Abstract
High-mobility group AT-hook 2 (HMGA2), a member of the high mobility group family, has been reported to correlate with cancer progression. However, there is no report concerning the correlation between HMGA2 and metastasis in renal cell carcinoma. In the present study, we found that HMGA2 was highly expressed in five renal cell carcinoma cell lines compared with that in the normal renal tubular epithelial HK2 cell line. Additionally, HMGA2 facilitated cell migration and invasion of renal cell carcinoma cells, as evidenced by wound healing and Transwell assays. Subsequently, our results revealed that the E-cadherin level was upregulated, while N-cadherin, Twist1 and Twist2 expression were downregulated in HMGA2-depleted ACHN cells. In contrast, overexpression of HMGA2 in 786-O cells enhanced epithelial-mesenchymal transition (EMT). In addition, analysis of the database Cancer Browser further validated the positive correlation between HGMA2 and Twist1 or Twist2 in renal cell carcinoma. Meanwhile, Kaplan-Meier analysis indicated that low HMGA2 expression was closely associated with an increased overall survival in renal cell carcinoma patients. To confirm the underlying mechanism of HMGA2-regulated EMT, our results revealed that silencing of HMGA2 downregulated the mRNA and protein levels of TGF-β and Smad2, while HMGA2 overexpression had the opposite effect. Furthermore, TGF-β overexpression could partially reverse the anti-metastatic effect and mesenchymal-epithelial transition (MET) by HMGA2 loss, while TGF-β deficiency impeded the pro-metastatic phenotype and high expression of EMT markers induced by HMGA2 overexpression. In summary, our results demonstrated that HMGA2 facilitated a metastatic phenotype and the EMT process in renal cell carcinoma cells in vitro through a TGF-β-dependent pathway. In addition, these data strongly suggest that HGMA2 may serve as a potential therapeutic target and prognostic biomarker against renal cell carcinoma in the future.
Keywords: HMGA2, renal cell carcinoma, epithelial-mesenchymal transition, TGF-β
Introduction
Renal cell carcinoma is the most common malignant tumor of the kidney, accounting for almost 3% of all human malignancies (1). Insensitivity to radiotherapy and chemotherapy are a great obstacle for the treatment of metastatic renal cell carcinoma. Epithelial-mesenchymal transition (EMT), a hallmark of metastasis, is a complicated process by which cells lose epithelial characteristics and acquire a mesenchymal phenotype (2). In the process of EMT, cancer cells escape from the primary site and invade to distant tissues through blood and lymphatic vessels. In addition, an EMT phenotype is often accompanied by the downregulation of epithelial markers E-cadherin and zonuela occludens 1 (ZO-1), and upregulation of mesenchymal markers such as N-cadherin, Twsit1 and Twist2, resulting in enhanced motility (3,4). Furthermore, the expression of E-cadherin or vimentin has been reported to be associated with tumor progression and overall survival (OS) in a variety of cancers, including lung cancer and nasopharyngeal carcinoma (5,6). Additionally, Slug and Snail are found to downregulate E-cadherin by binding to the promoter of E-cadherin (7). In view of this, there is an urgent need to identify potential therapeutic targets against renal cell carcinoma.
High-mobility group AT-hook 2 (HMGA2), a member of the high mobility group family, is a small non-histone nuclear-binding protein, which contains three AT-hook structural domains and an acid C-terminal tail. It has been reported that HMGA2 participates in proliferation and differentiation during embryonic development (8). In addition, HMGA2 was found to be overexpressed in various types of tumors, including ovarian cancer, hepatocellular carcinoma and gliomas (9–11). Moreover, a high level of HMGA2 was correlated with poor prognosis in breast cancer and lung cancer patients (12,13). Although numerous studies have validated that HMGA2 may play a crucial role in tumor progression, only few studies have shown a correlation between HMGA2 and renal cell carcinoma. In addition, the underlying role of HMGA2 in renal cell carcinoma has not yet been elucidated.
In the present study, we focused on the role of HMGA2 in cell migration, invasion and EMT of renal cell carcinoma and revealed the possible mechanism by which HMGA2 regulates EMT in vitro.
Materials and methods
Reagents
Rabbit monoclonal antibodies against HMGA2 (8179), E-cadherin (E-Ca; 3195), N-cadherin (N-Ca; 13116), Twist1 (46702), TGF-β (3709) phosphorylated-Smad2 (p-Smad2; 3108), Smad2 (5339), Gli1 (3538), phosphorylated-β-catenin (p-β-catenin; 4176) and β-actin (4970) were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Rabbit polyclonal antibody against Twist2 (ab66031) was purchased from Abcam (Cambridge, UK). The appropriate peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG secondary antibodies were purchased from Zhongshan Biotech (Beijing, China). ACHN stably transfected with sh-HMGA2, 786-O stably transfected with HMGA2 (OE-HMGA2), and their respective corresponding empty vector control sublines (ACHN-scramble, 786-O-vector) were previously constructed.
Cell lines and culture
Human renal tubular epithelial HK2 cell line, and five renal cell carcinoma cell lines 786-O, 769-P, OSRC-2, ACHN and Caki-1 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were maintained in RPMI-1640 medium and supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 µg/ml streptomycin and 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2.
Western blotting
For western blot analysis, cells were washed with phosphate-buffered saline (PBS) after the specific treatment, and proteins were extracted using a lysis buffer [10 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/l ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol tetraacetic acid, 0.3 mmol/l phenylmethylsulfonyl fluoride, 0.2 mmol/l sodium orthovanadate, 1% NP-40, 10 mg ml/1 leupeptin, and 10 mg/ml aprotinin]. Equal amounts of protein lysates (~40–60 µg) were loaded onto a 10 or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) for 2 h. The membranes were then blocked with 5% non-fat milk, and incubated with primary antibodies (1:1,000) against E-cadherin (E-Ca), N-cadherin (N-Ca), Twist1, Twist2, TGF-β, p-Smad2, Smad2, Gli1, p-β-catenin and β-actin at 4°C overnight. The membranes were subsequently washed with TBST (Tris-buffered saline with Tween) buffer and incubated with corresponding secondary antibodies (1:1,000) at room temperature (25°C) for 1 h. Ultimately, the bands were visualized by an enhanced chemiluminescence kit (Bio-Rad, Hercules, CA, USA) and analyzed using Image Lab 4.0 (Bio-Rad) imaging software.
Wound healing assay
Cells were seeded onto 6-well plates. After the cell density reached to complete cell culture medium, scratch wounds were made across the monolayer with the tip of a 200-µl pipette. Subsequently, the wounded cultures were visualized in serum-free medium at 0 and 24 h, and images (×100) were then captured by inverted microscopy (Olympus IX50; Olympus, Tokyo, Japan) to detect the migratory ability. The experiments were performed in triplicate.
Cell migration assay
The cell migration ability of renal cell carcinoma cells following different treatments was assessed using Transwell migration assay. Cells (ACHN, 2×104; 786-O, 1.5×104) in 200 µl serum-free medium were seeded into the upper chambers, and 800 µl of 10% fetal calf serum-containing medium was used as a chemoattractant in the lower chamber. After incubation for 24 h, the cells which migrated onto the bottom of the filter were then fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet (Beyotime, Shanghai, China). Cells were then washed and photographed in five independent visual fields using an inverted microscope (Olympus IX50; Olympus) at ×100 magnification.
Matrigel invasion assay
Cell invasive ability was detected by Matrigel invasion assay using a Millicell chamber (Millipore, Billerica, MA, USA). The Transwell chambers were precoated with 50 µl mixture (Matrigel:serum-free medium 1:5) and the stable clone cell lines (ACHN, 5×104; 786-O, 4×104) in 200 µl serum-free medium were seeded onto the upper chamber for 24 h according to the instructions of the Transwell migration assay. The difference between two groups was analyzed by Sudent's t-test (two-sided).
Quantitative real-time PCR assay
Total cellular RNA was extracted using TRIzol reagent (Invitrogen). The RNA sample was reversely transcribed by PrimerScript RT reagent kit (Takara, Dalian, China). Then the relative levels of target gene messenger RNA (mRNA) were evaluated by quantitative real-time PCR assay (qPCR) using FAST SYBR-Green Master Mix with the gene-specific primers: E-cadherin (119 bp) forward, 5′-CGAGAGCTACACGTTCACGG-3′; and reverse, 5′-GGGTGTCGAGGGAAAAATAGG-3′; N-cadherin (94 bp) forward, 5′-TCAGGCGTCTGTAGAGGCTT-3′ and reverse, 5′-ATGCACATCCTTCGATAAGACTG-3′; Twist1 (156 bp) forward, 5′-GTCCGCAGTCTTACGAGGAG-3′ and reverse, 5′-GCTTGAGGGTCTGAATCTTGCT-3′; Twist2 (200 bp) forward, 5′-GGAGTCCGCAGTCTTACGAG-3′; and reverse, 5′-TCTGGAGGACCTGGTAGAGG-3′; TGF-β (201 bp) forward, 5′-GGCCAGATCCTGTCCAAGC-3′ and reverse, 5′-GTGGGTTTCCACCATTAGCAC-3′; Smad2 (182 bp) forward, 5′-CGTCCATCTTGCCATTCACG-3′; and reverse, 5′-CTCAAGCTCATCTAATCGTCCTG-3′; β-actin (250 bp) forward, 5′-CATGTACGTTGCTATCCAGGC-3′; and reverse 5′-CTCCTTAATGTCACGCACGAT-3′. The n-fold change in mRNA expression was analyzed according to the method of 2−∆∆Ct.
Plasmid transfection
TGF-β cDNA was cloned into the pcDNA3.1 vector. The plasmid was transfected into renal cell carcinoma ACHN cells with sh-HMGA2 or 768-O cells with HMGA2 overexpression by Bo Kou using X-treme GENE HP DNA transfection reagent (Roche, Germany) for 48 h according to the manufacturer's instructions, and subsequently prepared for the mechanistic research.
Statistical analysis
All experimental data were analyzed by GraphPad Prism (San Diego, CA, USA) software and presented as means ± SD. Differences between two groups were analyzed by Student's t-test (two-sided). The correlations between HGMA2 and Twist1 or Twist2 were analyzed by Spearman's rank test. In addition, overall survival (OS) was assessed by log-rank test, while a Kaplan-Meier curve was generated. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of HMGA2 in human renal epithelial cells and renal cell carcinoma cell lines
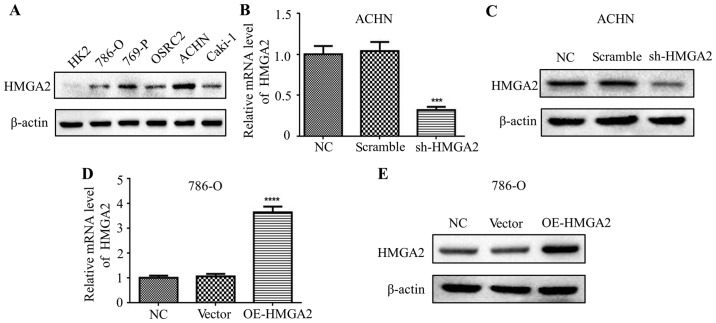
Firstly, we detected the expression of HMGA2 in several renal cell carcinoma cell lines using western blotting. The results demonstrated that HMGA2 was elevated in all renal cell carcinoma cell lines, compared with that noted in the human renal tubular epithelial HK2 cell line (Fig. 1A). Among these cell lines, HMGA2 was the most highly expressed in the renal cell carcinoma ACHN cell line, whereas it was lowly expressed in the 786-O cell line. In view of this, we constructed stable clone cell lines with knockdown or overexpression of HMGA2. In addition, we explored the HMGA2 expression level in ACHN cells with HMGA2 knockdown by qPCR and western blotting, and validated a significant decrease in HMGA2 expression in the sh-HMGA2 ACHN cells compared with that in the scramble or negative control (Fig. 1B and C). Conversely, the expression of HMGA2 was significantly increased in the 786-O cells overexpressing HMGA2 (OE-HMGA2) in comparison with that noted in cells transfected with the vector or negative control, as evidenced by qPCR and western blotting (Fig. 1D and E).
Figure 1.
Expression of HMGA2 in human renal epithelial cells and renal cell carcinoma cell lines. (A) Western blotting was used to detect the protein level of HMGA2 in the HK2 cell line and 5 renal cell carcinoma cell lines, respectively. The HMGA2 levels in ACHN cells transfected with with negative control, scramble, sh-HMGA2 (B and C), and 786-O cells transfected with the negative control, vector, OE-HMGA2 (D and E) were determined by qPCR and western blotting. Quantification from three independent experiments is shown with error bars representing standard deviation (SD). ***P<0.001 and ****P<0.0001.
HMGA2 regulates the metastatic phenotype of renal cell carcinoma
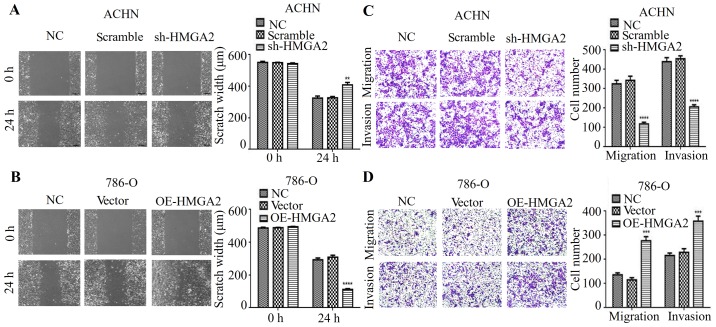
Studies showed that HMGA2 is associated with cancer metastasis in a variety of cancers (14,15). Thus, we hypothesized that HMGA2 is implicated in the migration and invasion of human renal cell carcinoma. To verify this hypothesis, wound healing and Transwell migration assays with statistical quantification were used. As expected, our results indicated that HMGA2 knockdown had a much wider scratch width and restrained cell migration when compared to the negative control or scramble in ACHN cell line (Fig. 2A and C). In contrast, HMGA2 overexpression promoted cell migration of renal cell carcinoma (Fig. 2B and D). Next, we determined whether HMGA2 affects invasive ability in vitro. As shown in Fig. 2C, the silencing of HMGA2 expression decreased the number of invaded cells, while overexpressing HMGA2 increased the invasive property of 786-O cell line compared with negative control (Fig. 2D). These data indicated that HMGA2 knockdown inhibited the metastatic phenotype of human renal cell carcinoma cells in vitro.
Figure 2.
HMGA2 regulates the metastatic phenotype of renal cell carcinoma cells. The ability of cell migration was evaluated by wound healing assay, and is expressed as the number of migrated cells. Representative photographs and statistical charts are shown for the HMGA2-knockdown ACHN (A) and HMGA2-overexpressing 786-O (B) cells compared with their corresponding control, respectively. Quantification from three independent experiments is shown with error bars representing standard deviation (SD). **P<0.01 and ****P<0.0001. Transwell migration and Matrigel invasion assays were used for the analysis of the migrated or invaded cells in the ACHN cells with HGMA2 knockdown (C) and 786-O with HMGA2 overexpression (D), respectively. Quantification from three independent experiments is shown with error bars representing standard deviation (SD). ***P<0.001 and ****P<0.0001.
HMGA2 regulates the EMT of renal cell carcinoma
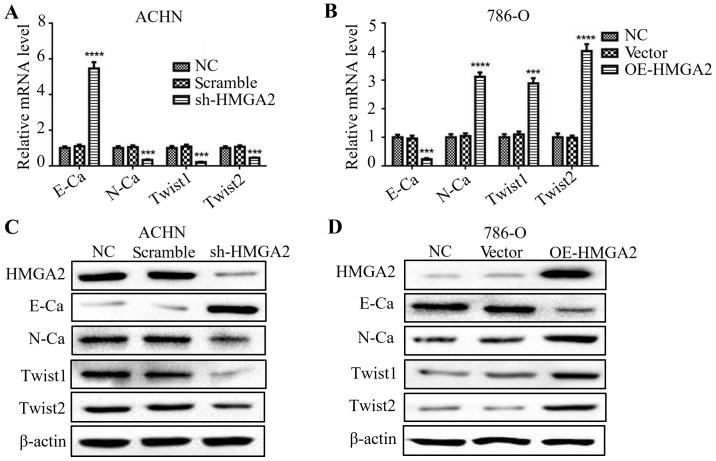
Several studies have stated that HMGA2 is involved in the process of EMT (16,17). To validate the function of HMGA2 in the EMT process of renal cell carcinoma cells, we evaluated the levels of both epithelial and mesenchymal markers by qPCR and western blotting. The deficiency in HMGA2 presented an elevated level of E-cadherin, while a reduction in N-cadherin, Twist1 and Twist2 (Fig. 3A and C). Conversely, 786-O cells with HMGA2 overexpression exhibited a significant decline in E-cadherin level, while an increase in N-cadherin, Twist1 and Twist2. (Fig. 3B and D). These results indicated that knockdown of HMGA2 reversed the EMT of human renal cell carcinoma.
Figure 3.
HMGA2 regulates the EMT of renal cell carcinoma cells. qPCR and western blotting were used to detect the expression of E-cadherin (E-Ca), N-cadherin (N-Ca), Twist1, Twist2 and β-actin in ACHN cells with HMGA2 knockdown (A and C) and 786-O cells with HMGA2 overexpression (B and D). Quantification from three independent experiments is shown as mean ± standard deviation (SD). Representative protein bands from three experiments are shown. ***P<0.001 and ****P <0.0001.
Correlation between HMGA2 and EMT markers based on Cancer Browser database
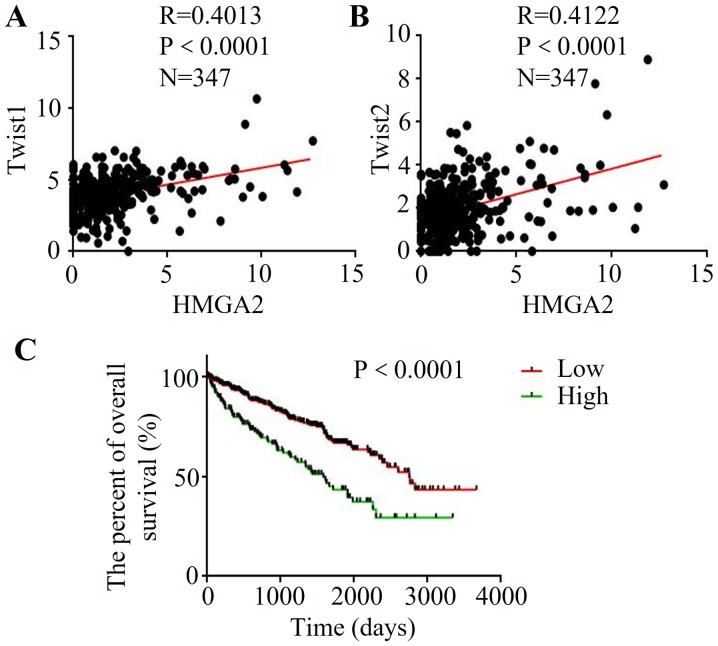
To further explore the association between HMGA2 and EMT in renal cell carcinoma, the clinical data and gene expression from database Cancer Browser (TCGA_KIRC_exp_HiSeqV2-2015-02-24) were extracted. The results demonstrated that high expression of HMGA2 was correlated with increased Twist1 expression (R=0.0.4013, P<0.0001) (Fig. 4A). Meanwhile, HMGA2 expression was positively correlated with the Twist2 level in renal cell carcinoma (R=0.0.4122, P<0.0001) (Fig. 4B). Then, we used Kaplan-Meier analysis to evaluate the prognostic value of HMGA2 in renal cell carcinoma. Importantly, the low HMGA2 patient group had a better OS than that of the high-expression group (Fig. 4C), indicating that high HMGA2 may be a poor prognostic predictor of renal cell carcinoma.
Figure 4.
Expression of HMGA2 is correlated with EMT markers and overall survival (OS) in renal cell carcinoma. Correlation between HMGA1 and Twist1 (A) or Twist2 (B) mRNA level in renal cell carcinoma based on a public available database (Cancer Browser, TCGA_KIRC_exp_HiSeqV2-2015-02-24). P-values were analyzed by Spearman's rank test. (C) The expression of HMGA2 is associated with OS using Kaplan-Meier analysis based on Cancer Browser database (TCGA_KIRC_exp_HiSeqV2-2015-02-24). x-axis indcates OS time (days) in patients with renal cell carcinoma, and the y-axis indicates the percentage of OS. P-value was analyzed by the log-rank test.
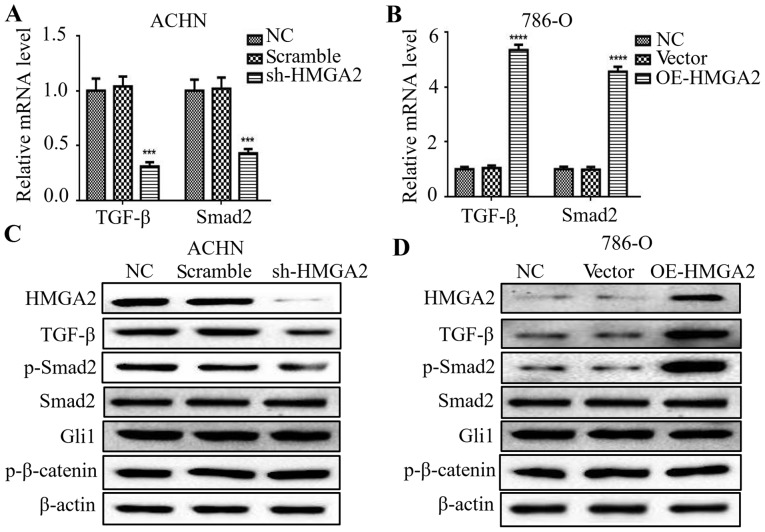
Silencing of HMGA2 decreases TGF-β and Smad2 expression in renal cell carcinoma
Previous studies have reported that the EMT process is governed by various regulatory networks, such as TGF-β, Wnt and Hedgehog signaling. To clarify the correlation among HMGA2 and several signals in renal cell carcinoma, we firstly evaluated that the change in TGF-β-, Wnt- and Hedgehog-related markers in HMGA2-knockdown ACHN cells or HMGA2-overexpressing 786-O cells. The mRNA levels of TGF-β and Smad2 were downregulated by silencing of HMGA2 (Fig. 5A), and upregulated in the 786-O cells with HMGA2 overexpression (Fig. 5B). To further examine the protein levels of the above markers, we found a marked decrease of TGF-β and phosphorylated-Smad2 in the HMGA2-depleted ACHN cells, and a marked increase in TGF-β and phosphorylated-Smad2 in the HMGA2-overexpressing 786-O cells (Fig. 5C and D). Meanwhile, the protein level of total Smad2, Gli1 and p-β-catenin had no significant change following HMGA2 knockdown or overexpression. These findings suggest that HMGA2 regulated TGF-β/Smad2 signaling in renal cell carcinoma.
Figure 5.
Silencing of HMGA2 decreased TGF-β and Smad2 expression in renal cell carcinoma cells. qPCR was used to detect the expression of TGF-β, Smad2 and β-actin in HMGA2-depleted ACHN (A) and HMGA2-overexpressing 786-O cells (B). Quantification from three independent experiments is shown as mean ± standard deviation (SD). ***P<0.001 and ****P<0.0001. Western blotting was used to detect the protein levels of HMGA2, TGF-β, phosphorylated-Smad2 (p-Smad2), Smad2, Gli1, phosphorylated-β-catenin (p-β-catenin) and β-actin in HMGA2-depleted ACHN cells (C) and HMGA2-overexpressing 786-O cells (D). Representative protein bands from three experiments are shown.
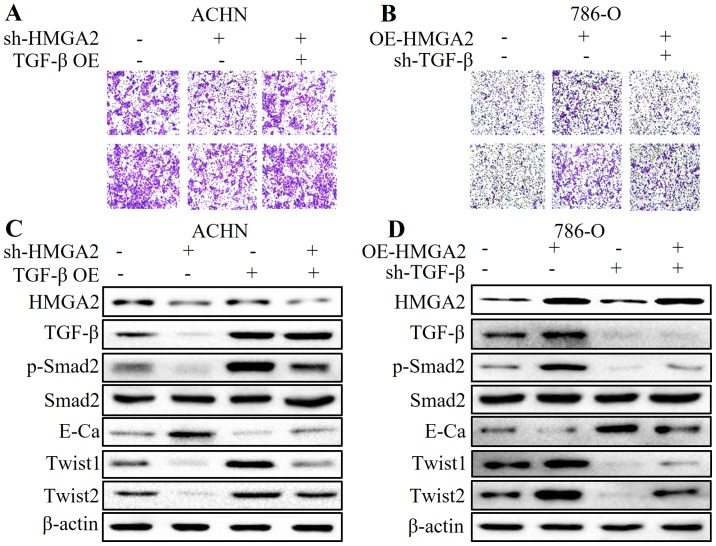
HMGA2 participates in the EMT process of renal cell carcinoma by regulating the TGF-β/Smad2 signaling pathway
To further elucidate the role of TGF-β in HMGA2-regulated EMT in renal cell carcinoma, we applied plasmid transfection to overexpress TGF-β in HMGA2-deficient ACHN cells, and to knock down TGF-β in HMGA2-overexpressing 786-O cells. We found that overexpression of TGF-β partially reversed the anti-metastatic effect and MET by HMGA2 loss (Fig. 6A and C). Conversely, the pro-metastatic phenotype and high expression of TGF-β- and EMT-related markers induced by HMGA2 overexpression were abolished by TGF-β deficiency (Fig. 6B and D). These results strongly suggest that the TGF-β/Smad2 signaling pathway is involved in the HMGA2-mediated EMT of renal cell carcinoma.
Figure 6.
HMGA2 participates in the EMT process of renal cell carcinoma by regulating the TGF-β/Smad2 signaling pathway. Transwell migration and Matrigel invasion assays were used for analysis of the migrated and invaded cells in the (A) HMGA2-depleted ACHN cells treated with TGF-β overexpression, or (B) HMGA2-overexressing 786-O cells with TGF-β deficiency (sh-TGF-β). Western blotting was used to detect the protein levels of HMGA2, TGF-β, phosphorylated-Smad2 (p-Smad2), Smad2, E-cadherin (E-Ca), Twist1, Twist2 and β-actin in the (C) HMGA2-depleted ACHN cells treated with TGF-β overexpression, or (D) the HMGA2-overexressing 786-O cells transiently transfected with the sh-TGF-β plasmid. Representative protein bands from three experiments are shown.
Discussion
Accumulating evidence indicates that HMGA2 is highly expressed in solid tumors and is regulated by complicated regulatory systems (18–20). Studies have shown that in renal cell carcinoma, HMGA2 expression is significantly higher than that in benign and normal renal tissues (21). Moreover, there is a positive correlation between HMGA2 and clinical staging and lymph node metastasis. Also, another study reported that the expression of HMGA2 was significantly associated with tumor size and Fuhrman grade in patients with clear cell renal cell carcinoma (ccRCC) (22). In the present study, we firstly confirmed that HMGA2 was highly expressed in five renal cell carcinoma cell lines compared with that in the normal renal tubular epithelial HK2 cell line. Recent studies have shown that HMGA2 may play an essential role in cancer proliferation, migration and metastasis (23–25). It was reported that deficiency of HMGA2 reduced the metastatic potential of breast cancer cells (26). Additionally, HMGA2 seems to have the potential of enhancing self-renewal capacity in cancer stem cells (27). The present study demonstrated that HMGA2 knockdown inhibited cell migration and invasion, while overexpression of HMGA2 facilitated the metastatic phenotype in renal cell carcinoma cells in vitro, as evidenced by wound healing and Transwell assays. These results emphasize the critical role of HGMA2 in renal cell carcinoma metastasis.
EMT is a complex process during which tumor cells gain more aggressive and metastatic ability (28). Previous studies have reported that silencing of HMGA2 may attenuate migration and invasion, and reverse epithelial-mesenchymal transition (EMT) in nasopharyngeal cancer cells (29). Furthermore, HMGA2 was found to promote EMT by inducing Slug expression in colon cancer (30). Our findings revealed that the E-cadherin level was upregulated, while N-cadherin, Twist1 and Twist2 were downregulated in HMGA2-depleted ACHN cells. In contrast, overexpression of HMGA2 in 786-O cells enhanced EMT. Subsequently, the analysis of database Cancer Browser further validated the positive correlation between HGMA2 and Twist1 or Twist2 in renal cell carcinoma. In addition, the Kaplan-Meier analysis indicated that low HMGA2 expression was closely associated with an increased overall survival (OS) in renal cell carcinoma, which was in accordance with a previous study (22).
Multiple signaling pathways are found to participate in the EMT process. Sonic hedgehog-Gli1 signals are considered to be critical for EMT in ovarian cancer (31). In addition, the Wnt/β-catenin signaling pathway is reported to promote EMT in oral squamous carcinoma stem cells (32). Studies have shown that TGF-β induces PLOD2 expression to promote EMT in cervical cancer (33). In the present study, there was no significant change in Gli1 and p-β-catenin by HMGA2 depletion or overexpression. In addition, our results showed that silencing of HMGA2 downregulated the mRNA and protein levels of TGF-β and Smad2, while HMGA2 overexpression had the opposite effect. In addition, TGF-β overexpression by transient transfection partially abolished the anti-metastatic phenotype and mesenchymal-epithelial transition (MET) by HMGA2 loss, whereas TGF-β deficiency reversed the pro-metastatic effect and high expression of TGF-β- and EMT-related markers by overexpression of HMGA2. These results confirmed the vital role of the TGF-β signaling pathway in HMGA2-mediated EMT.
In conclusion, our results revealed, for the first time, that HMGA2 facilitated a metastatic phenotype and the EMT process in renal cell carcinoma in vitro through a TGF-β-dependent pathway. In addition, our findings strongly suggest that HGMA2 may serve as a potential therapeutic target and prognostic biomarker against renal cell carcinoma in the future, although further investigation is needed.
Acknowledgements
This study was partially supported by the National Natural Science Foundation of China (no. 81602562) and the International Science and Technology Cooperative Project of Shaanxi Province (no. 2017KW-063).
References
- 1.Fayaz MS, Al-Qaderi AE, El-Sherify MS. Metastatic renal cell carcinoma with undetectable renal mass presenting as lymphadenopathy. CEN Case Rep. 2017;6:36–38. doi: 10.1007/s13730-016-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Zeng S, Jiang X, Lai D, Su Z. SOX4 induces tumor invasion by targeting EMT-related pathway in prostate cancer. Tumour Biol. 2017;39:1010428317694539. doi: 10.1177/1010428317694539. [DOI] [PubMed] [Google Scholar]
- 3.Chang JW, Gwak SY, Shim GA, Liu L, Lim YC, Kim JM, Jung MG, Koo BS. EZH2 is associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the epithelial-to-mesenchymal transition and chemosensitivity. Oral Oncol. 2016;52:66–74. doi: 10.1016/j.oraloncology.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Xia P, Xu XY. Epithelial-mesenchymal transition and gastric cancer stem cell. Tumour Biol. 2017;39:1010428317698373. doi: 10.1177/1010428317698373. [DOI] [PubMed] [Google Scholar]
- 5.Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M, Guo YH, Shi J, Gong XD, Zhu YL, Liu F, et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 2014;31:2660–2668. doi: 10.3892/or.2014.3129. [DOI] [PubMed] [Google Scholar]
- 6.Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;131:1863–1873. doi: 10.1002/ijc.27467. [DOI] [PubMed] [Google Scholar]
- 7.Naber HP, Drabsch Y, Snaar-Jagalska BE, ten Dijke P, van Laar T. Snail and Slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem Biophys Res Commun. 2013;435:58–63. doi: 10.1016/j.bbrc.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Kumar MS, Armenteros-Monterroso E, East P, Chakravorty P, Matthews N, Winslow MM, Downward J. HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature. 2014;505:212–217. doi: 10.1038/nature12785. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wu J, Wei JJ. HMGA2 and high-grade serous ovarian carcinoma. J Mol Med (Berl) 2013;91:1155–1165. doi: 10.1007/s00109-013-1055-8. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Li W, Liao H. HMGA2 induces epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Lett. 2013;5:1353–1356. doi: 10.3892/ol.2013.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B, Pang B, Hou X, Fan H, Liang N, Zheng S, Feng B, Liu W, Guo H, Xu S, et al. Expression of high-mobility group AT-hook protein 2 and its prognostic significance in malignant gliomas. Hum Pathol. 2014;45:1752–1758. doi: 10.1016/j.humpath.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Langelotz C, Schmid P, Jakob C, Heider U, Wernecke KD, Possinger K, Sezer O. Expression of high-mobility-group-protein HMGI-C mRNA in the peripheral blood is an independent poor prognostic indicator for survival in metastatic breast cancer. Br J Cancer. 2003;88:1406–1410. doi: 10.1038/sj.bjc.6600935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, Peng J, Loera S, et al. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res. 2011;17:2570–2580. doi: 10.1158/1078-0432.CCR-10-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi YN, Xin XY, Ye HM. Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Asian Pac J Trop Med. 2014;7:289–292. doi: 10.1016/S1995-7645(14)60040-7. [DOI] [PubMed] [Google Scholar]
- 15.Zou Q, Wu H, Fu F, Yi W, Pei L, Zhou M. RKIP suppresses the proliferation and metastasis of breast cancer cell lines through up-regulation of miR-185 targeting HMGA2. Arch Biochem Biophys. 2016;610:25–32. doi: 10.1016/j.abb.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL, Pan CB. Overexpression of HMGA2 promotes tongue cancer metastasis through EMT pathway. J Transl Med. 2016;14:26. doi: 10.1186/s12967-016-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J, Shen G, Liu S, Meng Q. Downregulation of HMGA2 inhibits cellular proliferation and invasion, improves cellular apoptosis in prostate cancer. Tumour Biol. 2016;37:699–707. doi: 10.1007/s13277-015-3853-9. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Sun B, Zhu D, Zhao X, Zhang Y, Dong X, Che N, Li J, Liu F, Zhao N, et al. HMGA2 regulates CD44 expression to promote gastric cancer cell motility and sphere formation. Am J Cancer Res. 2017;7:260–274. [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Wang R, Ren G, Li X, Wang J, Sun Y, Liang J, Nie Y, Wu K, Feng B, et al. HMGA2-FOXL2 axis regulates metastases and epithelial-to-mesenchymal transition of chemoresistant gastric cancer. Clin Cancer Res. 2017;23:3461–3473. doi: 10.1158/1078-0432.CCR-16-2180. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Jiang Z, Chen H, Wu X, Xiang J, Peng J. MicroRNA-495 inhibits gastric cancer cell migration and invasion possibly via targeting high mobility group AT-hook 2 (HMGA2) Med Sci Monit. 2017;23:640–648. doi: 10.12659/MSM.898740. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Liu Y, Fu QZ, Pu L, Meng QG, Liu XF, Dong SF, Yang JX, Lv GY. HMGA2 expression in renal carcinoma and its clinical significance. J Med Biochem. 2015;34:338–343. doi: 10.2478/jomb-2014-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na N, Si T, Huang Z, Miao B, Hong L, Li H, Qiu J, Qiu J. High expression of HMGA2 predicts poor survival in patients with clear cell renal cell carcinoma. Onco Targets Ther. 2016;9:7199–7205. doi: 10.2147/OTT.S116953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Z, Wu D, Tang R, Li X, Chen R, Xue S, Zhang C, Sun X. Silencing of HMGA2 promotes apoptosis and inhibits migration and invasion of prostate cancer cells. J Biosci. 2016;41:229–236. doi: 10.1007/s12038-016-9603-3. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis; Proc Natl Acad Sci USA; 2013; pp. 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natarajan S, Hombach-Klonisch S, Dröge P, Klonisch T. HMGA2 inhibits apoptosis through interaction with ATR-CHK1 signaling complex in human cancer cells. Neoplasia. 2013;15:263–280. doi: 10.1593/neo.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang E, Cisowski J, Nguyen N, O'Callaghan K, Xu J, Agarwal A, Kuliopulos A, Covic L. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene. 2016;35:1529–1540. doi: 10.1038/onc.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur H, Ali SZ, Huey L, Hütt-Cabezas M, Taylor I, Mao XG, Weingart M, Chu Q, Rodriguez FJ, Eberhart CG, et al. The transcriptional modulator HMGA2 promotes stemness and tumorigenicity in glioblastoma. Cancer Lett. 2016;377:55–64. doi: 10.1016/j.canlet.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micalizzi DS, Haber DA, Maheswaran S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol Oncol. 2017;11:770–780. doi: 10.1002/1878-0261.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia YY, Yin L, Jiang N, Guo WJ, Tian H, Jiang XS, Wu J, Chen M, Wu JZ, He X. Downregulating HMGA2 attenuates epithelial-mesenchymal transition-induced invasion and migration in nasopharyngeal cancer cells. Biochem Biophys Res Commun. 2015;463:357–363. doi: 10.1016/j.bbrc.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Zhao Z, Xu C, Zhou Z, Zhu Z, You T. HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer Lett. 2014;355:130–140. doi: 10.1016/j.canlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32:368. doi: 10.1007/s12032-014-0368-y. [DOI] [PubMed] [Google Scholar]
- 32.Qiao B, He BX, Cai JH, Tao Q, King-Yin Lam A. MicroRNA-27a-3p modulates the Wnt/β-catenin signaling pathway to promote epithelial-mesenchymal transition in oral squamous carcinoma stem cells by targeting SFRP1. Sci Rep. 2017;7:44688. doi: 10.1038/srep44688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Xu F, Zhang J, Hu G, Liu L, Liang W. Hypoxia and TGF-β1 induced PLOD2 expression improve the migration and invasion of cervical cancer cells by promoting epithelial-to-mesenchymal transition (EMT) and focal adhesion formation. Cancer Cell Int. 2017;17:54. doi: 10.1186/s12935-017-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]