Abstract
Background
Unintentional overdose involving opioid analgesics is a leading cause of injury-related death in the United States.
Objectives
To evaluate the feasibility and impact of implementing naloxone prescription to patients prescribed opioids for chronic pain.
Design
2-year non-randomized intervention study.
Setting
6 safety net primary care clinics in San Francisco.
Participants
1985 adults receiving long-term opioids for pain.
Intervention
Providers and clinic staff were trained and supported in naloxone prescribing.
Measurements
Outcomes were proportion of patients prescribed naloxone, opioid-related emergency department (ED) visits, and prescribed opioid dose based on chart review.
Results
38.2% of 1,985 patients on long-term opioids were prescribed naloxone. Patients on higher doses of opioids and with a past 12-month opioid-related emergency department (ED) visit were independently more likely to be prescribed naloxone. Patients who received a naloxone prescription had 47% fewer opioid-related ED visits per month six months after the receipt of the prescription (IRR=0.53, 95%CI=0.34–0.83, P=0.005) and 63% fewer visits after one year (IRR=0.37, 95%CI=0.22–0.64, P<0.001), compared to patients who did not receive naloxone. There was no net change over time in opioid dose among those who received naloxone compared to those who did not (IRR 1.03, 95% CI 0.91–1.27, P = 0.61).
Limitations
Results are observational and may not be generalizable beyond safety net settings.
Conclusion
Naloxone can be co-prescribed to primary care patients prescribed opioids for pain. When advised to offer naloxone to all patients on opioids, providers may prioritize those with established risk factors. Providing naloxone in primary care settings may have ancillary benefits such as reducing opioid-related adverse events.
Funding Source
National Institutes of Health grant R21DA036776
Introduction
In the United States, the opioid analgesic overdose death rate increased from 1.4 to 5.4 per 100,000 adults from 1999–2011.(1) Efforts to manage this increase in mortality have focused on modifying the prescribing practices of providers.(2) Mandated urine testing, pain agreements, and inspections of prescription drug monitoring program data have become standard practice, yet there are few data to support a link between such interventions and reduced opioid-related morbidity or mortality. In fact, while opioid analgesic deaths have recently plateaued, heroin use and overdose deaths have skyrocketed, suggesting possible unintended consequences of opioid stewardship initiatives.(3, 4)
Many communities have employed the targeted distribution of naloxone, the short-acting opioid antagonist, to address opioid-related mortality.(5) Provision of naloxone to those likely to witness or experience an opioid overdose, principally illicit drug users, has been associated with substantial reductions in community-level opioid overdose mortality relative to communities that did not implement naloxone distribution.(6) Other observational and ecologic analyses have demonstrated marked reductions in opioid overdose mortality in communities that distributed naloxone, including Chicago,(7) New York City,(8) and Scotland,(9) and a meta-analysis demonstrated a higher likelihood of survival in overdose situations when lay naloxone was administered.(10) Naloxone distribution to heroin users is remarkably cost-effective.(11)
In San Francisco, California, implementation and expansion of a targeted naloxone distribution program was temporally associated with a decline in heroin overdose deaths from approximately 180 to 10 per year through 2012. The number of deaths attributed to opioid analgesics, however, exceeded 100 annually from 2010–2012.(12) The majority of these decedents received primary care in safety net clinics, and most had received long-term opioids for pain. However, literature to support naloxone prescribing to this population is limited to early descriptive analyses(13) and anecdotal reports.(14) At U.S. Army Fort Bragg, overdoses seen in the emergency department declined from 8 per month to 0 after naloxone co-prescription was started,(14) suggesting that naloxone prescription may have affected the overdose event rate by influencing patient and/or provider behavior, rather than simply being available as a reversal agent. These results are consistent with some data suggesting that heroin users who receive naloxone reduce their heroin use.(15)
In response to these data, we developed and coordinated a standardized naloxone co-prescribing program at primary care clinics in a safety net system in San Francisco. To inform the larger scale implementation of naloxone prescribing for patients prescribed opioid medications, we assessed the feasibility of introducing and scaling up naloxone co-prescribing in these primary care clinics and conducted analyses to assess the association of naloxone co-prescribing with emergency department (ED) utilization and prescribed opioid dose.
Methods
Naloxone for Opioid Safety Evaluation (NOSE) staff coordinated the clinical program and conducted the evaluation. The study was approved by the Committee on Human Research of the University of California San Francisco (CHR#13-11168).
Clinical Program
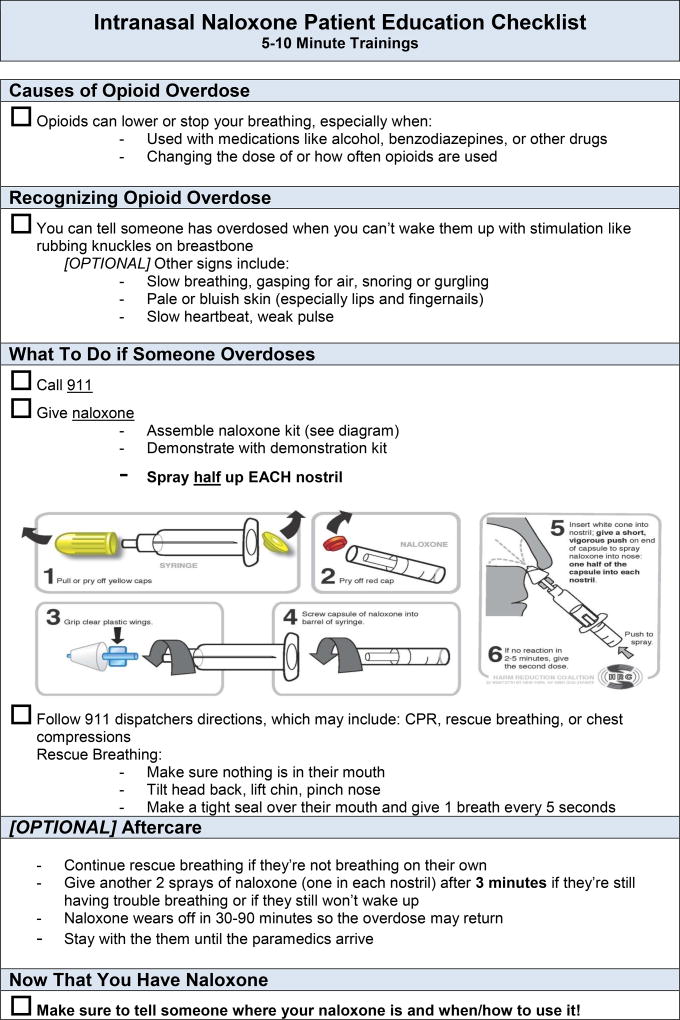
The clinical program was implemented in a rolling fashion from February 2013 to April 2014 at six clinics, all of which had lost patients to opioid overdose from 2010–2012. All clinics accepted only publicly- or un-insured patients and two were resident training sites. On-site leaders were selected and a consistent protocol was implemented across sites, beginning with training in naloxone prescribing for providers (MDs, NPs, PAs) and staff (see Methods Appendix and Appendix Table 1 for implementation plan and process outcomes). Trainings covered rationale and indications for prescribing naloxone (anyone who uses opioids chronically or is otherwise at risk for witnessing or experiencing an opioid overdose), language to approach patients (e.g., use phrases such as “bad reaction” instead of “overdose”), naloxone formulations, and pharmacy/payer coverage. Additionally, providers and staff were trained on how to educate patients about how to use naloxone, how to assemble the intranasal device (a device requiring no assembly has since been approved by the Food and Drug Administration [FDA] (16)), and to ensure caretakers know how and when to administer naloxone (Appendix Figure 1).
Initial training was provided to all sites approximately 30 days preceding initiation of naloxone co-prescription; after initiation, additional trainings were provided and at least one reminder email was sent to providers (Appendix Figure 2). As most providers opted to prescribe the intra-nasal formulation of naloxone and the mucosal atomization device was not readily available from pharmacies, clinics were able to order the device and patient brochures (Appendix Figure 3) in zip-lock bags from the clinic system’s central pharmacy. NOSE staff assisted with any logistical problems and a clinical pharmacist educated any pharmacies that encountered problems ordering, dispensing, or billing for naloxone (Appendix Figure 4).
Data Sources and Data Abstraction
Feasibility was assessed through chart reviews of all patients receiving opioids chronically by prescription. Patients receiving sufficient opioids to take at least one pill daily for over three months were added to a “Pain Management Registry” (PMR) by staff at each clinic. This list was downloaded every three months during the intervention period and a merged list of 3,138 patients with demographic data was generated in March 2015. A manual chart review was conducted to determine if patients were valid PMR patients during the study period and to collect the following data: (1) opioid name, dose, quantity per 30 days, and date prescribed at two clinic visits, the visit closest to the baseline date (either start of naloxone co-prescribing at the given clinic or the date the patient was added to the PMR, whichever was later) and the last visit at the clinic prior to chart review (i.e. follow-up date); (2) the date of initial naloxone prescription; and (3) dates of all ED visits at the county hospital and opioid-relatedness.
The ED visits were coded “opioid-related” in accordance with documentation for establishing drug-relatedness of ED visits from the Drug Abuse Warning Network.(17) Visits were opioid-related if considered by the documenting physician to be primarily due to an adverse event from an opioid or due to opioid-seeking behavior; a subset were coded “oversedation” if the assessment was an opioid poisoning or other complication attributed by the physician to opioid-induced sedation. Staff conducting chart reviews included a clinician who trained other staff and reviewed uncertain cases; 62.5% of charts were independently assessed by at least two reviewers (see Appendix for details). Death information was extracted from the California Electronic Death Record System on 14 July 2015.
Feasibility Analyses
We assessed bivariate relationships between all demographic and clinical characteristics presented in Table 1 and receipt of naloxone during the study period using Chi-square, Fisher’s exact (for comparisons with cell sizes <5), and Wilcoxon rank sum tests. Morphine equivalent daily dose in milligrams (MEQ) was calculated for each patient at their baseline and subsequent follow-up dates using standard conversion ratios from the literature.(18, 19)
Table 1.
Demographic and clinical characteristics of Pain Management Registry patients by receipt of a naloxone prescription
| Received Naloxone | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| No | Yes | Total | ||||
|
|
|
|||||
| N | (%) | N | (%) | N | (%) | |
|
|
|
|||||
| Total | 1226 | (61.8) | 759 | (38.2) | 1985 | (100.0) |
| Gender | ||||||
| Female | 503 | (61.2) | 319 | (38.8) | 822 | (41.4) |
| Male | 723 | (62.2) | 440 | (37.8) | 1163 | (58.6) |
| Age* | ||||||
| Mean Age (SD) | 57.3 | (10.8) | 55.7 | (10.7) | 56.7 | (10.8) |
| Race/Ethnicity* | ||||||
| White | 338 | (55.8) | 268 | (44.2) | 606 | (30.5) |
| Black | 622 | (64.8) | 338 | (35.2) | 960 | (48.4) |
| Hispanic/Latino | 175 | (66.0) | 90 | (34.0) | 265 | (13.4) |
| Other | 91 | (59.1) | 63 | (40.9) | 154 | (7.8) |
| Clinic* | ||||||
| Clinic A | 431 | (68.8) | 195 | (31.2) | 626 | (31.5) |
| Clinic B | 313 | (69.9) | 135 | (30.1) | 448 | (22.6) |
| Clinic C | 165 | (48.7) | 174 | (51.3) | 339 | (17.1) |
| Clinic D | 199 | (67.5) | 96 | (32.5) | 295 | (14.9) |
| Clinic E | 98 | (44.5) | 122 | (55.5) | 220 | (11.1) |
| Clinic F | 20 | (35.1) | 37 | (64.9) | 57 | (2.9) |
| Morphine Equivalent Daily Dose (MEQ)* | ||||||
| ≤20mg | 418 | (72.8) | 156 | (27.2) | 574 | (28.9) |
| 21–60mg | 338 | (66.9) | 167 | (33.1) | 505 | (25.4) |
| 61–120mg | 165 | (56.5) | 127 | (43.5) | 292 | (14.7) |
| 121–200mg | 109 | (54.2) | 92 | (45.8) | 201 | (10.1) |
| 201–400mg | 113 | (49.6) | 115 | (50.4) | 228 | (11.5) |
| ≥400mg | 83 | (44.9) | 102 | (55.1) | 185 | (9.3) |
| Prescribed Opioid | ||||||
| Codeine | 130 | (67.4) | 63 | (32.6) | 193 | (9.7) |
| Hydrocodone* | 361 | (70.0) | 155 | (30.0) | 516 | (26.0) |
| Oxycodone* | 523 | (57.0) | 394 | (43.0) | 917 | (46.2) |
| Morphine* | 269 | (53.6) | 233 | (46.4) | 502 | (25.3) |
| Methadone* | 106 | (53.3) | 93 | (46.7) | 199 | (10.0) |
| Hydromorphone | 33 | (54.1) | 28 | (45.9) | 61 | (3.1) |
| Fentanyl* | 20 | (41.7) | 28 | (58.3) | 48 | (2.4) |
| Other‡ | 12 | (63.2) | 7 | (36.8) | 19 | (1.0) |
| Opioid Dose Change During Study Period*† | ||||||
| Mean Dose Change in MEQ (SD) | −21.6 | (197.6) | −44.9 | (228.2) | −31 | (210.0) |
| Median Dose Change in MEQ (IQR) | 0 | (−15, 5) | 0 | (−50, 3) | 0 | (−25, 4.5) |
| Increase | 340 | (62.7) | 202 | (37.3) | 542 | (27.3) |
| No Change | 415 | (65.7) | 217 | (34.3) | 632 | (31.8) |
| Reduction | 279 | (53.4) | 243 | (46.6) | 522 | (26.3) |
| Discontinuation | 192 | (66.4) | 97 | (33.6) | 289 | (14.6) |
| ED Visits During 12 Months Prior to Baseline Date | ||||||
| Any visit* | 390 | (58.3) | 279 | (41.7) | 669 | (33.7) |
| Any opioid-related visit* | 59 | (46.5) | 68 | (53.5) | 127 | (6.4) |
| Any oversedation visit | 14 | (46.7) | 16 | (53.3) | 30 | (1.5) |
| ED Visits Between 1/1/13 and End of Follow-Up | ||||||
| Patients with any visit | 644 | (60.7) | 417 | (39.3) | 1061 | (53.5) |
| Patients with any opioid-related visit* | 130 | (52.8) | 116 | (47.2) | 246 | (12.4) |
| Patients with any oversedation visit* | 31 | (46.3) | 36 | (53.7) | 67 | (3.4) |
| Annual ED Visit Rate Between 1/1/13 and End of Follow-Up | ||||||
| Mean rate of any any type of visit (SD) | 0.87 | (2.0) | 0.99 | (2.0) | 0.91 | (2.0) |
| Mean rate of opioid-related visits (SD)† | 0.11 | (0.6) | 0.13 | (0.6) | 0.12 | (0.6) |
| Mean rate of oversedation visits (SD)† | 0.017 | (0.1) | 0.024 | (0.1) | 0.020 | (0.1) |
| Deaths During Study Period | ||||||
| All-cause | 40 | (67.8) | 19 | (32.2) | 59 | (3.0) |
| Opioid poisoning§ | 3 | (60.0) | 2 | (40.0) | 5 | (0.3) |
P < 0.05 from chi-squared or Fisher's exact test
P < 0.05 from Wilcoxon rank-sum test
Other opioids included buprenorphine for pain and meperidine
Bivariate relationship assessed with Fisher's exact test due to small cell sizes
We fit a normal-logistic regression model, with random effects for providers, to assess both patient- and provider-level predictors of naloxone prescription. All baseline patient characteristics assessed in bivariate analyses were included in the model, except for opioid type, which was excluded because relevant elements of formulations (e.g. presence of acetaminophen or duration of action) do not necessarily correspond to opioid type. Only baseline history of any opioid-related ED visit was included in the model because this category of visit was hypothesized to be most relevant to naloxone prescribing. The model also included provider type (attending physician or fellow, resident physician, or other provider) and the size of each provider’s panel of PMR patients, while controlling for time in days from February 1, 2013 (the earliest program initiation date) to patient baseline date, as well as time between the baseline and follow-up visit dates.
To characterize residual differences among providers in naloxone prescription rates, we calculated the odds-ratio for the difference between the 25th and 75th percentile values of the random provider effect. A descriptive summary of the PMR panel size, number of patients prescribed naloxone, and the percentage of patients prescribed naloxone per provider is presented in the Appendix (see Appendix Table 2).
Analysis of ED Use
In our pre-specified plan to assess the association of naloxone receipt with opioid-related ED visits, numbers of opioid-related ED visits were calculated for each patient in each month between January 2013 and the date of chart review (March to October 2015). For patients who died during the study period (N=59), follow-up ended at the date of death.
We then developed a multivariable Poisson regression model for the monthly number of opioid-related ED visits, using an offset to account for days of exposure in each month (ranging from one to 31 with an average of 30.0). This model used generalized estimating equations (GEE) with exchangeable working correlation and robust standard errors to account for clustering by patient as well as over-dispersion. The effect of receipt of a naloxone prescription was assessed using two time-dependent covariates: the first, an indicator for all months after the first naloxone prescription, models the immediate effect; and the second, the number of months since first naloxone prescription, captures subsequent increases or decreases in the prescription effect; this has value zero before receipt of naloxone. Patients never prescribed naloxone were assigned values of zero for both covariates.
The model adjusted for age, race/ethnicity, gender, MEQ at baseline date, history of any opioid-related ED visit between January 1, 2012 and December 31, 2012, and clinic. The model also flexibly controlled for secular trends in ED utilization using a 3-knot restricted cubic spline in calendar month, starting from January 2013; as a result, effect estimates for having received a naloxone prescription are net of any underlying secular trend.
To illustrate the estimated naloxone effects, we plotted the expected number of ED visits in each month for two patients, one who received naloxone, the other who did not, with the time scale for both trajectories centered on the median month of naloxone prescription; for both patients, expected values were evaluated at the mean values of all covariates. Similar plots stratified by clinic and models allowing modification of both the immediate naloxone prescription effect and subsequent changes in the effect over time by clinic are presented in the appendix (see Appendix Figure 5 for plots and Appendix Table 3 [regression results]). In a sensitivity analysis, we counted opioid overdose deaths that occurred during the study period as an event. In a second sensitivity analysis, we adjusted for whether or not the patient ever received naloxone during the study period, to control for unmeasured differences between individuals who were and were not prescribed naloxone that may not have been accounted for by the included demographic and clinical covariates. We conducted a third sensitivity analysis in which we excluded the variable indicating a history of any opioid-related ED visit between January 1, 2012 and December 31, 2012.
Analysis of Opioid Dose
We fit an adjusted GEE negative binomial model for the baseline and follow-up total MEQ values, set up in essentially the same way as the model for opioid-related ED visits. Negative binomial models accommodate severe right skewness and also zero values, observed at follow-up among participants whose opioids were discontinued. Specifically, we used the same two time-dependent covariates to model the immediate effect of having received a naloxone prescription as well as changes in this effect, net of the secular effect modeled using a 3-knot restricted cubic spline in months since February 1, 2013 (the program initiation date), and controlling for age, gender, race/ethnicity, history of any opioid-related ED visit, and clinic. However, in line with our sensitivity model for ED visits, we included an indicator for naloxone group as a fixed effect (i.e. whether or not the patient ever received naloxone during the study period), to capture the systematically higher total MEQ at baseline in the group that went on to receive a naloxone prescription; this difference could not be adequately controlled by the covariates available to us. This is analogous to an analysis of pre- and post-treatment values in a randomized trial using group, time, and their interaction, with the main effect for group capturing any baseline between-group differences.
Finally, as indicated by exploratory analysis, we allowed this baseline group effect to vary by clinic, using an interaction term. As in the analysis of ED visits, we illustrate the estimated naloxone effects by plotting expected MEQ dose for two patients, one of whom received naloxone, both with typical covariate levels, and the time scale centered on the median month of naloxone prescription. Similar plots stratified by clinic and models allowing modification of both the immediate naloxone prescription effect and subsequent changes in the effect over time by clinic are presented in the appendix (see Appendix Figure 6 for plots and Appendix Table 4 [regression results]).
Motivated by the hypothesis that naloxone prescription could lead providers to decrease total MEQ for some patients and increase it for others, we also categorized the change in prescribed opioid dose between the baseline and follow-up clinic visits as increased, decreased/discontinued, or unchanged, and used a multinomial logistic regression model to assess the association of naloxone prescription with this 3-level outcome, with no change in dose as the reference level of the outcome (see Appendix).
Role of the Funding source
The funder, the National Institute on Drug Abuse under grant R21DA036776, had no role in the design, conduct, or reporting of this study.
Results
Patient Characteristics
A total of 3,138 patient chart reviews identified 1,985 patients prescribed opioids chronically for pain management from the clinics during the time of naloxone prescribing (see Table 1). The excluded patients included those who, at the start of naloxone prescribing, were no longer in care at the clinics (600), not prescribed opioids (447), deceased (21), or prescribed opioids only for opioid use disorder treatment (85). There were more men than women and Blacks accounted for the plurality of patients. Baseline opioid dose ranged from 2–4,200MEQ/day, with a median dose of 53MEQ/day. Over one-quarter received <=20MEQ/day and nearly 10% received >400MEQ/day. Oxycodone was the most commonly prescribed opioid, followed by hydrocodone and morphine. Patient characteristics stratified by clinic are presented in the appendix (see Appendix Table 5).
Feasibility of Naloxone Prescribing
During the study period, naloxone was prescribed to 759 pain patients (38.2%) over 2,254 patient-years. Patients who received naloxone accounted for 19 (32.2%) of 59 deaths during the study period and 2 (40%) of 5 opioid poisoning deaths. Our logistic regression model assessing predictors of naloxone prescription included only the 1,805 (90.9%) patients for whom provider data was available. In this analysis, patients who were on a higher dose of opioids or seen in the county ED for an opioid-related visit in the 12 months preceding program initiation at their clinic were more likely to receive a naloxone prescription (see Table 2).
Table 2.
Multivariable logistic regression model assessing odds of naloxone prescription (N=1,805 patients)*
| aOR† | (95% CI) | P-Value | |
|---|---|---|---|
| Age (5 Year Units) | 0.94 | (0.89 – 1.00) | 0.036 |
| Race/Ethnicity | |||
| White | Reference | ||
| Black | 0.77 | (0.58 – 1.03) | 0.078 |
| Hispanic/Latino | 0.74 | (0.49 – 1.13) | 0.162 |
| Other | 0.74 | (0.45 – 1.22) | 0.239 |
| Gender | |||
| Female | Reference | ||
| Male | 0.99 | (0.77 – 1.27) | 0.945 |
| Log Morphine Equivalent Daily Dose (MEQ) | 1.73 | (1.57 – 1.92) | <0.001 |
| ED Visit During 12 Months Prior to Baseline Date‡ | 2.54 | (1.54 – 4.18) | <0.001 |
| Provider Type | |||
| Attending Physician / Fellow | Reference | ||
| Resident Physician | 1.84 | (0.98 – 3.45) | 0.058 |
| Other Provider | 0.83 | (0.41 – 1.68) | 0.606 |
| Number of PMR Patients Seen By Provider | 1.00 | (0.98 – 1.02) | 0.691 |
Adjusted for patient clinic, number of days elapsed between the earliest date of program initiation (February 1, 2013) and patient baseline date and number of years elapsed between patient baseline date and subsequent follow-up date.
aOR = Adjusted Odds Ratio
Includes only opioid-related ED visits
Older patients had lower odds of being prescribed naloxone. Receiving a naloxone prescription was also dependent on which clinic patients attended, with three clinics (including one of two resident training sites) prescribing naloxone to a substantially lower proportion of patients than the other clinics. Although statistically insignificant (p>0.05), there were trends towards a lower odds of being prescribed naloxone among Black patients compared to white patients and a higher odds of prescribing naloxone among resident physicians compared to attending physicians and fellows. The OR for the difference between the 25th and 75th percentiles of the provider random effect, our measure of residual between-provider variability in naloxone prescription rates not accounted for by the fixed effects in the model, was 5.06 (95% CI: 3.45–6.9).
Opioid-Related Emergency Department Visits
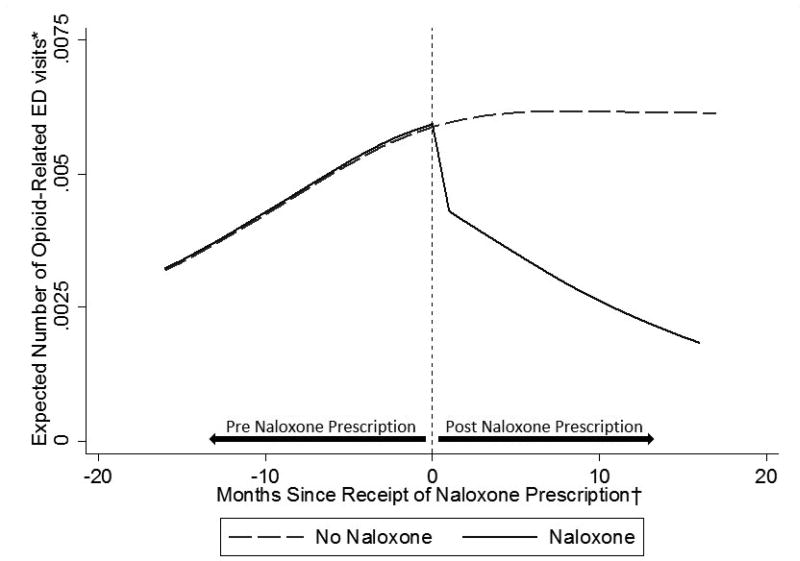
There were a total of 4,322 ED visits during the study period, 471 of which were opioid-related and 95 which were attributed to opioid-induced oversedation. On average, patients had 6% fewer opioid-related ED visits with each additional month since the receipt of a naloxone prescription (IRR = 0.94, 95% CI = 0.89–0.998, P = 0.044), controlling for all demographic and clinical covariates and secular trends in emergency department utilization. This monthly decrease in opioid-related ED visits after the receipt of a naloxone prescription corresponds to a 47% reduction in opioid-related ED visits per month six months after receipt of the prescription (IRR = 0.53, 95% CI = 0.34 – 0.83, P = 0.005) and a 63% reduction after one year (IRR = 0.37, 95% CI = 0.22 – 0.64, P < 0.001).
Figure 1 shows the pattern of expected ED visit rates for two typical patients, one of whom received naloxone. Results were essentially unchanged when the five opioid poisoning deaths that occurred during the study period were included as events (IRR = 0.95, 95% CI = 0.89–1.00, P = 0.050) and in our sensitivity analysis adjusting for ever receiving a naloxone prescription (IRR = 0.94, 95% CI = 0.89–1.00, P = 0.039). In our final sensitivity analysis excluding history of any opioid-related ED visit, the evidence for the relationship between months since naloxone prescription and the monthly number of ED visits was marginally insignificant (IRR = 0.94, 95% CI = 0.88–1.01, P = 0.080).
Figure 1.
Expected number of opioid-related emergency department visits per month by receipt of naloxone prescription
*Expected number of emergency department visits per month calculated for two patients, one who received a naloxone prescription and another who did not, both with mean values of all covariates
† For both trajectories, time was uniformly centered on April 2014, the median month of receipt of naloxone prescription during the study period among patients who received naloxone.
Prescribed Opioid Dose
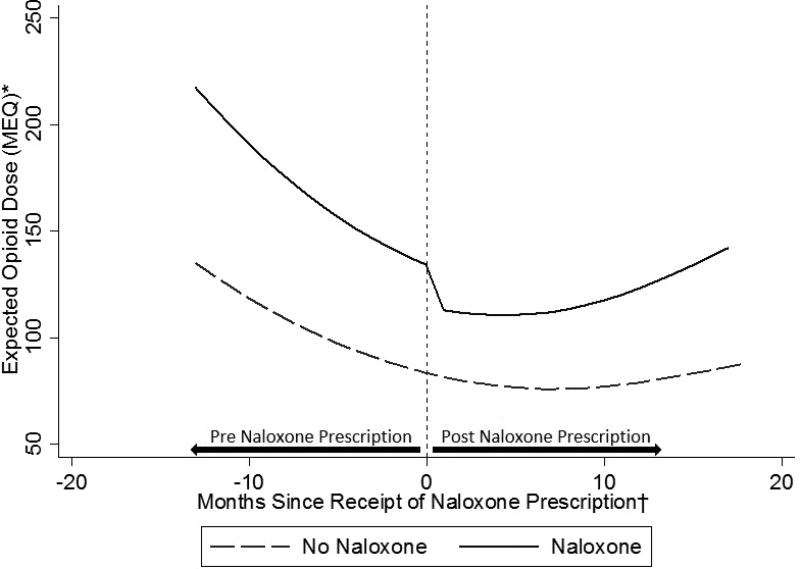
In the generalized estimating equation negative binomial model for expected MEQ, the baseline secular trend showed a rapid decrease followed by leveling off (P < 0.0005 for both the overall effect and its non-linearity), as well as strong baseline differences between the two groups, in particular at two of the six clinics. After controlling for demographic and clinical characteristics and secular trend, we found a nominal 15% decrease in total MEQ at the time of naloxone prescription (IRR = 0.85, 95% CI = 0.67–1.08, P = 0.191), followed by 1% monthly increases in dose (IRR 1.01, 95% CI = 0.996 – 1.03, P = 0.154), resulting in an estimated net effect at 18 months of nil (IRR 1.03, 95% CI = 0.91–1.27, P = 0.61; Table 4). These effects are illustrated for two typical patients in Figure 2. In our additional analysis using multinomial logistic regression, having received a naloxone prescription was associated with a decrease or discontinuation in opioid dose (RRR = 1.47, 95% CI = 1.17 – 1.86, P = 0.001) but not significantly associated with an increase in dose (RRR = 1.18, 95% CI = 0.92 – 1.52, P = 0.198) (see Appendix Table 6).
Table 4.
Multivariable negative binomial regression model fit with generalized estimating equations assessing opioid dose at baseline and follow-up (N=1,985 patients)*
| IRR† | (95% CI) | P-Value | |
|---|---|---|---|
| Immediate Naloxone Effect | 0.85 | (0.67 – 1.08) | 0.191 |
| Naloxone Trend Effect Per Additional Month After Naloxone Receipt | 1.01 | (1.00 – 1.03) | 0.154 |
| Age (5 Year Units) | 0.99 | (0.97 – 1.02) | 0.725 |
| Race/Ethnicity | |||
| White | Reference | ||
| Black | 0.83 | (0.71 – 0.98) | 0.031 |
| Hispanic/Latino | 0.63 | (0.50 – 0.79) | <0.001 |
| Other | 0.45 | (0.35 – 0.58) | <0.001 |
| Gender | |||
| Female | Reference | ||
| Male | 1.19 | (1.04 – 1.37) | 0.012 |
| ED Visit During 12 Months Prior to Baseline Date‡ | 1.43 | (1.11 – 1.83) | 0.005 |
Adjusted for patient clinic, a naloxone group indicator (i.e. whether or not patient ever received naloxone during the study period), and a cubic spline in calendar month. The model allowed for the effect of the naloxone group indicator to vary by clinic, using an interaction term.
IRR = Incidence Rate Ratio
Includes only opioid-related ED visits
Figure 2.
Expected opioid dose (MEQ – morphine equivalent daily dose in milligrams) by receipt of naloxone prescription
*Expected morphine equivalent daily dose in milligrams among two patients, one who received a naloxone prescription and another who did not, both with mean values of all covariates
† For both trajectories, time was uniformly centered on April 2014, the median month of receipt of naloxone prescription during the study period among patients who received naloxone.
Discussion
This non-randomized intervention study found that primary care providers prescribed naloxone to a substantial proportion of patients receiving chronic opioid therapy for pain management. When advised to offer naloxone to all patients on long-term opioids, clinicians were more likely to prescribe to those likely to be at higher risk for overdose, including patients on higher doses of opioids and those who have had opioid-related ED visits in the past. In the absence of guideline-based indications for naloxone co-prescribing, these may be reasonable metrics upon which to prioritize prescription of naloxone. In fact, the Centers for Disease Control and Prevention recently released guidelines on opioid prescribing that recommend considering naloxone prescription for patients with a prior history of overdose, a history of a substance use disorder, an opioid dose > 50 MEQ, or concurrent benzodiazepine use.(20)
Nonetheless, there may be hazards to risk stratifying patients for naloxone prescription, including stigma, medico-legal concerns about acknowledging a patient’s elevated risk of overdose, and failure to reach the high proportion of potential decedents who access intentionally or unintentionally diverted opioids.(21) Finally, there may be a behavioral impact of naloxone co-prescription in which patients become more aware of the hazards of these medications and engage in efforts to improve medication safety – a benefit hinted at by our analyses.
The proportion of patients prescribed naloxone both by clinic and by provider varied substantially both by clinic and by provider. In addition, older patients were less likely to receive naloxone prescriptions and there was weak evidence suggesting the same for Black patients. There are multiple possible explanations for this variation. As prescribing naloxone was not considered standard practice and lacked the wealth of data supporting most other routine preventive medical interventions, some providers may have opted to not follow the recommendations for naloxone prescribing and vocal “champions” at selected clinics may have been able to substantially influence other providers. With regard to patient-level factors, the median age of opioid overdose death in San Francisco is 50 years,(12) suggesting unmet need for naloxone among older patients. Similarly, Blacks were overrepresented among PMR patients in the safety net clinics (particularly in two of the low-prescribing clinics, representing 88.4% of patients at one and 42.5% of patients at another), as well as among opioid overdose decedents, relative to the San Francisco population.(12) Changes in clinic protocols and additional provider education may be needed to ensure access to naloxone to patients most at risk.
Receipt of naloxone was independently associated with a reduction in opioid-related ED visits over time, raising the possibility that providing naloxone impacted patient behavior with respect to opioids. This finding is consistent with prior observations of similar benefits with naloxone receipt among patients prescribed opioids at U.S. Army Fort Bragg(14) and among some heroin users trained in overdose prevention.(15) Such a change was not found in an interrupted time series of community distribution of naloxone,(6) suggesting that any associated behavioral modification may be dependent upon the mode of intervention delivery. In addition, we found no net effect of naloxone receipt on opioid dose over time, and a possible reduction in dose in an alternative analysis, alleviating potential concerns that providing naloxone could result in risk compensation via increased use of opioids. These potential benefits of naloxone provision should be targets for future research.
This study had several limitations. First, we cannot definitively infer causality from this observational study. Second, data collected by chart review may vary based on documentation patterns; however, the size of our sample should reduce the effect of such variation. Third, our data do not confirm that patients filled their naloxone prescription. Fourth, we were unable to ascertain if patients sought care outside of the safety net system. In addition, we were unable to assess details of patients’ substance use history and incarceration events, factors that may influence naloxone prescribing and overdose risk. Finally, results may not be generalizable outside of safety-net clinical care settings.
In summary, we demonstrated that naloxone can be successfully prescribed to a substantial proportion of patients on opioids for chronic pain in primary care practices. Naloxone co-prescribing was associated with reduced opioid-related ED visits, suggesting a possible ancillary benefit of reducing opioid-related adverse events, and no net change in opioid dose. Naloxone prescribing is now more straightforward, with recent FDA approval of naloxone devices designed for lay persons.(16)
Table 3.
Multivariable Poisson regression fit with generalized estimating equations assessing count of opioid-related emergency department visits per month (N=1,985 patients)*
| IRR† | (95% CI) | P-Value | |
|---|---|---|---|
| Immediate Naloxone Effect | 0.76 | (0.42 – 1.36) | 0.355 |
| Naloxone Trend Effect Per Additional Month After Naloxone Receipt | 0.94 | (0.89 – 0.998) | 0.044 |
| Age (5 Year Units) | 0.94 | (0.85 – 0.97) | 0.003 |
| Race/Ethnicity | |||
| White | Reference | ||
| Black | 0.91 | (0.50 – 1.66) | 0.769 |
| Hispanic/Latino | 1.21 | (0.46 – 3.17) | 0.702 |
| Other | 1.40 | (0.63 – 3.10) | 0.415 |
| Gender | |||
| Female | Reference | ||
| Male | 1.61 | (1.09 – 2.37) | 0.017 |
| Log Morphine Equivalent Daily Dose (MEQ) | 1.25 | (1.04 – 1.51) | 0.017 |
| ED Visit During 12 Months Prior to Baseline Date‡ | 9.65 | (5.68 – 16.40) | <0.001 |
Adjusted for patient clinic and a cubic spline of the sequential count of patient-months starting with a value of one for January 2013.
IRR = Incidence Rate Ratio
Includes only opioid-related ED visits
Acknowledgments
Authors would like to thank Michele Geier and the site leads at each clinic - Soraya Azari, Barbara Wismer, Jan Gurley, and Keith Seidel - for participation in coordination of this project.
Funding Source: National Institutes of Health grant R21DA036776
Methods Appendix
Clinical Program
NOSE staff provided initial and ongoing trainings at each clinic and provided ongoing support throughout the pilot. NOSE staff conducted on-site naloxone prescribing and education trainings at each clinic prior to program initiation and provided additional trainings intermittently throughout the study (see Appendix Table 1). Clinic-wide staff received information about the program at least once through in-person meetings and staff-wide emails; providers, nurses and medical assistants (MEAs) received additional specialized education through group-specific meetings and one-on-one trainings.
Meetings with providers focused on technical aspects of naloxone prescribing, including entering the prescription into the electronic medical record, interfacing with pharmacies, delegation of naloxone prescribing and education tasks, and fielding provider questions and concerns. These trainings also covered non-stigmatizing language to present naloxone to patients. Trainings were often conducted at provider-wide meetings or smaller provider “huddles” which varied in size and length. Provider trainings included from 5–30 providers and were 5 to 60 minutes in duration.
One-on-one trainings were also conducted with the nursing and MEA staff to discuss educating patients who were receiving naloxone prescriptions. These trainings were designed to ensure familiarity with the naloxone device, including its formulation, assembly, and indications for when and how to use it and to ensure comfort with the education guidelines as described in Appendix Figure 1. One-on-one trainings included roll-plays and lasted from 5–15 minutes.
After rollout, NOSE staff remained engaged with clinic activities and available to provide technical support, such as addressing problems with pharmacy access to naloxone and access to naloxone kit supplies, such as the atomizer and brochure.
Support for all six clinics combined required on average approximately 20% full-time effort per year provided by mid-level non-clinical staff.
Data Sources and Data Abstraction
A total of 3,138 charts were reviewed, identifying 1,985 patients eligible for inclusion in the study. Patients were excluded if, at the start of naloxone prescribing they were not in care (600), not prescribed opioids (447), on opioids for opioid use disorder treatment only (85), or deceased (21). At least 1,241 (62.5%) of the 1,985 eligible charts were assessed by one or more additional reviewers. These additional assessments occurred in several different manners. First, reviewers were instructed to mark “review” on any charts for which there was uncertainty about any data elements, resulting in a second assessment of at least 908 charts (an unquantified number of additional charts were assessed by a second reviewer in real-time when the initial reviewer had questions). Second, at the conclusion of data collection, to ensure charts assessed early on in the process were consistent with interpretations made later in the process, all 339 charts from the first clinic reviewed were assessed by a second reviewer. Third, at the conclusion of data collection, the 409 charts assessed by reviewers who had assessed less than 20% of the total charts were assessed by a second reviewer. Finally, at the conclusion of data collection, 63 additional charts not re-assessed through any of the prior processes were randomly selected for a final assessment. Data were not collected with regard to changes made during secondary reviews, with the exception of the final random review of 63 charts which resulted in no changes to any data elements. The total number of repeated assessments exceeds the total number of charts that were re-assessed because some charts marked for “review” were later selected for re-assessment.
Analysis of Opioid Dose
In an additional analysis, motivated by the hypothesis that naloxone prescription could lead providers to decrease total MEQ for some patients and increase it for others, depending on current dose as well as unmeasured patient characteristics, we categorized the change in prescribed opioid dose between the first and final clinic visits as increased, decreased/discontinued, or unchanged. We then used multinomial logistic regression to assess the association of naloxone prescription with this multinomial 3-level outcome, with no change in dose as the reference level of the outcome, and controlling for patient age, race/ethnicity, gender, and history of an opioid-related ED visit in the one year prior to baseline date. The model also flexibly adjusted for a linear secular trend as the time in days from February 1, 2013 (the earliest program initiation date) to patient baseline date, as well as time between the baseline and follow-up visits. Adjustment for baseline MEQ could induce collider-stratification bias if this potentially important confounder is a common effect of both unmeasured confounders as well as measurement error in both the baseline and follow-up dose(1); as result, we omit baseline MEQ from the model. The results from this analysis are presented in Appendix Table 6.
Appendix Figure 1.
Checklist for clinic staff to train patients receiving naloxone
Appendix Figure 2.
Email template to remind providers about naloxone prescribing
Appendix Figure 3.
Naloxone for Opioid Safety patient brochure
Appendix Figure 4.
Informational sheet for pharmacists on ordering, dispensing, counseling, and billing for naloxone
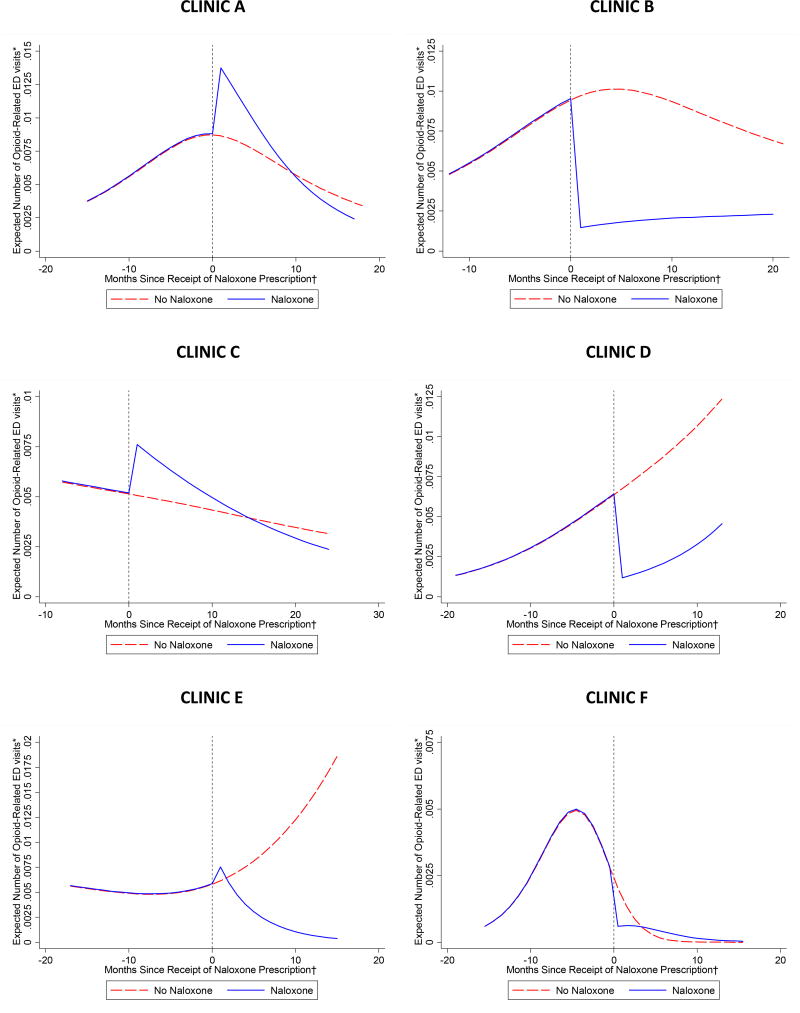
Appendix Figure 5.
Expected number of opioid-related emergency department visits per month by receipt of naloxone prescription, stratified by clinic
*Expected number of emergency department visits per month among two patients, one who received a naloxone prescription and another who did not, both with mean values of all covariates and stratified by clinic.
†For both trajectories, time was uniformly centered on April 2014, the median time of receipt of naloxone prescription during the study period among patients who received naloxone.
Appendix Figure 6.
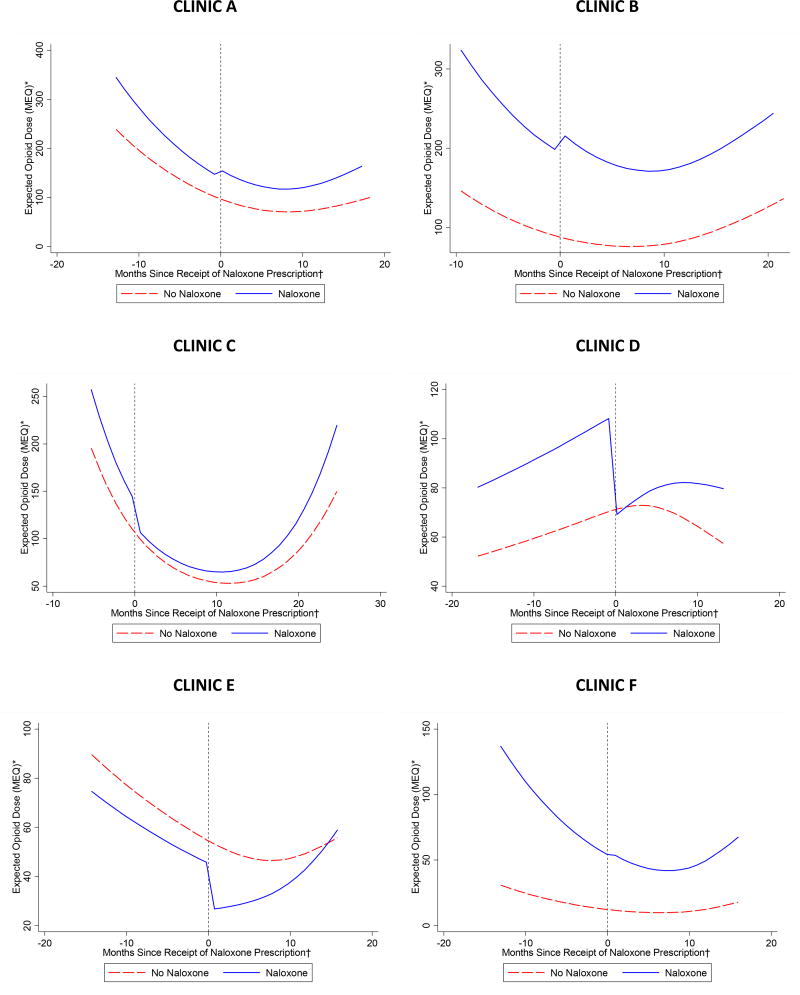
Expected opioid dose (MEQ – morphine equivalent daily dose in milligrams) by receipt of naloxone prescription, stratified by clinic
*Expected morphine equivalent daily dose in milligrams among two patients, one who received a naloxone prescription and another who did not, both with mean values of all covariates and stratified by clinic
† For both trajectories, time was uniformly centered on April 2014, the median time of receipt of naloxone prescription during the study period among patients who received naloxone.
Appendix Table 1.
Implementation plan and process outcomes for naloxone co-prescribing at safety net clinics
| Activity | # of occurrences |
Timeframe | Purpose | Personnel | # Conducted at clinics | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A Start- date 2/1/13 |
B Start- date 2/1/13 |
C Start- date 2/1/13 |
D Start- date 2/1/13 |
E Start- date 2/1/13 |
F Start- date 2/1/13 |
|||||
| NOSE introduction meeting |
1 | 2–3 months prior to program initiation | Introduce program to clinic leaders and discuss rollout logistics | NOSE study staff, clinic director, nurse manager and “champions” | 1 | 1 | 1 | 1 | 1 | 1 |
| Clinic-wide staff training |
≥1 | 1–2 months pre-initiation | Introduce / review program with all staff, disseminate naloxone education checklist* | NOSE study staff, all clinic staff | 3 | 1 | 1 | 4 | 3 | 2 |
| Naloxone kit material procurement, kit creation and site storage | Ongoing | 2 months pre-initiation; 1 month pre-initiation determine communal location for kit storage; restock and assemble kits on ongoing basis when supplies are low | Obtain and assemble naloxone prescribing materials for “kit” including: atomizers, plastic bags, and patient education brochures | Designated clinic staff | N/A | N/A | N/A | N/A | N/A | N/A |
| Provider trainings | ≥1 | 0–1 months pre-initiation | Answer questions related to provider-specific activities and remind providers of protocol | NOSE study staff, clinic providers (MD, NP, PA) | 2 | 6* | 11** | 1 | 1 | 1 |
| Nurse/MEA trainings | ≥1 | 0–1 months pre-initiation | Answer questions related to nurse/MEA-specific activities; Nurses/MEAs 1-on-1 roll-plays | NOSE study staff, clinic nurses and MEAs | 4 | 0 | 2 | 2 | 2 | 1 |
| Staff-wide emails† | ≥2 | At rollout; 3–4 months after initiation | Alert clinic staff of program start and refresh on protocol and purpose | Clinic director | 2 | 2 | 2 | 2 | 2 | 1 |
| Ongoing technical support | Ongoing | Ongoing | Provide technical assistance | NOSE study staff | N/A | N/A | N/A | N/A | N/A | N/A |
Five of these trainings included 5–10 providers each in pre-clinic “huddles”
Ten of these trainings included 5–10 chief residents (5) or 5–10 providers in pre-clinic “huddles” (5)
Email template provided by NOSE staff; see Appendix Figure 2.
Appendix Table 2.
Number of providers and demographic and clinical characteristic of patients by clinic
| Clinic A | Clinic B | Clinic C | Clinic D | Clinic E | Clinic F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||
| Total Number of Providers | 22 | 85 | 75 | 12 | 11 | 6 | |||||||
|
| |||||||||||||
| Total Number of Patients | 626 | 448 | 339 | 295 | 220 | 57 | |||||||
|
| |||||||||||||
| Prescribed Naloxone | 195 | (31.2) | 135 | (30.1) | 174 | (51.3) | 96 | (32.5) | 122 | (55.5) | 37 | (64.9) | |
| Sex | |||||||||||||
| Female | 211 | (33.7) | 181 | (40.4) | 181 | (53.4) | 124 | (42.0) | 99 | (45.0) | 26 | (45.6) | |
| Male | 415 | (66.3) | 267 | (59.6) | 158 | (46.6) | 171 | (58.0) | 121 | (55.0) | 31 | (54.4) | |
| Age | |||||||||||||
| Mean Age (SD) | 56.4 | (9.1) | 57.7 | (11.5) | 54.6 | (12.6) | 58.7 | (10.3) | 56.3 | (10.5) | 57.2 | (12.6) | |
| Race/Ethnicity | |||||||||||||
| White | 269 | (43.0) | 129 | (28.8) | 103 | (30.4) | 26 | (8.8) | 49 | (22.3) | 30 | (52.6) | |
| Black | 266 | (42.5) | 203 | (45.3) | 121 | (35.7) | 249 | (84.4) | 111 | (50.5) | 10 | (17.5) | |
| Hispanic/Latino | 59 | (9.4) | 82 | (18.3) | 74 | (21.8) | 11 | (3.7) | 34 | (15.5) | 5 | (8.8) | |
| Other | 32 | (5.1) | 34 | (7.6) | 41 | (12.1) | 9 | (3.1) | 26 | (11.8) | 12 | (21.1) | |
| Morphine Equivalent Daily Dose (MEQ) | |||||||||||||
| ≤20mg | 165 | (26.4) | 96 | (21.4) | 90 | (26.5) | 109 | (36.9) | 91 | (41.4) | 23 | (40.4) | |
| 21–60mg | 148 | (23.6) | 119 | (26.6) | 78 | (23.0) | 81 | (27.5) | 59 | (26.8) | 20 | (35.1) | |
| 61–120mg | 101 | (16.1) | 66 | (14.7) | 45 | (13.3) | 43 | (14.6) | 30 | (13.6) | 7 | (12.3) | |
| 121–200mg | 77 | (12.3) | 44 | (9.8) | 34 | (10.0) | 24 | (8.1) | 19 | (8.6) | 3 | (5.3) | |
| 201–400mg | 84 | (13.4) | 58 | (12.9) | 40 | (11.8) | 31 | (10.5) | 14 | (6.4) | 1 | (1.8) | |
| ≥400mg | 51 | (8.1) | 65 | (14.5) | 52 | (15.3) | 7 | (2.4) | 7 | (3.2) | 3 | (5.3) | |
| Prescribed Opioid | |||||||||||||
| Codeine | 52 | (8.3) | 36 | (8.0) | 43 | (12.7) | 29 | (9.8) | 27 | (12.3) | 6 | (10.5) | |
| Hydrocodone | 125 | (20.0) | 93 | (20.8) | 109 | (32.2) | 109 | (36.9) | 63 | (28.6) | 17 | (29.8) | |
| Oxycodone | 221 | (35.3) | 281 | (62.7) | 165 | (48.7) | 122 | (41.4) | 102 | (46.4) | 26 | (45.6) | |
| Morphine | 214 | (34.2) | 115 | (25.7) | 77 | (22.7) | 56 | (19.0) | 28 | (12.7) | 12 | (21.1) | |
| Methadone | 84 | (13.4) | 20 | (4.5) | 43 | (12.7) | 23 | (7.8) | 22 | (10.0) | 7 | (12.3) | |
| Hydromorphone | 17 | (2.7) | 22 | (4.9) | 10 | (2.9) | 5 | (1.7) | 7 | (3.2) | 0 | (0.0) | |
| Fentanyl | 13 | (2.1) | 17 | (3.8) | 12 | (3.5) | 3 | (1.0) | 3 | (1.4) | 0 | (0.0) | |
| Other* | 9 | (1.4) | 1 | (0.2) | 4 | (1.2) | 3 | (1.0) | 2 | (0.9) | 0 | (0.0) | |
| Opioid Dose Change During Study Period | |||||||||||||
| Mean Dose Change in MEQ (SD) | −34.8 | (202.5) | −38.4 | (216.1) | −58.2 | (327.2) | −9.0 | (73.8) | 5.7 | (102.2) | −7.1 | (64.5) | |
| Median Dose Change in MEQ (IQR) | 0.0 | (−33.8, 7.5) | 0.0 | (−45.0, 5.0) | −3.3 | (−45.0, 2.5) | 0.0 | (−15.0, 0.0) | 0.0 | (−2.3, 0.8) | 0.0 | (0.0, 2.3) | |
| Increase | 183 | (29.2) | 127 | (28.3) | 89 | (26.3) | 73 | (24.7) | 55 | (25.0) | 15 | (26.3) | |
| No Change | 172 | (27.5) | 134 | (29.9) | 72 | (21.2) | 114 | (38.6) | 110 | (50.0) | 30 | (52.6) | |
| Reduction | 166 | (26.5) | 127 | (28.3) | 120 | (35.4) | 63 | (21.4) | 38 | (17.3) | 8 | (14.0) | |
| Discontinuation | 105 | (16.8) | 60 | (13.4) | 58 | (17.1) | 45 | (15.3) | 17 | (7.7) | 4 | (7.0) | |
| ED Visits During 12 Months Prior to Baseline Date | |||||||||||||
| Any visit | 189 | (30.2) | 186 | (41.5) | 112 | (33.0) | 103 | (34.9) | 66 | (30.0) | 13 | (7.0) | |
| Any opioid-related visit | 36 | (5.8) | 34 | (7.6) | 17 | (5.0) | 13 | (4.4) | 21 | (9.5) | 6 | (7.0) | |
| Any oversedation visit | 11 | (1.8) | 11 | (2.5) | 4 | (1.2) | 3 | (1.0) | 1 | (0.5) | 0 | (7.0) | |
| ED Visits Between January 1, 2013 and End of Follow-Up | |||||||||||||
| Patients with any visit | 301 | (48.1) | 276 | (61.6) | 179 | (52.8) | 161 | (54.6) | 122 | (55.5) | 22 | (38.6) | |
| Patients with any opioid-related visit | 70 | (11.2) | 61 | (13.6) | 41 | (12.1) | 32 | (10.8) | 35 | (15.9) | 7 | (12.3) | |
| Patients with any oversedation visit | 22 | (3.5) | 23 | (5.1) | 9 | (2.7) | 9 | (3.1) | 4 | (1.8) | 0 | (0.0) | |
| Annual ED Visit Rate | |||||||||||||
| Mean rate of any any type of visit (SD) | 0.73 | (1.46) | 1.30 | (2.72) | 0.81 | (1.74) | 0.93 | (2.06) | 0.90 | (1.93) | 0.44 | (1.00) | |
| Mean rate of opioid-related visits (SD) | 0.09 | (0.38) | 0.13 | (0.60) | 0.07 | (0.23) | 0.13 | (0.82) | 0.23 | (1.06) | 0.07 | (0.19) | |
| Mean rate of oversedation visits (SD) | 0.02 | (0.15) | 0.03 | (0.17) | 0.01 | (0.06) | 0.02 | (0.11) | 0.01 | (0.09) | 0.00 | (0.00) | |
| Deaths During Study Period | |||||||||||||
| All-cause | 18 | (2.9) | 26 | (5.8) | 10 | (2.9) | 4 | (1.4) | 1 | (0.5) | 0 | (0.0) | |
| Opioid poisoning | 2 | (0.3) | 2 | (0.4) | 1 | (0.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |
Other opioids included buprenorphine for pain and meperidine
Appendix Table 3.
Provider-level data on total number of patients, number of patients prescribed naloxone, and percentage of patients prescribed naloxone
| Providers by Quartiles of Total Number of Patients | |||||
|---|---|---|---|---|---|
|
|
|||||
| All Providers | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
|
|
|
|
|
||
| Number of Providers | 186 | 63 | 34 | 45 | 44 |
| Number of PMR Patients Per Provider | |||||
| Mean (SD) | 9.7 (14.6) | 1.4 (0.5) | 3.4 (0.5) | 6.8 (1.7) | 29.3 (19.4) |
| Median (IQR) | 4 (2 – 10) | 1 (1 – 2) | 3 (3 – 4) | 7 (5 – 8) | 23 (13 – 44) |
| Range | 1 – 93 | 1 – 2 | 3 – 4 | 5 – 10 | 11 – 93 |
| Number of Patients Prescribed Naloxone Per Provider | |||||
| Mean (SD) | 3.8 (7.2) | 0.6 (0.6) | 1.7 (1.1) | 2.6 (2.1) | 11.1 (11.9) |
| Median (IQR) | 1 (1 – 4) | 1 (0 – 1) | 2 (1 – 2) | 2 (1 – 4) | 7 (5 – 11) |
| Percentage of Patients Prescribed Naloxone | |||||
| Mean (SD) | 42.4 (34.9) | 43.7 (43.5) | 50.7 (33.8) | 38.5 (29.6) | 38.3 (25.2) |
| Median (IQR) | (12.5 – 38.8 66.7) | 50.0 (0.0 – 100.0) | 58.3 (25 – 66.7) | 33.3 (14.3 – 85.7) | (19.2 – 27.6 58.9) |
PMR = pain management registry
Appendix Table 4.
Clinic-specific IRR values for post naloxone receipt and months since naloxone receipt on count of opioid-related emergency department visits per month*
| Post Naloxone Receipt | Months Since Naloxone Receipt | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| IRR† | (95% CI) | P-Value | Overall P-Value‡ |
IRR† | (95% CI) | P-Value | Overall P-Value‡ |
|
| Clinic A | 1.49 | (0.43 – 5.14) | 0.525 | 0.040 | 0.92 | (0.81 – 1.04) | 0.170 | 0.093 |
| Clinic B | 0.15 | (0.03 – 0.63) | 0.010 | 1.03 | (0.93 – 1.15) | 0.550 | ||
| Clinic C | 1.29 | (0.48 – 3.43) | 0.615 | 0.93 | (0.87 – 0.99) | 0.030 | ||
| Clinic D | 0.26 | (0.07 – 0.96) | 0.044 | 1.08 | (0.93 – 1.25) | 0.302 | ||
| Clinic E | 1.58 | (0.50 – 4.95) | 0.433 | 0.78 | (0.62 – 0.97) | 0.025 | ||
| Clinic F | 0.63 | (0.17 – 2.28) | 0.481 | 0.94 | (0.83 – 1.07) | 0.354 | ||
Calculated from multivariable Poisson regression, fit with generalized estimating equations, assessing count of opioid-related emergency department visits per month. Model adjusts for age, race/ethnicity, gender, log morphine equivalent daily dose, patient clinic, history of opioid-related ED visit, and a cubic spline of the sequential count of patient-months starting with a value of one for January 2013. The model includes interaction terms between patient clinic and the post naloxone receipt indicator variable as well as between patient clinic and the months since naloxone receipt continuous variable.
IRR = Incidence Rate Ratio
Overall P-Values correspond to global tests for significance of the interaction terms between clinic and either post naloxone receipt or months since naloxone receipt.
Appendix Table 5.
Clinic-specific IRR values for post naloxone receipt and months since naloxone receipt on opioid dose at baseline and follow-up*
| Post Naloxone Receipt | Months Since Naloxone Receipt | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| IRR† | (95% CI) | P-Value | Overall P-Value‡ |
IRR† | (95% CI) | P-Value | Overall P-Value‡ |
|
| Clinic A | 0.84 | (0.56 – 1.27) | 0.415 | 0.166 | 1.00 | (0.98 – 1.04) | 0.755 | 0.548 |
| Clinic B | 1.50 | (1.04 – 2.19) | 0.032 | 0.99 | (0.96 – 1.01) | 0.217 | ||
| Clinic C | 0.96 | (0.42 – 2.21) | 0.928 | 1.01 | (0.98 – 1.05) | 0.458 | ||
| Clinic D | 0.74 | (0.33 – 1.66) | 0.465 | 1.00 | (0.93 – 1.07) | 0.945 | ||
| Clinic E | 0.51 | (0.21 – 1.23) | 0.134 | 1.05 | (0.98 – 1.13) | 0.172 | ||
| Clinic F | 1.01 | (0.52 – 1.97) | 0.980 | 1.00 | (0.94 – 1.06) | 0.917 | ||
Calculated from multivariable negative binomial regression, fit with generalized estimating equations, assessing opioid dose at baseline and follow-up. Model adjusts for age, race/ethnicity, gender, patient clinic, history of opioid-related ED visit, a naloxone group indiciator (i.e. whether or not the patient ever recieved naloxone during the study period), and a cubic spline of the sequential count of patient-months starting with a value of one for January 2013. The model includes interaction terms between patient clinic and the naloxone group indicator variable, the post naloxone receipt indicator variable, the months since naloxone receipt continuous variable.
IRR = Incidence Rate Ratio
Overall P-Values correspond to global tests for significance of the interaction terms between clinic and either post naloxone receipt or months since naloxone receipt.
Appendix Table 6.
Multinomial logistic regression model assessing odds of increase in opioid dose and decrease in opioid dose relative to no change in opioid dose (N=1,985 patients)*
| Increase in Opioid Dose Relative to No Change in Dose |
Decrease in Opioid Dose Relative to No Change in Dose |
|||||
|---|---|---|---|---|---|---|
| RRR† | (95% CI) | P-Value | RRR† | (95% CI) | P-Value | |
| Naloxone Receipt | 1.18 | (0.92–1.52) | 0.198 | 1.47 | (1.17–1.86) | 0.001 |
| Age (5 Year Units) | 0.90 | (0.85–0.95) | <0.001 | 0.91 | (0.87–0.96) | 0.001 |
| Race/Ethnicity | ||||||
| White | Reference | Reference | ||||
| Black | 0.97 | (0.73–1.29) | 0.835 | 1.24 | (0.95–1.61) | 0.115 |
| Hispanic/Latino | 1.03 | (0.70–1.52) | 0.865 | 0.94 | (0.66–1.35) | 0.749 |
| Other | 1.17 | (0.73–1.86) | 0.517 | 0.99 | (0.63–1.55) | 0.966 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 1.05 | (0.82–1.34) | 0.696 | 1.00 | (0.80–1.25) | 0.990 |
| ED Visit During 12 Months | ||||||
| Prior to Baseline Date‡ | 1.89 | (1.16–3.08) | 0.011 | 1.39 | (0.86–2.25) | 0.182 |
Adjusted for patient clinic, number of days elapsed between the earliest date of program initiation (February 1, 2013) and patient baseline date and number of days elapsed between patient baseline date and subsequent follow-up date.
RRR = Relative Risk Ratio
Includes only opioid-related ED visits
- 1.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–6. [PubMed] [Google Scholar]
Footnotes
Disclaimer. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the San Francisco Department of Public Health.
References
- 1.Chen LH, Hedegaard H, Warner M. NCHS data brief, no 166. Hyattsville, MD: National Center for Health Statistics; 2014. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. [PubMed] [Google Scholar]
- 2.Khalid L, Liebschutz JM, Xuan Z, Dossabhoy S, Kim Y, Crooks D, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16(3):480–7. doi: 10.1111/pme.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd RA, Paulozzi LJ, Bauer MJ, Burleson RW, Carlson RE, Dao D, et al. Increases in heroin overdose deaths - 28 States, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Jones CM, Logan J, Gladden RM, Bohm MK. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–25. [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid Overdose Prevention Programs Providing Naloxone to Laypersons - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(23):631–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. Bmj. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25(3):89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 8.Paone D, Heller D, Olson C, Kerker B. NYC Vital Signs. New York NY: Department of Mental Health and Hygeine; 2010. Illicit drug use in New York City; pp. 1–4. [Google Scholar]
- 9.National Services Scotland. National Naloxone Programme Scotland – naloxone kits issued in 2013/14 and trends in opioid-related deaths. Glasgow, Scotland: 2014. [Google Scholar]
- 10.Giglio RE, Guohua L, DiMaggio CJ. Effectiveness of bystander naloxone administration and overdose education programs: a meta-analysis. Injury Epidemiology. 2015;2(10):1–9. doi: 10.1186/s40621-015-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158:1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Visconti AJ, Santos GM, Lemos NP, Burke C, Coffin PO. Opioid Overdose Deaths in the City and County of San Francisco: Prevalence, Distribution, and Disparities. J Urban Health. 2015 doi: 10.1007/s11524-015-9967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert S, Brason FW, 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 2011;12(Suppl 2):S77–85. doi: 10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartoszek M. Role of Naloxone in Opioid Overdose Fatality Prevention, 2012. Silver Spring MD: Food and Drug Administration; [Accessed 4 November 2015]. Operation OpioidSAFE, Ft Bragg NC. www.fda.gov/Drugs/NewsEvents/ucm277119.htm. [Google Scholar]
- 15.Seal KH, Thawley R, Gee L, Bamberger J, Kral AH, Ciccarone D, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study. J Urban Health. 2005;82(2):303–11. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naloxone (Narcan) nasal spray for opioid overdose. Med Lett Drugs Ther. 2016;58(1485):1–2. [PubMed] [Google Scholar]
- 17.Substance Abuse and Mental Health Services Administration: Office of Applied Studies. Drug Abuse Warning Network Emergency Department Reference Guide, Version 5. Vol. 178 Rockville MD: 2009. [Google Scholar]
- 18.Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25(7):725–32. doi: 10.1177/0269216311398300. [DOI] [PubMed] [Google Scholar]
- 19.Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016 doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch A, Proescholdbell SK, Bronson W, Dasgupta N. Prescription histories and dose strengths associated with overdose deaths. Pain Med. 2014;15(7):1187–95. doi: 10.1111/pme.12391. [DOI] [PubMed] [Google Scholar]