Abstract
Background:
Crops grown under elevated atmospheric concentrations () contain less protein. Crops particularly affected include rice and wheat, which are primary sources of dietary protein for many countries.
Objectives:
We aimed to estimate global and country-specific risks of protein deficiency attributable to anthropogenic emissions by 2050.
Methods:
To model per capita protein intake in countries around the world under , we first established the effect size of on the protein concentration of edible portions of crops by performing a meta-analysis of published literature. We then estimated per-country protein intake under current and anticipated future using global food balance sheets (FBS).
We modeled protein intake distributions within countries using Gini coefficients, and we estimated those at risk of deficiency from estimated average protein requirements (EAR) weighted by population age structure.
Results:
Under , rice, wheat, barley, and potato protein contents decreased by 7.6%, 7.8%, 14.1%, and 6.4%, respectively. Consequently, 18 countries may lose of their dietary protein, including India (5.3%). By 2050, assuming today’s diets and levels of income inequality, an additional 1.6% or 148.4 million of the world’s population may be placed at risk of protein deficiency because of . In India, an additional 53 million people may become at risk.
Conclusions:
Anthropogenic emissions threaten the adequacy of protein intake worldwide. Elevated atmospheric may widen the disparity in protein intake within countries, with plant-based diets being the most vulnerable. https://doi.org/10.1289/EHP41
Introduction
Globally, 76% of the population derives most of their daily protein from plants (FAO 2014a). With projected population growth to 9.5 billion by 2050 (UN 2013), alongside dietary and demographic changes, future nutritional demands may overwhelm global crop production (Alexandratos 1999). Compounding the strain on food supply, plant nutrient content changes under elevated atmospheric carbon dioxide concentrations () (Myers et al. 2014).
Under the concentrations predicted in the next 50 y, crops with photosynthesis, such as rice and wheat, may experience up to 15% decreases in grain protein content (Myers et al. 2014). The effects of are less on crops, such as maize and sorghum, and on nitrogen-fixing plants, such as legumes (Myers et al. 2014). Thus, the impacts of on dietary protein intake will depend on which staples a country consumes, their dependence on the staple for protein, and their current risk of protein deficiency.
Protein deficiency usually co-occurs with energy and micronutrient deficiencies (Millward and Jackson 2004). Insufficient protein intake limits growth, tissue repair, and turnover (Gropper and Smith 2008). Few controlled studies investigate protein deficiency syndromes in otherwise energy and nutrient sufficient diets. In renal disease, isocaloric protein reduction decreased lean body mass and lymphocyte count (Ihle et al 1989; Klahr et al. 1994). In elderly women, these diets reduced cell mass and protein synthesis while impairing muscle function and immune status (Castaneda et al. 1995). Low protein intake contributes to wasting, stunting, intrauterine growth restriction, and low birth weight (Black et al. 2008). Together with protein–energy malnutrition syndromes, this causes an estimated 90.9 million disability-adjusted life years (DALYs) and 2 million deaths annually (Black et al. 2008).
Previous meta-analyses conducted on the effects of on plant nutrient contents (Taub et al. 2008; Loladze 2014) have not assessed impacts on edible protein from a global dietary context, nor did they consider distributional effects within countries. We aimed to estimate impacts on global protein intake, and on the proportion of the population by country at risk of protein deficiency. We aimed to expand on the meta-analysis by Myers et al. (2014), including all available studies reporting impacts on the edible portions of crop plants, including lesser-studied foods and studies in (sub)tropical locations. Then, using published food balance sheets (FBS) and measures of economic inequality within countries, we aimed to estimate dietary protein intake under current and future atmospheric . We thereby tested the sensitivity of global protein intake and inequality of intake to rising atmospheric , identifying key regions to target with nutritional interventions.
Methods
Systematic Review and Raw Data
We conducted ISI Web of Knowledge (https://pcs.webofknowledge.com/) literature searches in July–September 2014 and in January 2016 for the effects of on the protein content of all plants listed in the FAO FBS. This study supplements the meta-analysis of common European/U.S. staples conducted by Myers et al. (2014). Because estimates of plant protein are commonly derived by multiplying measured plant nitrogen (N) by a conversion factor, we considered published changes in N and protein to be equivalent (Taub et al. 2008). For the full search string and exclusions, see “Part 1” and “Part 2” in the Supplemental Material. A total of 119 citations were used. For references, see “Part 3” in the Supplemental Material.
We included raw data from free-air enrichment (FACE) and open-top chamber studies, with data from European wheat, barley, and potato Changing Climate and Potential Impacts on Potato Yield and Quality (CHIP) and the European Stress Physiology and Climate Experiment (ESPACE) studies (A. Fangmeier, unpublished data, 1994–1999) and Australian wheat and pea Australian Grains Free Air Enrichment (AGFACE), Japanese rice, American soy, corn, and sorghum Soybean Free Air Concentration Enrichment (SoyFACE) and Arizona FACE (data from Myers et al. 2014). Raw data included free-to-air carbon dioxide elevation (FACE) and open-top chamber studies, 41 cultivars, nitrogen fertilizer, watering, and time of sowing treatments over multiple years.
Response ratios (RRs) and standard errors (SEs) for protein response to were calculated from each study’s reported error terms. When studies indicated merely significant at or not significant, the SE was calculated from p-values of 0.049 and 0.1, respectively.
Metaregression
Metaregression was performed individually for each commodity where data were available from four or more experiments and for commodity groups listed in the FAO FBS (Table 1). We used the statistical package Metafor (version 1.9-4 Wolfgang Viechtbauer) in R (version 3.0.3; R Development Core Team). For each commodity or group, the difference between ambient () and treatments was tested as a modifier. We used multivariate linear (mixed-effects) models (the function rma.mv) with outcomes being percent decrease in protein, and modifiers being the difference between and in parts per million. Models included variance and were weighted by replicate facilities (e.g., number of FACE rings or growth cabinets) with random effects being year within site, and each cultivar (and unless tested as a modifier, each watering and nitrogen fertilizer treatment) was treated as a separate experiment. We performed Q tests to assess heterogeneity.
Table 1.
Percent change in protein content by commodity class.
| Commodity (n) | Estimate [mean (95% CI)] |
|---|---|
| grains (257) | (, ) |
| Wheat (166) | (, ) |
| Rice (66) | (, ) |
| Barley (21) | (, ) |
| grains (12) | 2.07 (, 7.35) |
| Maize (8) | 3.08 (, 11.35) |
| Sorghum (4) | 0.26 (, 6.84) |
| Root vegetable (15) | (, 1.78) |
| Potato (9) | (, ) |
| Pulses, legumes (26) | (, 1.04) |
| Peas (15) | (, 0.18) |
| Beans (7) | (, 3.2) |
| Chickpea (4) | (, ) |
| Oil crops (54) | (, 3.47) |
| Soy (44) | (, 1.95) |
| Rapeseed/mustard seed (5) | 0.92 (, 10.74) |
| Vegetables (32) | (, ) |
| Fruit (5) | (, 8.24) |
Note: , crops with photosynthesis; , crops with photosynthesis; CI, confidence interval; , number of experiments, where each treatment/cultivar/experiment was treated as a separate experiment, yet experiments at the same location for the same crop were grouped together.
Meta-Analysis
Because there was no reliable dose-dependent decrease in protein content with degree of elevation, we used meta-analysis to derive average response ratios comparing plants grown in with plants grown in , where was in the range of . We used the rma.mv function as for metaregression, but without the modifier term. Both meta-analysis and metaregression tested fixed effects of pot- versus field-grown plants and a qualitative measure of nitrogen fertilizer treatment, categorized as low, adequate, or high, based on descriptions in each study’s experimental design. Neither modifier changed the magnitude of the response, and neither was used in subsequent analyses.
We minimized publication bias by including unpublished data. Furthermore, we tested sensitivity to publication bias. For each commodity, we incrementally added experiments with no effect of on protein content (RR 1, variance 0.5) until confidence intervals for RR crossed 1. Some commodities, including rice, were sensitive to null results, but wheat was insensitive to null results (see Table S3).
Food Balance Sheets
The FAO FBS estimate per capita availability of each food-based commodity (including energy and protein contents). We averaged data over 2009–2013 FAO FBS. We assumed that protein availability equals protein intake, corrected for digestibility (FAO 2014a). Per convention, we assumed that plant-based protein was 80% digestible and that animal-based protein was 95% digestible (Millward and Jackson 2004).
The “Vegetables, other” and “Cereals, other” categories were large contributors to protein intake in some countries, and contained both and plants, and for vegetables, nitrogen fixers. We produced weighted estimates of the contributions of each these categories, using re-calculated 2009 FBS from the FAOstat classic platform (described fully by Smith et al. 2015). We converted from total grams to grams protein, using food composition tables (Abdel-Aal et al 1997; USDA 2011; FAO 2012; Ballogou et al. 2013; New Zealand Ministry of Health 2014). We assumed that the “Cereals, other, not elsewhere specified” category within the “Cereals, other” category was derived from grains in sub-Saharan Africa, but from grains elsewhere.
To estimate the effect of on protein intake in each country, we assumed constant mass-based consumption of each commodity over time, with declining protein content predicted by our meta-analyses. We used commodity-based averages when available, and otherwise applied the averages from the commodity group to each commodity (Table 1). We found no studies on response of tree nuts, thus conservatively assumed no change in their protein content. Likewise, we assumed no effect on animal protein.
Plant-Based Diets
Within a population, the lowest protein consumers also frequently consume the least meat (see World Food Programme household surveys; e.g, Santacroce 2008). For an extreme scenario, we reran the models, removing all animal-sourced foods (including eggs and dairy) from the diet, assuming no other changes in dietary fractional composition.
Intake Distribution
We assumed a lognormal distribution of protein intake within countries (FAO 2014b), a cumulative distribution function, with the mean,
and the standard deviation,
where is the national mean protein intake, as estimated above, and CV is its coefficient of variation. Because protein intake is likely to be related to household income, we estimated the CV of protein intake () from the Gini coefficient of national household income inequality. The national Gini coefficient for household income describes a Lorenz curve plotting the cumulative percentages of total income against the cumulative number of households from poorest to richest. Using linear regression, we compared per-household from household surveys across 36 countries (FAO 2014a) with contemporaneous national Gini coefficients (Arneberg and Pedersen 2001; Garcia et al. 2001; Kim and Kim 2007; OECD 2009; Liberati 2013; USAID 2012; CIA 2014; Solt 2014; World Bank 2014). The FAO uses Gini coefficients, gross domestic product (GDP), and food prices to estimate CV for caloric intake (FAO 2015). We then estimated the national from the country’s Gini coefficient in the year closest to 2011. Owing to high uncertainty among future economic projections, we assumed each country’s future would remain constant.
Estimated Average Requirements
We calculated a weighted estimated average requirement (EAR) (grams per day) for absorbed protein from the published EAR for adults () and for children by age and sex, using current and mid-range 2050 demographic projections (IOM 2005; UN 2013). For adults, the minimum safe protein intake in grams per day is based on the minimum healthy body weight calculated from the lowest 5th percentile of body mass index (BMI), this being (WHO 1995). We calculated average height from national surveys (OECD 2009; Hatton and Bray 2010; USAID 2012). Where male height was unavailable, it was calculated as , based on the median male-to-female height ratio across all countries. For child weight, the ideal body mass was the 50th percentile by age from growth tables (WHO 2006). We adjusted EAR to include the increased protein requirements of pregnant and lactating women (IOM 2005) with demographics estimated from projected birth rates, 2009 stillbirth rates and infant mortality, and breastfeeding prevalence and duration (McDowell et al. 2008; AIHW 2011; CDC 2011; UN 2013; USAID 2012; Liu et al. 2013).
Risk of Protein Deficiency
From each country’s 2050 population, we calculated the proportion and the number of people whose intake fell below the EAR under current and scenarios, with the difference between these populations being our measure of impact.
We used Monte Carlo methods to propagate error from the SE of the meta-analysis results, and for modeled , through the model, using 10,000 random draws from normal distributions of mean national protein intakes, and again for error around linear regression of on Gini coefficient. From these two parameters, we calculated the means () and standard deviations () of 10,000 lognormal distributions. These were used to estimate the probability for each country of protein intake being below the calculated EAR.
We summarize data based on regional classifications from the reporting regions of the Global Burden of Disease Study 2010 (Lim et al. 2012), but we present India and the greater China region separately because of their large population sizes.
At each stage of analysis, where country-specific data were unavailable, data were derived from regional estimates, which were in turn derived from weighted means by population size of each available country represented within the region (see Table S4 for regions).
Protein–Energy Ratio
Assuming all calories lost from declines in food protein contents were replaced as carbohydrates (as supported by the stoichiometry of Loladze 2014), we calculated the ratio of protein to total energy in current diets and projected diets under . Because commodity-based digestibility of energy is less easily estimated, digestibility was not included in these estimates.
Results
Our analysis was based on 99 experiments and 48 crops, and it included 54 field experiments. Of the 64 experimental sites, 37 were elsewhere than Europe or North America (see Table S1).
In maize, peas, and mustard seed, we found a linear dose response when the RR of protein content was compared with the degree of elevation above ambient (see Tables S1 and S2). Metaregression for other crops was not significant, partly because of insufficient statistical power. Maize protein content under was not significantly below that under when considered overall from meta-analyses or when predicted for an atmospheric increase of from metaregression. Metaregression predicted a decrease in pea protein content of 4.1% (1.6–6.7%) with an atmospheric increase of , and overall, meta-analyses showed no significant declines in pea protein. National changes in dietary protein content were on average 0.04% less when modeled for a increase in atmospheric based on metaregression results compared with meta-analyses. This difference was small enough to warrant the use of meta-analyses rather than metaregression. Comparisons between field and pot-based experiments, and between nitrogen fertilizer treatments were largely nonsignificant (; see Table S2).
Meta-analyses confirmed lower protein content of grains (including barley, 14.1% lower), tubers (including potato, 6.4% lower), fruit (23.0% lower), and vegetables (17.3% lower) under , with no significant change in the protein content of grains, nitrogen-fixing pulses, or oil crops (Table 1).
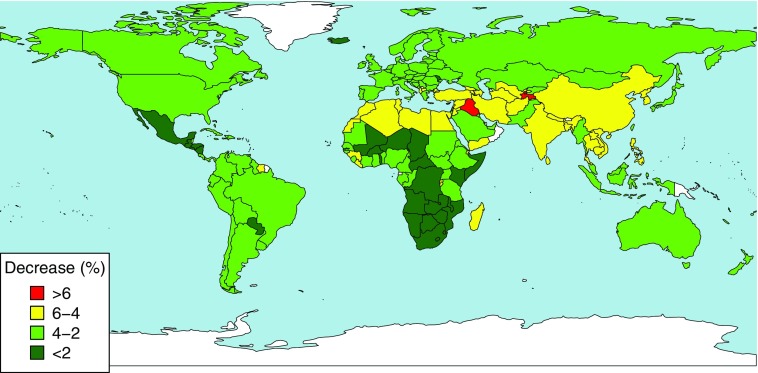
When these effect sizes were translated to FBS-standardized commodity intakes, the mean protein intake decreased under by in 18 countries, including India, Bangladesh, Turkey, Egypt, Iran, and Iraq. Particularly large declines are expected through the Middle East and India, where a 5.3% decrease in dietary protein is predicted (Table 2, Figure 1).
Table 2.
Change in dietary protein.
| Region | Mean change in protein intake (%) | Mean change in protein intake (%), plant-based diet | Difference in protein–energy ratio ( minus , %) |
|---|---|---|---|
| CALACA | (, ) | (, ) | (, ) |
| CANAME | (, ) | (, ) | (, ) |
| CEEAEU | (, ) | (, ) | (, ) |
| CHINAR | (, ) | (, ) | (, ) |
| ESEASP | (, ) | (, ) | (, ) |
| HIGHIN | (, ) | (, ) | (, ) |
| India | (, ) | (, ) | (, ) |
| SOASIA | (, ) | (, ) | (, ) |
| SOTRLA | (, ) | (, ) | (, ) |
| SUSAAF | (, ) | (, ) | (, 0.00) |
| World | (, ) | (, ) | (, ) |
Note: Figures represent population-weighted averages (and 95% confidence intervals) globally and for each region (2050 populations). Protein–energy ratio is the percentage of dietary energy (calories) that is derived from protein. CALACA, Central and Andean Latin America and the Caribbean; CANAME, Central Asia, North Africa and the Middle East; CEEAEU, Central and Eastern Europe; CHINAR, Greater China; ESEASP, East and Southeast Asia and the Pacific excluding China; HIGHIN, high income countries; SOASIA, South Asia excluding India; SOTRLA, Southern and Tropical Latin America; SUSAAF, sub-Saharan Africa. See Table S4 for country grouping.
Figure 1.
Per-country change in dietary protein intake under elevated carbon dioxide [ (%)]. Baseline intake is based on Food and Agriculture Organisation of the United Nations Food Balance Sheets (FAO FBS) estimates, and changes are calculated from decreases in protein content in the edible portions of crops when grown under . Data were plotted using the Rworldmap package in R (version 3.2.4; R Development Core Team).
Globally, decreases in protein intake are predicted for plant-based diets under , with countries dependent on staples particularly affected (Table 2), including Central Asia, North Africa and the Middle East (7.9%), Central and Eastern Europe (8.2%), and China (8.9%).
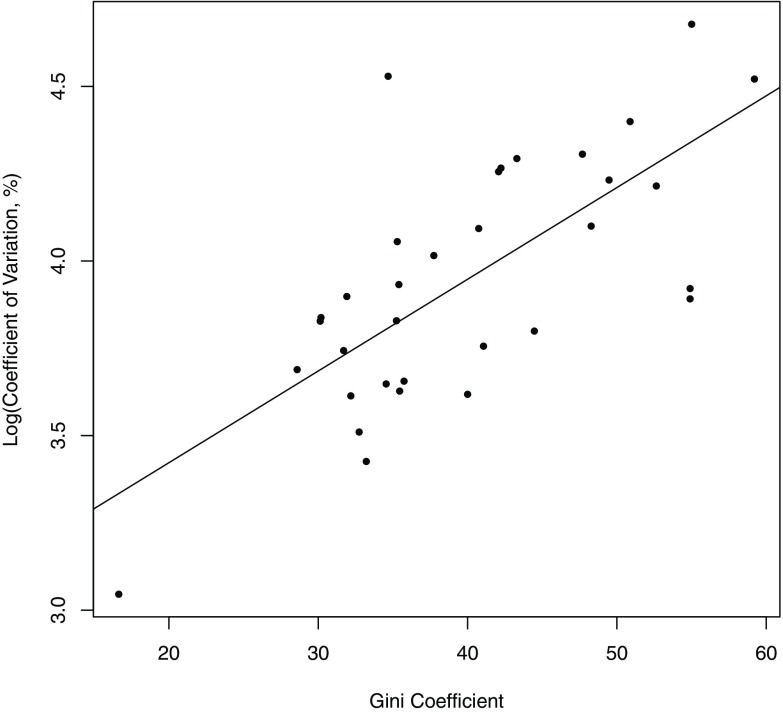
A significant positive linear relationship existed between the natural log of and income-based Gini coefficients (slope 0.026, ; Figure 2). Income inequality explained half of within-country variation in protein intake ().
Figure 2.
Coefficient of variation in protein intake derived from household surveys plotted against the income-based Gini coefficient for the year closest to the year household surveys were conducted (slope 0.026, ).
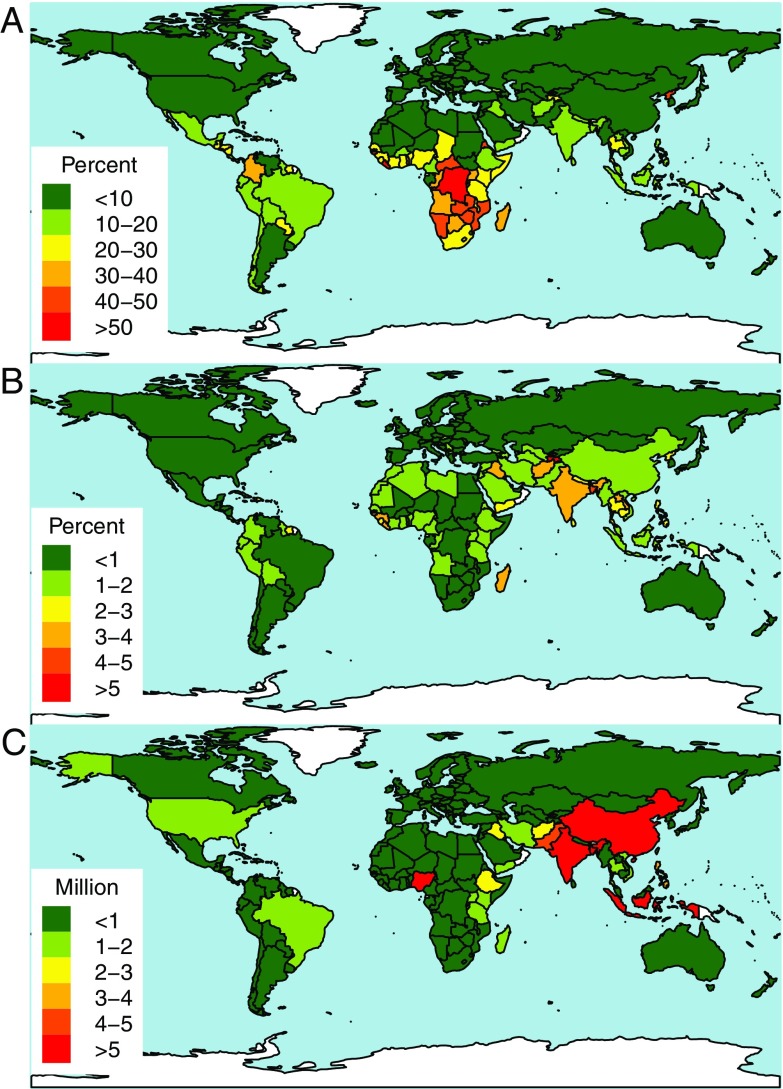
Estimates indicated a 12.2% current risk of protein deficiency globally. With constant atmospheric concentrations, we predict that globally, 15.1% or 1.4 billion people will be at risk of protein deficiency by 2050 because of demographic changes. This estimate includes 613.6 million people at risk in sub-Saharan Africa, 276.4 million in India, 131.7 million in Eastern and Southeast Asia and the Pacific, 84.4 million in Central Latin America and the Caribbean, and 77.8 million elsewhere in South Asia (Figure 3, Table 3).
Figure 3.
Risk of protein deficiency as defined by protein intake below estimated average protein requirements (EAR). Estimates of (A) current percentage of the population at risk of deficiency, (B) percent of the population newly at risk of deficiency under elevated carbon dioxide (), and (C) millions of people estimated to be newly at risk of deficiency under , based on 2050 population projections. Data were plotted using the Rworldmap package in R (version 3.2.4; R Development Core Team).
Table 3.
Populations at risk of protein deficiency under and (population-weighted averages and 95% confidence intervals).
| Region | EAR, 2050 (g/d) | At risk of protein deficiency, (%) | At risk of deficiency in 2050, (%) | At risk of deficiency in 2050, (millions) | Additionally at risk with : 2050 (%) | Additionally at risk with : 2050 (millions) |
|---|---|---|---|---|---|---|
| CALACA | 30.49 | 17.05 (11.64, 24.65) | 18.4 (12.84, 25.64) | 84.37 (58.90, 117.61) | 0.86 (0.57, 1.19) | 3.94 (2.64, 5.46) |
| CANAME | 31.46 | 6.22 (4.00, 9.32) | 7.32 (4.88, 10.46) | 57.2 (38.09, 81.71) | 1.53 (1.14, 1.94) | 11.97 (8.93, 15.12) |
| CEEAEU | 33.44 | 3.58 (1.40, 9.84) | 3.52 (1.37, 9.79) | 9.64 (3.74, 26.77) | 0.56 (0.27, 0.95) | 1.52 (0.73, 2.61) |
| CHINAR | 31.68 | 5.25 (0.16, 21.01) | 5.38 (0.16, 21.31) | 74.96 (2.27, 297) | 1.14 (0.11, 2.04) | 15.94 (1.47, 28.45) |
| ESEASP | 29.47 | 15.35 (9.59, 22.78) | 15.79 (9.68, 23.56) | 131.67 (80.71, 196.44) | 1.92 (1.40, 2.43) | 15.99 (11.68, 20.28) |
| HIGHIN | 33.05 | 2.62 (1.01, 6.86) | 2.6 (0.99, 7.36) | 28.39 (10.83, 80.47) | 0.31 (0.16, 0.53) | 3.41 (1.75, 5.78) |
| India | 30.06 | 16.27 (3.73, 32.49) | 17.06 (4.46, 33.36) | 276.42 (72.32, 540.41) | 3.30 (1.93, 4.55) | 53.41 (31.22, 73.71) |
| SOASIA | 30.41 | 13.23 (6.15, 21.98) | 13.74 (6.91, 22.59) | 77.76 (39.10, 127.88) | 2.80 (1.88, 3.74) | 15.86 (10.64, 21.20) |
| SOTRLA | 31.35 | 11.46 (2.80, 27.14) | 12.03 (3.25, 27.40) | 38.14 (10.30, 86.87) | 0.68 (0.26, 1.07) | 2.17 (0.81, 3.39) |
| SUSAAF | 30.53 | 27.12 (23.07, 31.46) | 28.89 (24.73, 33.44) | 613.56 (525.07, 710.24) | 1.16 (0.73, 1.59) | 24.64 (15.44, 33.83) |
| World | 30.99 | 12.18 (9.07, 16.32) | 15.06 (12.11, 18.71) | 1424.59 (1145.89, 1770.20) | 1.57 (1.26, 1.86) | 148.37 (119.06, 176.09) |
Note: Calculations use 2011 populations and 2050 population projections. , ambient atmospheric carbon dioxide; CALACA, Central and Andean Latin America and the Caribbean; CANAME, Central Asia, North Africa and the Middle East; CEEAEU, Central and Eastern Europe; CHINAR, Greater China; EAR, estimated average protein requirement based on 2050 demographic projections; elevated atmospheric carbon dioxide; ESEASP, East and Southeast Asia and the Pacific excluding China; HIGHIN, high income countries; SOASIA, South Asia excluding India; SOTRLA, Southern and Tropical Latin America; SUSAAF, sub-Saharan Africa. See Table S4 for country grouping.
With predicted atmospheric concentrations by 2050, we estimate an additional 1.57% of the world’s population (148.4 million) will be at risk of protein deficiency, compared with 2050 scenarios. In particular, an additional 53.4 million people in India, 15.9 million elsewhere in South Asia and 24.6 million in sub-Saharan Africa are estimated to become newly at risk (Table 3, Figure 3). An additional 15.9 million people in the China region and 12.0 million in Central Asia, North Africa, and the Middle East are expected to become at risk with . The greatest increases in percent at risk of protein deficiency are expected in Tajikistan, Bangladesh, Burundi, Liberia, Occupied Palestinian Territory, Iraq, and Afghanistan (Figure 3B).
Globally, we predict the protein–energy ratio (protein caloric contribution as a percent of total calories) to decrease under by 0.41%; in individual countries and regions, we predict this ratio to decrease by 0.6% in 17 counties including China, Iran, Iraq, Morocco, and Turkey. We expect decreases in China of 0.57% (Table 2).
Discussion
Our study highlights the potential impact of on dietary protein intake globally. Wheat and rice, among the most sensitive crops to , are primary protein sources for 71% of the world’s population (FAO 2014a). By 2050, 148.4 million people worldwide may become at risk of protein deficiency from rising . In India, expected to be the world’s most populous country (UN 2013), and a country that is highly dependent on rice, 53.4 million people may be newly at risk of protein deficiency. Additionally, the protein deficiency in roughly 1.4 billion people globally (predicted under in 2050) is anticipated to become more severe under scenarios. Although estimates of current protein intake and income inequality highlight the current risk of deficiency in sub-Saharan Africa and South America, their dependence on less-sensitive crops make these diets less sensitive to .
Importantly, we incorporated into the risk assessment different distributions of protein intake in countries based on income inequality from the association of income-based Gini coefficients with variability in protein intake from national dietary surveys. We find it equally plausible that would decrease or increase by 2050. We therefore provide the most conservative estimate of future protein intake distributions, namely that within countries will remain unchanged. We also assume unchanged duration and prevalence of breastfeeding, and unchanged adult height.
Although our calculations assume no change in the shape of the intake distribution, we anticipate a worsening of inequality in protein intake within populations because a larger decrease in protein content is observed in plant-based than in omnivore diets under (Table 2). Some changes in meat quality are anticipated owing to increased fat content under lower-protein diets (Blome et al. 2003), but this is likely to be negligible compared with the effects on plant-based protein sources. Those who consume the least protein have diets more dependent on plant protein, and these people are more vulnerable to effects on plant protein. This is likely to extend the lower tail of the intake distribution, increasing the severity and prevalence of protein inadequacy. Our estimates are worst-case scenarios where no substitution of animal-sourced protein sources for other high-protein foods is allowed. In particular, the predicted large decreases in protein content of plant-based diets in high income countries may be overestimates, where plant-based diets are likely to be supplemented with other protein sources.
The countries that we estimated to be currently most at risk of protein deficiency are also those with the greatest estimated prevalence of undernourishment (FAO 2014b), increasing confidence in our estimates; however, energy balance and nitrogen balance interact (Garza et al. 1976). For simplicity, we modeled overall protein intake and risk of deficiency based on the EAR, which assumes adequate energy intake. Published EARs are defined for zero protein balance, which is a conservative estimate of protein requirements (IOM 2005). Older, sedentary people and those suffering from or recovering from illness are likely to be at greater risk of deficiency in any population (Ghosh 2013). We have not accounted for current or future patterns of illness in our estimates of EAR. Furthermore, we have not considered changes in protein quality; however, several studies have shown that essential amino acids tend to be relatively preserved at the expense of nonessential amino acids under , and degradability may decrease (Högy et al. 2009; Wroblewitz et al. 2013). Bioavailability may change, for example, if meal composition and thus digestibility changes. Furthermore, levels of secondary metabolites, including toxins, tend to increase under elevated (Cavagnaro et al. 2011), which could decrease protein bioavailability.
In addition to increasing the risk of protein deficiency, there may be other nutritional consequences of changing the stoichiometry of carbohydrate-to-protein ratios in staple food crops. For example, replacing dietary carbohydrate with protein has been shown in interventional trials and observational studies to 15-y duration, and in diverse countries including Japan, China, the United States, and Chile, to improve cardiovascular disease risk through lowering blood pressure and changing lipid profiles (Hu et al. 1999; Obarzanek et al. 1996; Appel et al. 2005; Altorf-van der Kuil et al. 2010; Rebholz et al. 2012). Improvements are often greatest with plant-rather than animal-sourced protein (Altorf-van der Kuil et al. 2010). These experiments underscore the need for additional investigation into whether replacing plant-sourced protein with plant-sourced carbohydrate could exacerbate the already concerning pandemic of metabolic disease driving increased cardiovascular morbidity and mortality globally.
It is unclear how trends in dietary quality will be counterbalanced by the effects of population growth and climate change. That is why, for our analysis, we assume no future change to food composition of diets or to per capita food intake and no dietary substitution to compensate for deficits. Agricultural production will need to roughly double to match increasing demand by 2050 (Alexandratos 1999). Climate change may pose the greatest challenge to this need. Climate change-induced reductions in crop yield are expected to be greatest in lower-latitude regions, including developing countries and those dependent on crops (Rosenzweig et al. 2014). Resulting economic changes may shape future diets, and changes to water, soils, and weather in these areas may affect crops in ways that may overwhelm, or exacerbate, the effects of . For example, decreases in yield under drought and warming temperatures may counteract the effects of rising on protein concentrations (Kimball et al. 2001). Only 37 of 99 study sites in our meta-analysis were in countries outside of Europe and North America, and only just over half of the studies were performed in the field, with only 10% involving watering experiments (see Table S1). Most experiments were undertaken over 1 y only, and effects on crop nutrient content may not match those under the next 50 y of gradual atmospheric increase. The consistent decreases in protein contents across crop cultivars, including 47 wheat cultivars and 27 rice cultivars, reassure us that our results are generalizable to other cultivars. Nevertheless, to better predict the dietary impacts of , we need more long-term field-based experiments involving plants and cultivars grown under the climates and farming practices applicable to the developing world.
We also assumed that global population growth and future demographic trends will match UN projections, which include declining fertility rates, and migration from developing to developed countries (UN 2013). However, the greatest population growth is projected to occur in areas most vulnerable to climate change (Watts et al. 2015). Climate, economic, and demographic changes will likely interact, producing a global population distribution that we are not yet able to fully comprehend. In the absence of conclusive projections of future food production, we believe it is the most conservative, albeit perhaps optimistic, assumption that per capita food intake will remain constant despite sharp increases in global demand.
In predicting the nutritional consequences of , other nutrients must be considered. Zinc and iron concentrations are greatly decreased in plants grown under (Myers et al. 2014). Zinc is a cofactor for protein synthesis, and protein inadequacy decreases uptake and availability of other nutrients (Gropper and Smith 2008). A recent analysis predicts strong increases in the risk of global zinc deficiency with (Myers et al. 2015). Identifying the countries most vulnerable to future malnutrition requires a targeted synthesis of crop research on climate and responses. This information can then be applied to global climate and atmospheric models.
To our knowledge, this is the first global comparison of dietary protein that estimates a country-specific CV. Like energy consumption, the variability of protein consumption in a population relates to the Gini coefficient (Raubenheimer et al. 2015). Our use of this metric would be expected to produce more accurate estimates than the previously used 25% CV (Ghosh 2013). The WHO continues to refine its models of energy intake variability based on gross domestic product (GDP), Gini, and food prices, using skew log rather than lognormal distributions. As this methodology becomes available, future work could incorporate these considerations to produce better estimates of protein consumption.
Because added fertilizer did not predictably mitigate the effects of on crop protein, and with the production and application of fertilizer being a principal contributor to agricultural greenhouse gas emissions (Vermeulen et al. 2012), we cannot simply add more fertilizer to reduce the protein deficit. As populations increase, and with livestock production being resource-intensive (Vermeulen et al. 2012), eating more meat is not a practical solution. Cultivars could be selected or bred based on their nutritional content under . In addition to efforts to mitigate emissions, nutritious and resilient crops should be promoted, for example legumes, which will withstand the effects of on protein content. Because may have the greatest effect on the protein intake of those with the poorest diets, more equitable food distribution, and poverty reduction measures should be a focus for minimizing risk of deficiency.
Conclusions
Anthropogenic emissions, via their impact on the protein content of staples, may threaten the adequacy of protein intake for many populations. Although quantifying protein deficiency is notoriously difficult, we have estimated current and future risk of protein deficiency by country and region, suggesting enduring challenges for sub-Saharan Africa and growing challenges for South Asia, including India. For nutritionally sensitive agriculture, the high effects on crop nutrient contents must be incorporated into future food security policies.
Supplemental Material
Acknowledgments
We thank A. Fangmeier for additional data generously shared, M. Smith for valuable comments and data, M. Stefan and R. Wessells for statistical advice, W. Willett for help with project conception, the Harvard University Center for the Environment for hosting D.E.M., and C. Hotz for her insights into the literature on protein deficiency.
Financial support was provided by the Bill & Melinda Gates Foundation and by the Winslow Foundation. Funding sources had no input into study design, data collection, analysis, data interpretation, report writing, or decision to submit the manuscript for publication.
References
- Abdel-Aal ESM, Hucl PJ, Sosulski FW. 1997. Structural and compositional characteristics of canaryseed (Phalaris canariensis L.). J Agric Food Chem 45(8):3049–3055, 10.1021/jf970100x. [DOI] [Google Scholar]
- AIHW (Australian Institute of Health and Welfare). 2011. Australian National Infant Feeding Survey 2010: Indicator Results. Canberra, Australia:AIHW. http://www.aihw.gov.au/publication-detail/?id=10737420927 [accessed 23 February 2015].
- Alexandratos N. 1999. World food and agriculture: outlook for the medium and longer term. Proc Natl Acad Sci U S A 96(11):5908–5914, PMID: 10339517, 10.1073/pnas.96.11.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, et al. . 2010. Dietary protein and blood pressure: a systematic review. PLoS One 5(8):e12102, PMID: 20711407, 10.1371/journal.pone.0012102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, et al. . 2005. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 294(19):2455–2464, PMID: 16287956, 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- Arneberg MW, Pedersen J. 2001. Urban Households and Urban Economy in Eritrea: Analytical, Report from the Urban Eritrean Household Income and Expenditure Survey 1996/97. Oslo, Norway:Fafo Institute for Applied Social Science. [Google Scholar]
- Ballogou VB, Soumanou MM, Toukourou F, Hounhouigan JD. 2013. Structure and nutritional composition of Fonio (Digitaria exilis) grains: a review. Int Res J Biol Sci 2(1):73–79. [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. . 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371(9608):243–260, PMID: 18207566, 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Blome RM, Drackley JK, McKeith FK, Hutjens MF, McCoy GC. 2003. Growth, nutrient utilization, and body composition of dairy calves fed milk replacers containing different amounts of protein. J Anim Sci 81(6):1641–1655, PMID: 12817512, 10.2527/2003.8161641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda C, Charnley JM, Evans WJ, Crim MC. 1995. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 62(1):30–39, PMID: 7598064. [DOI] [PubMed] [Google Scholar]
- Cavagnaro TR, Gleadow RM, Miller RE. 2011. Plant nutrient acquisition and utilisation in a high carbon dioxide world. Funct Plant Biol 38(2):87–96, 10.1071/FP10124. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2011. Breastfeeding Report Card—United States. Atlanta GA:Centers for Disease Control and Prevention. https://www.cdc.gov/breastfeeding/pdf/2011breastfeedingreportcard.pdf [accessed 12 August 2014].
- CIA (U.S. Central Intelligence Agency). 2014. The World Factbook 2013–2014. Washington, DC:CIA. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2172rank.html [accessed 11 November 2014].
- FAO (Food and Agriculture Organization of the United Nations). 2012. West African Food Composition Table. Rome, Italy:FAO. http://www.fao.org/docrep/015/i2698b/i2698b00.pdf [accessed 28 August 2014].
- FAO. 2014a. Food Balance Sheets, 1970–2011. Rome, Italy:FAO. http://www.fao.org/faostat/en/#data/FBS/visualize [accessed 15 January 2016].
- FAO. 2014b. The State of Food Insecurity in the World 2014. Rome, Italy:FAO. [Google Scholar]
- FAO. 2015. The State of Food Insecurity in the World 2015. Rome, Italy:FAO. [Google Scholar]
- Garcia YT, Garcia AG, Oo M, Hossain M. 2000. Income distribution and poverty in irrigated and rainfed ecosystems: the Myanmar case. Econ Polit Wkly 35(52–53):4670–4676. [Google Scholar]
- Garza C, Scrimshaw NS, Young VR. 1976. Human protein requirements: the effect of variations in energy intake within the maintenance range. Am J Clin Nutr 29(3):280–287, PMID: 1258818. [DOI] [PubMed] [Google Scholar]
- Ghosh S. 2013. Assessment of protein adequacy in developing countries: quality matters. Food Nutr Bull 34(2):244–246, PMID: 23964401, 10.1177/156482651303400217. [DOI] [PubMed] [Google Scholar]
- Gropper SS, Smith JL. 2008. Advanced Nutrition and Human Metabolism. Belmont CA:Wadsworth Cengage Learning. [Google Scholar]
- Hatton TJ, Bray BE. 2010. Long run trends in the heights of European men, 19th–20th centuries. Econ Hum Biol 8(3):405–413, PMID: 20399715, 10.1016/j.ehb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Högy P, Wieser H, Köhler P, Schwadorf K, Breuer J, Franzaring J, et al. . 2009. Effects of elevated CO2 on grain yield and quality of wheat: results from a 3-year free-air CO2 enrichment experiment. Plant Biol 11(s1):60–69, PMID: 19778369, 10.1111/j.1438-8677.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Speizer FE, et al. . 1999. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr 70(2):221–227, PMID: 10426698. [DOI] [PubMed] [Google Scholar]
- Ihle BU, Becker GJ, Whitworth JA, Charlwood RA, Kincaid-Smith PS. 1989. The effect of protein restriction on the progression of renal insufficiency. N Engl J Med 321(26):1773–1777, PMID: 2512486, 10.1056/NEJM198912283212601. [DOI] [PubMed] [Google Scholar]
- IOM [Institute of Medicine (U.S.)]. 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC:National Academies Press. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim T. 2007. Economic assimilation of North Korean refugees in South Korea: survey evidence. KDI School of Pub Policy & Management. No. 06-19. http://dx.doi.org/10.2139/ssrn.960640 [accessed 8 February 2016].
- Kimball BA, Morris CF, Pinter PJ Jr, Wall GW, Hunsaker DJ, Adamsen FJ, et al. . 2001. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol 150(2):295–303, 10.1046/j.1469-8137.2001.00107.x. [DOI] [Google Scholar]
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. . 1994. The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 330(13):877–884, PMID: 8114857, 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- Liberati P. 2013. The world distribution of income and its inequality, 1970–2009. Rev Income Wealth 61(2):248–273, 10.1111/roiw.12088. [DOI] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. . 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260, PMID: 23245609, 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Qiao L, Xu F, Zhang M, Wang Y, Binns CW. 2013. Factors associated with breastfeeding duration: a 30-month cohort study in Northwest China. J Hum Lact 29(2):253–259, PMID: 23504474, 10.1177/0890334413477240. [DOI] [PubMed] [Google Scholar]
- Loladze I. 2014. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 3:e02245, PMID: 24867639, 10.7554/eLife.02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MM, Wang CY, Kennedy-Stephenson J. 2008. Breastfeeding in the United States: Findings from the National Health and Nutrition Examination Surveys, 1999–2006. NCHS Data Briefs, No. 5. Atlanta, GA:U.S. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Health Statistics. https://www.cdc.gov/nchs/products/databriefs/db05.htm [accessed 8 February 2016].
- Millward DJ, Jackson AA. 2004. Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: implications for recommended protein and amino acid intakes. Public Health Nutr 7(3):387–405, PMID: 15153271, 10.1079/PHN2003545. [DOI] [PubMed] [Google Scholar]
- Ministry of Health (New Zealand). 2014. New Zealand Food Composition Database. http://www.foodcomposition.co.nz/ [accessed 28 August 2014].
- Myers SS, Wessells KR, Kloog I, Zanobetti A, Schwartz J. 2015. Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: a modelling study. Lancet Glob Health 3(10):e639–e645, PMID: 26189102, 10.1016/S2214-109X(15)00093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey AD, Bloom AJ, et al. . 2014. Increasing CO2 threatens human nutrition. Nature 510(7503):139–142, PMID: 24805231, 10.1038/nature13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obarzanek E, Velletri PA, Cutler JA. 1996. Dietary protein and blood pressure. JAMA 275(20):1598–1603, PMID: 8622252, 10.1001/jama.1996.03530440078040. [DOI] [PubMed] [Google Scholar]
- OECD (Organization For Economic Co-Operation and Development). 2009. Society at a Glance 2009: OECD Social Indicators. Paris, France:OECD Publishing. [Google Scholar]
- Raubenheimer D, Machovsky-Capuska GE, Gosby AK, Simpson S. 2015. Nutritional ecology of obesity: from humans to companion animals. Br J Nutr 113(suppl):S26–S39, PMID: 25415804, 10.1017/S0007114514002323. [DOI] [PubMed] [Google Scholar]
- Rebholz CM, Friedman EE, Powers LJ, Arroyave WD, He J, Kelly TN. 2012. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am J Epidemiol 176(suppl 7):S27–S43, PMID: 23035142, 10.1093/aje/kws245. [DOI] [PubMed] [Google Scholar]
- Rosenzweig C, Elliott J, Deryng D, Ruane AC, Müller C, Arneth A, et al. . 2014. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci U S A 111(9):3268–3273, PMID: 24344314, 10.1073/pnas.1222463110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacroce P. 2008. Kingdom of Cambodia: Comprehensive Food Security and Vulnerability Analysis (CFSVA). Rome, Italy:United Nations World Food Programme. [Google Scholar]
- Smith MR, Singh GM, Mozaffarian D, Myers SS. 2015. Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. Lancet 386(10007):1964–1972, PMID: 26188748, 10.1016/S0140-6736(15)61085-6. [DOI] [PubMed] [Google Scholar]
- Solt F. 2014. The Standardized World Income Inequality Database. SWIID Version 5.0. http://myweb.uiowa.edu/fsolt/swiid/swiid.html [accessed 12 November 2014).
- Taub DR, Miller B, Allen H. 2008. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol 14(3):565–575, 10.1111/j.1365-2486.2007.01511.x. [DOI] [Google Scholar]
- UN (United Nations). 2013. World Population Prospects: The 2012 Revision. http://www.un.org/en/development/desa/publications/world-population-prospects-the-2012-revision.html [accessed 15 February 2015].
- USAID (U.S. Agency for International Development). 2012. STATcompiler. The DHS Program. http://www.statcompiler.com [accessed 28 August 2014].
- USDA (U.S. Department of Agriculture). 2011. National Nutrient Database for Standard Reference. http://ndb.nal.usda.gov/ [accessed 28 August 2014].
- Vermeulen SJ, Campbell BM, Ingram JSI. 2012. Climate Change and Food Systems. Annu Rev Environ Resour 37:195–222, 10.1146/annurev-environ-020411-130608. [DOI] [Google Scholar]
- Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, et al. . 2015. Health and climate change: policy responses to protect public health. Lancet 386(10006):1861–1914, PMID: 26111439, 10.1016/S0140-6736(15)60854-6. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1995. “Physical Status: The Use and Interpretation of Anthropometry.” WHO Technical Report Series 854. Geneva, Switzerland:WHO. [PubMed] [Google Scholar]
- WHO. 2006. The WHO Child Growth Standards. Geneva, Switzerland:WHO. http://www.who.int/childgrowth/standards/en/ [accessed 10 August 2014].
- World Bank. 2014. World Bank Open Data. World Bank. http://data.worldbank.org/ [accessed 5 September 2014].
- Wroblewitz S, Hüther L, Manderscheid R, Weigel HJ, Wätzig H, Dänicke S. 2013. The effect of free air carbon dioxide enrichment and nitrogen fertilisation on the chemical composition and nutritional value of wheat and barley grain. Arch Anim Nutr 67(4):263–278, PMID: 23870025, 10.1080/1745039X.2013.821781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.