Abstract
Background
Although elderly men, particularly patients with low-risk prostate cancer and a life expectancy fewer than 10-years, are unlikely to benefit from prostate cancer active therapy, treatment rates in this group are high.
Methods
Using the population-based Surveillance, Epidemiology and End Results (SEER) linked to Medicare data from 2004 to 2005, we examined the effects of clinical and non-clinical factors on the selection of prostate cancer active therapy (i.e. radical prostatectomy, external beam radiation therapy, brachytherapy, or androgen deprivation therapy) in men ≥ 75 years newly diagnosed with localized prostate cancer. Multivariate logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for receiving prostate cancer active therapy.
Results
The majority of men 75 years or older were treated with prostate cancer active therapy (81.7%), which varied by disease risk level: low, 72.2%, intermediate, 83.7%, and high-risk disease, 86.4%. Overall, in older men, the percentage of the total variance in the use of prostate cancer active therapy attributable to clinical and non-clinical factors was minimal, 5.1% and 2.6%, respectively. In men with low-risk disease, co-morbidity status did not impact treatment selection, such that patients with 1 or 2+ co-morbidities were as likely to receive prostate cancer active therapy as healthy men (OR = 0.98; 95% CI: 0.76, 1.27) and (OR = 1.19; 95% CI: 0.84, 1.68), respectively. Geographic location was the most powerful predictor of treatment selection [Northeast vs. Greater California (OR = 2.41; 95% CI: 1.75, 3.32)].
Conclusions
Clinical factors play a limited role in treatment selection among elderly patients with localized prostate cancer.
Keywords: treatment, prostatic neoplasms, aged, region, Surveillance, Epidemiology, and End Results program, Medicare
INTRODUCTION
Prostate cancer is the most common non-cutaneous malignancy in men with an estimated 186,320 new cases of prostate cancer diagnosed in 2008.1 The treatment options for prostate cancer include prostate cancer active therapy (e.g. radical prostatectomy, radiation therapy, or androgen deprivation therapy (ADT)) and conservative management. Ideally, treatment choice should take into account the possible risks of disease progression, disease characteristics, competing risks, life expectancy, and treatment-related complications including the potential to alter disease progression. As a result, medical guidelines do not recommend screening hence treating prostate cancer with prostate cancer active therapy in men 75 years and older.2–6
In particular, elderly patients diagnosed with incident, low-risk prostate cancer may benefit the least from being treated with prostate cancer active therapy as low-risk prostate cancer confers a low probability of disease progression, recurrence, and clinically symptomatic disease. 7–9 For example, in several studies pertaining to the natural progression of prostate cancer, it was observed that men with low-risk cancer can survive up to twenty years without the occurrence of clinically symptomatic disease.8, 9 Despite the indolent nature of low-risk prostate cancer, the prevalence of prostate cancer active therapy is high.10 In a community-based study, Cooperberg et al. found that treatment increased from 48% to 76% from 1993–1995 to 1999–2001 among elderly men with low-risk disease.11
However, it remains unclear which factors are influencing the use of attempted curative treatment instead of conservative management in elderly patients diagnosed with low-risk prostate cancer. Understanding which clinical as well as non-clinical characteristics most contribute to treatment selection is important because radiation therapy, radical prostatectomy, or ADT could engender more immediate harm (e.g. erectile dysfunction and bladder injury) than long-term benefit, especially in elderly patients with low-risk disease.
Thus, in a population-based sample we sought to examine the clinical and nonclinical characteristics that are associated with the receipt of prostate cancer treatment in patients 75 years and older diagnosed with incident, localized prostate cancer.
METHODS
Setting and Participants
Data for men, 75 years or older, diagnosed with localized prostate cancer from 2004–2005 was obtained from the latest Surveillance, Epidemiology and End Results (SEER) program database and linked Medicare administrative claims. SEER is a population-based cancer registry, which encompasses approximately 26% of the United States, and has 98% completeness in case ascertainment.12
In order to examine the factors that influence first course of therapy within the first year of diagnosis in men with localized prostate cancer, men with T3 or T4 prostate cancer (derived AJCC T, 6th edition)13 were excluded (n =1,168). Men with Health Maintenance Organization coverage or without both Medicare Part A and Part B during the study period were not included (n = 6,025). Patients whose prostate cancer diagnosis was obtained from autopsy or death certificate (n = 669) or had at least one prior cancer (n =2,451) were removed. After these exclusions, participants with unknown poverty rate (n = 1,186), Gleason score (n= 1,672), tumor stage (n=1,751), or prostate-specific antigen (PSA) values (n = 3,431) were also excluded. The final sample size consisted of 8,323 men ≥ 75 years with incident localized prostate cancer.
Outcome Variables
Primary treatment selected within one year following initial diagnosis for prostate cancer was defined as the receipt of either prostate cancer active therapy (i.e. radical prostatectomy, external beam radiation therapy (EBRT), brachytherapy, or ADT) or conservative management (i.e. no active therapy).
Explanatory Variables
Demographic, non-clinical, and clinical factors were measured at the time of diagnosis. Men ≥ 75 years of age were selected for this study because on average they will live for less than ten years14 and therefore are the least likely to benefit from local therapy due to the indolent nature of this disease and competing causes of death15 The non-clinical variables included census tract poverty level, marital status, registry region/city, and urban/rural residency. The percent of residents in each U.S. census tract living below the poverty line accounting for race and age was dichotomized at the median. Marital status was categorized as “Married”, “Unmarried” (i.e. separated, divorced, or widowed), and “Unknown”. In order to assess the regional differences in treatment, the 16 SEER registries were grouped into four distinct geographical regions as designated by the SEER program16: Northeast (Connecticut and New Jersey); North Central (Metropolitan Detroit and Iowa); South (Kentucky, Louisiana, Rural Georgia, and Metropolitan Atlanta); and West (Los Angeles County, San Jose-Monterey, Utah, Seattle-Puget Sound Area, New Mexico, San-Francisco-Oakland, Hawaii, and Greater California). The West was further divided into three groups based on men in these registries having a similar likelihood of receiving attempted curative (Group 1: Seattle, New Mexico, and Hawaii; Group 2: San Francisco and Los Angeles Group3: Other registries in California). Counties with a population less than 20,000 people and not adjacent to a metro area were considered to be rural.17
The clinical covariates consisted of PSA, Gleason score, clinical tumor classification, and the Charlson co-morbidity score. The co-morbidity score was derived from Medicare claims during the year prior to prostate cancer diagnosis by using a validated algorithm.18 Risk, a measure of disease progression and PSA failure, was based on the risk classification developed by D’Amico et al.7 and recommended by the National Comprehensive Cancer Network.19 Risk is a composite of clinical stage classification, highest serum PSA level prior to diagnostic biopsy or treatment, and Gleason score: low-risk (T1–T2a and PSA level <10 ng/mL and Gleason score 2–6), intermediate-risk (T2b-T2c or 10 ≤ PSA ≤ 20 ng/mL or Gleason score = 7) and high-risk (PSA level >20ng/mL or Gleason score 8–10).19
Statistical Analysis
Chi-square tests were used to assess the statistical difference in treatment selection according to co-morbidity score and region. Unconditional multivariate logistic regression was used to estimate the relative odds of receiving prostate cancer active therapy adjusted for demographic, non-clinical, and clinical risk factors.
In order to determine whether the clinical factors collectively exert more impact on the outcome than non-clinical variables, we evaluated the maximum rescaled R2. The maximum rescaled R2 assesses the percentage of variance in the use of prostate cancer active therapy that was attributable to the clinical and non-clinical characteristics. It was calculated with the partial generalized coefficient of determination with simultaneous adjustment for the other factors.20
All tests of significance were two-sided and p-values of <0.05 were considered statistically significant. The analyses were conducted using SAS, version 9.1.3 (SAS Institute Inc., Cary, NC). The study protocol was approved by the institutional review board of the University of Medicine and Dentistry of New Jersey.
RESULTS
Treatment assignment, demographic, non-clinical, and clinical characteristics are shown in Table 1 according to disease risk level. In this study of men ≥ 75 years of age, only 18.3% were assigned conservative management (Table 1).
Table 1.
Characteristics of men ≥75 years with incident, localized prostate cancer by risk* strata, SEER-Medicare 2004–2005.
| Characteristic | All (n = 8,323) | Low (n = 2,069) | Intermediate (n = 3,575) | High (n = 2,679) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| Age (median (IQR) years | 78 (76, 82) | 77 (76, 80) | 78 (76, 81) | 80 (77, 83) | ||||
| Race | ||||||||
| White | 6766 | 81.3 | 1753 | 84.7 | 2882 | 80.6 | 2131 | 79.5 |
| Black | 786 | 9.4 | 155 | 7.5 | 339 | 9.5 | 292 | 10.9 |
| Other | 771 | 9.3 | 161 | 7.8 | 354 | 9.9 | 256 | 9.6 |
| Treatment | ||||||||
| Radical Prostatectomy | 328 | 3.9 | 67 | 3.2 | 175 | 4.9 | 86 | 3.2 |
| Brachytherapy | 963 | 11.6 | 457 | 22.1 | 397 | 11.1 | 109 | 4.1 |
| Androgen Deprivation Therapy | 2215 | 26.6 | 312 | 15.1 | 823 | 23.0 | 1080 | 40.3 |
| EBRT | 3295 | 39.6 | 657 | 31.8 | 1597 | 44.7 | 1041 | 38.9 |
| Conservative Management | 1522 | 18.3 | 576 | 27.8 | 583 | 16.3 | 363 | 13.6 |
| % Below Poverty Line† | ||||||||
| Low | 4146 | 49.8 | 1076 | 52.0 | 1781 | 49.8 | 1289 | 48.1 |
| High | 4177 | 50.2 | 993 | 48.0 | 1794 | 50.2 | 1390 | 51.9 |
| Residency | ||||||||
| Urban | 7550 | 90.7 | 1898 | 91.7 | 3249 | 90.9 | 2403 | 89.7 |
| Rural | 773 | 9.3 | 171 | 8.3 | 326 | 9.1 | 276 | 10.3 |
| Marital Status | ||||||||
| Married | 5607 | 67.4 | 1423 | 68.8 | 2445 | 68.4 | 1739 | 64.9 |
| Unmarried‡ | 1792 | 21.5 | 379 | 18.3 | 760 | 21.3 | 653 | 24.4 |
| Unknown | 924 | 11.1 | 267 | 12.9 | 370 | 10.4 | 287 | 10.7 |
| Region/ Registry City§ | ||||||||
| Greater CA + SJ | 1865 | 22.4 | 398 | 19.2 | 837 | 23.4 | 630 | 23.5 |
| LA + SF | 1232 | 14.8 | 316 | 15.3 | 556 | 15.6 | 360 | 13.4 |
| Other West | 1014 | 12.2 | 250 | 12.1 | 434 | 12.1 | 330 | 12.3 |
| South | 1470 | 17.7 | 345 | 16.7 | 642 | 18.0 | 483 | 18.0 |
| North Central | 1063 | 12.8 | 273 | 13.2 | 445 | 12.5 | 345 | 12.9 |
| Northeast | 1679 | 20.2 | 487 | 23.5 | 661 | 18.5 | 531 | 19.8 |
| PSA (ng/mL) | ||||||||
| 0.1 < 2.5 | 396 | 4.8 | 175 | 8.5 | 154 | 4.3 | 67 | 2.5 |
| 2.5 – ≤4 | 420 | 5.1 | 168 | 8.1 | 181 | 5.1 | 71 | 2.7 |
| 4 – ≤ 10 | 4141 | 49.8 | 1726 | 83.4 | 1777 | 49.7 | 638 | 23.8 |
| 10 – ≤ 20 | 1937 | 23.3 | 0.0 | 0.0 | 1463 | 40.9 | 474 | 17.7 |
| >20 | 1429 | 17.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1429 | 53.3 |
| Gleason Score | ||||||||
| 2–6 | 3315 | 39.8 | 2069 | 100 | 953 | 26.7 | 293 | 11 |
| 7 | 3156 | 37.9 | 0.0 | 0.0 | 2622 | 73.3 | 534 | 19.9 |
| 8–10 | 1852 | 22.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1852 | 69.1 |
| Stage | ||||||||
| T1 | 4113 | 49.4 | 1264 | 61.1 | 1692 | 47.3 | 1157 | 43.2 |
| T2 | 4210 | 50.6 | 805 | 38.9 | 1883 | 52.7 | 1522 | 56.8 |
| Charlson Co-Morbidity Score | ||||||||
| 0 | 5767 | 69.3 | 1460 | 70.6 | 2493 | 69.7 | 1814 | 67.7 |
| 1 | 1638 | 19.7 | 405 | 19.6 | 707 | 19.8 | 526 | 19.6 |
| 2+ | 918 | 11.0 | 204 | 9.9 | 375 | 10.5 | 339 | 12.7 |
Patients were categorized into three risk groups on the basis of clinical classification, PSA level and Gleason score: low-risk (T1–T2a and PSA level <10 ng/mL and Gleason score 2–6), intermediate-risk (T2b–T2c or 10 ≤ PSA ≤ 20 ng/mL or Gleason score = 7) and high-risk (≥ T3a or PSA level >20ng/mL or Gleason score 8–10).
Percent of residents living below poverty level was obtained from the 2000 Census tracts and dichotomized at the median = 6.47%.
Unmarried = Separated, Divorced, or Widowed
The Western registries were divided into three groups based on similar odds of receiving prostate cancer active therapy vs. conservative management in unadjusted analyses. Group 1: Other West = Utah, Seattle, New Mexico, and Hawaii; Group 2: Los Angeles (LA) and San Francisco (SF); and Group 3: San Jose (SJ) and Greater California (CA)
Charlson co-morbidity score was derived from Medicare claims during the year before prostate cancer diagnosis using a validated algorithm.18
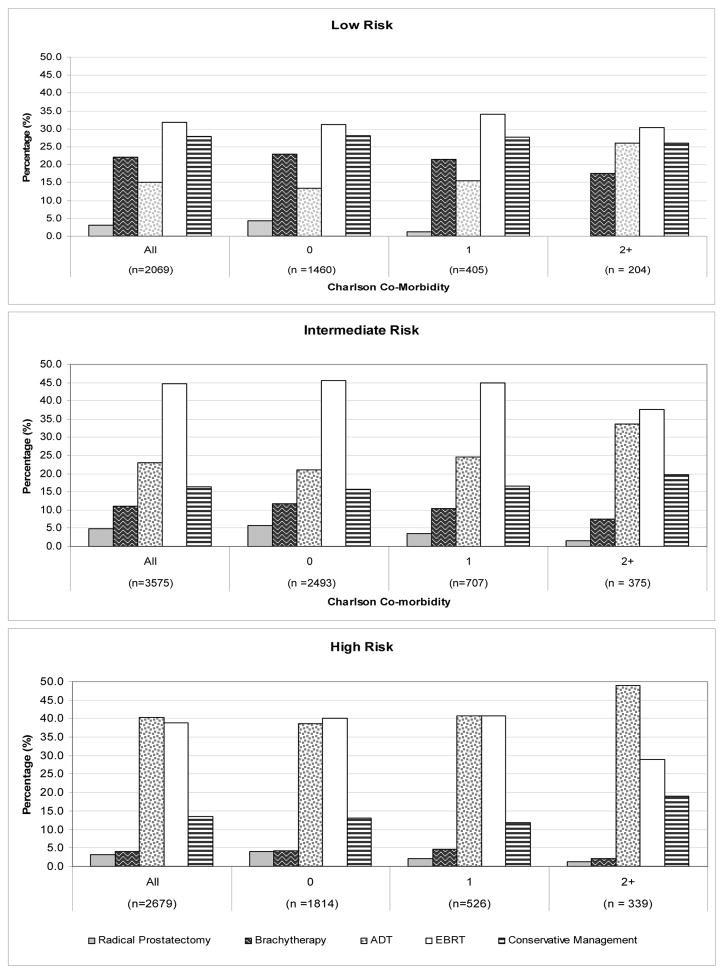
The prevalence of radical prostatectomy, brachytherapy, ADT, EBRT and conservative management by Charlson co-morbidity score is provided in Figure 1. Overall, the proportion of low-risk men receiving prostate cancer active therapy did not vary by co-morbidity status (Figure 1). The use of radical prostatectomy, brachytherapy, and EBRT each declined as the number of co-morbidities increased whereas the use of ADT increased in men with low, intermediate, or high-risk disease.
Figure 1.
The prevalence of radical prostatectomy, brachytherapy, androgen deprivation therapy (ADT), external beam radiation therapy (EBRT), and conservative management by Charslon co-morbidity score in men diagnosed with localized prostate cancer, SEER-Medicare 2004–2005.
Charlson co-morbidity score was derived from Medicare claims during the year before prostate cancer diagnosis using a validated algorithm.18
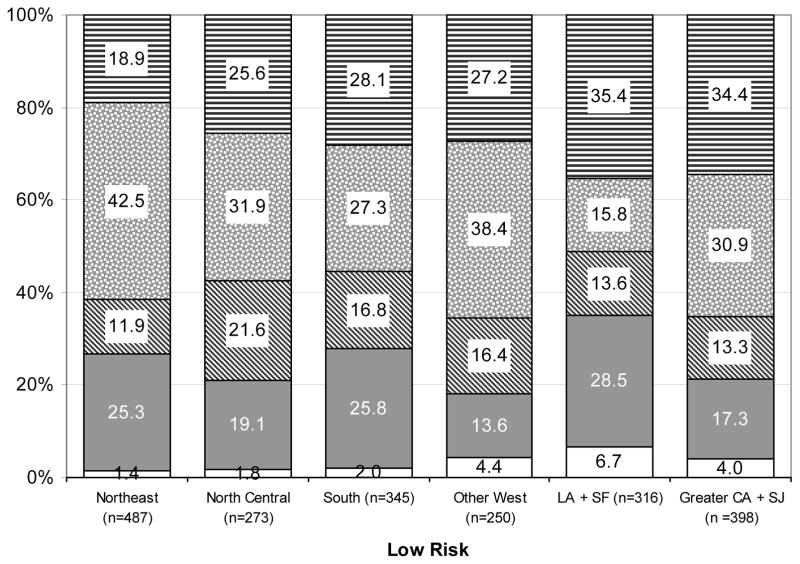
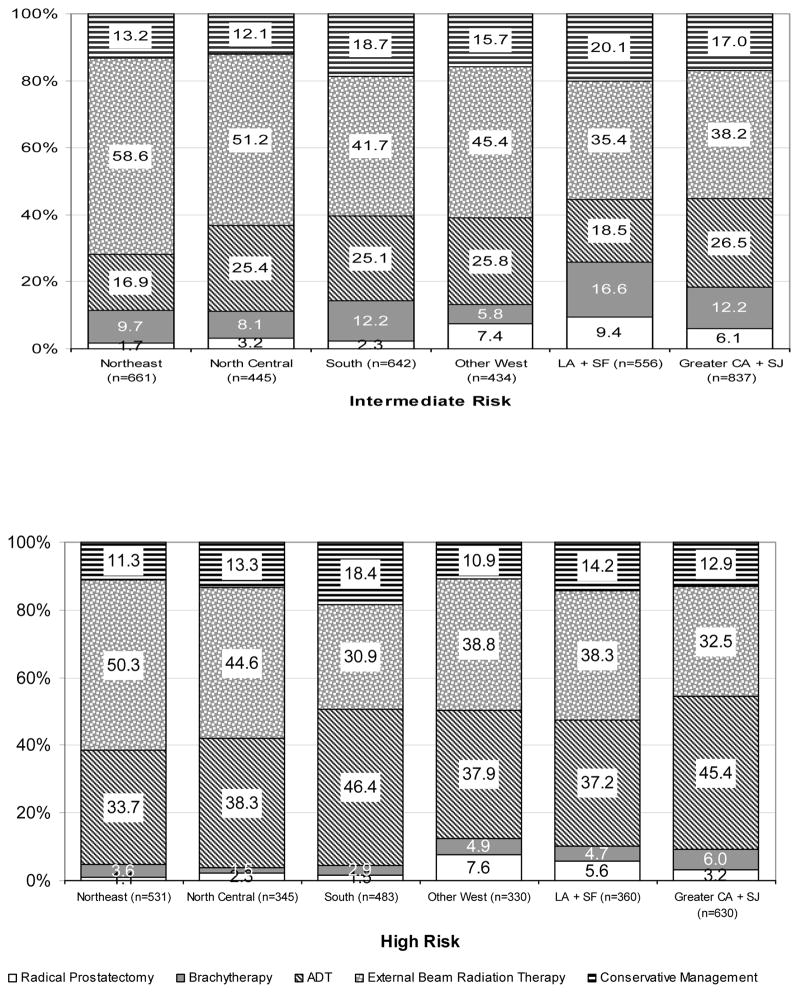
The prevalence of initial prostate cancer treatment stratified by risk across regions for men ≥ 75 years of age are presented in Figure 2. Generally, for men with intermediate or high-risk disease, the use of treatment was consistent across regions with EBRT being the most used therapy followed by ADT, conservative management, brachytherapy, and then radical prostatectomy.
Figure 2.
Distribution of treatment by age and geographic region in men with low-risk, intermediate-risk, and high-risk localized prostate cancer, SEER-Medicare 2004–2005. Geographical differences in treatment were significant (p-value <0.001) within each risk group.
* The Western registries were divided into three groups based on similar odds of receiving prostate cancer active therapy vs. conservative management in unadjusted analyses. Group 1: Other West = Utah, Seattle, New Mexico, and Hawaii; Group 2:Los Angeles (LA) and San Francisco (SF); and Group 3: San Jose (SJ) and Greater California (CA)
In the entire study population, age, race, marital status, and geographic region were significantly associated with receiving prostate cancer active therapy while the clinical factors were marginally related (Table 2). For men with low-risk disease, demographic and non-clinical factors (i.e. age, marital status, and geographic region) were related to treatment. Notably, geographic location was the most influential predictor in men ≥ 75 years of age with low-risk disease [Northeast vs. Greater California (OR = 2.41; 95% CI =1.75, 3.32). Having T1 stage cancer increased the likelihood of low-risk patients receiving therapy. Receiving prostate cancer therapy was not influenced by PSA level (0.1 – ≤ 4 ng/ml vs. 4– ≤ 10 ng/ml), Gleason score (2–4 vs. 5–6), or co-morbidity status in patients with low-risk disease. The results for men with intermediate or high-risk disease were similar as for the entire population (Results not shown).
Table 2.
Adjusted* odds ratios (95% Confidence Intervals) for prostate cancer active therapy versus conservative management stratified by risk† in men ≥75 years with localized prostate cancer, SEER-Medicare (2004–2005)
| Characteristic | All (n = 8,323) | Low Risk (n=2,069) |
|---|---|---|
| Demographic: | ||
|
| ||
| Age (yrs) | 0.92 (0.91, 0.94) | 0.91 (0.88, 0.93) |
| Race | ||
| Black | 0.57 (0.47, 0.68) | 0.76 (0.52, 1.12) |
| Other | 1.34 (1.07, 1.66) | 1.33 (0.90, 1.97) |
| White | 1.00 | 1.00 |
|
| ||
| Non-Clinical: | ||
|
| ||
| Percent Below Poverty Line‡ | ||
| High | 0.97 (0.86, 1.09) | 0.99 (0.80, 1.22) |
| Low | 1.00 | 1.00 |
| Marital Status | ||
| Married | 1.37 (1.19, 1.58) | 1.19 (0.92, 1.55) |
| Unknown | 0.73 (0.61, 0.89) | 0.54 (0.38, 0.77) |
| Unmarried§ | 1.00 | 1.00 |
| Residency | ||
| Rural | 0.94 (0.76, 1.17) | 1.13 (0.76, 1.68) |
| Urban | 1.00 | 1.00 |
| Registry Region/City|| | ||
| Northeast | 1.74 (1.44, 2.09) | 2.41 (1.75, 3.32) |
| North Central | 1.61 (1.30, 1.99) | 1.83 (1.27, 2.64) |
| South | 1.09 (0.90, 1.32) | 1.41 (1.00, 1.99) |
| LA and SF | 0.87 (0.72, 1.05) | 1.13 (0.82, 1.57) |
| Other West | 1.24 (1.01, 1.53) | 1.45 (1.01, 2.08) |
| Greater CA and SJ | 1.00 | 1.00 |
|
| ||
| Clinical: | ||
|
| ||
| PSA (ng/mL) | ||
| 0.1– ≤4 | 0.82 (0.65, 1.04) | 0.95 (0.73, 1.25) |
| 4 – ≤ 10 | 0.89 (0.75, 1.05) | 1.00 |
| 10 – ≤ 20 | 1.21 (1.00, 1.47) | --- |
| >20 | 1.00 | --- |
| Gleason Score | ||
| 2–4 | 0.34 (0.44, 1.63) | 0.86 (0.45, 1.66) |
| 5–6 | 0.40 (0.36, 0.45) | 1.00 |
| 7–10 | 1.00 | |
| Tumor Stage | ||
| T1 | 0.93 (0.83, 1.05) | 1.35 (1.09, 1.66) |
| T2 | 1.00 | 1.00 |
| Charlson Comorbidity¶ | ||
| 2+ | 0.87 (0.73, 1.05) | 1.19 (0.84, 1.68) |
| 1 | 1.01 (0.87, 1.17) | 0.98 (0.76, 1.27) |
| 0 | 1.00 | 1.00 |
Adjusted for other variables in the model.
Patients were categorized into low risk group on the basis of clinical classification, PSA level and Gleason score: low-risk (T1–T2a and PSA level <10 ng/mL and Gleason score 2–6).
Percent of residents living below poverty level was obtained from the 2000 Census tracts and dichotomized at the median = 6.47%.
Other = Separated, Divorced, or Widowed
The Western registries were divided into three groups based on similar odds of receiving prostate cancer active therapy vs. conservative management in unadjusted analyses. Group 1: Other West = Utah, Seattle, New Mexico, and Hawaii; Group 2: Los Angeles (LA) and San Francisco (SF); and Group 3: San Jose (SJ) and Greater California (CA) (excluding San Francisco, Los Angeles, and San Jose-Monterey)
Overall, the clinical factors, which included PSA, Gleason Score, tumor stage, and co-morbidity, explained merely 5.1% of the variance in the use of prostate cancer active therapy. In aggregate, the non-clinical variables factors (i.e. Census track poverty level, marital status, residency, and registry region/city) also contributed minimally to the variance in the selection of prostate cancer active therapy at 2.6%. The demographic characteristics (i.e. age and race) attributed 2.2% of the total variance.
To examine the effect of co-morbidity, we conducted a sensitivity analysis of healthy men (i.e. zero co-morbidity), and the results were similar as observed for the entire study cohort.
DISCUSSION
It is important to understand which factors may contribute to treatment decisions in elderly men because approximately one quarter of all diagnosed prostate cancers occur in men 75 years and older.21 To our knowledge, this is the first population-based study to analyze potential factors that may influence the receipt of prostate cancer active therapy in elderly men by risk group. Clinical characteristics including PSA, Gleason Score, tumor stage, and co-morbidity contributed only 5.1% to the total variance in the use of prostate cancer active therapy. Geographic location was the most powerful predictor of cancer therapy whereas tumor characteristics and co-morbidity status had little bearing in treatment decisions. It is disturbing to find that low-risk patients with 1 or 2+ co-morbidities were as likely to receive prostate cancer active therapy as healthy men. This raises the concern of overtreatment among low-risk elderly patients with multiple co-morbidities.
Receiving active therapy instead of conservative management is particularly a concern for low-risk elderly men as the clinical tradeoffs involved in the treatment of intermediate- or high-risk disease differs considerably. Despite a growing body of evidence which suggests that prostate cancer active therapy produces a lower quality of life without improving survival in elderly low-risk patients 19, 22 more than three-quarters (81.7%) of low-risk patients aged 75 years or older in this study received prostate cancer active therapy. Consequently, the majority of elderly men with low-risk disease may incur avoidable treatment related complications and side-effects. In addition, to reducing one’s quality of life, the healthcare costs of treatment are high. The 5-year net cost to care for elderly patients diagnosed with prostate cancer is estimated to be approximately $59.1 million.23 Thus, educational programs are needed to emphasize the risks and benefits of conservative management 8, 24 in order to reduce treatment related complications, side-effects, and costs in elderly men with limited life expectancies and low-risk disease. This is supported by findings from several studies that have shown that most prostate cancer patients have poor knowledge of treatment options and unrealistic expectations of prostate cancer active therapy.25–27
To our knowledge the association between co-morbidity and prostate cancer therapy in multivariate analyses has not been reported. Concerning PSA screening, previous studies have shown that the number of screenings does not vary by worsening co-morbidity,28 this is likely is because PSA screening is non-invasive. However, treatment can be invasive. The lack of effect that co-morbidity may have on being treated with prostate cancer active therapy in low-risk patients may be in part explained by disease severity. More studies are needed to understand the role of co-morbidity in selecting prostate cancer active therapy instead of conservative management in older men with limited life expectancies.
Our data does suggest that geographic region and marital status may be used to identify groups to target for intervention. The wide variation in utilization of potentially curative therapies across geographic regions and risk strata suggests that there is still a lack of consensus concerning the appropriate treatment of prostate cancer within the United States. Fowler and colleagues reported that although urologists and radiation oncologists share similar recommendations in the detection of prostate cancer that these specialists primarily suggest the prostate cancer treatment of their profession.29 Thus, these regional differences in treatment may reflect an uneven distribution of urologists30 and radiation oncologists in the United States or different regional preferences as shown in previous studies.22–24
Marital status was consistently a strong predictor of treatment selection in elderly men 31–33. Our findings confirm and extend previous studies which have indicated that a higher proportion of men married at the time of diagnosis receive potentially curative therapy when compared to their unmarried counterparts.31–33 Being married influences treatment decisions in elderly men and therefore spouses should also be educated about the risks and benefits of different treatment alternatives.
As reported in previous studies, the use of prostate cancer active therapy is common in elderly men.10, 11 An advantage of this study over previous studies in estimating the prevalence of treatment in older men is that we were able to incorporate Gleason score, tumor stage, and PSA values in order to generate modern risk profile.10 In addition, previous studies were limited by a small sample size and older datasets.10, 11
Our findings likely reflect clinical practice in the U.S. because the SEER database covers 26% of the U.S. population; however there are several limitations. The total variance of treatment choice explained by the variables in this study is 10%, which implies that treatment choice is random or dependent on unknown or unmeasured factors. For instance, PSA values over time (i.e. PSA velocity) or provider characteristics may contribute to the variations in observed treatment patterns. However, it is unlikely that including additional patient characteristics or tumor features would have changed the results given that adjustment for other important factors, such as poverty level, stage, Gleason score, had little influence on the variation. In addition, some men ≥ 75 years may have a life expectancy more than 10 years, but we were unable to estimate each patient’s individual predicted life expectancy. Furthermore, missing data could have biased our results because a greater proportion of men with missing data were older, had T1ab cancer, had lower Gleason Scores and PSA, and were treated with conservative management compared to men with non-missing data.
The results from this study provide insights into the prevalence and trends in treatment among elderly men with localized prostate cancer research. We found that a large proportion of older men with low-risk disease continue to receive prostate cancer active therapy. This finding raises concerns surrounding overtreatment, especially in light of recent findings from several published randomized trials, which suggest that older men are unlikely to benefit from PSA screening hence prostate cancer active therapy.34, 35 We also report that clinical factors explain only a small portion of the variance in treatment selection, and that co-morbidity status does not predict treatment selection. These findings suggest that for many patients their risk profile and life expectancy has minimum impact on treatment decisions. Effective educational interventions may be needed to facilitate informed treatment decisions.
Acknowledgments
Funding: The study was supported by the following grants and awards: National Cancer Institute grant # RO1 CA 116399, Cancer Institute of New Jersey core grant NCI CA-72720-10 and Robert Wood Johnson foundation grant # 60624. The funding source had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: None to be reported.
Authorship/Disclaimer: All authors had access to the data, and a role in writing the manuscript. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not reflect the position or policy of the Government or the employers, and no official endorsement should be inferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002 Dec 3;137(11):917–929. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007 Jun;177(6):2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.U. S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 5.Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA) Oncology. 2000 Feb;14(2):267–272. 277–268, 280. [PubMed] [Google Scholar]
- 6.Screening for prostate cancer. American College of Physicians. Ann Intern Med. 1997 Mar 15;126(6):480–484. [PubMed] [Google Scholar]
- 7.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 8.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005 May 4;293(17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 9.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004 Jun 9;291(22):2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 10.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006 Aug 16;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004 Jun 1;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002 Jul;87(7):13–15. [PubMed] [Google Scholar]
- 14.Arias E. United States Life Tables, 2004. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Reports NVS. [PubMed] [Google Scholar]
- 15.Welch HG, Albertsen PC, Nease RF, et al. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996 Mar 15;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance Epidemiology and End Results (SEER) Number of Persons by Race and Hispanic Ethnicity for SEER Participants. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 17.Measuring Rurality: Rural-Urban Continuum Codes. 2009 Jan 10; http://www.ers.usda.gov/briefing/rurality/ruralurbcon/
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.The NCCN 1.2010 Prostate Cancer Clinical Practice Guidelines in Oncology. 2010 To view the most recent and complete version of the guideline, go online to www.nccn.org. Published Last Modified Date|. Accessed Dated Accessed|.
- 20.Nagelkerke N. A note on a general definition of the coefficient of determination. Biometrika. 1991 Sep 1;78(3):691–692. [Google Scholar]
- 21.Horner M, Ries L, Krapcho M, et al. Institute NC, editor. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: 2009. [Google Scholar]
- 22.Ollendorf D, Hayes J, McMahon P, et al. Management options for low-risk prostate cancer: a report on comparative effectiveness and value. Boston, MA: Dec, 2009. [Google Scholar]
- 23.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008 May 7;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 24.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009 Sep 16;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry DL, Ellis WJ, Woods NF, Schwien C, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003 Mar-Apr;21(2):93–100. doi: 10.1016/s1078-1439(02)00209-0. [DOI] [PubMed] [Google Scholar]
- 26.Davison BJ, Oliffe JL, Pickles T, Mroz L. Factors influencing men undertaking active surveillance for the management of low-risk prostate cancer. Oncol Nurs Forum. 2009 Jan;36(1):89–96. doi: 10.1188/09.ONF.89-96. [DOI] [PubMed] [Google Scholar]
- 27.Steginga SK, Occhipinti S, Gardiner RA, et al. Making decisions about treatment for localized prostate cancer. BJU Int. 2002 Feb;89(3):255–260. doi: 10.1046/j.1464-4096.2001.01741.x. [DOI] [PubMed] [Google Scholar]
- 28.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006 Nov 15;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 29.Fowler FJ, McNaugton M, Albertsen PC, et al. Comparison of Recommendations by Urologists and Radiation Oncologists for Treatment of Clinically Localized Prostate Cancer. JAMA. 2000;283(24):3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 30.Odisho AY, Fradet V, Cooperberg MR, et al. Geographic distribution of urologists throughout the United States using a county level approach. J Urol. 2009 Feb;181(2):760–765. doi: 10.1016/j.juro.2008.10.034. discussion 765–766. [DOI] [PubMed] [Google Scholar]
- 31.Lai S, Lai H, Krongrad A, Lamm S, et al. Radical prostatectomy: geographic and demographic variation. Urology. 2000 Jul;56(1):108–115. doi: 10.1016/s0090-4295(00)00557-4. [DOI] [PubMed] [Google Scholar]
- 32.Lai S, Lai H, Lamm S, Obek C, et al. Radiation therapy in non-surgically-treated nonmetastatic prostate cancer: geographic and demographic variation. Urology. 2001 Mar;57(3):510–517. doi: 10.1016/s0090-4295(00)01034-7. [DOI] [PubMed] [Google Scholar]
- 33.Harlan LC, Potosky A, Gilliland FD, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001 Dec 19;93(24):1864–1871. doi: 10.1093/jnci/93.24.1864. [DOI] [PubMed] [Google Scholar]
- 34.Andriole GL, Grubb RL, 3rd, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009 Mar 26;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009 Mar 26;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]