Abstract
Introduction:
A commonly used approach to adjust for urine dilution in analyses of biomarkers is to adjust for urinary creatinine. However, creatinine is a product of muscle mass and is therefore associated with body mass. In studies of urinary analytes and obesity or obesity-related outcomes, controlling for creatinine could induce collider stratification bias. We illustrate this phenomenon with an analysis of urinary arsenic.
Objective:
We aimed to evaluate various approaches of adjustment for urinary dilution on the associations between urinary arsenic concentration and measures of obesity.
Methods:
Using data from the National Health and Nutrition Examination Survey, we regressed body mass index (BMI) and waist-to-height ratios on urinary arsenic concentrations. We compared eight approaches to account for urine dilution, including standardization by urinary creatinine, osmolality, and flow rates, and inclusion of these metrics as independent covariates. We also used a recently proposed method known as covariate-adjusted standardization.
Results:
Inverse associations between urinary arsenic concentration with BMI and waist-to-height ratio were observed when either creatinine or osmolality were used to standardize or as covariates. Not adjusting for dilution, standardizing or adjusting for urinary flow rate, and using covariate-adjusted standardization resulted in null associations observed between arsenic concentration in relation to BMI and waist-to-height ratio.
Conclusions:
Our findings suggest that arsenic exposure is not associated with obesity, and that urinary creatinine and osmolality may be colliders on the causal pathway from arsenic exposure to obesity, as common descendants of hydration and body composition. In studies of urinary biomarkers and obesity or obesity-related outcomes, alternative metrics such as urinary flow rate or analytic strategies such as covariate-adjusted standardization should be considered. https://doi.org/10.1289/EHP1202
Introduction
Exposure concentrations assessed from spot urine samples are typically adjusted to a constant creatinine concentration to correct for dilution of the samples due to the variable hydration status of the participants. However, recent studies have suggested that this commonly used approach of correcting urinary analyte concentrations by creatinine could result in biased estimates in some instances (Christensen et al. 2014; Hoet et al. 2016; Middleton et al. 2016). Urine specimens are susceptible to inter- and intra-individual variations in dilution due to a variety of factors such as water intake, circadian fluctuations, physical activity, temperature, humidity, age, and disease status (Aylward et al. 2014; Hoet et al. 2016). As a result, measurement error can bias analyte concentrations in the form of under- or overestimations if adjustments for dilution are not made. Creatinine is a byproduct of muscle metabolism excreted from the body primarily through glomerular filtration, in addition to active secretion by peritubular capillaries of the kidney (Barr et al. 2005). In healthy individuals, urinary creatinine concentrations are correlated with anthropometric measurements including body mass and body mass index (BMI); lean body mass, rather than adiposity, likely explains these associations (Baxmann et al. 2008; Gerchman et al. 2009; Yeh et al. 2015). These relationships have implications for epidemiologic analyses of biomarkers and obesity, in which creatinine correction may be inappropriate.
With more than one-third of the U.S. adult population obese, interest in understanding how exposure to environmental chemicals may contribute is increasing (Maull et al. 2012; Ogden et al. 2014; Thayer et al. 2012). However, a limited number of studies have focused on the association of arsenic exposure with obesity. These studies have been heterogeneous in terms of study populations, biomarkers of exposure, and results (Ettinger et al. 2014; Grashow et al. 2014; Lin et al. 2014; Ronco et al. 2010). In an occupational study of welders in the U.S. (), toenail arsenic concentrations were inversely associated with BMI (, ) after adjusting for age, caloric intake, alcohol intake, smoking status, and season of toenail clipping (Grashow et al. 2014). A cross-sectional analysis of young adults of African descent () across five countries (Ghana, South Africa, Seychelles, Jamaica, and the United States) found blood arsenic concentrations greater than the median () were associated with significantly lower odds of being overweight [, 95% CI: 0.09–0.81], but not with obesity (; 95% CI: 0.24–3.07), after adjustment for age, sex, site location, marital status, education, paid employment, smoking, alcohol use, and fish intake (Ettinger et al. 2014). A cross-sectional study of Chilean women of childbearing age () found no crude associations between urinary arsenic concentrations and BMI or fat mass percentage () (Ronco et al. 2010). Average urinary arsenic concentrations when expressed as per gram of creatinine were (95% CI: 8.9–20.3) among women with a , (95% CI: 6.2–16.1) among women with a BMI between 19 and , and (95% CI: 6.9–16.5) among women with a (Ronco et al. 2010). Last, a cross-sectional study of Taiwanese adolescents () found a significant age- and sex-adjusted inverse relationship with BMI regardless of creatinine correction (p-value for ; 0.03 when creatinine corrected), but the urinary arsenic concentrations observed (mean: ) suggested higher exposures than would be expected in the United States (Su et al. 2012).
It has been hypothesized that urinary creatinine may be a collider, or common descendent of two variables, in causal pathways (Greenland et al. 1999; O'Brien et al. 2016). Specifically, urinary creatinine concentrations are influenced by both the body’s hydration status and body composition. Crude measures of obesity like BMI poorly discriminate between fat and muscle; as such, controlling for urinary creatinine in regression models could induce spurious associations (Greenland 2003). Given the biological process of creatinine excretion and the equivocal epidemiological evidence of a relationship between low-level arsenic exposure and obesity, a thorough investigation of appropriate urinary dilution metrics and analytic techniques is warranted. Thus, using data from the National Health and Nutrition Examination Survey (NHANES), we evaluated the association between arsenic exposure and obesity using various dilution adjustment approaches, including creatinine, urinary osmolality (the amount of solute particles contained in urine), and urinary flow rate (the quantity of urine produced over a specified period of time) as alternative metrics of urine dilution (Hays et al. 2015). We also employed a novel method (covariate-adjusted standardization) to obtain an estimate of arsenic exposure that is independent of demographic factors and anthropometric measures, and solely attributable to hydration status (O'Brien et al. 2016; O'Brien et al. 2017).
Methods
We used data from the 2009–2012 NHANES survey cycles. Conducted by the National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention (CDC), NHANES is a complex survey design that surveys a representative sample of the civilian, noninstitutionalized U.S. population (NHANES 2014). We limited our analysis to adults (), and complete data for the measures are described below (). Females who self-reported being pregnant or breastfeeding, or who had a positive urine pregnancy test at the time of exam, were excluded. We also excluded participants with chronic kidney disease, defined as an estimated glomerular filtration rate less than using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, or self-report of receiving dialysis in the past 12 mo (Levey et al. 2009). NHANES is approved by the National Center for Health Statistics Research Ethics Review Board. All NHANES participants provide informed consent before taking part in the survey.
Arsenic Biomarkers
Participants were asked to provide a spot urine sample at the mobile examination center (CDC 2009a, 2011c). A random one-third subsample was selected for urinary total arsenic and speciated arsenic laboratory measurements. Detection limits varied by survey cycle. For the 2009–2010 survey, limits were 0.74, 0.4, 0.6, 1.2, 1.0, 0.9, and for total arsenic, arsenobetaine, arsenocholine, arsenite, arsenate, monomethylarsonic acid, and dimethylarsonic acid, respectively. For the 2011–2012 survey, corresponding limits were 1.25, 1.19, 0.28, 0.48, 0.87, 0.89, and . To be conservative, we used the higher limit of detection across the survey cycles. We substituted the limit divided by the square root of 2 for nondetectable values (Table 1).
Table 1.
Detection limit and proportion undetectable for total and speciated arsenic.
| Arsenic species | LOD () | (Weighted %) |
|---|---|---|
| Total arsenic | 1.25 | 3.2 |
| Arsenobetaine | 1.19 | 1.2 |
| Arsenocholine | 0.6 | 97.6 |
| Arsenite | 1.2 | 95.5 |
| Arsenate | 1.0 | 97.4 |
| Monomethylarsonic acid | 0.9 | 74.0 |
| Dimethylarsonic acid | 1.80 | 23.0 |
We estimated urinary arsenic concentrations (estimated urinary arsenic 1) as total arsenic minus arsenobetaine and arsenocholine (nontoxic forms of organic arsenic); this method previously has been proposed for modeling NHANES arsenic data (Cardenas et al. 2015; Davis et al. 2012; Steinmaus et al. 2009). After subtracting arsenobetaine and arsenocholine, some estimates were negative due to measurement error. We substituted a constant of for these samples (). An alternative estimate of arsenic (estimated urinary arsenic 2), calculated as the sum of arsenite, arsenate, monomethylarsonic acid, and dimethylarsonic acid, was also assessed to allow for comparisons with the study of Taiwanese adolescents (Su et al. 2012).
Obesity Ascertainment
Participants were examined for anthropometric measures using standardized techniques and equipment (CDC 2009b, 2011a). Body weight was measured using an electronic digital scale; standing height was measured using a stadiometer. Obesity was assessed using BMI, calculated as body weight in kilograms divided by height in meters-squared (). Secondary analyses were performed using waist-to-height ratio as an additional indicator of obesity because BMI has been criticized for not adequately distinguishing between adipose tissue and muscle mass (Ashwell et al. 1996a; Ashwell et al. 1996b; Ashwell et al. 2012; Flegal et al. 2009; Gomez-Ambrosi et al. 2012; Heymsfield et al. 2009; Nevill et al. 2006). Waist circumference was measured following a standard protocol using a measuring tape on standing participants (CDC 2009b, 2011a). Waist-to-height ratio was calculated as waist circumference in centimeters divided by height in centimeters.
Urinary Dilution Metrics
Participants were asked to void the bladder completely and to provide the time of their previous urinary void before the examination (CDC 2009a, 2011c). If the volume of urine provided at the mobile clinical examination was insufficient (i.e., for females of childbearing age to allow for pregnancy testing, and for all other participants), up to two additional voids were collected. The volumes and time of voids were recorded, which allowed for the calculation of urinary flow rates. We summed the total volume of urine collected in milliliters, and divided that total by the total time in hours between voids (i.e., the time between the void prior to examination and the last collected void during the examination) (Hays et al. 2015).
Osmolality was measured on collected urine samples using freezing point depression osmometry (CDC 2009a, 2011c). Results were reported as milliosmoles per kilogram of water (mOsm/kg), with higher values indicating a more concentrated sample (Yeh et al. 2015). Urinary creatinine was measured on a Roche/Hitachi Modular P Chemistry Analyzer using an enzymatic (creatininase) reaction (CDC 2011b). Results were reported as milligrams per deciliter (mg/dL), with higher values also indicating a more concentrated sample (WHO 1996).
Covariates
Participants completed demographic questionnaires. Self-reported data on age (years), gender (male, female), and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial) were utilized in these analyses.
Statistical Methods
We considered several approaches for urine dilution adjustment of arsenic concentrations: 1) no adjustment; 2) creatinine as an independent covariate in the regression model; 3) arsenic concentration divided by creatinine concentration (); 4) covariate-adjusted standardization of arsenic concentration divided by creatinine concentration (); 5) osmolality as an independent covariate in the regression model; 6) arsenic concentration divided by osmolality (); 7) urinary flow rate as an independent covariate in the regression model; and 8) arsenic concentration multiplied by urinary flow rate to obtain excretion rate ().
Model 4 employed a newly developed method known as covariate-adjusted standardization (O'Brien et al. 2016). Creatinine corrections assume that urinary creatinine excretion is constant. To avoid this assumption, entering urinary creatinine as a separate variable has been recommended (Barr et al. 2005). However, if urinary creatinine is a collider (Figure 1), neither method is appropriate because it will introduce collider stratification bias (Greenland 2003). As an alternative, we performed covariate-adjusted standardization. In this multistep approach, log-transformed creatinine is first regressed on variables known to directly and chronically affect urine dilution. We included age, gender, race/ethnicity, and BMI as predictors in our model. In this capacity, BMI was used as a proxy for muscle mass and stature. Observed creatinine concentrations were then divided by the fitted creatinine values obtained from this model, producing a ratio representing the covariate-independent residual effect of hydration on creatinine. We then standardized urinary arsenic concentrations by dividing the biomarker concentrations by the ratio of observed to fitted creatinine.
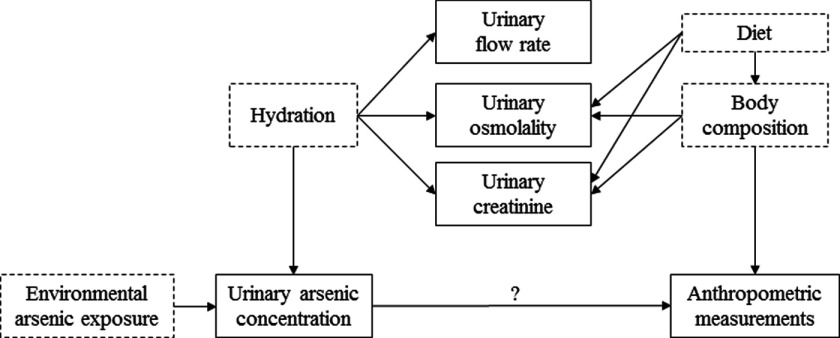
Figure 1.
Directed acyclic graph of the causal pathway between arsenic exposure and anthropometric measurements (BMI and waist-to-height ratio). Variables with solid outlines are observed; variables with dashed lines are unobserved. Body composition refers to the amounts of fat, bone, water, and muscle in human bodies. Note: Age, gender, race/ethnicity, and survey cycle, which were hypothesized to directly affect arsenic exposure, urine dilution, and anthropometric measures, are not pictured.
Statistical analyses accounted for the complex survey design of NHANES by incorporating sampling weights, primary sampling units, and strata. We used the laboratory subsample weights multiplied by one-half because we combined two survey cycles. Selected characteristics, including urinary arsenic, dilution metrics, BMI, waist-to-height ratio, and demographics, were compared using least-square geometric means. Urinary arsenic concentrations and dilution-adjusted arsenic concentrations were categorized into quartiles and modeled as categorical variables to evaluate dose-response and ordinal variables to test for linear trends. BMI, which was positively skewed, was log-transformed prior to being modeled as a continuous dependent variable using linear regression. Estimates and 95% confidence intervals were back-transformed to provide geometric means. Age (continuous), gender (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial), and survey cycle (2009–2010 or 2011–2012) were included as potential confounders in all regression models. All analyses were performed in SAS (version 9.4). were considered statistically significant.
Results
Complete data were available for 3,097 adult NHANES participants. Geometric means of the urinary arsenic concentrations, dilution metrics, BMI, and waist-to-height ratio across covariate groups are presented in Table 2. Urinary arsenic concentrations were positively associated with urinary creatinine and osmolality, and inversely associated with urinary flow rates. BMI and waist-to-height ratio were also positively associated with urinary creatinine and osmolality, but no associations were observed with urinary flow rate.
Table 2.
Geometric means for selected characteristics ().
| Characteristic | Estimated urinary arsenic 1a () | Estimated urinary arsenic 2b () | Urinary creatinine () | Urinary osmolality | Urinary flow rate () | BMI () | Waist-to-height ratio |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| 20–39 (reference) | 3.7 | 6.5 | 101.6 | 567.6 | 53.6 | 27.1 | 0.54 |
| 40–59 | 3.6 | 6.3 | 89.3* | 524.0* | 54.5 | 28.3* | 0.58* |
| 3.5 | 6.4 | 76.7* | 494.8* | 49.5 | 28.1* | 0.60* | |
| Gender | |||||||
| Female (reference) | 3.0 | 6.1 | 73.6 | 482.0 | 50.8 | 27.6 | 0.58 |
| Male | 4.4* | 6.8* | 111.5* | 589.6* | 55.3* | 28.0 | 0.57* |
| Race/ethnicity | |||||||
| Non-Hispanic white (reference) | 2.9 | 5.8 | 85.4 | 510.7 | 55.5 | 27.6 | 0.57 |
| Non-Hispanic black | 4.8* | 7.1* | 128.6* | 599.6* | 44.1* | 30.1* | 0.59* |
| Mexican American | 4.9* | 6.8* | 97.5* | 616.2* | 48.3* | 28.8* | 0.60* |
| Other Hispanic | 5.5* | 7.8* | 92.8 | 575.1* | 51.4 | 28.1 | 0.58 |
| Other race/multi-racial | 7.9* | 10.2* | 85.1 | 533.3 | 52.3 | 25.3* | 0.54* |
| Survey cycle | |||||||
| 2009-2010 (reference) | 5.1 | 6.7 | 94.5 | 532.7 | 53.3 | 27.8 | 0.57 |
| 2011-2012 | 2.6* | 6.1* | 87.2* | 534.0 | 52.8 | 27.8 | 0.58 |
| Estimated urinary arsenic 1a () | |||||||
| 0.0–2.3 (reference) | 0.6 | 3.8 | 47.2 | 337.6 | 86.3 | 27.7 | 0.57 |
| 2.4–5.3 | 3.7* | 5.2* | 93.2* | 545.2* | 51.2* | 27.6 | 0.57 |
| 5.4–10.7 | 7.5* | 7.4* | 126.5* | 689.0* | 41.6* | 28.0 | 0.57 |
| 21.7* | 14.6* | 149.0* | 733.8* | 37.1* | 27.9 | 0.57 | |
| Estimated urinary arsenic 2b () | |||||||
| 3.5-4.3 (reference) | 0.7 | 3.6 | 45.4 | 325.6 | 89.7 | 27.5 | 0.57 |
| 4.4–6.1 | 3.3* | 5.1* | 92.1* | 551.5* | 51.4* | 27.8 | 0.57 |
| 6.2–9.6 | 7.1* | 7.5* | 127.3* | 674.1* | 43.1* | 28.2* | 0.58 |
| 18.7* | 15.9* | 151.7* | 753.7* | 34.8* | 27.8 | 0.57 | |
| BMI () | |||||||
| (reference) | 3.5 | 6.3 | 76.8 | 475.9 | 53.2 | 22.1 | 0.49 |
| 25–29 | 3.6 | 6.5 | 92.9* | 528.6* | 52.6 | 27.3* | 0.57* |
| 3.8 | 6.5 | 104.6* | 602.7* | 53.3 | 35.5* | 0.68* | |
| Waist-to-height ratio | |||||||
| (reference) | 3.7 | 6.2 | 84.0 | 492.0 | 53.5 | 22.0 | 0.47 |
| 0.51–0.57 | 3.6 | 6.5 | 86.6 | 511.1 | 55.3 | 25.6* | 0.54* |
| 3.6 | 6.4 | 97.2* | 569.5* | 51.7 | 32.6* | 0.65* |
Estimated as [] with negative values set to .
Estimated as [].
compared with reference.
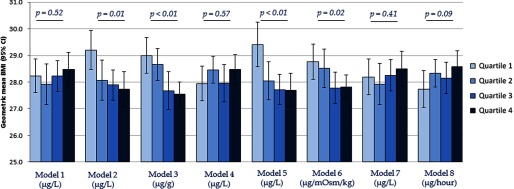
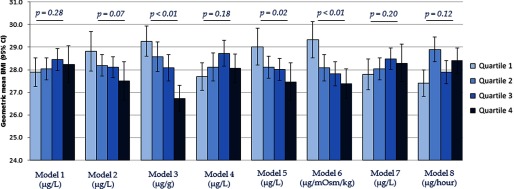
The associations between estimated urinary total arsenic 1 and BMI are summarized in Figure 2. Strong inverse associations between urinary total arsenic and BMI were observed when creatinine was included as an independent covariate in the regression model (Model 2), arsenic concentration was standardized by creatinine (Model 3), osmolality was included as an independent covariate in the regression model (Model 5), and arsenic concentration was standardized by osmolality (Model 6). Null associations were observed between urinary total arsenic and BMI when no adjustment for urinary dilution was made (Model 1), arsenic concentration was covariate-adjusted standardized by creatinine (Model 4), and urinary flow rate was included as an independent covariate in the regression model (Model 7). A marginally positive association was observed between urinary total arsenic concentration and BMI when arsenic was standardized to urinary flow rate through multiplication to obtain excretion rates (Model 8). Appreciably similar associations were observed between estimated urinary total arsenic 2 and BMI (Figure 3).
Figure 2.
Geometric mean (95% CI) of BMI across estimated urinary arsenic 1a quartiles by urine dilution adjustment approaches. aEstimated as [] with negative values set to . are for assessment of linear trends across urinary arsenic quartiles. Models refer to: 1) no adjustment for urine dilution; 2) creatinine as an independent covariate in the regression model; 3) arsenic concentration divided by creatinine concentration (); 4) covariate-adjusted standardization of arsenic concentration (); 5) osmolality as an independent covariate in the regression model; 6) arsenic concentration divided by osmolality (); 7) urinary flow rate as an independent covariate in the regression model; and 8) arsenic concentration multiplied by urinary flow rate to obtain excretion rate (). All models were additionally adjusted for age (continuous), gender (male/female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial), and survey cycle (2009–2010 or 2011–2012).
Figure 3.
Geometric mean (95% CI) of BMI across estimated urinary arsenic 2a quartiles by urine dilution adjustment approaches. a Estimated as []. are for assessment of linear trends across urinary arsenic quartiles. Models refer to: 1) no adjustment for urine dilution; 2) creatinine as an independent covariate in the regression model; 3) arsenic concentration divided by creatinine concentration (); 4) covariate-adjusted standardization of arsenic concentration (); 5) osmolality as an independent covariate in the regression model; 6) arsenic concentration divided by osmolality (); 7) urinary flow rate as an independent covariate in the regression model; and 8) arsenic concentration multiplied by urinary flow rate to obtain excretion rate (). All models were additionally adjusted for age (continuous), gender (male/female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial), and survey cycle (2009–2010 or 2011–2012).
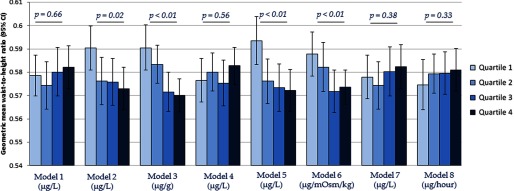
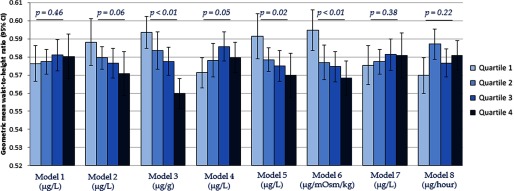
The associations between estimated urinary total arsenic 1 and waist-to-height ratio are summarized in Figure 4. Inverse associations between urinary total arsenic and waist-to-height ratio were observed when creatinine was included as an independent covariate in the regression model (Model 2), arsenic concentration was standardized by creatinine (Model 3), osmolality was included as an independent covariate in the regression model (Model 5), and arsenic concentration was standardized by osmolality (Model 6). Null associations were observed between urinary total arsenic and waist-to-height ratio when no adjustment for urinary dilution was made (Model 1), arsenic concentration was covariate-adjusted standardized by creatinine (Model 4), urinary flow rate was included as an independent covariate in the regression model (Model 7), and arsenic was standardized to urinary flow rate through multiplication (Model 8). Appreciably similar associations were observed between estimated urinary total arsenic 2 and weight-to-height ratio (Figure 5).
Figure 4.
Geometric mean (95% CI) of waist-to-height ratio across estimated urinary arsenic 1a quartiles by urine dilution adjustment approaches. a Estimated as [total arsenic in ] with negative values set to . are for assessment of linear trends across urinary arsenic quartiles. Models refer to: 1) no adjustment for urine dilution; 2) creatinine as an independent covariate in the regression model; 3) arsenic concentration divided by creatinine concentration (); 4) covariate-adjusted standardization of arsenic concentration (); 5) osmolality as an independent covariate in the regression model; 6) arsenic concentration divided by osmolality ( ); 7) urinary flow rate as an independent covariate in the regression model; and 8) arsenic concentration multiplied by urinary flow rate to obtain excretion rate (). All models were additionally adjusted for age (continuous), gender (male/female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial), and survey cycle (2009–2010 or 2011–2012).
Figure 5.
Geometric mean (95% CI) of waist-to-height ratio across estimated urinary arsenic 2a quartiles by urine dilution adjustment approaches. aEstimated as []. are for assessment of linear trends across urinary arsenic quartiles. Models refer to: 1) no adjustment for urine dilution; 2) creatinine as an independent covariate in the regression model; 3) arsenic concentration divided by creatinine concentration (); 4) covariate-adjusted standardization of arsenic concentration (); 5) osmolality as an independent covariate in the regression model; 6) arsenic concentration divided by osmolality (); 7) urinary flow rate as an independent covariate in the regression model; and 8) arsenic concentration multiplied by urinary flow rate to obtain excretion rate (). All models were additionally adjusted for age (continuous), gender (male/female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other race/multiracial), and survey cycle (2009–2010 or 2011–2012).
Discussion
The epidemiologic literature on arsenic and obesity is scant, but limited evidence exists suggesting arsenic exposure may be inversely related to BMI (Ettinger et al. 2014; Grashow et al. 2014; Ronco et al. 2010; Su et al. 2012). In contrast, our findings do not support an association between arsenic exposure, as measured in urine, and obesity. It is possible that low-level arsenic exposure, as seen among the general population of the United States, has no effects on adiposity; occupational studies and studies conducted outside of the United States may be capturing exposures above a potential threshold. Our findings further suggest that urinary creatinine and osmolality may be colliders on the causal pathway from arsenic exposure to obesity as common descendants of hydration and body composition. When urinary creatinine and osmolality were accounted for in our analyses, we observed significant inverse associations with BMI and waist-to-height ratios. In other words, controlling for these variables may have opened a previously blocked backdoor path between urinary arsenic concentrations and obesity. Using covariate-adjusted standardization, adjusting for urine dilution using urinary flow rates, or modeling arsenic excretion rates resulted in null associations between urinary arsenic concentrations and measures of obesity.
Nearly all the creatinine excreted in urine is produced in skeletal muscle (Barr et al. 2005). Skeletal muscle is positively associated with stature, as taller individuals have longer bones and muscles (Janssen et al. 2000). Similarly, skeletal muscle mass is correlated with body weight; not only is muscle denser than adipose tissue, but heavier individuals require more muscle mass for movement (Janssen et al. 2000). In comparison with normal-weight individuals, obese individuals have more skeletal muscle, albeit with lesser amounts of muscle relative to body fat (Janssen et al. 2000). As such, obese individuals excrete more creatinine than their normal-weight counterparts (Gerchman et al. 2009). It is unclear whether more refined methods of measuring adiposity (e.g., bioelectrical impedance, dual-energy X-ray absorptiometry) would be useful in disentangling fat from muscle mass rendering creatinine a noncollider. Urine osmolality and creatinine are highly correlated (Yeh et al. 2015). Although osmolality is generally considered more robust than creatinine because it is less influenced by individual characteristics (e.g., demographics or medical conditions), we observed that both factors similarly introduced bias (Middleton et al. 2016; Yeh et al. 2015). Multiple studies have observed that osmolality tends to be higher in urine samples from obese individuals, indicating more concentrated urine, than in normal-weight individuals (Chang et al. 2016; Hays et al. 2015; Yeh et al. 2015). However, osmolality has been considered the optimal metric of urine concentration, because it provides a measure of the number of all solutes present regardless of their molecular mass or structure (Chadha et al. 2001; Dossin et al. 2003). The solutes that contribute to total osmolality include urea, sodium, potassium, chloride, and creatinine, among others (Chadha et al. 2001). Studies have observed that urinary concentrations of sodium, chloride, and creatinine solutes have been positively associated with obesity (Al-Hayek et al. 2013; Fotheringham et al. 2014; Fram et al. 2015; Ge et al. 2016; Taylor and Curhan 2006). With the exception of creatinine, however, these urinary components are not derived from muscle; rather, urinary solute concentrations likely reflect dietary intakes (Bingham 2003; Taylor and Curhan 2006).
Urinary flow rate has been proposed as a direct metric of hydration status (Hays et al. 2015). In the present analyses, urinary flow rate was observed to be independent of BMI and waist-to-height ratio, suggesting that it is not a collider of the causal path between urinary arsenic and obesity. Flow-rate calculations assume that the bladder is entirely emptied and requires both the total void volume and times of the prior and current void. To calculate excretion rates, analyte concentrations are multiplied by urinary flow rates. However, the time between voids is rarely collected in large-scale epidemiologic studies and may be difficult to accurately obtain in certain populations, such as children.
In the present analysis, we evaluated a general population sample; however, analytical approaches in high-risk populations should also be considered. For example, kidney disease is qualitatively associated with metrics of urine dilution. Urinary creatinine is elevated in individuals with chronic kidney disease, whereas osmolality and urinary flow rates are significantly lower among diseased individuals. This contrast suggests that urinary flow rate, although not related to measures of obesity among healthy individuals, may be a collider among persons with chronic kidney disease. Thus, covariate-adjusted standardization may be the most suitable approach for high-risk populations, such as individuals with chronic kidney disease. Children were not included in our analyses because the obesity metrics used are systematically lower in this population. We have no reason to believe, however, that methods to account for urine dilution would perform differentially among children. In fact, a recently published study utilized the covariate-adjusted standardization approach to account for urine dilution in a study of urinary phenols and childhood fat mass in a birth cohort (Buckley et al. 2016).
We acknowledge several limitations of our analyses. We did not evaluate specific gravity, a measure of the number of solute particles in urine as well as their size, because it was not measured as part of the NHANES 2009–2012 surveys. Although specific gravity is considered a more robust measure of urine dilution than creatinine (Pearson et al. 2009), it is associated with muscle mass (Hamouti et al. 2010) and would have likely yielded results similar to those observed with creatinine and osmolality in these analyses. Additionally, conditions like diabetes can alter solute concentrations (e.g., glucose and protein) in urine, further disqualifying specific gravity adjustments (Voinescu et al. 2002). Urinary flow rates were used independently and to calculate arsenic excretion rates. The accuracy of the urinary flow rates is questionable, as participants were asked to self-report the time of their last void. However, we have no reason to believe that any inaccuracies would be related to urinary arsenic concentrations or anthropometric measures. We adjusted for age, gender, race/ethnicity, and survey cycle in all regression models, but as in all observational studies, residual confounding could partially explain our findings. Specifically, we did not assess muscle wasting and diuretic usage, which could directly affect urinary creatinine and osmolality, as well as body mass or size, although we expect their effect to be minimal, given the low prevalence in the general population. Further, we did not evaluate total water or protein intake, which could be common causes of urine diluteness and anthropometric measures (Krieger et al. 2006; Rosinger et al. 2016). Lastly, we simplified the causal diagram by not showing the involvement of the one carbon metabolism pathway. Evidence has been found that suggests that arsenic exposure alters creatinine excretion via folate-mediated single-carbon metabolism and that obesity may alter the body’s ability to metabolize arsenic (Gamble and Hall 2012; Hudgens et al. 2016). Regardless of these relationships, urinary creatinine and osmolality would remain colliders in our hypothesized causal scenario.
Conclusions
We recommend investigators draw a directed acyclic graph of a biomarker of exposure–outcome relationships prior to making adjustments for urine dilution. For studies of arsenic specifically, such adjustments may be unnecessary as 24-h concentrations do not significantly differ from spot urine samples (Hinwood et al. 2002). However, if measurement error is a concern, performing covariate-adjusted standardization, adjusting for urinary flow rates (if collected), or modeling analyte excretion rates may be good options, depending on the specific causal scenario. Given that metrics of urine dilution are often affected by body composition, studies of urinary biomarkers of environmental exposures and obesity or obesity-related conditions should carefully consider how to best correct for variations in urine dilution at the time of measurement.
Acknowledgments
We thank K.M. O’Brien of the National Institute of Environmental Health Sciences (NIEHS) and L. Aylward of Summit Toxicology for helpful comments on this analysis. This work was supported by the National Institutes of Health (NIH) grant numbers T32 HL125294 and R01 ES024423. C.B. was supported by the National Heart, Lung, and Blood Institute (NHLBI) T32 HL125294. M.A. was supported by the NIEHS R01 ES024423.
References
- Al-Hayek S, Schwen ZR, Jackman SV, Averch TD. 2013. The impact of obesity on urine composition and nephrolithiasis management. J Endourol 27(3):379–383, PMID: 22967041, 10.1089/end.2012.0275. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Cole TJ, Dixon AK. 1996a. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. BMJ 313(7056):559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M, Lejeune S, McPherson K. 1996b. Ratio of waist circumference to height may be better indicator of need for weight management. BMJ 312(7027):377, PMID: 8611847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M, Gunn P, Gibson S. 2012. Waist-to-height ratio is a better screening tool than waist circumference and bmi for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev 13(3):275–286, PMID: 22106927, 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- Aylward LL. et al. 2014. Sources of variability in biomarker concentrations. J Toxicol Environ Health B Crit Rev 17(1):45–61, PMID: 24597909, 10.1080/10937404.2013.864250. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: 15687057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. 2008. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3(2):348–354, PMID: 18235143, 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham SA. 2003. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 133(suppl 3):921S–924S, PMID: 12612177. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. 2016. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study. Environ Int 91:350–356, PMID: 27037776, 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Smit E, Houseman EA, Kerkvliet NI, Bethel JW, Kile ML. 2015. Arsenic exposure and prevalence of the varicella zoster virus in the United States: NHANES (2003-2004 and 2009-2010). Environ Health Perspect 123(6):590–596, PMID: 25636148, 10.1289/ehp.1408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2009a. National health and nutrition examination survey (NHANES) laboratory procedures manual. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf. [accessed 26 July 2016].
- CDC (Centers for Disease Control and Prevention). 2009b. National health and nutrition examination survey (NHANES) anthropometry procedures manual. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/BodyMeasures_09.pdf. [accessed 27 July 2016].
- CDC (Centers for Disease Control and Prevention). 2011a. National health and nutrition examination survey (NHANES) anthropometry procedures manual. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf. [accessed 27 July 2016].
- CDC (Centers for Disease Control and Prevention). 2011b. Creatinine in urine. NHANES 2011-2012. Laboratory procedure manual. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/alb_cr_g_met_creatinine.pdf. [accessed 26 July 2016].
- CDC (Centers for Disease Control and Prevention). 2011c. National health and nutrition examination survey (NHANES) laboratory procedures manual. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_Laboratory_Procedures_Manual.pdf. [accessed 26 July 2016].
- Chadha V, Garg U, Alon US. 2001. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol 16(4):374–382, PMID: 11354785. [DOI] [PubMed] [Google Scholar]
- Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. 2016. Inadequate hydration, BMI, and obesity among us adults: NHANES 2009-2012. Ann Fam Med 14(4):320–324, PMID: 27401419, 10.1370/afm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Sobus J, Phillips M, Blessinger T, Lorber M, Tan YM. 2014. Changes in epidemiologic associations with different exposure metrics: a case study of phthalate exposure associations with body mass index and waist circumference. Environ Int 73:66–76, PMID: 25090576, 10.1016/j.envint.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. 2012. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect 120(10):1418–1424, PMID: 23008276, 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossin O, Germain C, Braun JP. 2003. Comparison of the techniques of evaluation of urine dilution/concentration in the dog. J Vet Med A Physiol Pathol Clin Med 50(6):322–325, PMID: 12887626. [DOI] [PubMed] [Google Scholar]
- Ettinger AS, Bovet P, Plange-Rhule J, Forrester TE, Lambert EV, Lupoli N, et al. 2014. Distribution of metals exposure and associations with cardiometabolic risk factors in the “Modeling the Epidemiologic Transition Study”. Environ Health 13:90, PMID: 25374160, 10.1186/1476-069X-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. 2009. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 89(2):500–508, PMID: 19116329, 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham J, Weatherley N, Kawar B, Fogarty DG, Ellam T. 2014. The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int 86(6):1221–1228, PMID: 24717300, 10.1038/ki.2014.112. [DOI] [PubMed] [Google Scholar]
- Fram EB, Agalliu I, DiVito J, Hoenig DM, Stern JM. 2015. The visceral fat compartment is independently associated with changes in urine constituent excretion in a stone forming population. Urolithiasis 43(3):213–220, PMID: 25903669, 10.1007/s00240-015-0770-8. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Hall MN. 2012. Relationship of creatinine and nutrition with arsenic metabolism. Environ Health Perspect 120(4):A145–A146, PMID: 22469551, 10.1289/ehp.1104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Zhang J, Chen X, Yan L, Guo X, Lu Z, et al. 2016. Are 24 h urinary sodium excretion and sodium: potassium independently associated with obesity in Chinese adults? Public Health Nutr 19(6):1074–1080, PMID: 26228639, 10.1017/S136898001500230X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerchman F, Tong J, Utzschneider KM, Zraika S, Udayasankar J, McNeely MJ, et al. 2009. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J Clin Endocrinol Metab 94(10):3781–3788, PMID: 19584179, 10.1210/jc.2008-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millan D. et al. 2012. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes Relat Metab Disord 36(2):286–294, PMID: 21587201, 10.1038/ijo.2011.100. [DOI] [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Kile ML, et al. 2014. Inverse association between toenail arsenic and body mass index in a population of welders. Environ Res 131:131–133, PMID: 24721130, 10.1016/j.envres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: 9888278. [PubMed] [Google Scholar]
- Greenland S. 2003. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology 14(3):300–306, PMID: 12859030. [PubMed] [Google Scholar]
- Hamouti N, Del Coso J, Avila A, Mora-Rodriguez R. 2010. Effects of athletes' muscle mass on urinary markers of hydration status. Eur J Appl Physiol 109(2):213–219, PMID: 20058021, 10.1007/s00421-009-1333-x. [DOI] [PubMed] [Google Scholar]
- Hays SM, Aylward LL, Blount BC. 2015. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect 123(4):293–300, PMID: 25625328, 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Scherzer R, Pietrobelli A, Lewis CE, Grunfeld C. 2009. Body mass index as a phenotypic expression of adiposity: quantitative contribution of muscularity in a population-based sample. Int J Obes (Lond) 33(12):1363–1373, PMID: 19773739, 10.1038/ijo.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood AL, Sim MR, de Klerk N, Drummer O, Gerostamoulos J, Bastone EB. 2002. Are 24-hour urine samples and creatinine adjustment required for analysis of inorganic arsenic in urine in population studies? Environ Res 88(3):219–224, PMID: 12051800, 10.1006/enrs.2002.4339. [DOI] [PubMed] [Google Scholar]
- Hoet P, Deumer G, Bernard A, Lison D, Haufroid V. 2016. Urinary trace element concentrations in environmental settings: is there a value for systematic creatinine adjustment or do we introduce a bias? J Expo Sci Environ Epidemiol 26(3):296–302, PMID: 25827313, 10.1038/jes.2015.23. [DOI] [PubMed] [Google Scholar]
- Hudgens EE. et al. 2016. Biological and behavioral factors modify urinary arsenic metabolic profiles in a U.S. population. Environ Health 15(1):62, PMID: 27230915, 10.1186/s12940-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. 2000. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 89(1):81–88, PMID: 10904038. [DOI] [PubMed] [Google Scholar]
- Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. 2006. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr 83(2):260–274, PMID: 16469983. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612, PMID: 19414839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Huang YK, Shiue HS, Chen LS, Choy CS, Huang SR, et al. 2014. Arsenic methylation capacity and obesity are associated with insulin resistance in obese children and adolescents. Food Chem Toxicol 74:60–67, PMID: 25241017, 10.1016/j.fct.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien a, Pi j, et al. 2012. Evaluation of the association between arsenic and diabetes: a national toxicology program workshop review. Environ Health Perspect 120(12):1658–1670, PMID: 22889723, 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton DR, Watts MJ, Lark RM, Milne CJ, Polya DA. 2016. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ Health 15(1):68, PMID: 27286873, 10.1186/s12940-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill AM, Stewart AD, Olds T, Holder R. 2006. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol 129(1):151–156, PMID: 16270304, 10.1002/ajpa.20262. [DOI] [PubMed] [Google Scholar]
- NHANES (National Health and Nutrition Examination Survey). 2014. NHANES ― about the national health and nutrition examination survey. Available: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [accessed 11 March 2015].
- O'Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227, PMID: 26219104, 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KM, Upson K, Buckley JP. 2017. Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Curr Environ Health Rep 4(1):44–50, PMID: 28097619, 10.1007/s40572-017-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311(8):806–814, PMID: 24570244, 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. 2009. Evaluation of physiological measures for correcting variation in urinary output: implications for assessing environmental chemical exposure in children. J Expos Sci Environ Epidemiol 19(3):336–342, PMID: 18841168, 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Gutierrez Y, Gras N, Munoz L, Salazar G, Llanos MN. 2010. Lead and arsenic levels in women with different body mass composition. Biol Trace Elem Res 136(3):269–278, PMID: 19851721, 10.1007/s12011-009-8546-z. [DOI] [PubMed] [Google Scholar]
- Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL. 2016. The role of obesity in the relation between total water intake and urine osmolality in U.S. adults, 2009-2012. Am J Clin Nutr 104(6):1554–1561, PMID: 27935519, 10.3945/ajcn.116.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Liaw J, Smith AH. 2009. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology 20(6):807–815, PMID: 19652600, 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- Su CT, Lin HC, Choy CS, Huang YK, Huang SR, Hsueh YM. 2012. The relationship between obesity, insulin and arsenic methylation capability in Taiwan adolescents. Sci Total Environ 414:152–158, PMID: 22104380, 10.1016/j.scitotenv.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Curhan GC. 2006. Body size and 24-hour urine composition. Am J Kidney Dis 48(6):905–915, PMID: 17162145, 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ Health Perspect 120(6):779–789, PMID: 22296744, 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinescu GC, Shoemaker M, Moore H, Khanna R, Nolph KD. 2002. The relationship between urine osmolality and specific gravity. Am J Med Sci 323(1):39–42, PMID: 11814141. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1996. Biological monitoring of chemical exposure in the workplace: Guidelines. Geneva, Switzerand:World Health Organization; http://www.who.int/iris/handle/10665/41856 [accessed 20 March 2017]. [Google Scholar]
- Yeh HC. et al. 2015. Urine osmolality in the us population: implications for environmental biomonitoring. Environ Res 136:482–490, PMID: 25460670, 10.1016/j.envres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]