Abstract
Background:
Numerous chemicals are capable of disrupting androgen production, but the possibility that they might act together to produce effects greater than those of the most effective component in the mixture has not been studied directly in human tissues. Suppression of androgen synthesis in fetal life has been associated with testis maldescent, malformations of the genitalia at birth, and poor semen quality later in life.
Objectives:
Our aim was to investigate whether chemicals can act together to disrupt androgen production in human fetal testis explants and to evaluate the importance of mixture effects when characterizing the hazard of individual chemicals.
Methods:
We used an organotypic culture system of human fetal testes explants called FEtal Gonad Assay (FEGA) with tissue obtained at 10 and 12 gestational wk (GW 10–12), to screen 27 chemicals individually for their possible anti-androgenic effect. Based on the results of the screen, we selected 11 compounds and tested them as mixtures.
Results:
We evaluated mixtures composed of four and eight antiandrogens that contained the pharmaceuticals ketoconazole and theophylline and several previously untested chemicals, such as the pesticides imazalil and propiconazole. Mixtures of antiandrogens can suppress testosterone synthesis in human fetal testicular explants to an extent greater than that seen with individual chemicals. This revealed itself as a shift towards lower doses in the dose–response curves of individual antiandrogens that became more pronounced as the number of components increased from four to eight.
Conclusions:
Our results with the FEGA provide the foundations of a predictive human mixture risk assessment approach for anti-androgenic exposures in fetal life. https://doi.org/10.1289/EHP1014
Introduction
In chemical hazard characterizations for pesticides, biocides, and other potentially toxic chemicals, single substances are tested in relevant animal studies with the aim of estimating exposures that are without apparent effects. These estimates form the basis for deriving human reference doses commonly regarded as safe levels of exposure. Normally, this exercise is conducted by considering one chemical at a time, but this single chemical approach does not account for the possibility that simultaneous exposures to other chemicals may also contribute to the toxicity under consideration. As a consequence, single chemical exposures judged to be safe in isolation may in reality pose significant risks, if there is co-exposure to mixtures of substances with similar toxicities. However, the impact of not considering such co-exposures on risk estimates as well as the relevance of co-exposures on the safe use of drugs remains poorly defined.
Potentially higher risks of mixtures need to be taken into account, particularly when the health effects are irreversible, such as those arising from disrupting the action of hormones during key stages of development. Here we investigate the combined effects of chemicals that interfere with androgen action in human testicular tissue. During the first trimester of pregnancy, fetal androgens play a key role in the development and growth of the male reproductive tract, specifically by regulating testicular descent, penile development, and organization of seminiferous tubules (Huhtaniemi 1994). Animal toxicology studies and human epidemiology studies have shown that disruption of androgen action at this time can have irreversible consequences at birth, including testis maldescent (cryptorchidism) and penile malformations where the urethral opening is placed on the underside of the penis (hypospadias). Analysis of secular trends in humans have revealed increasing prevalence of these disorders in some industrialized countries; furthermore, cryptorchidism and hypospadias are increased risk factors for testicular cancer (Cook et al. 2010; Serrano et al. 2013; Skakkebaek et al. 2016).
Numerous chemicals have been identified as antiandrogens in experimental animals and cell-based systems and have been shown to induce androgen insufficiency by several modes of action: suppressing androgen synthesis, blocking the androgen receptor, or altering the signaling of local mediators such as prostaglandins. Prominent examples of anti-androgenic chemicals include certain phthalates used as plasticizers, pesticides, and mild analgesics (Albert and Jégou 2014; Ben Maamar et al. 2017; Kristensen et al. 2016; Mazaud-Guittot et al. 2013; Orton et al. 2011; van den Driesche et al. 2015a).
The combined effects of anti-androgenic chemicals have been assessed in cell-based assays that capture responses close to the molecular events of androgen receptor activation or steroid synthesis (Ermler et al. 2011; Orton et al. 2014; Taxvig et al. 2013) and in animal studies by investigating endpoints indicative of disruption of androgen action at the physiological level (Christiansen et al. 2012; Hass et al. 2007; Howdeshell et al. 2015). This body of work demonstrated that chemicals with greatly varying structural features and modes of action can act together to produce anti-androgenic effects such that the toxicity of the mixture is greater than that of the most effective single component.
However, it is difficult to apply this evidence directly in human risk assessment. Mixture studies with cell-based assays can provide clues as to the possibility of combination effects at the molecular level of hormone signaling, but do not necessarily predict how such effects will play out in vivo at concentrations to which humans may be exposed. Cell-based assays help inform the design of animal studies on the consequences of androgen insufficiency in vivo, but animal studies do not necessarily reflect the human situation. For example, the androgen synthesis-suppressing effects of phthalates and bisphenol A (BPA) in the rat might not be predictive of responses in fetal human tissues (Ben Maamar et al. 2015; van den Driesche et al. 2015b). Furthermore, human health studies cannot provide direct answers about causation, whether for single chemicals or mixtures of chemicals (Braun et al. 2016).
Here we use a novel alternative approach that exposes isolated human fetal testicular tissue to specific mixtures of chemicals in vitro, the FEtal Gonad Assay (FEGA) (Ben Maamar et al. 2015, 2017; Mazaud-Guittot et al. 2013). FEGA maintains the complexity of human fetal testicular tissues during the time of the culture, including its ability to synthesize testosterone. Therefore, it offers the possibility of investigating chemical effects on testosterone synthesis in human fetal testis tissue obtained toward the end of the first trimester of pregnancy, a time when testosterone plays key roles in human reproductive tract development. Furthermore, we applied a predictive modeling approach to mixtures that reduces the need for testing every conceivable combination of chemicals one by one.
Methods
Chemicals
We selected the following chemicals for our studies: bisphenol A (BPA, 239658, CAS No. 80-05-7), ketoconazole (K1003, CAS No. 65277-42-1), bisphenol S (BPS, 103039, CAS No. 80-09-1), clomiphene (C6272, CAS No. 50-41-9, analytical standard), theophylline (T1633, CAS No. 58-55-9), prochloraz (45631, CAS No. 67747-09-5, analytical standard), imazalil (32007, CAS No. 35554-44-0, analytical standard), bitertanol (45349, CAS No. 55179-31-2, analytical standard), propiconazole (45642, CAS No. 60207-90-1, analytical standard), valproic acid (P4543, CAS No. 99-66-1), and chlordecone (SC-394278, CAS No. 143-50-0, Santa Cruz, Dallas, TX, USA). All chemicals were obtained from Sigma-Aldrich, except chlordecone.
Collection of Human Samples
First trimester human fetal testes were obtained from pregnant women after legally induced abortions in collaboration with the University Hospital of Rennes (France); all women received information and gave written consent in accordance with the French national guidelines (Agence de la Biomédecine, authorization PFS09–011). We obtained approval from the local ethics committee of Rennes (advice # 11–48). Women did not receive any financial compensation for donating tissue. The testes were recovered from the aspiration product by examination with a binocular microscope (Olympus SZX7) and immediately placed in cold phosphate buffered saline (PBS). Testes were selected only from GW 10–12 because, in our experience, they are well suited to test multiple concentrations of chemicals in the same testis (Ben Maamar et al. 2015, 2017; Mazaud-Guittot et al. 2013). Also, this is a likely pertinent window of sensitivity for the action of fetal testosterone in directing male reproductive tract development in humans (Welsh et al. 2008).
Fetal Testis Culture
The fetal testes were cultured as previously described (Ben Maamar et al. 2015). Briefly, testes were cut into approximately pieces and each explant was cultured separately. At least two explants per fetus were cultured in control conditions. Explants were placed onto culture inserts () in 24-well companion plates. Each well was filled with phenol red–free medium 199 (21157-029, Invitrogen Life Technologies, Saint Aubin, France) supplemented with gentamicin (G1272, Sigma-Aldrich, Saint-Quentin, France) and Fungizone (A2942, Sigma-Aldrich). Human chorionic gonadotrophin (CG5, Sigma-Aldrich) was added to a final concentration of , which was found in our previous experiments to sustain steroidogenic responsiveness and the integrity of the tissue (Hallmark et al. 2007; Mazaud-Guittot et al. 2013). Cultures were incubated at 37°C for 96 h under a humidified atmosphere of 95% air and 5% . The medium was changed every 24 h and immediately snap-frozen on dry ice and stored at . The first 24 h of culture were conducted without addition of test chemicals or their mixtures. This served as a baseline for hormone production, allowing normalization of explant secretory capacity before the addition of chemicals alone or as mixtures. Dimethylsulfoxyde (DMSO, 34943, CAS No. 67-68-5, Sigma-Aldrich) was used to dilute chemicals [except for the BPA stock solution, which was diluted in ethanol (UN1170, CAS No. 64-17-5, TechniSolv, Fontenay-sous-bois, France)] and as a solvent control to a final concentration of 0.1%. The final concentration of ethanol was (v:v).
Immunostaining and Cytotoxicity
To ensure that inherent toxicity of our tested chemicals and their mixtures did not induce a downturn in testosterone synthesis, thereby confounding any genuine anti-androgenic effects, we assessed the functional integrity of the testosterone-producing Leydig cell population by immunolabeling of cytochrome P450 (CYP11A1), a key enzyme of the androgen biosynthesis pathway. We also monitored cell apoptosis by immunolabeling of cleaved caspase 3. After fixation in paraformaldehyde or Bouin solution and paraffin embedding, the randomly collected exposed explants were cut, mounted into slides, and labeled with either a rabbit anti-cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) antibody (1/200; HPA016436, Sigma-Aldrich) or a rabbit anticleaved capsase-3 antibody 1/200; 9661S (Cell Signaling Technology), as described by Ben Maamar et al. 2015). Cleaved caspase-3 immunostaining required a step of antigen retrieval performed by incubation in preheated (pH 9.0) at for 40 min and cooled at room temperature (Ben Maamar et al. 2015). All the microscopic observations were made at low and high magnifications, and representative photos were taken at magnification. Taken alone or in combination, both the testicular histopathology, the Leydig cell labeling and the cleaved caspase-3 positive cells staining provided a qualitative overall assessment of the integrity of the explant for each chemical at any concentration.
Testosterone Assay
The testosterone levels measured in the FEGA are influenced by maternal factors, the intrinsic properties of the fetal testes, their age, the size of the explants, and the precise culture conditions. These factors inevitably introduce considerable variations of the measured hormone levels. Out of concerns that this variability might complicate dose–response analyses and create serious difficulties during the interpretation of mixture experiments, we took steps to improve the reproducibility of assay responses by working with a tightly controlled age range of fetal testes (GW 10–12). We also obtained several explants per testes, which enabled us to test multiple concentrations of a chemical with the same material. The size of the explants was rigorously controlled and a normalization procedure allowed us to increase statistical power by pooling the results from multiple experimental sessions.
Testosterone levels from each testis explant were measured at the beginning of the experiment [day 0 (D0), that is, after 24 h of culture] and after 72 h in culture media (D3). Measurements at D0 were used as reference baseline for the normalization of hormone production per testis. This procedure was adhered to for all treated and untreated samples, and each sample was assayed in duplicate in a radioimmunoassay (RIA) according to the kit manufacturer’s instructions [testosterone direct RIA; Beckman Coulter, Villepinte, France; intra-assay coefficient of variation (CV) of 5.6% and interassay (CV) of 15%].
Experimental Testing
Range-finding experiments were performed for each compound to identify nontoxic concentration ranges. Definite studies were then conducted to obtain data for the entire effect range of testosterone production, with individual compounds and mixtures run on explants from 3 to 12 different testis. At last three different concentrations were tested. All experiments were run with a fixed DMSO concentration of 0.1% among the tested doses range. A positive control of ketoconazole was used in each study (Mazaud-Guittot et al. 2013).
Design of Mixture Experiments
Multi-component mixtures were evaluated by comparing their experimentally observed responses with those predicted by a dose-addition model (DA) (Loewe and Muischnek 1926). This pharmacological model uses the dose–response information from all individual compounds that are present in the mixture. We used a stepwise approach: a) Compounds were tested individually to provide sufficient data for the statistical dose–response regression analysis; b) pooled data from each compound were used to establish a statistical best-fitting regression model; c) equi-potent mixtures were designed according to the “fixed mixture ratio design” (Faust et al. 2001), where mixture ratios were chosen in line with the potencies of the mixture components and additivity expectations were calculated; and d) mixtures were tested under exactly the same experimental conditions as the individual compounds and their observed effects were compared to the predicted responses. For details of the composition of the mixtures, see Table 1.
Table 1.
Model parameters for all tested single compounds and composition of the tested mixtures.
| Chemical | Dose–response model | Mixture compositiona | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | (M) | Toxicity (M) | Mix I | Mix II | Mix III | Mix IV | |||
| Ketoconazole | Weibull | 0.20 | 0.20 | 0.025 | |||||
| BPA | Weibull | 0.45 | 0.45 | 0.053 | 0.057 | ||||
| Valproic acid | Weibull | 0.16 | 0.30 | 0.021 | |||||
| Clomiphene | Logit | 0.19 | 0.026 | ||||||
| Theophylline | Logit | 0.05 | 0.002 | ||||||
| BPS | Weibull | 0.355 | 0.302 | ||||||
| Chlordecone | Logit | 0.243 | 0.277 | ||||||
| Imazalil | Logit | 0.275 | 0.337 | ||||||
| Bitertanol | Logit | 0.003 | |||||||
| Prochloraz | Logit | 0.001 | |||||||
| Propiconazole | logit | 0.023 | |||||||
Note: and are estimates of the unknown model parameters and . represents the mean molar concentration (M, ) of a chemical that provokes a 50% suppression of testosterone levels in the FEGA. Mix, mixture.
Expressed as the fraction of the dose of each single chemical in the total mixture dose.
Mixture effects were predicted according to the model of DA (Loewe and Muischnek 1926), which is defined for a mixture of components by
| [1] |
where are the effect concentrations of the single compounds in the mixture that each on their own produces the same quantitative effect as the mixture, and defined as the prevalence of a mixture component in the mixture, that is, the ratio of its concentration to the total mixture concentration (see mixture ratio in Table 1). The statistical uncertainty for the predicted mixture effects and effect concentration was estimated using the bootstrap method (Efron and Tibshirani 1994) and expressed as 95% confidence limits for the predicted mean estimate. Differences between predicted and observed effect concentrations were considered as statistically significant when the 95% confidence belts of the prediction did not overlap with those of the experimentally observed mixture effects.
Mixture Testing
Four different mixtures were investigated. They were composed of ketoconazole, BPA, valproic acid, and clomiphene (mixture I); ketoconazole, BPA, valproic acid, and theophylline (mixture II); ketoconazole, BPA, valproic acid, clomiphene, theophylline, chlordecone, BPS, and imazalil (mixture III); and bitertanol, BPA, prochloraz, propiconazole, theophylline, chlordecone, BPS, and imazalil (mixture IV). The mixture concentrations to be tested were chosen according to the DA expectations, by establishing a geometric sequence of concentrations bounded by the lowest concentration predicted to inhibit the testosterone production only minimally and the highest concentration predicted to stop the production altogether. All mixture solutions were prepared in DMSO for a final concentration of DMSO equal to 0.1% in each cultured well.
Data Normalization during Dose–Response Analysis
The baseline testosterone production depends on various factors, such as age of the fetus, pregnancy circumstances, and lifestyle pattern of the mothers, and because these factors are not controlled (or are unknown), they might lead to considerable variability between the individual control measurements. This suggests that whenever possible, individual testosterone measurements should be referenced only to the same fetus origin, and not the sample (or population) mean. Furthermore, testosterone levels in untreated samples are expected to drop by approximately 50% over the 3 d, suggesting that these differences must be taken into account during data treatment and analysis. As a pragmatic solution to these difficulties we normalized testosterone measurements in the following way, with only data used from the same testis explants:
| [2] |
Here and refer to the treated measurements from the beginning () and after 3 d (). A testosterone change of 1 refers to no change in the testosterone production, and 0 to a complete suppression.
Statistical regression analysis was carried out by using a best fit approach (Scholze et al. 2001) on the basis of two nonlinear (sigmoidal) concentration–response functions (logit, Weibull), with both considered as flexible enough to accurately describe the experimental concentration–response data for the mixture predictions. The best-fitting model of these two was then selected for the mixture modeling. All regression analysis was performed using Graphpad prism software (GraphPad Software, Inc., La Jolla, California, USA).
Results
Selection and Effects of Chemicals Screened for Inclusion in Mixtures
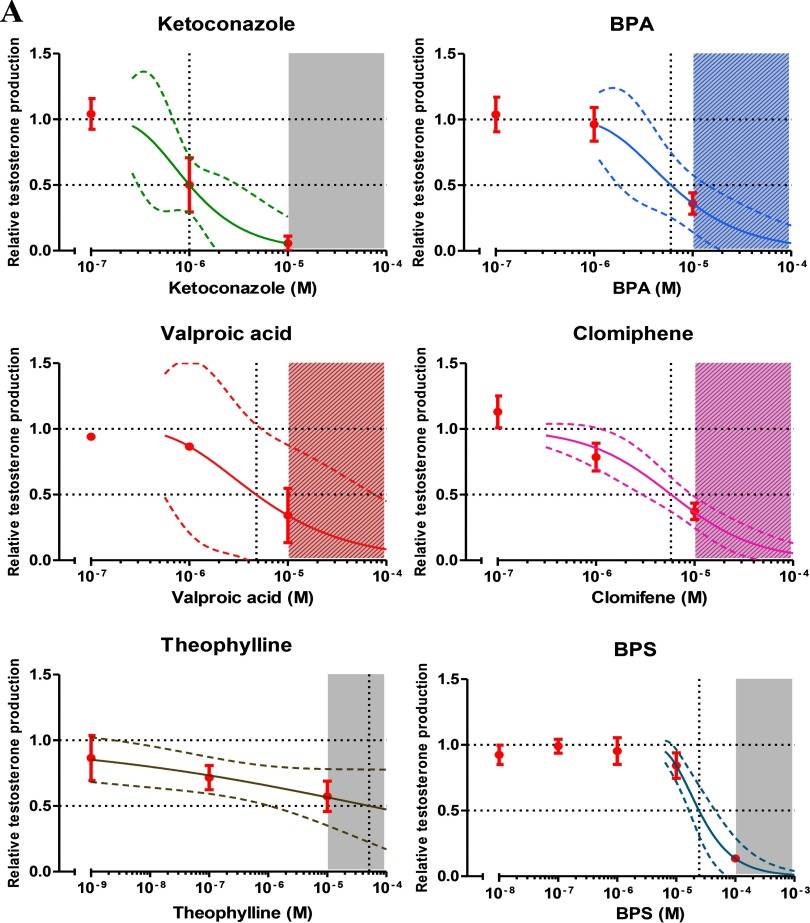
Candidate chemicals were selected for inclusion in our multi-component mixtures if they met the following criteria: a) They had to be able to suppress testosterone synthesis in the FEGA, and b) they had to show dose–response curves with almost complete inhibition of testosterone synthesis. Compliance with these two criteria ensured that expected combination effects could be calculated over a large range of responses on the basis of the effects of the single mixture components. Furthermore, all selected chemicals had to be of relevance to human exposures. Because too few chemicals were known that fulfilled all these criteria when we began our work, we engaged in a screening exercise in which 27 chemicals from a wide range of use categories and applications, and to which pregnant women might be exposed, were tested (see Table S1). Of these 27 chemicals, 11 compounds qualified for inclusion in our mixtures (Table 1; see also Figure S1). Not only did this effort provide detailed dose–response data about many chemicals already known to be active in the FEGA (Ben Maamar et al. 2015; Eladak et al. 2015; Mazaud-Guittot et al. 2013; Pont et al. 1982), it also uncovered several new chemicals whose capability of suppressing testosterone synthesis in humans was not previously recognized: the pesticide imazalil, which had been described before as an androgen receptor antagonist in the MDA-kb2 cell line (Orton et al. 2011); the pesticides propiconazole and bitertanol, which had been previously shown to decrease the testosterone production in the NCI-H295R cell line (Taxvig et al. 2013), and the fungicide prochloraz and the selective estrogen receptor modulator clomifen, both known to be anti-androgenic in the rat (Laier et al. 2006; Fontenot et al. 2015). Theophylline, a caffeine metabolite and pharmaceutical used in the treatment of asthma had previously been shown to impair Leydig cell development in the rat (Pollard et al. 2001). Our data also revealed anti-androgenic properties of the teratogenic drug valproic acid and the pesticide chlordecone (Figure 1A,B, Table 1).
Figure 1.
Concentration–response data for individual chemicals from the organotypic culture system in human fetal testes. (A,B) The graphs show the experimental data as of at least three independent studies together with the regression curves (solid lines) and their respective 95% confidence intervals (CI) (dashed lines). Testosterone production is represented as relative to the first day of culture (D0) production and the control level, see text for more details. Gray areas indicate the cytotoxic concentration ranges, the vertical dashed line the concentrations expected to inhibit testosterone secretion in human fetal testis by 50%.
Dose–Response Analysis of Individual Chemicals
For all 11 chemicals included in the mixture experiments, we determined the best-fitting regression models by nonlinear regression analysis, together with the 95% confidence intervals of these estimates (Figure 1A,B). The regression model parameters and estimated levels associated with a 50% inhibitory response are listed in Table 1. Judging by the concentrations required to induce effects of this magnitude, the potencies of the 11 selected chemicals differed approximately 500-fold. With , the pesticide prochloraz was the most potent of the tested chemicals, and the drug theophylline the weakest ().
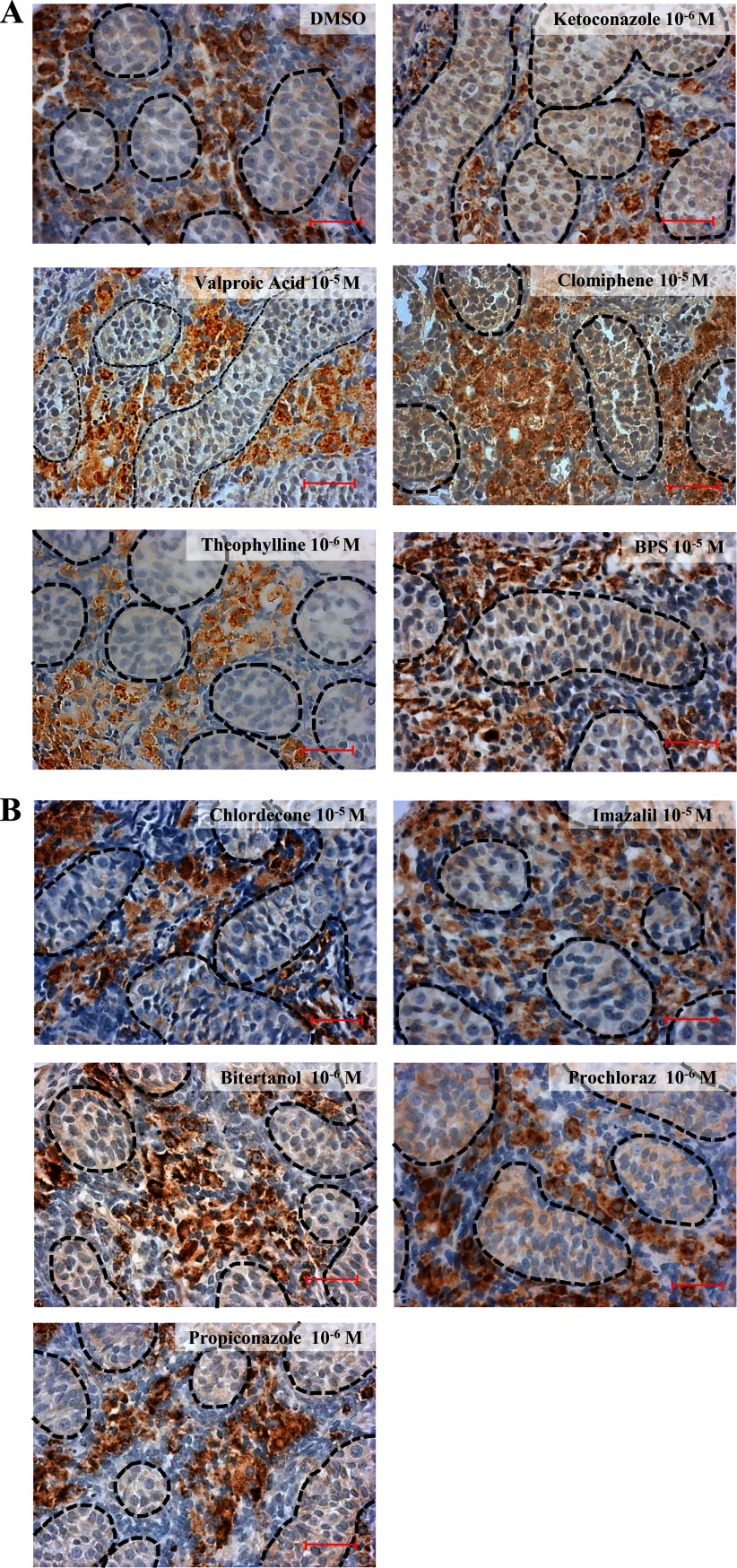
General Toxicity of Antiandrogens Tested
At levels equivalent to, or even exceeding, half-maximal suppressions of testosterone synthesis by all of the 11 chemicals, the tissues appeared intact and well organized, with the expression of CYP11A1 clearly visible (Figure 2A,B). We also monitored possible apoptotic effects by immunolabelling of cleaved caspase-3 positive cells. Our results showed that the tested chemicals did not induce tissue toxicity at doses equal to, or even higher than, those used in the mixture experiments described below (see Figure S2).
Figure 2.
Single compound histopathology of treated human fetal testes explants at the highest nontoxic concentrations after a culture of 96 h. (A,B) Steroidogenic Leydig cells were labeled by immunostaining of CYP11A1 in cultured explants of gestational week (GW) 10–12. The 3,3′-diaminobenzidine tetrahydrochloride staining appears brown in all photos, and sections were counterstained with hematoxylin. Testis cords and interstitial tissue could be easily identified in all the sections (dashed lines represent testis cords). Bar.
Mixture Experiments
With the intention of analyzing whether combination effects could be predicted accurately on the basis of the effects of its components, we investigated four different mixtures composed of four and eight components. A second aim was to assess the impact of co-exposures on the dose–response curve of a specific chemical and to assess how this varies with the number of mixture components. To realize this aim, there had to be one chemical common to all the mixtures; we selected BPA for this purpose, in view of widespread concerns about its endocrine disrupting potential (Ben Maamar et al. 2015) and its ubiquity as an environmental pollutant.
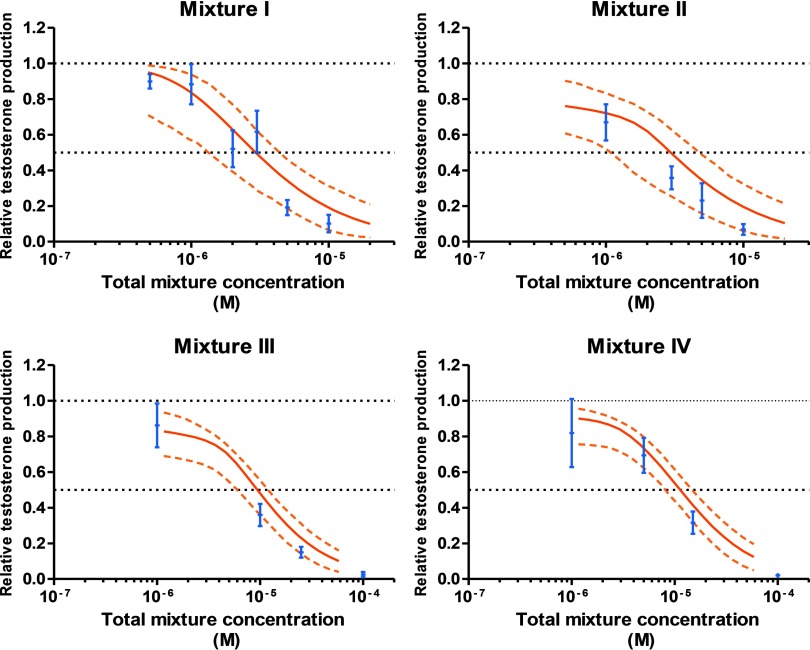
In each case, we began by calculating the mixture dose–response curves that were to be expected if all components acted together according to the principles of dose addition. These calculations relied on the best-fitting regression models for each individual mixture component (Table 1, Figure 1A,B) and the mixture ratios chosen for each mixture. The choice of mixture ratio was driven by the need to ensure that each single chemical contributed significantly to the overall combined effect of the mixture. Mixture ratios were set to reflect the potencies of all mixture components in the FEGA (Table 1). We then predicted the expected combination effects together with their 95% bootstrap confidence intervals. These predictions defined the boundaries of the dose ranges that were examined in the mixture experiments (Figure 3).
Figure 3.
Predicted and observed testosterone secretion in human fetal testis by four chemical mixtures. Experimental data are shown as (blue) of at least four independent experiments. Testosterone production is represented as relative to the first day of culture (D0) production and the control level, see text for more details. The mixture effects were predicted according to dose addition (DA) (thick red curve), with dashed curves the respective 95% confidence intervals (CIs) (dotted orange lines).
As the screening process of the selected compounds was progressing, we began by testing two four-component mixtures that, apart from BPA, contained pharmaceutical drugs. Mixture I comprised the antifungal ketoconazole, the selective estrogen receptor modulator clomiphene, the anticonvulsant valproic acid, and BPA. In mixture II, clomiphene was replaced with theophylline, a metabolite of caffeine, which is used in the therapy of asthma and other respiratory diseases. Thus, mixture II was composed of ketoconazole, BPA, valproic acid, and theophylline. Both mixtures I and II showed a clear dose–response pattern for declining testosterone levels (Figure 3). Even at mixture doses associated with 80% suppression of testosterone levels there were no signs of impairment of histopathology or of cleaved caspase-3 immunostaining, demonstrating that confounding toxicity did not influence the observed anti-androgenic effects of the mixture (Figure 4). In both cases, the experimentally observed effects stayed well within the statistical 95% confidence belt of the additivity prediction curves, which led us to conclude that the mixture effect was additive (Table 2, Figure 3).
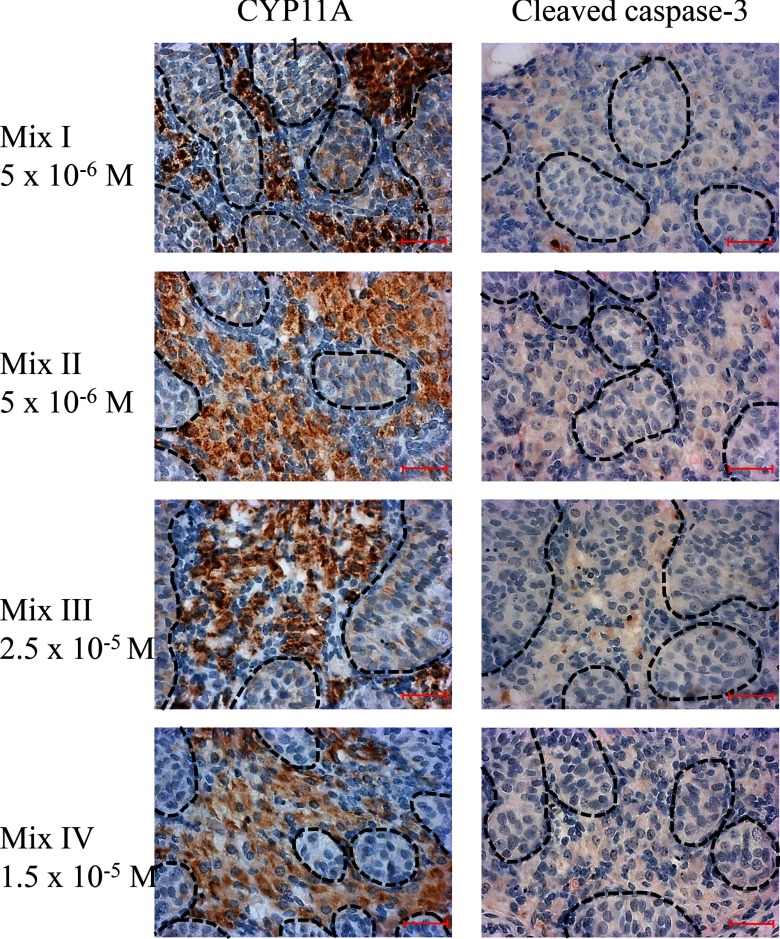
Figure 4.
Histopathology of explants at high-effect concentrations of the mixtures. Steroidogenic Leydig cells and apoptotic cells were labeled in cultured explants of GW 10–12 human fetal testis with an immunostaining of CYP11A1 (left panel) and an anticleaved caspase-3 antibody (right panel), respectively. The 3,3′-diaminobenzidine tetrahydrochloride staining appears brown, and sections were counterstained with hematoxylin. Testis cords are highlighted by dashed lines, and the Leydig cells within the interstitial tissue can be identified by their brown labeling. Bar.
Table 2.
Statistical uncertainty of predicted and observed effect concentrations for mixtures [effect concentration (M)].
| Observed | Predicted | |||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Mixture I | , | , | ||
| Mixture II | , | , | ||
| Mixture III | , | , | ||
| Mixture IV | , | , | ||
These observations motivated us to extend our studies to assess whether additivity can be considered as the default assumption for the joint action of compounds on disrupting testosterone production in the human male fetus. This required investigations of mixtures that contained a larger number of components and apart from drugs also included other chemicals. Accordingly, we composed mixture III by adding theophylline, BPS, and the pesticides chlordecone and imazalil to the ingredients of mixture I. Mixture IV contained BPA, theophylline, BPS, and the pesticides chlordecone, imazalil, bitertanol, prochloraz, and propiconazole (Table 1). As seen previously with the four-component mixtures, the experimentally observed suppressions of testosterone synthesis of both these eight-component mixtures fell within the 95% confidence belts of the prediction curves and agreed well with the expectation that the combined effects of these agents are additive. Thus, the predictability of mixture effects did not diminish as we increased both number and variety of mixture components (Table 2, Figure 3). As before with the four-component mixtures, there was no evidence of confounding toxicity with either of the eight-component mixtures (Figure 4).
The Impact of Co-Exposures on the Apparent Potency of Anti-Androgenic Chemicals
Finally, we investigated the magnitude of errors that might be introduced by a disregard of co-exposures to similarly acting chemicals during the process of hazard characterizations. We approached this by adopting the perspective of a risk assessor attempting to define safe exposures for a specific chemical in isolation, when in reality there is co-exposure to several other chemicals. To this end, we compared the dose–response curves of all the single components of mixture III tested on their own (right-hand curves in Figure 5A) with those obtained in the presence of the other seven mixture constituents (left-hand curves in Figure 5A). These latter curves were constructed by plotting each chemical’s dose in the mixture against the observed mixture effects. In all cases, the curves shifted to the left, towards lower concentrations, indicating increased potency relative to the single chemical, but the extent of these shift depended on the chemical considered in these analyses. Potencies (judged on the basis of the concentrations required to achieve half-maximal effects) increased by a factor of 10 (imazalil) and up to a factor of 10,000 (theophylline) due to co-exposure to the seven other chemicals in the mixture.
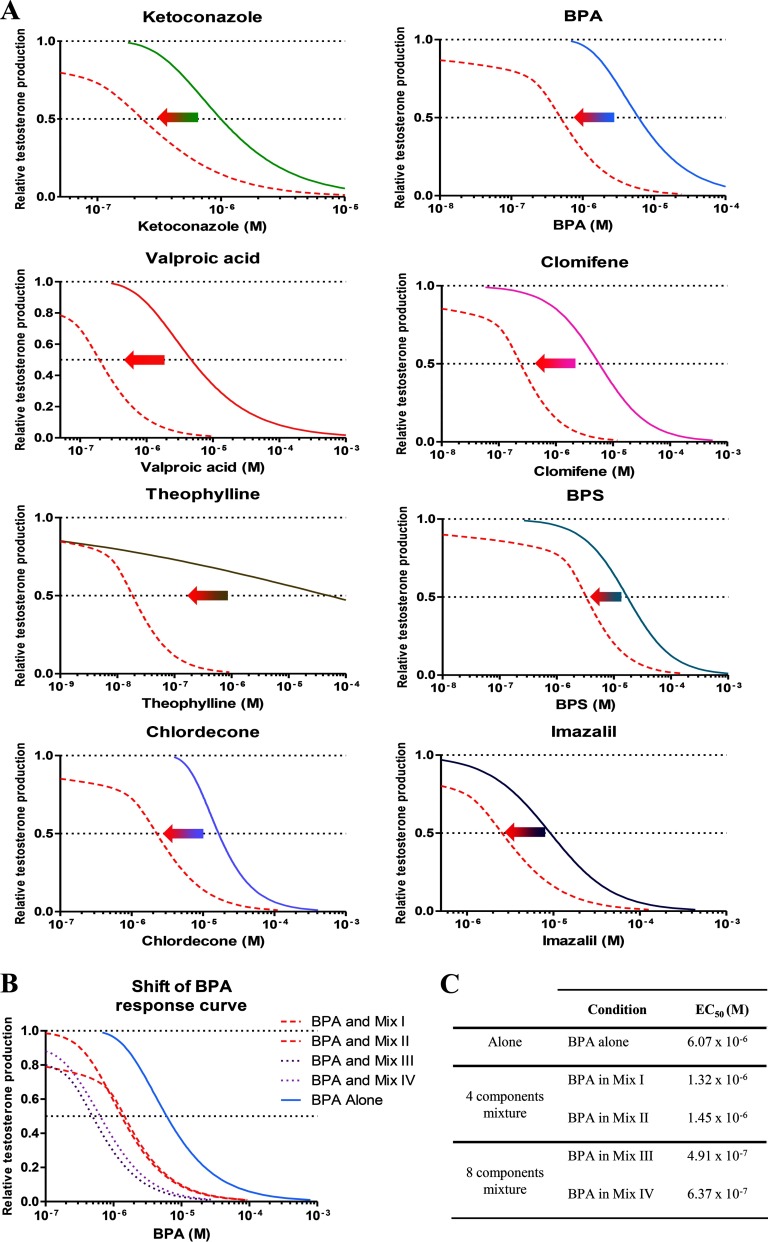
Figure 5.
The impact of co-exposures on the dose–response curves of individual mixture components. A) The concentration–response curves on the right in each panel show the responses for each compound tested on its own, and the curves on the left, the response if tested in combination with seven other compounds in mixture III (red solid lines). Testosterone production is represented as relative to the D0 production and the control level, see text for more details. B) The shift in the concentration–response curve of BPA in the presence of mixtures I–IV. C) Comparison of the concentrations of BPA associated with 50% testosterone synthesis suppression in the presence of mixtures I–IV.
Because BPA was present in all four mixtures, this offered the opportunity of investigating how these potency shifts are influenced by the number of chemicals in the mixtures. As shown in Figure 5B, the apparent potency of BPA increased by a factor of about 3 in the presence of three compounds, and by a factor of 10 in the presence of seven compounds. A BPA level judged to be without effect on its own () produced a 75% suppression of testosterone synthesis in the presence of the seven other chemicals that made up mixture III.
For mixture III, we also compared the concentrations of each component that produced a 20% testosterone suppression (EC20) with the concentrations present in a mixture causing a 50% effect. As shown in Table 3, the concentrations of every chemical in the mixture were by a factor of 2–3 lower than their EC20. This suggests that in combination, the components of this mixture are able to work together at levels that had they been given singly would not have produced observable effects.
Table 3.
Comparison of values of the components of mixture III with the concentrations present in a mixture causing 50% suppression of testosterone.
| Chemical | (M) | Individual dose in mix III at |
|---|---|---|
| BPA | ||
| Clomifene | ||
| Ketoconazole | ||
| Valproic acid | ||
| BPS | ||
| Chlordecone | ||
| Imazalil | ||
| Theophylline | ||
| Mixture III effect | 53% reduction in the testosterone production |
Note: is the concentration (M, ) of each substance associated with a 20% reduction of testosterone production. Mix, mixture.
Discussion
Concerns that the traditional focus of chemical risk assessment on single chemical exposures might underestimate the risks associated with adverse effects of multiple chemicals have been expressed earlier (Kortenkamp 2014), but the impact on risk estimates has been proven difficult to define. This is partly due to incomplete information about the complexity of combined human exposures and to a lack of clarity about the approaches and methods that should be used for mixture risk assessment. Our study provides important advances in improving the scientific basis for human mixture risk assessment. To our knowledge, we demonstrate for the first time that the mixture assessment concept of dose addition is applicable to human tissues. This not only enabled us to avoid certain uncertainties associated with animal-to-human extrapolations, but also enabled us to use a predictive approach. Rather than studying every conceivable combination of chemicals within a mixture, the joint effects of anti-androgenic chemicals in the FEGA can now be approximated on the basis of the effects of each single component by using dose addition as the default assumption.
To utilize the FEGA in multi-component mixture studies required making a leap from qualitative studies to quantitative dose–response analyses. Due to the inhomogeneity of the material and the variations inevitably introduced through the age differences of the fetal testes, the assay outcome (fetal testosterone production) shows high variability, which we had to deal with by rigorously controlling experimental conditions. We achieved good reproducibility, which was essential for realizing our goal of analyzing whether the combined effects of multiple chemicals can be predicted accurately on the basis of the effects of individual mixture components and of assessing the impact of co-exposures on the dose–response curves of single chemicals.
A difficulty in using the FEGA as a screening method for the identification of chemicals with endocrine disruptive properties is the limited availability of human fetal tissue. An additional challenge is in the requirement of collecting tissues of comparable age.
Our study provides direct evidence that co-exposures should be considered when evaluating the risk of a single chemical. We show that effects of a single chemical are underestimated when co-exposure to related chemicals are not considered, and that this underestimation is driven by the number, type, and potency of co-occurring chemicals. In this study, overlooking co-exposures to only seven chemicals led to an underestimation of the potency of BPA by a factor of 10. A corollary of the principles of dose addition is that co-exposure to a larger number of chemicals will drive up the extent of such underestimations if these chemicals are present at levels equipotent with the components we used in our experiments. Alternatively, replacement of some components with larger numbers of other chemicals, but at lower levels, may lead to similar underestimations. More studies using the FEGA are needed to establish these assumptions.
Based on our findings, we suggest that the impact of mixture effects on male sexual differentiation during the first trimester of pregnancy may be considerable. However, although in this study the selection of chemicals was empirically based on the results obtained in our dose–response study, analysis of individual chemicals, assessment of the extent of adverse effects in human fetuses will require more knowledge about the spectrum of chemicals capable of suppressing testosterone synthesis. Future FEGA studies will help close this knowledge gap, especially if based on companion studies that identify all of the exogenous chemicals found in maternal and fetal tissues.
Supplemental Material
Acknowledgments
We thank all the staff of the Department of Obstetrics and Gynecology and the Department of Pediatric Surgery of the Rennes Sud Hospital (Rennes, France) and the participating women, without whom this study would not have been possible.
We acknowledge the financial supports from the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES); CHEMIX-EST-12-171, ChemPSy-EST-13-081, Institut National de la Santé et de la Recherche Médicale (Inserm). P.G. is a recipient of a stipend from the Fondation pour la Recherche Médicale.
References
- Albert O, Jégou B. 2014. A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum Reprod Update 20(2):231–249, PMID: 24077978, 10.1093/humupd/dmt050. [DOI] [PubMed] [Google Scholar]
- Ben Maamar M, Lesné L, Desdoits-Lethimonier C, Coiffec I, Lassurguère J, Lavoué V, et al. . 2015. An investigation of the endocrine-disruptive effects of bisphenol A in human and rat fetal testes. PLoS One 10(2):e0117226, PMID: 25706302, 10.1371/journal.pone.0117226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Maamar M, Lesné L, Hennig K, Desdoits-Lethimonier C, Kilcoyne KR, Coiffec I, et al. . 2017. Ibuprofen results in alterations of human fetal testis development. Sci Rep 7:44184, PMID: 28281692, 10.1038/srep44184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 124(1):A6–A9, PMID: 26720830, 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Kortenkamp A, Axelstad M, Boberg J, Scholze M, Jacobsen PR, et al. . 2012. Mixtures of endocrine disrupting contaminants modelled on human high end exposures: an exploratory study in rats. Int J Androl 35(3):303–316, PMID: 22372636, 10.1111/j.1365-2605.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, McGlynn KA. 2010. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer–experiences of the son. Int J Epidemiol 39(6):1605–1618, PMID: 20660640, 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. 1994. An Introduction to the Bootstrap. Boca Raton, FL:Chapman & Hall. [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, et al. . 2015. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103(1):11–21, PMID: 25475787, 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A. 2011. The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicol Appl Pharmacol 257(2):189–197, 10.1016/j.taap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Faust M, Altenburger R, Backhaus T, Blanck H, Boedeker W, Gramatica P, et al. . 2001. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aquat Toxicol 56:13–32, PMID: 11690628, 10.1016/S0166-445X(01)00187-4. [DOI] [PubMed] [Google Scholar]
- Fontenot GK, Wiehle RD, Podolski JS. 2015. Differential effects of isomers of clomiphene citrate on reproductive tissues in male mice. BJU Int 117(2):344–350, PMID: 26220499, 10.1111/bju.13244. [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, et al. . 2007. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect 115(3):390–396, PMID: 17431488, 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, et al. . 2007. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect 115(suppl 1):122–128, PMID: 18174960, 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Furr JR, Lambright CR, Gray LE. 2015. Dose addition models based on biologically relevant reductions in fetal testosterone accurately predict postnatal reproductive tract alterations by a phthalate mixture in rats. Toxicol Sci 148(2):488–502, PMID: 26350170, 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I. 1994. Fetal testis—a very special endocrine organ. Eur J Endocrinol 130(1):25–31, PMID: 8124476, 10.1530/eje.0.1300025. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. 2014. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr Opin Pharmacol 19:105–111, PMID: 25244397, 10.1016/j.coph.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Mazaud-Guittot S, Gaudriault P, Lesné L, Serrano T, Main KM, et al. . 2016. Analgesic use—prevalence, biomonitoring and endocrine and reproductive effects. Nat Rev Endocrinol 12(7):381–93, PMID: 27150289, 10.1038/nrendo.2016.55. [DOI] [PubMed] [Google Scholar]
- Laier P, Metzdorff SB, Borch J, Hagen ML, Hass U, Christiansen S, et al. . 2006. Mechanisms of action underlying the antiandrogenic effects of the fungicide prochloraz. Toxicol Appl Pharmacol 213(2):160–171, PMID: 16375936, 10.1016/j.taap.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Loewe S, Muischnek H. 1926. Über Kombinationswirkungen [in German]. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 114:313–326, 10.1007/BF01952257. [DOI] [Google Scholar]
- Mazaud-Guittot S, Nicolas Nicolaz C, Desdoits-Lethimonier C, Coiffec I, Ben Maamar M, Balaguer P, et al. . 2013. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J Clin Endocrinol Metab 98(11):E1757–E1767, PMID: 24030937, 10.1210/jc.2013-2531. [DOI] [PubMed] [Google Scholar]
- Orton F, Ermler S, Kugathas S, Rosivatz E, Scholze M, Kortenkamp A. 2014. Mixture effects at very low doses with combinations of anti-androgenic pesticides, antioxidants, industrial pollutant and chemicals used in personal care products. Toxicol Appl Pharmacol 278(3):201–208, PMID: 24055644, 10.1016/j.taap.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Orton F, Rosivatz E, Scholze M, Kortenkamp A. 2011. Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ Health Perspect 119(6):794–800, PMID: 21310686, 10.1289/ehp.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard I, Locquet O, Solvar A, Magre S. 2001. Effects of caffeine and its reactive metabolites theophylline and theobromine on the differentiating testis. Reprod Fertil Dev 13(5–6):435–441, PMID: 11833941. [DOI] [PubMed] [Google Scholar]
- Pont A, Williams PL, Azhar S, Reitz RE, Bochra C, Smith ER, et al. . 1982. Ketoconazole blocks testosterone synthesis. Arch Intern Med 142(12):2137–2140, PMID: 6291475. [PubMed] [Google Scholar]
- Scholze M, Boedeker W, Faust M, Backhaus T, Altenburger R, Grimme LH. 2001. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem 20(2):448–457, PMID: 11351447. [PubMed] [Google Scholar]
- Serrano T, Chevrier C, Multigner L, Cordier S, Jégou B. 2013. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum Reprod 28(7):1974–1986, PMID: 23670171, 10.1093/humrep/det111. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. . 2016. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 96(1):55–97, PMID: 26582516, 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxvig C, Hadrup N, Boberg J, Axelstad M, Bossi R, Bonefeld-Jørgensen EC, et al. . 2013. In vitro – in vivo correlations for endocrine activity of a mixture of currently used pesticides. Toxicol Appl Pharmacol 272(3):757–766, PMID: 23954766, 10.1016/j.taap.2013.07.028. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Macdonald J, Anderson RA, Johnston ZC, Chetty T, Smith LB, et al. . 2015a. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model. Sci Transl Med 7(288):288ra80, 10.1126/scitranslmed.aaa4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, McKinnell C, Calarrão A, Kennedy L, Hutchison GR, Hrabalkova L, et al. . 2015b. Comparative effects of di(n-butyl) phthalate exposure on fetal germ cell development in the rat and in human fetal testis xenografts. Environ Health Perspect 123(3):223–230, PMID: 25514601, 10.1289/ehp.1408248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, et al. . 2008. Identification in rats of a programing window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118(4):1479–1490, PMID: 18340380, 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.