioned series by Hayashi et al that stratified patients at age 85, there was an overall reported 11.1% grade 2+ RP rate. Interestingly in this series, they found a statistically significantly higher rate of grade 2+ RP in patients ≥85 years old (30.0%) compared with patients younger than 85 years old (4.9%). On MVA of patient and dosimetric factors, only age remained associated with RP.25 This finding that age plays a role in increased toxicity contradicts the previously mentioned series by Mancini et al examining 251 patients with NSCLC treated with lung SBRT, where 126 patients were ≥75 years old represented their elderly cohort.22 They found no differences in acute or late grade 3+ toxicity between elderly and non-elderly patients. In terms of acute grade 3+ toxicity, rates were 11.1% in patients ≥75 years old and 8.0% in patients < 75 years old, and similarly found no statistical difference in grade 3+ late toxicity with rates of 10.3% in patients ≥75 years old and 7.2% in patients < 75 years old. In this series, no elderly patient experienced chest wall pain. The results of our series, with an older cohort, found similarly low rates of grade 3+ RP (3.5%) and low rates of any other grade 3+ toxicity (5.2%). In summary, most studies report acceptable grade 3+ toxicity rates of around 10%, regardless of the varying definitions of an “elderly cohort.”22,23,25,29 Our series is also the first to report an association between elderly patients not on an ACE-I during lung SBRT (OR, 5.83; 95% CI, 1.29-26.32) and higher rates of RP. This is hypothesis generating, and supports the findings of recently published work looking at a broader patient population managed with lung SBRT.17
Our series has several strengths and limitations. The major strength of this series is the large number of patients aged 80 or older at the time of treatment in this specific clinical scenario of definitive lung SBRT, the largest such series reported in the United States. We have detailed information on patient characteristics, treatment type, outcome, and toxicity. Our series is one of the few in the literature, and the first examining elderly patients, to incorporate prior cancer history, prior lung cancer history, and previous thoracic radiation in variables analyzed. We felt this was important given the many complexities surrounding the management of octogenarians, including their higher likelihood for previous cancer diagnoses and therapies. The major limitation of our series is the inherent bias of retrospective reviews, including the reliance on medical record for accurate information on follow-up. Although we attempted to document many known variables related to outcomes, including KPS, smoking status, and previous malignancy, there are undoubtedly confounding factors that are not accounted for given the retrospective nature of our study. Additionally, we do not report on the central or peripheral location of the lung tumors, a factor known to be related to toxicity outcomes.3
Conclusions
This series summarizes a large academic, multicenter experience in the management of octogenarians and nonagenarians treated with definitive lung SBRT for early-stage NSCLC. We demonstrate tumor control and survival outcomes similar to other series, including those with younger patients. Importantly we found that grade 3+ toxicity rates were low, indicating that this therapy is well-tolerated even in patients ≥80 years old. Using recursive partitioning analysis, we were able to find that patients with KPS ≥75 experience improved CSS and OS, suggesting that definitive lung SBRT is a reasonable option for octogenarians and nonagenarians meeting this criterion.
Clinical Practice Points.
SBRT is the standard of care for medically inoperable early stage NSCLC.
Octogenarians and nonagenarians have not been well-represented on prospective trials of SBRT; however, this is becoming a more common clinical scenario with an aging cancer population and patient preference.
The present study found that SBRT for patients ≥80 years old with NSCLC treated across 4 academic centers is generally well-tolerated with low rates of grade 3+ toxicity, despite our population having a high rate of previous cancer diagnoses as well as previous courses of thoracic radiation.
Tumor control was excellent with SBRT, and patients with a KPS of ≥75 derived the most significant survival benefit with therapy.
This series, in conjunction with series internationally, demonstrates that the use of SBRT should be considered for NSCLC in octogenarians and nonagenarians with reasonable performance status.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental tables accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.cllc.2017.03.006.
References
- 1.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmerman RD, Hu C, Michalski J, et al. Long-term results of RTOG 0236: a phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with medically inoperable stage I non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90:S30. [Google Scholar]
- 3.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 5.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereo-tactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7:1394–9. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 6.Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–25. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 8.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–9. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 9.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–96. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 11.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–9. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 12.Kent EE, Malinoff R, Rozjabek HM, et al. Revisiting the Surveillance Epidemiology and End Results Cancer Registry and Medicare Health Outcomes Survey (SEER-MHOS) linked data resource for patient-reported outcomes research in older adults with cancer. J Am Geriatr Soc. 2016;64:186–92. doi: 10.1111/jgs.13888. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB. American Joint Committee on Cancer AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman RD. RTOG 0618: a phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with operable stage I/II non–small-cell lung cancer. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0618. Accessed: October 1, 2016.
- 16.Karnofsky DA, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer Evaluation of chemotherapeutic agents. In: MacLeod C, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 17.Harder EM, Park HS, Nath SK, Mancini BR, Decker RH. Angiotensin-converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e643–9. doi: 10.1016/j.prro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–43. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Hu XJ, Lagakos SW. Nonparametric estimation of the mean function of a stochastic process with missing observations. Lifetime Data Anal. 2007;13:51–73. doi: 10.1007/s10985-006-9030-0. [DOI] [PubMed] [Google Scholar]
- 20.Therneau T, Atkinson B, Ripley B. Recursive Partitioning and Regression Trees, R package version 4.1-10. Cary, NC: R Software Project; 2015. [Google Scholar]
- 21.Kassambara A, Kosinski M, Biecek P. Drawing Survival Curves using ‘ggplot2’, R package version 0.2.2. Cary, NC: R Software Project; 2016. [Google Scholar]
- 22.Mancini BR, Park HS, Harder EM, et al. Elderly patients undergoing SBRT for inoperable early-stage NSCLC achieve similar outcomes to younger patients. Lung Cancer. 2016;97:22–7. doi: 10.1016/j.lungcan.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I non-small cell lung cancer in patients aged > or =75 years: outcomes after stereotactic radiotherapy. Cancer. 2010;116:406–14. doi: 10.1002/cncr.24759. [DOI] [PubMed] [Google Scholar]
- 24.Asai K, Shioyama Y, Nakamura K, et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: risk factors and dose-volume relationship. Int J Radiat Oncol Biol Phys. 2012;84:768–73. doi: 10.1016/j.ijrobp.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi S, Tanaka H, Kajiura Y, Ohno Y, Hoshi H. Stereotactic body radiotherapy for very elderly patients (age, greater than or equal to 85 years) with stage I non-small cell lung cancer. Radiat Oncol. 2014;9:138. doi: 10.1186/1748-717X-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezjak A, Paulus R, Gaspar LE, et al. Efficacy and toxicity analysis of NRG Oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2016;96:S8. [Google Scholar]
- 27.Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: a National Cancer Data Base analysis. Cancer. 2015;121:4222–30. doi: 10.1002/cncr.29640. [DOI] [PubMed] [Google Scholar]
- 28.Nath SK, Sandhu AP, Kim D, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol. 2011;99:12–7. doi: 10.1016/j.radonc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Takeda A, Sanuki N, Eriguchi T, et al. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86:257–63. doi: 10.1016/j.ijrobp.2013.01.006. [DOI] [PubMed] [Google Scholar]


