Abstract
OBJECTIVE
To determine the scope, source, and mode of transmission of a multifacility outbreak of extensively drug-resistant (XDR) Acinetobacter baumannii.
DESIGN
Outbreak investigation.
SETTING AND PARTICIPANTS
Residents and patients in skilled nursing facilities, long-term acute-care hospital, and acute-care hospitals.
METHODS
A case was defined as the incident isolate from clinical or surveillance cultures of XDR Acinetobacter baumannii resistant to imipenem or meropenem and nonsusceptible to all but 1 or 2 antibiotic classes in a patient in an Oregon healthcare facility during January 2012–December 2014. We queried clinical laboratories, reviewed medical records, oversaw patient and environmental surveillance surveys at 2 facilities, and recommended interventions. Pulsed-field gel electrophoresis (PFGE) and molecular analysis were performed.
RESULTS
We identified 21 cases, highly related by PFGE or healthcare facility exposure. Overall, 17 patients (81%) were admitted to either long-term acute-care hospital A (n = 8), or skilled nursing facility A (n = 8), or both (n = 1) prior to XDR A. baumannii isolation. Interfacility communication of patient or resident XDR status was not performed during transfer between facilities. The rare plasmid-encoded carbapenemase gene blaOXA-237 was present in 16 outbreak isolates. Contact precautions, chlorhexidine baths, enhanced environmental cleaning, and interfacility communication were implemented for cases to halt transmission.
CONCLUSIONS
Interfacility transmission of XDR A. baumannii carrying the rare blaOXA-237 was facilitated by transfer of affected patients without communication to receiving facilities.
Extensively drug-resistant (XDR) Acinetobacter baumannii causes significant patient morbidity and mortality but remains uncommon in the northwestern United States relative to other U.S. regions and Europe.1–4 Acinetobacter baumannii is transmitted by contaminated hands, fomites, and possibly an airborne route.5,6 Extensively drug-resistant A. baumannii can harbor several chromosomal and plasmid-mediated antibiotic resistance genes, which can be transmitted to other gram-negative organisms.7,8
The transfer of patients between acute and chronic health-care facilities increases opportunities for disease dissemination if interfacility communication is not robust.9,10 Clear, timely, and complete communication of patient infection or colonization by organisms of public health significance that have potential for person-to-person transmission (eg, multidrug-resistant organisms [MDROs], Clostridium difficile, norovirus, influenza, Mycobacterium tuberculosis) can prevent transmission between healthcare facilities.11–14 We investigated an outbreak of XDR A. baumannii infections in Oregon—where the pathogen had been rare—in an effort to prevent it from establishing endemicity.
Since January 2012, participating clinical laboratories voluntarily report carbapenem-resistant A. baumannii isolates from sterile sites (eg, blood, urine) among residents of the Portland tricounty metropolitan area (ie, Multnomah, Washington, and Clackamas) to the Oregon Public Health Division (OPHD) for the Centers for Disease Control and Prevention (CDC) Multidrug-resistant Gram-negative organism Surveillance Initiative (MuGSI).15 During June 2012, an isolated case of XDR A. baumannii (patient ORAB01, ie, Oregon A. baumannii patient 1) was reported by a participating laboratory. The isolate was resistant to penicillins, cephalosporins, extended-spectrum cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides. The isolate showed intermediate susceptibility to tetracyclines, and was susceptible to tigecycline (using the Enterobacteriaceae breakpoint) and to colistin. Because this was an isolated case, the healthcare facility was notified and educated to prevent transmission, but no further investigation was performed. In November 2012, a hospital infection preventionist notified the OPHD of 2 other XDR A. baumannii infections (patients ORAB02 and ORAB03).
Our initial review revealed that all 3 cases had 2 healthcare facility exposures in common: a skilled nursing facility (SNF-A) and an outpatient wound and infusion clinic (OPC-A) outside the MuGSI surveillance area. During December 2012, we conducted simultaneous resident and outpatient point-prevalence surveys on a single day at SNF-A and OPC-A. Facility staff obtained groin, axilla, and wound swabs for surveillance; specimens were processed by the clinical laboratory.16,17 We identified 3 additional cases of XDR A. baumannii colonization among the 42 residents of SNF-A (ie, patients ORAB04, ORAB05, and ORAB6a), and none among 15 patients at OPC-A. An outbreak linked to SNF-A was suspected, so we launched what became a 2-year, multifacility investigation to determine the scope of the problem, to identify a source, and to intervene to prevent further spread.
METHODS
Case Definition
A case was defined as the incident isolate from clinical or surveillance cultures of A. baumannii resistant to imipenem or meropenem and nonsusceptible to all but 1 or 2 antibiotic classes in a patient in an Oregon healthcare facility during January 2012–December 2014.18 Case patient medical records were abstracted using a standardized form.11
Case Finding
For this investigation, the OPHD requested that 6 large, hospital-based, clinical microbiology laboratories retrospectively report since June 2012 and prospectively report and submit any isolates from sterile (eg, blood, urine) or nonsterile sites (eg, respiratory, wound) meeting the case definition. Together, these laboratories process ~90% of Oregon clinical microbiology specimens. Involved facilities were instructed to report A. baumannii cases since January 2012. The Washington State Department of Health was notified of A. baumannii cases among Washington residents.
Case and Environmental Investigations
Following the December 2012 point-prevalence surveillance study at SNF-A, we conducted an environmental survey at SNF-A during June 2013. The OPHD staff obtained environmental samples by swabbing high-touch areas in the resident rooms, clinical and common areas, bathroom fixtures, and sinks; all environmental samples were processed by the Oregon State Public Health Laboratory (OSPHL).19 In response to 2 outbreak cases, Hospital-2 performed surveillance on the 2 affected units during July 2013. Hospital staff obtained groin, axilla, and rectal swabs for surveillance; specimens were processed at OSPHL.16,17
Susceptibility Testing and Definitions
Routine susceptibility testing was performed in clinical microbiology laboratories using Microscan or Vitek, and results were interpreted using the 2012 Clinical Laboratory Standards Institute (CLSI) clinical parameters.20 Patient and environmental specimens sent to OSHPL were plated on chromogenic media to select for multidrug-resistant A. baumannii (CHRO-Magar Acinetobacter, bioMérieux, France); API 20NE (France) was used for initial identification. Whole-genome sequence analysis later confirmed the species.
Pulsed-Gel Electrophoresis and Advanced Molecular Testing
Isolates meeting the case definition underwent pulsed-field gel electrophoresis (PFGE) analysis at OSPHL using the ApaI restriction enzyme. PFGE band patterns were compared using BioNumerics 5.1 software (Applied Maths, Belgium). A case was considered part of the outbreak if the isolate’s PFGE pattern had ≥92% homology with ORAB0121; or if no isolate was available for testing, the case shared a common healthcare facility exposure and resistance phenotype. The Louis Stokes Cleveland Department of Veterans Affairs Medical Center provided testing for the following: antimicrobial susceptibility; blaOXA carbapenemase genes; other bla and aminoglycoside resistance genes; molecular typing by repetitive-sequence-based PCR (rep-PCR) and multilocus sequence type (MLST) Pasteur scheme; and whole-genome and plasmid sequencing.22,23
RESULTS
Case Descriptions
We identified 21 cases (18 clinical, 3 surveillance) of XDR A. baumannii among Oregon (n = 16) and southwest Washington (n = 5) residents during the study period who shared healthcare facility exposures or isolates highly related by PFGE (Figures 1 and 2). Case patients were aged 25–92 years (median, 71 years); 13 patients (62%) were male. Patients were admitted to 37 medical facilities in Oregon and Washington; 7 patients (33%) received care in both states. In addition, 17 patients (81%) reported admissions to either a long-term acute-care hospital (LTACH-A, n = 8), SNF-A (n = 8), or both (n = 1) prior to XDR A. baumannii isolation. Index clinical specimen sources were: blood (n = 2), wound (n = 2), urine (n = 5), and sputum (n = 9); surveillance sources were axillary (n = 1) and groin (n = 2). In 4 patients, additional isolates were obtained ≥30 days apart: patient ORAB01 (190 days: blood and axilla), patient ORAB03 (36 days: urine and urine), patient ORAB6b (563 days: axilla and wound), and patient ORAB08 (31 days: unknown and bronchoalveolar lavage).
FIGURE 1.
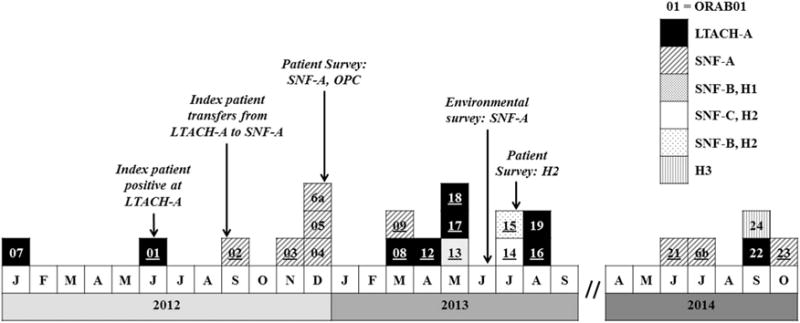
Extensively drug-resistant Acinetobacter baumannii outbreak cases by initial specimen collection date, facility, and significant events—Oregon healthcare facilities, 2012–2014 (N = 21 individuals). Patient ORAB06 had 2 isolates. Patient ORAB07 did not have an available isolate. LTACH, long-term acute care hospital; SNF, skilled nursing facility; OPC, outpatient clinic; H, hospital; Survey, surveillance cultures obtained at listed facilities; underline, blaOXA-237-carbapenemase gene sequenced from clinical isolate available for molecular study.
FIGURE 2.
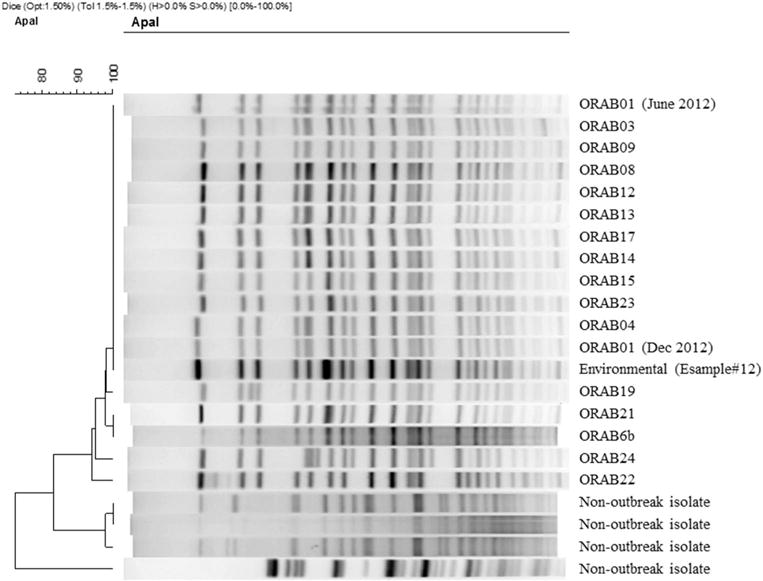
Pulsed-field gel electrophoresis of selected outbreak isolates of extensively drug-resistant Acinetobacter baumannii—Oregon healthcare facilities, 2012–2014. Patient ORAB01 had 2 isolates. ORAB, case; Environmental, sample obtained during SNF-A environmental survey during June 2013; Non-outbreak isolate, an isolate of carbapenem-resistant A. baumannii collected during the investigation, but unrelated to the outbreak.
Outbreak Investigation
During May 2012, the index-case patient, ORAB01, was admitted to LTACH-A with a peripherally inserted central catheter. She developed sepsis 25 days later and was diagnosed with an XDR A. baumannii catheter-associated bloodstream infection. The patient’s comorbidities included morbid obesity (BMI = 60.3 kg/m2), chronic nonhealing abdominal and lower extremity wounds, tracheostomy, and a chronic indwelling urinary catheter. Subsequently, XDR A. baumannii was identified in 7 more LTACH-A patients over 27 months: patients ORAB08, ORAB12, and ORAB22 (bronchoalveolar lavage); patients ORAB17, ORAB18, and ORAB19 (other respiratory); and patient ORAB16 (urine). The LTACH-A facility declined repeated requests by the OPHD to perform an inpatient point-prevalence surveillance study. Instead, the facility initiated admission surveillance cultures for evidence of MDROs; no cases were identified (personal communication, G.L.B.). During an internal review prompted by the outbreak, LTACH-A identified lapses in its bronchoscopy and ventilator reprocessing protocols (ex post facto personal communication, G.L.B.).
In September 2012, the index-case patient ORAB01 was transferred to SNF-A for rehabilitation (Figure 3). SNF-A did not receive notification that the case patient carried XDR A. baumannii. Subsequently, 5 symptomatic cases with matching PFGE patterns (patient ORAB02 [sputum]; patients ORAB03, ORAB09, ORAB21, and ORAB23 [urine]) and 3 asymptomatic carriers with matching PFGE patterns (patients ORAB04 and ORAB05 [groin]; patient ORAB06b [axilla]) were identified at SNF-A over a 25-month period. Extremely drug-resistant A. baumannii was isolated twice from patient ORAB6: first on surveillance culture (patient ORAB6a [axilla]) during the December 2012 point-prevalence survey, and 18 months later from a chronic ankle wound (ORAB6b). The June 2013 environmental survey identified 1 PFGE-matching XDR A. baumannii in 1 of 173 specimens, namely, from the handle of a linen cart in the unit where 4 cases resided during the outbreak: ORAB01, ORAB02, ORAB04, and ORAB06 (Figure 2, ESample#12).
FIGURE 3.
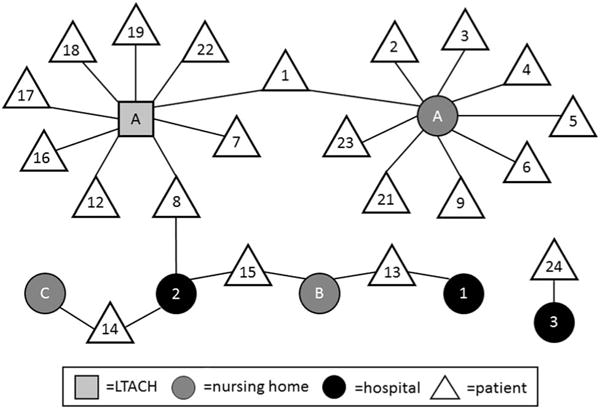
Exposure network of outbreak of extensively drug-resistant Acinetobacter baumannii—Oregon healthcare facilities, 2010–2014. Square, LTACH; light circle, nursing home; dark circle, hospital; triangle, patient.
The same strain also spread within Hospital-2. Case patient ORAB08 had a 2-month LTACH-A admission complicated by tracheostomy, dialysis, and decubitus ulcers. Seven months after discharge, patient ORAB08 was admitted to the medical-surgical unit and the intensive care unit (ICU) at Hospital-2, and a bronchoalveolar lavage specimen grew XDR A. baumannii identical by PFGE to that of ORAB01. Four months later, 2 additional PFGE-matching cases were identified at Hospital-2: ORAB14 from the oncology unit and ORAB15 from the medical-surgical unit and ICU; neither patient had prior exposure to SNF-A or LTACH-A. Surveillance cultures among patients in the ICU (n = 5) and the oncology unit (n = 26) at Hospital-2 identified no further transmission events.
Public Health Recommendations and Facility Response
While all licensed Oregon healthcare facilities must have infection control policies in place, adherence to such practices is not routinely externally validated. During the outbreak, the OPHD instructed affected facilities to audit hand hygiene, personal protective equipment use, and environmental cleaning; to reinforce universal precautions for contact with body fluids or wounds; and to request and provide interfacility communication upon admission and transfer of patients or residents with a history of Clostridium difficile, MDRO, including XDR A. baumannii, or other communicable disease. For facilities with current XDR A. baumannii cases, the OPHD recommended the following measures: single rooms or cohort; daily chlorhexidine baths or wipes to decrease colonization burden; hand hygiene; gloves and gown use by healthcare workers during patient care; daily enhanced environmental cleaning; and patient wound coverage when in common areas.24 Case patients with A. baumannii colonization at skilled nursing facilities were encouraged to participate in rehabilitation and social activities if hand hygiene was ensured, clean clothing was donned, and secretions were contained. The OPHD created guidelines for education, active surveillance, and augmented environmental cleaning of high-touch surfaces; the OPHD assisted facilities to develop a form and process for interfacility communication during admission and transfer.25
Advanced Molecular Testing
One resident surveillance isolate (ORABSC) and 16 clinical outbreak isolates (17 of 21 cases; 81%) were available for molecular characterization (Figure 1, ORABSC not shown). All isolates had rep-PCR patterns that clustered with >95% similarity, suggesting clonality.23 Multilocus sequence type analysis revealed all isolates as ST2, which corresponds to international clone 2, the most prevalent carbapenem-resistant A. baumannii lineage found worldwide.26 Importantly, whole-genome and plasmid sequencing revealed that all isolates except that in patient ORAB19 carried the plasmid-encoded blaOXA-237, an uncommon OXA carbapenemase related to OXA-235.27,28 Details are reported by Hujer et al.23
DISCUSSION
Given the relatedness of outbreak isolates by PFGE and rep-PCR, carriage of a rare blaOXA-237 carbapenemase gene, and the distribution of cases, we suggest that introduction and rapid dissemination of a single XDR Acinetobacter clone occurred across several northwestern US healthcare facilities because patients and residents with risk factors for prolonged carriage were transferred without interfacility communication. We combined a detailed epidemiologic investigation with whole-genome molecular analysis, thereby providing insight on how XDR A. baumannii carrying a blaOXA-237 carbapenemase could be introduced and disseminated in a geographical region with historically low XDR A. baumannii incidence.29–31 Epidemiologic, microbiologic, and molecular data suggest that case patients with persistently positive cultures (eg, from patients ORAB01, ORAB06, and ORAB08) could have transmitted the outbreak strain to at least a dozen other individuals. Evidence demonstrating that cases were related increased the commitments of facilities to further transmission prevention and compliance with public health recommendations.24,32
Risk factors for prolonged A. baumannii and other MDRO carriage include chronic wounds, morbid obesity, tracheostomies, or indwelling urine catheters.5,10,14 These characteristics favor both a heavy bioburden of colonizing bacteria and a need for more frequent healthcare contact, thereby increasing the likelihood of transmission via healthcare worker hands or fomites to other patients. Infection control deficiencies, (eg, improper bronchoscope reprocessing or inadequate hand washing) in the long-term-care chronic- and acute-care settings may have amplified transmission within the facilities. However, not all cases in this investigation were directly connected, suggesting other unidentified vectors: unidentified carriers, a tenacious environmental reservoir, or even airborne transmission.6
Based on our investigation, we suspect more widespread transmission of A. baumannii isolates carrying blaOXA-237 in the Pacific Northwest because 1 in 3 patients received health-care in both Oregon and Washington. For example, case investigation at LTACH-A identified case patient ORAB07, a patient with multiple comorbidities and multiple admissions to several southwest Washington and Oregon healthcare facilities who had been admitted several weeks before case patient ORAB01; unfortunately, no isolates were available. Phenotypic XDR A. baumannii was isolated from the sputum and urine of patient ORAB07 multiple times during January–March 2012, suggesting that she may have been the source patient for patient ORAB01. Furthermore, evidence that patients did not carry evidence of XDR A. baumannii on admission to LTACH-A suggests that acquisition occurred after admission by internal transmission.
This outbreak might have been missed if not for a new, limited, voluntary surveillance system in Oregon and an astute infection preventionist. Reporting of XDR A. baumannii infection is not required by most public health jurisdictions in the United States, and clinical laboratories do not routinely test for underlying resistance mechanisms like blaOXA-237 carbapenemase genes. Collaboration between public health surveillance systems and research laboratories, as in this investigation, could facilitate characterization of uncommon transmission pathways and new resistance mechanisms suggested by unusual clinical outbreaks.23,27,33,34 For example, a search for uncharacterized plasmid-encoded carbapenem resistance genes in 10 A. baumannii isolates from Utah, Illinois, California, and Nevada identified 3 novel OXA enzymes (OXA-235, OXA-236, and OXA-237), including an isolate of blaOXA-237 from California.27 Furthermore, Ou et al35 used whole-genome sequencing to show that a clone of A. baumannii can persist over time and across healthcare facilities.
Our investigation and analysis have limitations. First, surveillance for XDR A. baumannii was voluntary and likely incomplete. LTACH-A declined inpatient surveillance and cases were likely missed; more importantly, this refusal may have impeded identification and mitigation of an intrafacility source. This limitation was mitigated by interstate communication and extensive case finding with Oregon microbiology laboratories, which collectively test ~90% of clinical samples. Second, surveillance case identification could have been underestimated because of culture techniques: skin, sputum, stool, urine, and wound are preferred for A. baumannii.16,17,19 However, healthcare facilities requested a surveillance method that was easy, fast, and noninvasive. To optimize compliance, we accepted axilla, groin, and wound specimens as well as urine and sputum, when a catheter or tracheostomy was present. Third, we were unable to obtain some isolates (eg, from patient ORAB07) and healthcare records (eg, outside of Oregon). Despite these limitations, epidemiologic and molecular analyses provide compelling evidence of rapid, regional transmission of a rare, plasmid-encoded carbapenemase OXA-237 in a pervasive XDR A. baumannii clone.
This outbreak highlights the need for timely and transparent communication of patient and resident histories of infection or colonization by MDROs.36 Interfacility communication of MDRO status can enable receiving facilities to institute appropriate contact precautions immediately upon patient arrival. Interventions focused on decreasing fomite and healthcare worker hand carriage can decrease XDR A. baumannii intrafacility transmission.14,24 An entire chain of transmission at SNF-A might have been prevented if its staff had been notified by LTACH-A of the MDRO status of patient ORAB01. The findings from this outbreak helped to inspire the creation of Oregon Administrative Rule 333-019-0052, which mandates written communication of MDRO status for interfacility patient transfer, effective January 1, 2014.25
In conclusion, we have described a widespread, 2-year multifacility cluster of blaOXA-237 carbapenemase-carrying XDR A. baumannii in the northwestern United States. Lack of MDRO interfacility communication protocols and a heavy colonized patient likely facilitated transmission. Clinical and molecular epidemiology were key to linking cases, creating transmission hypotheses, and convincing healthcare facilities to implement interfacility transfer communication and heightened infection control processes.
Acknowledgments
The authors wish to acknowledge Jeannie Tierheimer, Oregon State Public Health Laboratory, who performed all pulsed-field electrophoresis analyses and Marisa d’Angeli MD, Washington Department of Health, who provided case information for Washington residents. Funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention, the National Institutes of Health, or the Department of Veterans Affairs.
Financial support; This work was funded by the Epidemiology and Laboratory Capacity Cooperative Agreement (no. NU50CK000484-01-01 to G.L.B., P.M.C, M.C.C., R.V., J.P.F., C.D.P., and Z.G.B.) with the Centers for Disease Control and Prevention. This work was also supported in part by funds and facilities provided by the Cleveland Department of Veterans Affairs Merit Review Program (grant no. 1I01BX001974 to S.R., A.M.H., S.H.M., P.G.H., M.R.J., M.S.W., M.D.A., and R.A.B.) from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to R. A. B. This work was also supported by funds from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant nos. R01AI063517, R01AI072219, and R01AI100560 to R.A.B and grant no. U19AI110819 to J.C.V.I.).
Footnotes
PREVIOUS PRESENTATION. These data were presented in part as “Investigation of First Carbapenem-Resistant Acinetobacter baumannii Outbreak in Oregon— Multi-Facility, 2012–2013” at the 2014 Council for State and Territorial Epidemiologists Annual Conference on June 23, 2014, in Nashville, Tennessee.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanism and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 2.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. Extensively drug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2009;15:980–981. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thom KA, Maragakis LL, Richards K, et al. Assessing the burden of Acinetobacter baumannii in Maryland: a statewide cross-sectional period prevalence survey. Infect Control Hosp Epidemiol. 2012;33:883–888. doi: 10.1086/667376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuan Anh N, Nga TV, Tuan HM, et al. The molecular epidemiology and antimicrobial resistance phenotypes of Acinetobacter baumannii isolated from patients in three hospitals in Southern Vietnam. J Med Microbiol. 2017;66:46–53. doi: 10.1099/jmm.0.000418. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DJ, Liang SY, Smith CL, et al. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol. 2010;31:716–721. doi: 10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spellberg B, Bonomo RA. “Airborne assault”: a new dimension in Acinetobacter baumannii transmission. Crit Care Med. 2013;41:1–3. doi: 10.1097/CCM.0b013e31829136c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert T. Acinetobacter and other antibiotics. In: Courvalin P, Leclercq R, Rice LB, editors. Antibiogram. Portland, OR: ESKA Publishing, ASM Press; 2010. pp. 421–424. [Google Scholar]
- 8.Durante-Mangoni E, Zarrilli R. Global spread of drug-resistant Acinetobacter baumannii. Future Microbiol. 2011;6:407–422. doi: 10.2217/fmb.11.23. [DOI] [PubMed] [Google Scholar]
- 9.Huang SS, Avery TR, Song Y, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31:1160–1169. doi: 10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortensen E, Trivedi KK, Rosenberg J, et al. Multidrug-resistant Acinetobacter baumannii infection, colonization, transmission related to a long-term care facility providing subacute care. Infect Control Hosp Epidemiol. 2014;35:406–411. doi: 10.1086/675612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer CD, Cunningham MC, Poissant T, et al. Establishment of a statewide network for carbapenem-resistant Enterobacteriaceae prevention in a low-incidence region. Infect Control Hosp Epidemiol. 2014;35:356–361. doi: 10.1086/675605. [DOI] [PubMed] [Google Scholar]
- 12.Trick We, Lin MY, Cheng-Leidig R, et al. Electronic public health registry of extensively drug-resistant organisms, Illinois, USA. Emerg Infect Dis. 2015;21:1725–1732. doi: 10.3201/eid2110.150538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin MY, Trick WE. Informatics in infection control. Infect Dis Clin N Am. 2016;30:759–770. doi: 10.1016/j.idc.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Dumyati G, Stone ND, Nace DA, Crnich CJ, Jump RLP. Challenges and strategies for prevention of multidrug-resistant organism transmission in nursing homes. Curr Infect Dis Rep. 2017;19:18. doi: 10.1007/s11908-017-0576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magill SS, Dumyati G, Ray SM, Fridkin SK. Evaluating epidemiology and improving surveillance of infections associated with health care, United States. Emerg Infect Dis. 2015;21:1537–1542. doi: 10.3201/eid2109.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajao AO, Robinson G, Lee MS, et al. Comparison of culture media for detection of Acinetobacter baumannii in surveillance cultures of critically-ill patients. Eur J Clin Microbiol Infect Dis. 2011;30:1425–1430. doi: 10.1007/s10096-011-1237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol. 2012;33:1193–1198. doi: 10.1086/668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Thom KA, Howard T, Sembajwe S, et al. Comparison of swab and sponge methodologies for identification of Acinetobacter baumannii from the hospital environment. J Clin Microbiol. 2012;50:2140–2141. doi: 10.1128/JCM.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Performance standards for antimicrobial susceptibility testing twenty-second informational supplement. Clin Lab Stnd Inst. 2012;32(3):M100–S22. [Google Scholar]
- 21.Tenovar FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiett KL, Seal BS. Use of repetitive element palindromic PCR (rep-PCR) for the epidemiologic discrimination of foodborne pathogens. Methods Mol Biol. 2009;551:49–58. doi: 10.1007/978-1-60327-999-4_5. [DOI] [PubMed] [Google Scholar]
- 23.Hujer AM, Higgins PG, Rudin S, et al. A nosocomial outbreak of extensively drug resistant (XDR) Acinetobacter baumannii isolates containing blaOXA-237 encoded on a plasmid. Antimicrob Agents Chemother. doi: 10.1128/AAC.00797-17. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guide to the Elimination of Multidrug-resistant Acinetobacter baumannii Transmission in Healthcare Settings. Association of Professionals in Infection Control; 2010. website. http://www.apic.org/Resource_/EliminationGuideForm/b8b0b11f-1808-4615-890b-f652d116ba56/File/APIC-AB-Guide.pdf. Published 2010. Accessed April 7, 2017. [Google Scholar]
- 25.Communication During Patient Transfer of Multidrug Resistant Organisms, Oregon Administrative Rule 333-019-0052. Oregon Health Authority; website. http://arcweb.sos.state.or.us/pages/rules/oars_300/oar_333/333_019.html. Published 2014. Accessed April 7, 2017. [Google Scholar]
- 26.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 27.Higgins PG, Perez-Llarena FJ, Zander E, Fernandez A, Bou G, Seifert H. OXA-235. A novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans BA, Amyes SGB. OXA β-lactamases. Clin Microbiol Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lolans K, Rice TW, Munoz-Price LS, Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother. 2006;50:2941–2945. doi: 10.1128/AAC.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams-Haduch JM, Onuoha EO, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49:3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2011;53:532–540. doi: 10.1093/cid/cir482. [DOI] [PubMed] [Google Scholar]
- 32.Halachev MR, Chan JZM, Constantinidou CI, et al. Genomic epidemiology of a protracted hospital outbreak caused by multidrug-resistant Acinetobacter baumannii in Birmingham, England. Genome Med. 2014;6:70–83. doi: 10.1186/s13073-014-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:5036–5038. doi: 10.1128/AAC.00856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decker BK, Perez F, Hujer AM, et al. Longitudinal analysis of the temporal evolution of Acinetobacter baumannii strains in Ohio, USA, by using rapid automated typing methods. PLoS ONE. 2012;7:e33443. doi: 10.1371/journal.pone.0033443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou H, Kuang SN, He X, et al. Complete genome sequence of hypervirulent and outbreak-associated Acinetobacter baumannii strain LAC-4: epidemiology, resistance genetic determinants and potential virulence factors. Scientific Reports. 2015;5:8643. doi: 10.1038/srep08643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuno JP, Hebden JN, Standiford HC, et al. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008;36:468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


