Abstract
FUT1 is a key rate-limiting enzyme in the synthesis of Lewis y, a membrane-associated carbohydrate antigen. The aberrant upregulation of FUT1 and Lewis y antigen is related to proliferation, invasion and prognosis in malignant epithelial tumors. A c-Fos/activator protein-1 (AP-1) binding site was found in the FUT1 promoter. However, the mechanisms of transcriptional regulation of FUT1 remain poorly understood. TGF-β1 is positively correlated to Lewis y. In the present study, we investigated the molecular mechanism of FUT1 gene expression in response to TGF-β1. We demonstrated that c-Fos was highly expressed in 77.50% of ovarian epithelial carcinoma cases and was significantly correlated with Lewis y. Using luciferase activity and chromatin immunoprecipitation (ChIP) assay, we further revealed that c-Fos interacted with the FUT1 promoter in ovarian cancer cells and transcriptional capacity of the heterodimer formed by c-Fos and c-Jun was stronger than that of the c-Fos or c-Jun homodimers. Then, we demonstrated that TGF-β1 induced dose-dependent c-Fos expression, which was involved in TGF-β1-induced ovarian cancer cell proliferation. In addition, inhibition of MAPK activation or TGF-β1 receptor by pharmacological agents prevented TGF-β1-induced c-Fos and Lewis y expression. Silencing of c-Fos prevented TGF-β1-induced Lewis y expression. Collectively, the results of these studies demonstrated that TGF-β1 regulated FUT1 and Lewis y expression by activating the MAPK/c-Fos pathway.
Keywords: TGF-β1, c-Fos, MAPK, α1, 2-fucosyltransferase, Lewis y, ovarian cancer
Introduction
Ovarian cancer is a common malignant tumor of the female reproductive system, and is the major cause of cancer-related deaths in women. In the US, in 2013 alone, there were 22,240 new cases of ovarian cancer and 14,030 deaths due to this disease (1). Due to the inconspicuous behavior of ovarian cancer and lack of effective measures for early diagnosis, ~75% of epithelial ovarian cancer patients are diagnosed at an advanced stage (III or IV) (2). Even with standard ovarian cancer cytoreductive surgery and chemotherapy, the 5-year survival rate is still lower than 30% due to drug resistance and relapse (3). An important feature of the malignant transformation of tumors is the change in cell surface glycosylation, which affects the function of adhesion molecules, alters mutual interactions between cells and the matrix and subsequently leads to the chemoresistance of cells (4–6).
Glycosylated proteins have been used as markers in cancer diagnosis and in evaluation of therapeutic effects (7,8). Lewis y antigen, a type 2 carbohydrate antigen, exhibited high expression in malignant epithelial tumors and was related to the prognosis of the disease (9–11).
The expression of cancer-associated carbohydrate antigens was modified by abnormal control by glycosyltransferase. FUT 1 is a key enzyme for Lewis y synthesis (12–16). Overexpression of FUT1 led to a marked increase in the expression of Lewis y and promoted the proliferation, invasion (17) and drug-resistance (6,18) of ovarian cancer cells transfected with the FUT1 gene. Knockdown of FUT1 expression attenuated cell proliferation in a HER2-overexpressing cancer cell line (16). Therefore, understanding the molecular mechanism of FUT1 expression in ovarian cancer is critical for early diagnosis and searching for better treatment options.
AP-1 is a classic nucleus transcription factor, including c-Fos, Fos-B, Fra-1 and Fra-2 of the Fos family and c-Jun, Jun-B and Jun-D of the Jun family. They bind to DNA target sequences in the form of homologous or heterologous dimers, which regulate gene expression in response to a variety of stimuli, including cytokines, growth factors, bacterial and viral infections. As an important downstream target of the MAPK signaling pathway, AP-1 is essential for normal cell differentiation and survival. However, overexpression of c-Jun and c-Fos also promoted the malignant transformation of cells (19). Previous investigations revealed that silencing of c-Fos sensitized glioma cells to radiation and c-Fos overexpression was correlated with poor prognosis in malignant glioma patients (20). c-Fos transcriptionally controled MMP10 and S100A15 expression in keratinocytes in skin squamous cell carcinoma, promoting epidermal hyperplasia (21). In ovarian cancer cells, c-Jun was found to be a transcriptional activator of FUT1, which facilitated FUT1 transcription and enhanced Lewis y biosynthesis (22). However, the underlying mechanism of c-Fos and the interaction between c-Fos and c-Jun in the regulation of FUT1 transcription is still unclear. In the present study, we investigated the expression of c-Fos in ovarian epithelial carcinoma and the role of c-Fos in the activation of the FUT1 promoter.
TGF-β1 is a multifunctional cytokine that regulates cell growth, motility, differentiation, apoptosis and plays an important role in tumor cell proliferation, migration and invasion (23–25). TGF-β1 is frequently overexpressed in multiple malignancies, colorectal (26), pancreatic (27), liver (28) and ovarian cancer (29–31). Canonical TGF-β signaling pathways begin with activation of serine/threonine kinase receptors, followed by the phosphorylation of Smad2/3 and Smad4, which formed into a complex. The activated Smads complex accumulates in nucleus together with other nuclear factors to act as transcription factors, regulating target genes (32). In addition to the canonical Smad pathways, the Smad-independent pathways, including the MAPK pathway, has been reported to be involved in TGF-β-mediated gene expression (33,34). Previous studies demonstrated that TGF-β1 was positively correlated to Lewis y in malignant tumors (35,36). The underlying mechanism involved in the association between TGF-β1 and Lewis y remain unknown. It was reported that TGF-β1 promoted the transcription of triphosphopyridine nucleotide oxidase gene through the AP-1 binding site in triphosphopyridine nucleotide oxidase gene promoter (37). Previously, we demonstrated that the FUT1 promoter had an AP-1 response element. Therefore, it was hypothesized that TGF-β1 may regulate FUT1 by activating AP-1 in ovarian cancer.
In the present study, we demonstrated that the FUT1 gene was a transcriptional target of TGF-β1 through the MAPK pathway in ovarian cancer cells. Furthermore, we revealed that c-Fos played a critical role in mediating TGF-β1-induced FUT1 expression, which eventually facilitated Lewis y biosynthesis and proliferation of ovarian cancer cells. This data provided insight into the molecular mechanisms of c-Fos in FUT1 gene expression and identified c-Fos as a potentially novel therapeutic target for TGF-β1/FUT1/Lewis y-overexpressing ovarian cancer.
Materials and methods
Collection of human samples
We selected 160 resected paraffin specimens obtained from the Department of Gynecology and Obstetrics of Shengjing Hospital Affiliated to China Medical University (Shenyang, China), from 2000 to 2013. All tissues were re-diagnosed by pathologists. According to the pathological results, there were 80 cases of epithelial ovarian cancer (including 30 cases of mucinous cystadenocarcinoma and 30 cases of serous cystadenocarcinoma, 10 cases of ovarian endometrioid carcinoma and 10 cases of clear-cell carcinoma), 30 cases of borderline ovarian epithelial tumors, 30 cases of benign ovarian tumors, and 20 cases of normal ovarian tissue (resected from cervical cancer patients during the same period). The mean age of the patients was 47 years (15–73 years) and the median age was 44 years. The age of ovarian cancer patients ranged from 36 to 73 years with a median age of 51 years. Borderline patients ranged from 22 to 55 years with a median age of 35 years. Benign patients ranged from 15 to 72 years with a median age of 44 years. Normal ovarian patients ranged from 37 to 52 years with a median age of 42 years. The age difference between patients in the different groups was not significant (P>0.05). The ovarian cancer group was pathologically classified as follows, 41-well-differentiated cases, 18 moderately differentiated cases and 21 poorly differentiated cases. Pathology stage: according to the standards of the International Federation of Gynecology and Obstetrics: 56 cases were stage I and II; 24 cases were stage III and IV. Of these, 15 cases had pelvic lymph node metastasis. All cases were primary tumors with complete clinical and pathological data and without preoperative radiotherapy or chemotherapy. The present study was approved by the hospital Ethics Committee.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Cell culture
The human ovarian cancer cell lines CAVO3, SKOV3, ES-2 and human embryonic kidney 293 (293) were purchased from the Shanghai Institute of Life Sciences of the Chinese Academy of Sciences. The cells were conventionally cultured in Roswell Park Memorial Institute formulation (RPMI)-1640 and McCoy's 5A medium (Gibco by Invitrogen) with 10% fetal bovine serum (HyClone, Logan, UT, USA).
Transient transfection and luciferase assay
A luciferase reporter vector of the FUT1 promoter, pGL4-FUT1, including one binding site for AP-1 (−3,000 to −1) was constructed as previously described (22). Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Cells were transferred to 24-well plates at a density of 4×104 cells/well 1 day before transfection. pcDNA3-c-Fos, pCMV6-c-Jun, pcDNA3-c-Fos/pCMV6-c-Jun or vector pCMV6 and pcDNA3 vector (1 µg/ml) (OriGene, Beijing, China) along with the human FUT1 promoter reporter gene were co-transfected in 293, SKOV3, CAVO3 and ES-2 cells. Forty hours after transfection, the activity of the promoters was detected by the Dual-Luciferase Assay System (Promega, Fitchburg, WI, USA). The luciferase data of the FUT1 promoter reporter constructs were calculated and normalized with Renilla luciferase activity. All transfections were carried out in triplicate.
Immunohistochemistry analysis
Histological sections from each group of ovarian tissues was 4 µm. Each tissue had two serial sections. The expression of c-Fos and Lewis y in paraffin sections were detected via immunohistochemical strepavidin-peroxidase staining. The sections were dewaxed and rehydrated by rinsing with xylene followed by graded ethanol washing. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 15 min before antigen retrieval by high pressure treatment in 10 mmol/l citrate phosphate buffer for 1.5 min. The sections were then incubated with a protein block in 10% normal goat serum for 10 min. The sections were incubated overnight at 4°C with polyclonal rabbit anti-human c-Fos (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-Lewis y antibodies (1:200; Abcam, Cambridge, UK). After being washed with phosphate-buffered saline (PBS), the sections were incubated with horseradish peroxidase-labelled secondary antibodies for 2 h at 37°C. Two cases of strongly positive sections and 2 cases of negative sections served as the positive control and negative control for c-Fos and Lewis y antigen, respectively. Rabbit IgG (BIOSS, Beijing, China) was used as the negative control. In addition, the blank control was incubated with PBS instead of a primary antibody. The empirical procedure was performed base on the manufacturer's protocol of UltraSensitive™ S-P (mouse/rabbit) IHC and DAB kits (both from MaiXin Bio, Fuzhou, China).
Five high power fields (magnification, ×400) were randomly selected in each section according to the staining intensity and the percentage of positive cells. The degree of staining was defined as follows: 0 points represented no staining; 1 point represented faint yellow; 2 points represented yellowish brown; 3 points represented brown. Percentage of stained cells was as follows, 0 points represented <5%; 1 point represented 5–25%; 2 points represented 26–50%; 3 points represented 51–75%; 4 points represented >75%. When both scores were multiplied: 0–2 indicated negative (−); 3–4 indicated weakly positive (+); 5–8 indicated moderately positive (++) and 9–12 indicated strongly positive (+++).
Chromatin immunoprecipitation (ChIP) assay
ChIP experiments were conducted according to the ChIP kit instructions (Upstate, Charlottesville, VA, USA). CAVO3 cells were fixed in 1% formaldehyde for 10 min for crosslinking reaction which was quenched with 125 nM glycin. The nuclei were pelleted by centrifugation at 3,000 rpm for 5 min at 4°C and sonicated to chromatin fragments between 100 and 1,000 bp. The sonicated lysate was centrifuged at 10,000 rpm for 5 min at 4°C. Supernatant (500 µl) was incubated with anti-c-Jun (1:50; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-c-Fos (1:50) antibody followed by an isolation procedure using protein A/G magnetic beads (both from Santa Cruz Biotechnology, Inc.). A normal rabbit IgG was used as a control. The crosslinking was reversed by incubation at 65°C for 10 h. Primers were designed according to the binding site of AP-1 in the FUT1 promoter and the sequence was as follows: F, 5′-CTAGCACTCAAGGTCCTGGTC-3′ and R, 5′-GCAAGATGAGGAAACTGAGGC-3′. The PCR conditions were as follows, 98°C for 5 min, 98°C for 30 sec, 60°C for 20 sec and 72°C for 5 min for 30 cycles. PCR products were resolved by electrophoresis on a 1% agarose gel and visualized after ethidium bromide staining.
Western blotting
Protein was extracted with lysis buffer [150 mM NaCl, 1% v/v NP-40, 0.1% v/v SDS, 2 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF)]. Protein (50 µg) was subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes. The membranes were blocked for 2 h at room temperature with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and subsequently incubated overnight at 4°C with the primary antibodies: anti-c-Fos (1:500; Santa Cruz Biotechnology, Inc.), β-actin (1:20,000; Sigma, St. Louis, MO, USA), anti-Lewis y (1:100; Abcam), anti-phospho-p38 (1:500), anti-(total p38) (1:500), anti-phospho-JNK (1:500), anti-(total JNK) (1:500), anti-phospho-ERK (1:500), anti-(total ERK) (1:500) (all from Cell Signaling Technology, Inc.). The samples were then washed 3 times for 15 min with TBS-T and incubated with appropriate horseradish peroxidase-conjugated IgG (1:5,000; Sigma) for 2 h at 37°C. The immunocomplex bands were detected with an enhanced chemiluminescence HRP substrate for western blotting (Pierce, Rockford, IL, USA) using the Molecular Imager system GDS8000b (UVP, Inc., Upland, CA, USA).
Interfering RNA transfection
When the cells reached 60% confluence, scramble siRNA, c-Jun siRNA and c-Fos siRNA obtained from Santa Cruz Biotechnology, Inc. were transfected into the cell line, respectively, using Lipofectamine RNA iMAX transfection reagent (Invitrogen). After 72 h of transfection, proteins were harvested for subsequent tests.
Cell proliferation assay
Twenty-four hours after CAVO3 cells had been transfected with pcDNA3-c-Fos, pcDNA3 or c-Fos siRNA, the transfected cells were inoculated into a 96-well plate at a density of 2×104 cells/ml (100 µl/well). Methyl thiazolyl tetrazolium (MTT; 20 µl) (5 mg/ml) was added 24, 48, 72 and 96 h later and after another 4 h. Dimethyl sulfoxide solution (150 µl) was added to each well to dissolve the crystals. The absorbance in each well was determined with enzyme-linked immunosorbent analyzer at 550 nm.
Colony formation
Cells in the logarithmic growth phase were titrated into single cells and inoculated into a 6-cm culture dish (200 cells/well) and the medium was discarded after 24 h when the cells adhered. The c-Fos interference, c-Fos overexpression and the corresponding control group were established. Using the control group as the reference standard, the original culture medium was discarded when visible cell clusters in the control group reached 50 cells under the microscope, and the cells were fixed using methanol. An appropriate amount of Giemsa pigment was added for 5 min to stain the nucleus. The dish was then inverted, placed under the microscope for clone counting, and cell clusters containing >50 cells were considered one clone, followed by calculation of the clone formation rate.
Statistical analysis
Quantitative data were expressed in terms of the means ± SD. Qualitative data were expressed in terms of the composition ratio. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The Pearson χ2 and likelihood ratio tests were used to analyze the correlation between the expression of Lewis y and c-Fos and clinicopathological factors. Spearman rank correlation analysis was employed to analyze the correlation between Lewis y and c-Fos. A P-value <0.05 indicated statistical significance.
Results
Overexpression of c-Fos in ovarian epithelial carcinoma and its correlation with clinicopathological characteristics
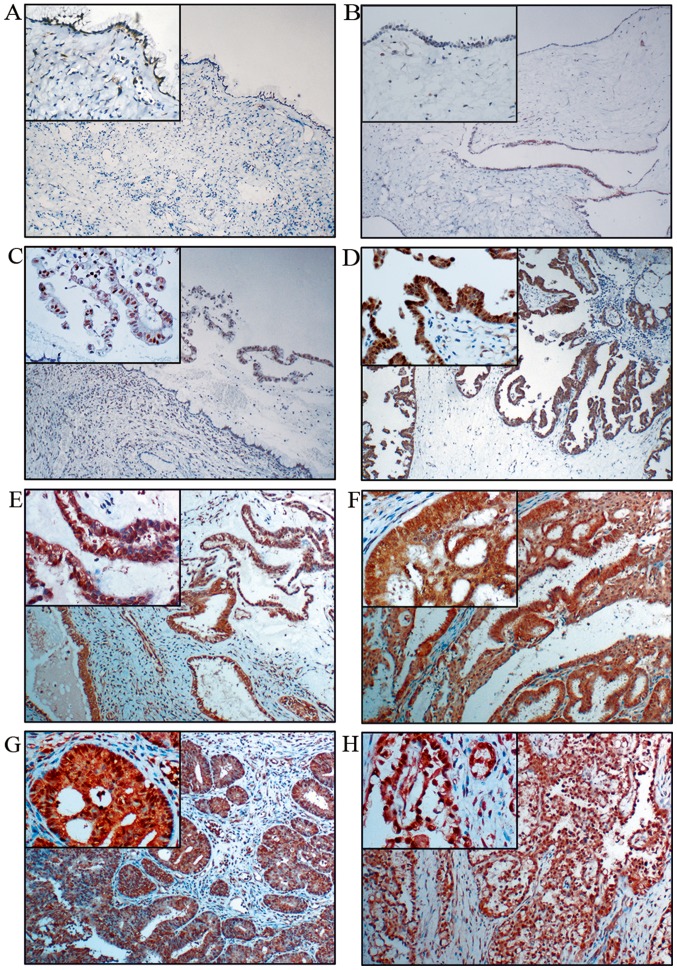
To determine the role of c-Fos in the progression of ovarian epithelial carcinoma, we examined c-Fos expression and its correlation with clinicopathological characteristics in 140 primary ovarian tumor samples and 20 normal ovarian tissue samples using immunohistochemistry. The 140 primary ovarian tumor samples included 30 mucinous and serous cystadenoma (benign), 30 borderline mucinous and borderline serous cystadenoma (borderline), 60 mucinous and serous cystadenocarcinoma, 10 endometrioid and 10 clear cell carcinoma (malignant). We observed that c-Fos was hardly expressed in the benign (Fig. 1A and B) and normal ovarian tissues (data not shown), only expressed in the nucleus of borderline mucinous cystadenoma (Fig. 1C), moderately expressed in the nucleus and the cytoplasm of borderline serous cystadenoma (Fig. 1D) and significantly overexpressed in both the nucleus and the cytoplasm of different malignant ovarian epithelial tumor cells, including mucinous (Fig. 1E) and serous cystadenocarcinoma (Fig. 1F), endometrioid (Fig. 1G) and clear cell carcinoma (Fig. 1H). The degree of the staining were defined as follows, negative (−), weakly positive (+), moderately positive (++) and strongly positive (+++) (see Materials and methods for details) and the cases were further divided into high (++ or +++) and low (− or +) c-Fos expression groups. The expression rate of c-Fos was positively associated with the degree of ovarian tumor malignancy (P<0.05) (Table I).
Figure 1.
Immunohistochemical staining of c-Fos in ovarian tumors. (A) Mucinous, (B) serous, (C) borderline mucinous and (D) borderline serous cystadenoma. (E) Mucinous cystadenocarcinoma and (F) serous cystadenocarcinoma. (G) Endometrioid and (H) clear cell carcinoma. (Original magnification ×100 and ×400 for the small box at the top-left corner).
Table I.
Expression of c-Fos in different ovarian tissues.
| c-Fos | |||
|---|---|---|---|
| Characteristics | Low n (%) | High n (%) | P-value |
| Malignant group (n=80) | 18 (22.50) | 62 (77.50) | <0.01a,b,c |
| Borderline group (n=30) | 20 (66.67) | 10 (33.33) | 0.136d, 0.091e |
| Benign group (n=30) | 25 (83.33) | 5 (16.67) | 0.687f |
| Normal group (n=20) | 18 (90.00) | 2 (10.00) | |
Compared with borderline group.
Compared with benign group.
Compared with normal tissue group. n, the number of cases.
The high expression rates of c-Fos in mucinous and serous ovarian cystadenocarcinoma were 83.33% (25/30) and 80% (24/30), respectively, which were higher than that in ovarian endometrioid carcinoma and clear cell carcinoma (60%, 6/10; and 70%, 7/10), but no significant difference was detected (P>0.05). The high expression rates of c-Fos in ovarian cancer tissues with high, middle and low differentiation were 63.41% (26/41), 88.89% (16/18) and 95.24% (20/21), respectively, and the high expression rate of c-Fos gradually increased with the decreasing degree of differentiation (P<0.05). The expression of c-Fos in advanced cancer (stage III–IV) was 91.67% (22/24), which was significantly higher than that in early-stage ovarian cancer (stage I–II) (71.43%, 40/56) (P<0.05). Expression of c-Fos was not correlated with lymph node metastasis (P>0.05) (Table II).
Table II.
Summary of the correlation in Lewis y and c-Fos expression with clinicopathological characteristics in ovarian carcinoma.
| Lewis y n (%) | c-Fos n (%) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Low | High | P-value | Low | High | P-value |
| Histology | ||||||
| Mucinous (n=30) | 4 (13.30) | 26 (86.67) | 0.221 | 5 (16.67) | 25 (83.33) | 0.463 |
| Serous (n=30) | 3 (10) | 27 (90) | 6 (20) | 24 (80) | ||
| Endometrioid (n=10) adenocarcinoma | 4 (40) | 6 (60) | 4 (40) | 6 (60) | ||
| Clear cell carcinoma (n=10) | 2 (20) | 8 (80) | 3 (30) | 7 (70) | ||
| FIGO stage | ||||||
| I+II (n=56) | 11 (19.64) | 45 (80.36) | 0.355 | 16 (28.57) | 40 (71.43) | 0.047* |
| III+IV (n=24) | 2 (8.33) | 22 (91.67) | 2 (8.33) | 22 (91.67) | ||
| Differentiation | ||||||
| High (n=41) | 10 (24.39) | 31 (75.61) | 0.01a | 15 (36.59) | 26 (63.41) | 0.004* |
| Middle (n=18) | 3 (16.67) | 15 (83.33) | 2 (11.11) | 16 (88.89) | ||
| Low (n=21) | 0 (0) | 21 (100) | 1 (4.76) | 20 (95.24) | ||
| Nodal status | ||||||
| No (n=65) | 11 (16.92) | 54 (83.08) | 0.729 | 16 (24.62) | 49 (75.38) | 0.323 |
| Yes (n=15) | 2 (13.33) | 13 (86.67) | 2 (13.33) | 13 (86.67) | ||
Indicate statistical significance. n, the number of cases. FIGO, International Federation of Gynecology and Obstetrics.
Overexpression of Lewis y in ovarian epithelial carcinoma and its association with c-Fos expression
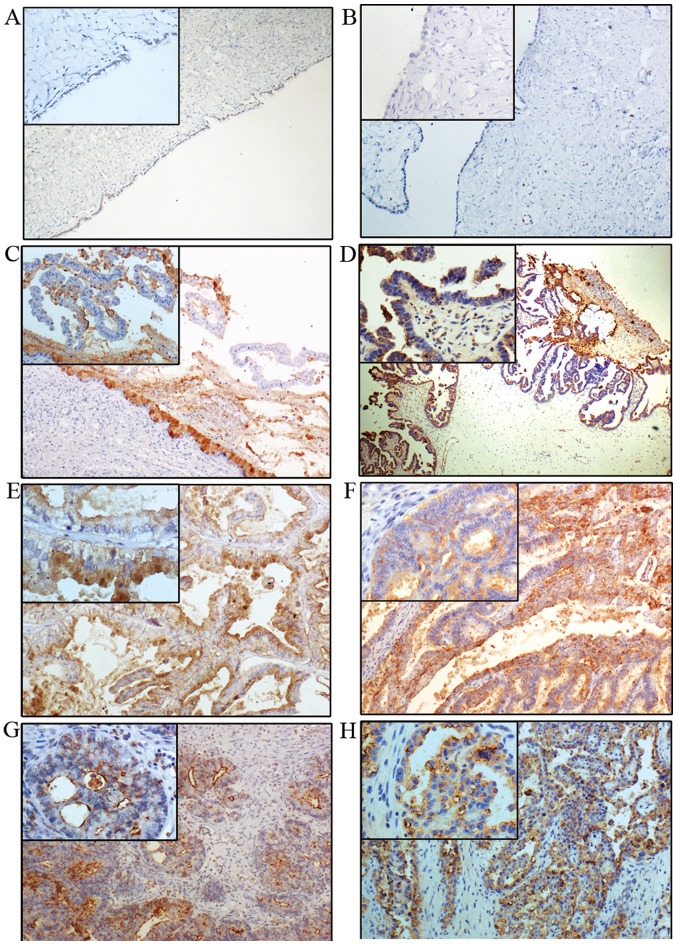
The expression of Lewis y is generally increased in ovarian epithelial carcinoma, exhibiting mainly cell membrane staining with occasional cytoplasm staining. Lewis y expression is barely detectable in normal ovarian tissue, and it was considered as low expression (−). The high expression rate of Lewis y (+/++/+++) in benign (Fig. 2A and B), borderline (Fig. 2C and D) and ovarian malignant tumor (Fig. 2E and F),) were 33.33% (10/30), 60% (18/30) and 83.75% (67/80), respectively. No expression was observed in 20 cases of normal ovarian tissue (Table III). The expression rate of Lewis y in ovarian epithelial cancer was significantly higher than that in borderline and benign tumors (P<0.05). The high expression rate of Lewis y in borderline ovarian tumors was higher than that in benign tumors (P<0.05).
Figure 2.
Immunohistochemical staining of Lewis y in ovarian tumors. (A) Mucinous, (B) serous, (C) borderline mucinous and (D) borderline serous cystadenoma. (E) Mucinous cystadenocarcinoma and (F) serous cystadenocarcinoma. (G) Endometrioid carcinoma and (H) clear cell carcinoma (original magnification ×100 and ×400 for the small box at the top-left corner).
Table III.
Expression of Lewis y in different ovarian tissues.
| Lewis y | |||
|---|---|---|---|
| Characteristics | Low n (%) | High n (%) | P-value |
| Malignant group (n=80) | 13 (16.25) | 67 (83.75) | <0.01a,b,c |
| Borderline group (n=30) | 12 (40.00) | 18 (60.00) | 0.038d, <0.01e |
| Benign group (n=30) | 20 (66.67) | 10 (33.33) | 0.003f |
| Normal group (n=20) | 20 (100.00) | 0 (0.00) | |
Compared with borderline group.
Compared with benign group.
Compared with normal tissue group. n, the number of cases.
The high expression rates of Lewis y in mucinous (Fig. 2E) and serous ovarian cancer tissues (Fig. 2F) were 86.67% (26/30) and 90% (27/30), respectively, which were higher than those in ovarian endometrioid (60%, 6/10) and clear cell carcinoma (80%, 8/10), but the difference was not statistically significant (P>0.05). The positive expression of Lewis y in stage III–IV ovarian cancer was 91.67% (22/24), higher than that in stage I–II (80.36%, 45/56), and there was no significant difference between the two groups (P>0.05). The expression rates of Lewis y in high, middle and low differentiation ovarian cancer were 75.61% (31/41), 83.33% (15/18) and 100% (21/21), respectively, with the high expression rate significantly increased with decreasing degree of differentiation (P<0.05). The expression of Lewis y was not correlated with lymph node metastasis (P>0.05) (Table II).
Of the 80 cases with ovarian cancer, 55 cases had high expression of both c-Fos and Lewis y, and 6 cases had double low expression. The expression of c-Fos and Lewis y in ovarian cancer exhibited significant correlation, with a correlation coefficient of 0.250, P<0.05 (Table IV).
Table IV.
Correlation between Lewis y and c-Fos in ovarian carcinoma.
| Lewis y expression | |||||
|---|---|---|---|---|---|
| Low (n) | High (n) | P-value | Correlation coefficient | ||
| c-Fos | Low (n) | 6 | 12 | 0.026a | 0.250 |
| expression | High (n) | 7 | 55 | ||
Indicate statistical significance. n, the number of cases.
Binding of c-Fos to TPA response element (TRE) of the FUT1 promoter enhances the activation of FUT1 transcription by c-Jun
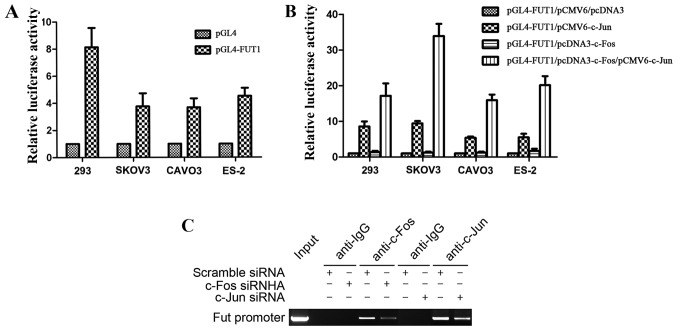
We previously constructed the FUT1 (−3,000 to −1) promoter luciferase reporter gene vector, which had one binding site for AP-1, and revealed that c-Jun transactivated FUT1 via the AP-1 binding site (22). The FUT1 promoter activity was first tested in 293 cells, and further confirmed in 3 ovarian cancer cell lines SKOV3, CAVO3 and ES-2, with the strongest enzyme activity achieved in 293 cells (Fig. 3A). Furthermore, to investigate the effect of c-Fos in the regulation of FUT1 transcription, we co-transfected ovarian cancer cells with the transcription factor expression vector c-Fos, c-Jun, c-Fos/c-Jun or empty vector (pCMV6 and pcDNA3) along with the human FUT1 promoter reporter gene. Compared with the empty vector transfection, c-Fos did not significantly affect the activity of the FUT1 promoter, whereas c-Jun transcription factor expression vector increased promoter activity. Compared with the single transfection of c-Jun expression vector, co-transfection with c-Fos and c-Jun expression vector significantly increased promoter activity, and the activity was increased 3.5-fold in SKOV3 cells (Fig. 3B). These results revealed that the transcriptional activation ability of the heterodimer formed by c-Fos and c-Jun was significantly enhanced in comparison with that of the homodimer with two c-Juns.
Figure 3.
Role of c-Fos in the regulation of FUT1. (A) The activity of human FUT1 promoter construct in 293 cells and ovarian cancer cells lines was detected. (B) The luciferase reporter assay of FUT1 promoter constructs in 293 cells and ovarian cancer cells. Cell lines 293, SKOV3, CAVO3 and ES-2 were co-transfected with pGL4-FUT1, with empty vectors, or pCMV6-c-Jun or pcDNA3-c-Fos or both. (C) ChIP assay detected c-Fos and c-Jun interacted with FUT1 promoter in CAVO3 cells. Data represent the mean ± SD of 3 independent experiments performed in triplicate; *P<0.05.
To determine whether c-Fos and c-Jun interacted with TRE of the FUT1 promoter (−1,908 to −1,914), ChIP assay was performed by transfection of c-Fos or c-Jun siRNA into CAVO3 cells. MNase cleavage was used to cut cell chromatin into 100–1,000 bp fragments, with an indwelling portion of the cell suspension used as the DNA input positive control, rabbit IgG precipitation as the negative control. In the input and c-Fos, c-Jun-specific antibody precipitation groups, PCR amplification obtained FUT1 promoter fragments of 147 bp, while the corresponding fragment was not amplified in the IgG precipitation group (Fig. 3C). The precipitated FUT1 promoter fragment expression was significantly decreased after c-Fos or c-Jun siRNA interference. These results revealed that c-Fos and c-Jun were bound to the FUT1 promoter region containing the AP-1 binding site.
c-Fos mediates TGF-β1-induced ovarian cancer cell proliferation
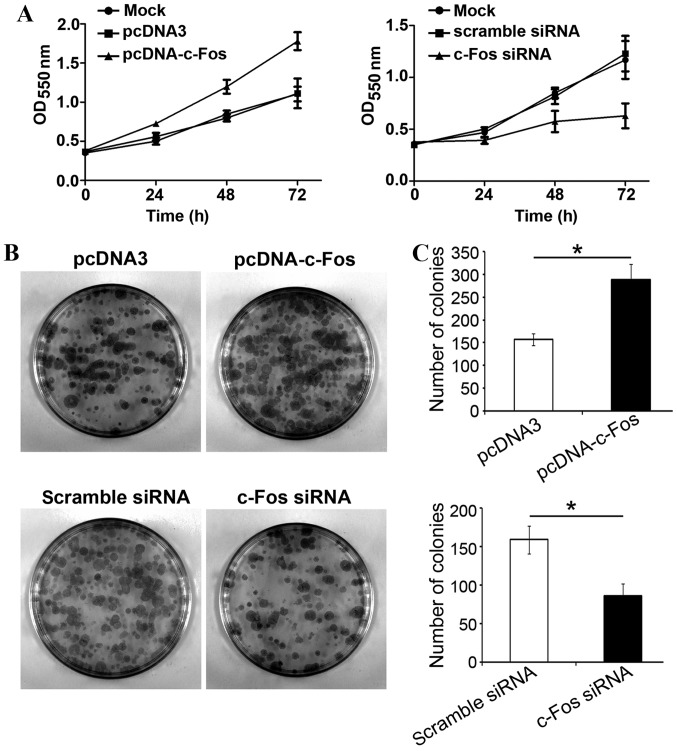
In order to examine whether c-Fos participated in TGF-β1-mediated proliferation of tumor cells, c-Fos or c-Fos siRNA were transfected into ovarian cancer CAVO3 cells, respectively. Upon TGF-β1 stimulation, cells were collected and inoculated onto a 96-well plate and 24 h later, cell proliferation status was analyzed. Transfection with c-Fos significantly increased CAVO3 proliferation with TGF-β1 treatment. Transfection with c-Fos siRNA strongly suppressed CAVO3 growth induced by TGF-β1 (P<0.05). The effects of both c-Fos and c-Fos siRNA were most significant on the third day (Fig. 4A). Likewise, the clone formation rate of ovarian cancer cells overexpressing c-Fos was significantly higher than that of cells transfected with the empty vector, and 1.8-folds that of the latter. The colony number of cells transfected with c-Fos siRNA was significantly reduced by 51% compared with that of the control group (Fig. 4C). Our results revealed that c-Fos was involved in TGF-β1-induced ovarian cancer cell proliferation.
Figure 4.
TGF-β1 promotes cell proliferation via c-Fos in ovarian cancer cells. (A) Cell viability was detected using MTT cell proliferation assay in CAVO3 cells. CAVO3 cells were transfected with pcDNA3-c-Fos, pcDNA3 or c-Fos siRNA for 24 h, followed by pretreatment with TGF-β1. (B and C) Colony formation assessed cell proliferation ability. The cells and processing were the same as shown in a. The data are expressed as the mean ± SD of 3 independent experiments; *P<0.05, compared with the control.
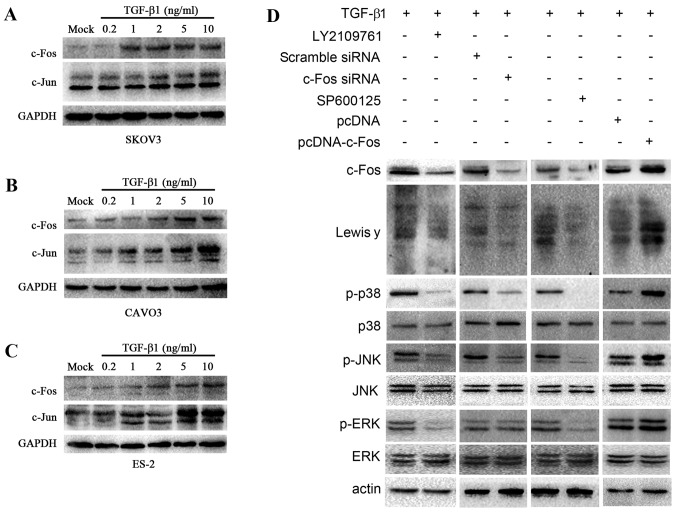
TGF-β1 activates c-Fos via the MAPK signaling pathway, regulates FUT1 transcription, and promotes Lewis y expression
TGF-β1 is positively correlated to Lewis y in ovarian cancer (35). To determine whether TGF-β1 increased AP-1 expression, we assessed c-Jun and c-Fos expression in response to TGF-β1 in 3 ovarian cancer cell lines. The ovarian cancer cells SKOV3, CAVO3 and ES-2 were treated with various concentrations of TGF-β1 (0.2, 1, 2, 5 and 10 ng/ml) for 5 min, and subsequently the change in c-Jun and c-Fos expression was detected by western blotting (Fig. 5A-C). TGF-β1 induced the expression of c-Jun and c-Fos in all 3 types of cells. In CAVO3 and SKOV3 cells, the expression of c-Fos and c-Jun was strongly dependent on the concentration of TGF-β1 used, and was highest when 10 ng/ml TGF-β1 was added. These results indicated that TGF-β1 induced the expression of c-Fos and c-Jun in ovarian cancer cells in a dose-dependent manner.
Figure 5.
TGF-β1 activates c-Fos through the MAPK/AP-1 pathway. Upon TGF-β1 treatment, western blotting was employed to determine c-Jun and c-Fos expression in 3 ovarian cells. (A) SKOV3, (B) CAVO3, (C) ES-2. c-Fos and c-Jun expression is related to TGF-β1 in a concentration manner in ovarian cells pretreated with various concentrations of TGF-β1 (0.2, 1, 2, 5 and 10 ng/ml) for 5 min. (D) Western blot analysis was carried out to determine the expression of c-Fos, Lewis y and the downstream signal elements of the MAPK signaling pathway, JNK, p38 and ERK, and the change in the phosphorylation level of JNK, p38 and ERK. CAVO3 cells were treated with pcDNA-c-Fos, c-Fos siRNA, inhibitor of TGF-β1 receptor (LY2109761) and JNK (SPF600125) upon TGF-β1 stimulation.
TGF-β1 and the members of TGF-β-independent MAPK signaling pathway act as key determinants of carcinoma cell behavior
AP-1 transcriptional activity is regulated by the activation of the MAPK signaling pathway, involving extracellular-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 pathways (38,39). To study the role of MAPK pathway in mediating TGF-β1-induced Lewis y expression, we applied specific JNK inhibitor (SP600125) and TGF-β receptor inhibitor (LY2109761). Upon TGF-β1 stimulation, CAVO3 was treated with LY2109761 and SP600125, respectively. Western blot analysis revealed that the expression of c-Fos, Lewis y, the phosphorylation levels of p38, JNK and ERK decreased significantly (Fig. 5D).
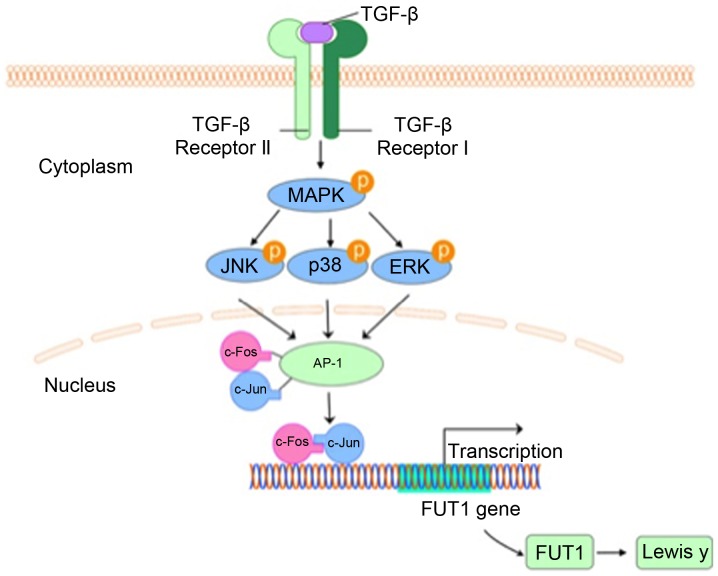
Furthermore, to address whether the increased expression of c-Fos contributed to Lewis y expression, we transfected CAVO3 cells with c-Fos and c-Fos-siRNA upon TGF-β1 stimulation. c-Fos transfection promoted TGF-β1-induced Lewis y expression and phosphorylated (p)-p38 and p-JNK. Consistent with these results, the silencing of c-Fos prevented TGF-β1-induced Lewis y expression and also suppressed p-p38 and p-JNK (Fig. 5D). Whereas, silencing or overexpression of c-Fos did not exert any effect on p-ERK. These results indicate that as a downstream effector of MAPK pathway, c-Fos is also capable of modulating p-p38 and p-JNK, but not p-ERK in response to TGF-β1. Collectively, our findings support that MAPK pathway palys a critical role in the mechanism of TGF-β1-induced Lewis y expression in ovarian cells (Fig. 6).
Figure 6.
Pathway of c-Fos-mediated Lewis y in response to TGF-β1. TGF-β1 activates c-Fos/AP-1 through the MAPK pathway. As a transcriptional factor, c-Fos together with c-Jun upregulated FUT1 expression by binding to its promoter, consequently leading to the enhanced synthesis of Lewis y.
Discussion
Lewis y antigen, a tumor-associated carbohydrate antigen, is overexpressed in malignant epithelial tumors and closely associated with the prognosis of the disease. Previously, in ovarian cancer cells, we identified an AP-1 binding site in the promoter of FUT1, which was a rate-limiting enzyme of Lewis y. AP-1, a dimer composed of Fos (c-Fos, Fos-B, Fra-1 and Fra-2) and Jun (c-Jun, Jun-B and Jun-D) family members, modulates gene transcription and cellular pathophysiological functions. It has been demonstrated that MMP9 promoter was activated by the IL-1β/p38/c-Fos pathway, which was correlated with gastric adenocarcinoma metastasis (40). Furthermore, c-Fos overexpression enhanced the epithelial-mesenchymal transition (EMT) state in head and neck squamous cell carcinoma (41). In the present study, we observed high levels of expression of c-Fos in 140 ovarian epithelial tumor cases. Overexpression of c-Fos in ovarian cancer was as high as 77.5% (62/80) and its expression rate and intensity increased with decreasing degree of differentiation. In malignant ovarian epithelial tumors, high expression of c-Fos was observed in both the nucleus and the cytoplasm. c-Fos expression was only detected in the nucleus in benign and normal ovarian tissues. These findings indicated that c-Fos expression was aberrantly upregulated in malignant ovarian tumors and significantly correlated with malignant behaviors.
TGF-β1 plays an important role in carcinogenesis and is associated with the proliferation and progression in advanced malignancies. TGF-β1 promotes tumor growth via regulating downstream targets of its signaling pathway. It was reported that c-Fos overexpression is positively correlated with carcinoma growth (42). Knockdown of c-Fos greatly suppressed tumor cell proliferation and invasion and downregulated CD44 and cyclin D1 expression (43). In the present study, we examined whether c-Fos was involved in TGF-β1-induced cell proliferation in ovarian cancer cells. These findings indicated that c-Fos expression was increased and contributed to the TGF-β1-induced tumor promoting effect in ovarian epithelial cells.
We previously revealed that c-Jun transactivated FUT1 through the AP-1 binding site in ovarian cancer cells (22). Thus, we wondered how c-Fos plays its role and how do the different components of AP-1 interact with each other in the regulation of FUT1 transcription. The underlying mechanism is still unclear. To answer this question, we performed ChIP assays to determine whether c-Fos and c-Jun bind to the FUT1 promoter. Furthermore, using luciferase activity analysis, we observed that the homodimer of c-Fos hardly influenced the activity of the FUT1 construct, suggesting that c-Fos activation alone was insufficient for the activation of FUT1 promoter activity. The activity of FUT1 promoter interacted with heterodimer of c-Fos and c-Jun increased 3.5-folds, compared with interaction with c-Jun alone, further illustrating the interaction and coordination between c-Fos and c-Jun. It was reported that c-Fos could not bind to TRE, but the DNA binding force of the heterodimer formed by c-Fos and c-Jun was 25 times that of the homodimer with the two c-Juns (44). However, DebRoy et al reported that the p38 pathway induced stoma interaction molecule 1 (STIM1) expression through c-Fos rather than c-Jun in endothelial cells (45). Collectively, these results revealed that co-presence and cooperation of c-Fos and c-Jun are essential in regulating FUT1 promoter activity in ovarian cancer cells.
In the present study it was also demonstrated that in the absence of TGF-β1 or stimulation with low concentrations of TGF-β1, the c-Fos protein expression level in ovarian cancer cells was significantly lower than that of c-Jun, however with increased TGF-β1 concentration, the protein expression levels of c-Fos significantly increased, reaching or even exceeding the expression level of c-Jun. The protein concentration and activity of c-Fos are extremely low under normal circumstances, while c-Jun has strong potential transcriptional competence. When cells are stimulated, levels of c-Fos protein are temporarily and quickly increased. Fos/Jun heterodimers instead of stable homodimers are formed, with rapid increased DNA binding and transcription-inducing ability. Following degradation of c-Fos which has a short half-life, AP-1 returns to basal levels and an inert state (46,47). Previous studies demonstrated that c-Fos was highly expressed in cervical cancer, and almost never expressed in normal tissues and precancerous lesions (48). c-Fos expression was upregulated following TGF-β1 treatment in immortalized liver cells, which was correlated with cell migration and invasion (49). Therefore, we proposed that increased TGF-β1 promoted expression of c-Fos and the formation of heterodimers, and upregulated FUT1 transcription. Inflammatory cytokines regulated the expression of FUTs involved in the biosynthesis of tumor-associated sialylated Lewis antigen in pancreatic cancer cells (50). To elaborate whether c-Fos is activated with TGF-β1 treatment and whether c-Fos promotes FUT1 and Lewis y synthesis in ovarian epithelial cancer, we focused on the MAPK signaling pathway. Inhibition of the JNK pathway with pharmacologic inhibitor resulted in marked reduction in c-Fos and Lewis y expression in ovarian cancer cells upon TGF-β1 stimulation. The JNK pathway inhibitor not only suppressed JNK phosphorylation, but also downregulated the phosphorylation level of p38 and ERK. Therefore, the transcriptional regulation is likely to involve the crosstalk between the JNK, p38 and ERK pathways in response to TGF-β1 stimulation. To determine whether there is a relationship between c-Fos and Lewis y expression, we knocked down c-Fos and examined the resultant expression of Lewis y. The result revealed that Lewis y expression was positively related to c-Fos expression. In addition, we observed that knockdown of c-Fos also diminished the phosphorylation of JNK and p38. It has been reported that TGF-β1 promoter regions had an AP-1 binding site, where c-Jun and c-Fos are essential (51–54). TGF-β1 can activate its own mRNA transcription, promoting its own secretion. The expression of TGF-β1 was significantly increased in osteosarcoma tissues of c-Fos transgenic mice and c-Fos transfected osteosarcoma cell lines (55). These data indicated that there may be mutual regulation between TGF-β1 and AP-1 through the JNK and p38 pathways. As our results revealed, the MAPK pathway plays a vital role in Lewis y expression induced by c-Fos in response to TGF-β1.
In conclusion, we demonstrated overexpression of c-Fos and its positive correlation with Lewis y in ovarian epithelial carcinoma. The transcriptional activity of the heterodimer formed by c-Fos and c-Jun was stronger than that of the homodimer with two c-Juns in FUT1 promoter activation. Notably, we revealed that TGF-β1 activated c-Fos via the MAPK pathway, promoting FUT1 transcription, and enhancing Lewis y biosynthesis in ovarian cancer cells.
In conclusion, the results of our studies in combination with those of other researchers, suggest that in ovarian cancer cells, TGF-β1 activates c-Fos through the MAPK signaling pathway, regulates FUT1 transcription, and eventually promotes Lewis y expression.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (nos. 81072118, 81172491, 81101527, 81472437 and 81402129), the Research Fund for Doctoral Program of Higher Education of China (nos. 20112104110016 and 20112104120019), and the Outstanding Scientific Fund of Shengjing Hospital (201303).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Salani R, Bristow RE. Surgical management of epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:75–95. doi: 10.1097/GRF.0b013e31824b4629. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaczmarek R. Alterations of Lewis histo-blood group antigen expression in cancer cells. Postepy Hig Med Dosw (Online) 2010;64:87–99. (In Polish) [PubMed] [Google Scholar]
- 5.Iwamori M, Iwamori Y, Kubushiro K, Ishiwata I, Kiguchi K. Characteristic expression of Lewis-antigenic glycolipids in human ovarian carcinoma-derived cells with anticancer drug-resistance. J Biochem. 2007;141:309–317. doi: 10.1093/jb/mvm031. [DOI] [PubMed] [Google Scholar]
- 6.Gao S, Liu Q, Wang X, Lin B, Zhang S. Effects of Lewis y antigen on the gene expression of multiple drug resistance-associated proteins in human ovarian cancer RMG-I-H cells. Med Oncol. 2010;27:960–967. doi: 10.1007/s12032-009-9317-6. [DOI] [PubMed] [Google Scholar]
- 7.Ricardo S, Marcos-Silva L, Valente C, Coelho R, Gomes R, David L. Mucins MUC16 and MUC1 are major carriers of SLea and SLex in borderline and malignant serous ovarian tumors. Virchows Arch. 2016;468:715–722. doi: 10.1007/s00428-016-1929-6. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima K, Satoh T, Baba S, Yamashita K. α1,2-Fucosylated and β-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology. 2010;20:452–460. doi: 10.1093/glycob/cwp197. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Liu S, Lin B, Yan L, Wang Y, Wang C, Zhang S. Expression and correlation of Lewis y antigen and integrins α5 and β1 in ovarian serous and mucinous carcinoma. Int J Gynecol Cancer. 2010;20:1482–1489. doi: 10.1111/IGC.0b013e3181ea7ecb. [DOI] [PubMed] [Google Scholar]
- 10.Madjd Z, Parsons T, Watson NF, Spendlove I, Ellis I, Durrant LG. High expression of Lewisy/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res. 2005;7:R780–R787. doi: 10.1186/bcr1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakabayashi M, Shiro T, Seki T, Nakagawa T, Itoh T, Imamura M, Shiozaki Y, Inoue K, Okamura A. Lewis y antigen expression in hepatocellular carcinoma. An immunohistochemical study. Cancer. 1995;75:2827–2835. doi: 10.1002/1097-0142(19950615)75:12<2827::AID-CNCR2820751207>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Larsen RD, Ernst LK, Nair RP, Lowe JB. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci USA. 1990;87:6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins WM. Biochemistry and genetics of the ABO, Lewis, and P blood group systems. Adv Hum Genet. 1980;10(1–136):379–185. doi: 10.1007/978-1-4615-8288-5_1. [DOI] [PubMed] [Google Scholar]
- 14.Oriol R. Genetic control of the fucosylation of ABH precursor chains. Evidence for new epistatic interactions in different cells and tissues. J Immunogenet. 1990;17:235–245. doi: 10.1111/j.1744-313X.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RJ, Ernst LK, Larsen RD, Bryant JG, Robinson JS, Lowe JB. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc Natl Acad Sci USA. 1994;91:5843–5847. doi: 10.1073/pnas.91.13.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai S, Kato S, Imai H, Okada Y, Ishioka C. Suppression of FUT1 attenuates cell proliferation in the HER2-overexpressing cancer cell line NCI-N87. Oncol Rep. 2013;29:13–20. doi: 10.3892/or.2012.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao YY, Lin B, Zhao Y, Zhang YH, Li FF, Diao B, Ou YL, Zhang SL. Alpha1,2-fucosyltransferase gene transfection influences on biological behavior of ovarian carcinoma-derived RMG-I cells. FFen Zi Xi Bao Sheng Wu Xue Bao. 2008;41:435–442. (In Chinese) [PubMed] [Google Scholar]
- 18.Liu Q, Lin B, Wang PL, Yan LM, Hao YY, Li FF, Zhu LC, Zhang SL. Effect of Lewis y antigen on regulating gene expression of partial drug resistance associated proteins in human ovarian cancer cell line RMG-I-H. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31:481–487. (In Chinese) [PubMed] [Google Scholar]
- 19.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZG, Jiang G, Tang J, Wang H, Feng G, Chen F, Tu Z, Liu G, Zhao Y, Peng MJ, et al. c-Fos over-expression promotes radioresistance and predicts poor prognosis in malignant glioma. Oncotarget. 2016;7:65946–65956. doi: 10.18632/oncotarget.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briso EM, Guinea-Viniegra J, Bakiri L, Rogon Z, Petzelbauer P, Eils R, Wolf R, Rincón M, Angel P, Wagner EF. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013;27:1959–1973. doi: 10.1101/gad.223339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao N, Liu J, Liu D, Hao Y, Yan L, Ma Y, Zhuang H, Hu Z, Gao J, Yang Z, et al. c-Jun transcriptionally regulates alpha 1, 2-fucosyltransferase 1 (FUT1) in ovarian cancer. Biochimie. 2014;107(Pt B):286–292. doi: 10.1016/j.biochi.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 24.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto A, Okitaka M, Takagi S, Takami M, Sato S, Nishio M, Okumura S, Fujita N. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep. 2017;7:42186. doi: 10.1038/srep42186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Liu H, Gong XL, Wu LY, Wen B. Effect of evodiamine and berberine on the interaction between DNMTs and target microRNAs during malignant transformation of the colon by TGF-β1. Oncol Rep. 2017;37:1637–1645. doi: 10.3892/or.2017.5379. [DOI] [PubMed] [Google Scholar]
- 27.Witte D, Zeeh F, Gädeken T, Gieseler F, Rauch BH, Settmacher U, Kaufmann R, Lehnert H, Ungefroren H. Proteinase-activated receptor 2 is a novel regulator of TGF-β signaling in pancreatic cancer. J Clin Med. 2016;5:5. doi: 10.3390/jcm5120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ru NY, Wu J, Chen ZN, Bian H. HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal transition and hepatocellular carcinoma invasion. Cell Biol Int. 2015;39:44–51. doi: 10.1002/cbin.10341. [DOI] [PubMed] [Google Scholar]
- 29.Rafehi S, Ramos Valdes Y, Bertrand M, McGee J, Préfontaine M, Sugimoto A, DiMattia GE, Shepherd TG. TGFβ signaling regulates epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr Relat Cancer. 2016;23:147–159. doi: 10.1530/ERC-15-0383. [DOI] [PubMed] [Google Scholar]
- 30.Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AE, et al. A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res. 2014;20:711–723. doi: 10.1158/1078-0432.CCR-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsina-Sanchis E, Figueras A, Lahiguera Á, Vidal A, Casanovas O, Graupera M, Villanueva A, Viñals F. The TGFβ pathway stimulates ovarian cancer cell proliferation by increasing IGF1R levels. Int J Cancer. 2016;139:1894–1903. doi: 10.1002/ijc.30233. [DOI] [PubMed] [Google Scholar]
- 32.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ST, Liu JJ, Wang CZ, Lin B, Hao YY, Wang YF, Gao S, Qi Y, Zhang SL, Iwamori M. Expression and correlation of Lewis y antigen and TGF-β1 in ovarian epithelial carcinoma. Oncol Rep. 2012;27:1065–1071. doi: 10.3892/or.2011.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Yu L, Hishikawa Y, Yamanoi A, Kubota H, Nagasue N. Growth kinetic study of human hepatocellular carcinoma using proliferating cell nuclear antigen and Lewis y antigen: Their correlation with transforming growth factor-alpha and beta 1. Oncology. 1997;54:245–251. doi: 10.1159/000227696. [DOI] [PubMed] [Google Scholar]
- 37.Bai G, Hock TD, Logsdon N, Zhou Y, Thannickal VJ. A far-upstream AP-1/Smad binding box regulates human NOX4 promoter activation by transforming growth factor-β. Gene. 2014;540:62–67. doi: 10.1016/j.gene.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 39.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]
- 40.Huang Q, Lan F, Wang X, Yu Y, Ouyang X, Zheng F, Han J, Lin Y, Xie Y, Xie F, et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 2014;13:18. doi: 10.1186/1476-4598-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muhammad N, Bhattacharya S, Steele R, Phillips NJ, Ray RB. Involvement of c-Fos in the promotion of cancer stem-like cell properties in head and neck squamous cell carcinoma. Clin Cancer Res. 2017;23:3120–3128. doi: 10.1158/1078-0432.CCR-16-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motrich RD, Castro GM, Caputto BL. Old players with a newly defined function: Fra-1 and c-Fos support growth of human malignant breast tumors by activating membrane biogenesis at the cytoplasm. PLoS One. 2013;8:e53211. doi: 10.1371/journal.pone.0053211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong C, Ye DX, Zhang WB, Pan HY, Zhang ZY, Zhang L. Overexpression of c-fos promotes cell invasion and migration via CD44 pathway in oral squamous cell carcinoma. J Oral Pathol Med. 2015;44:353–360. doi: 10.1111/jop.12296. [DOI] [PubMed] [Google Scholar]
- 44.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988;55:917–924. doi: 10.1016/0092-8674(88)90147-X. [DOI] [PubMed] [Google Scholar]
- 45.DebRoy A, Vogel SM, Soni D, Sundivakkam PC, Malik AB, Tiruppathi C. Cooperative signaling via transcription factors NF-κB and AP1/c-Fos mediates endothelial cell STIM1 expression and hyperpermeability in response to endotoxin. J Biol Chem. 2014;289:24188–24201. doi: 10.1074/jbc.M114.570051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basbous J, Jariel-Encontre I, Gomard T, Bossis G, Piechaczyk M. Ubiquitin-independent-versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: Is there a unique answer? Biochimie. 2008;90:296–305. doi: 10.1016/j.biochi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 48.Prusty BK, Das BC. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int J Cancer. 2005;113:951–960. doi: 10.1002/ijc.20668. [DOI] [PubMed] [Google Scholar]
- 49.Güller MC, André J, Legrand A, Setterblad N, Mauviel A, Verrecchia F, Daniel F, Bernuau D. c-Fos accelerates hepatocyte conversion to a fibroblastoid phenotype through ERK-mediated upregulation of paxillin-Serine178 phosphorylation. Mol Carcinog. 2009;48:532–544. doi: 10.1002/mc.20492. [DOI] [PubMed] [Google Scholar]
- 50.Bassagañas S, Allende H, Cobler L, Ortiz MR, Llop E, de Bolós C, Peracaula R. Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine. 2015;75:197–206. doi: 10.1016/j.cyto.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/MCB.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy S, Khanna S, Azad A, Schnitt R, He G, Weigert C, Ichijo H, Sen CK. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc Res. 2010;87:647–655. doi: 10.1093/cvr/cvq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med. 2009;13:1866–1876. doi: 10.1111/j.1582-4934.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SJ, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989;264:402–408. [PubMed] [Google Scholar]
- 55.Kunita A, Kashima TG, Ohazama A, Grigoriadis AE, Fukayama M. Podoplanin is regulated by AP-1 and promotes platelet aggregation and cell migration in osteosarcoma. Am J Pathol. 2011;179:1041–1049. doi: 10.1016/j.ajpath.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]