Abstract
Background:
Prior studies suggest neurodevelopmental impacts of polybrominated diphenyl ethers (PBDEs), but few have examined diagnosed developmental disorders.
Objectives:
Our aim was to determine whether prenatal exposure to brominated flame retardants (BFRs) is associated with autism spectrum disorder (ASD) or intellectual disability without autism (ID).
Methods:
We conducted a population-based case–control study including children with ASD () and ID () identified from the California Department of Developmental Services and general population (GP) controls () from state birth certificates. ASD cases were matched to controls by sex, birth month, and birth year. Concentrations of 10 BFRs were measured in maternal second trimester serum samples stored from routine screening. Logistic regression was used to calculate crude and adjusted odds ratios (AOR) for associations with ASD, and separately for ID, compared with GP controls, by quartiles of analyte concentrations in primary analyses.
Results:
Geometric mean concentrations of five of the six congeners with of samples above the limit of detection were lower in mothers of children with ASD or ID than in controls. In adjusted analyses, inverse associations with several congeners were found for ASD relative to GP (e.g., quartile 4 vs. 1, BDE-153: , 95% CI: 0.38, 0.84). When stratified by child sex (including 99 females with ASD, 77 with ID, and 73 with GP), estimates were consistent with overall analyses in boys, but in the opposite direction among girls, particularly for BDE-28 and -47 (, 95% CI: 0.86, 7.79 and , 95% CI: 0.97, 7.19, respectively). Similar patterns overall and by sex were observed for ID.
Conclusions:
Contrary to expectation, higher PBDE concentrations were associated with decreased odds of ASD and ID, though not in girls. These findings require confirmation but suggest potential sexual dimorphism in associations with prenatal exposure to BFRs. https://doi.org/10.1289/EHP1079
Introduction
Autism spectrum disorder (ASD) is defined according to deficits in social communication and presence of restricted behaviors. This neurodevelopmental condition currently affects about 1 in 68 U.S. children, and is approximately four times more common in males than females (Christensen et al. 2016). The etiology of ASD remains largely unexplained; however, evidence supports the influence of both genetic and environmental factors acting in the prenatal period (Rodier 2000; Newschaffer et al. 2007). We recently reported that prenatal exposure to certain polychlorinated biphenyl (PCBs) congeners was positively associated with odds of ASD (Lyall et al. 2017). Like PCBs, polybrominated diphenyl ethers (PBDEs) and the chemical 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153), known as brominated flame retardants (BFRs), are another class of endocrine-disrupting chemicals (EDCs). Developmental exposure to BFRs, and EDCs in general, has emerged as a suspected risk factor for ASD (Hertz-Picciotto et al. 2011; Grandjean and Landrigan 2014; Lyall et al. 2014) given known influences of hormone disruptions on brain development (de Escobar et al. 2004; Morreale de Escobar et al. 2004; Costa and Giordano 2007).
Although many BFRs have been phased out of use in the United States, levels of these chemicals are still detected in human blood samples due to their lipophilic nature and the continued existence of products such as furniture and plastics that contain them (Hites 2004; Sjödin et al. 2004, 2014). Developmental concerns arose following evidence in animal studies that BFRs at levels within the range of high exposure in humans in recent decades impact brain development and neurodevelopmental outcomes relevant to ASD (Eriksson et al. 2001, 2002; Birnbaum and Staskal 2004; Costa and Giordano 2007; Suvorov et al. 2009). Further, BFRs can transfer from maternal to fetal circulation in humans (Mazdai et al. 2003; Frederiksen et al. 2010; Gómara et al. 2007; Chen et al. 2014b), have been associated with thyroid hormone in humans (Chevrier et al. 2010, 2011; Vuong et al. 2015) and markers of immune function in humans and animal studies (Ashwood et al. 2009; Liu et al. 2012), and could suggest potential pathways through which these chemicals may influence fetal neurodevelopment and ASD.
To our knowledge, no published studies to date have considered prenatal BFR levels in association with risk of ASD diagnoses specifically. However, gestational exposure in some human epidemiological studies has been shown to be associated with evidence of cognitive deficits, behavioral impairments, and attention problems (Herbstman et al. 2010; Dingemans et al. 2011; Czerska et al. 2013; Chen et al. 2014a; Herbstman and Mall 2014; Cowell et al. 2015; Sagiv et al. 2015). Only one study (Braun et al. 2014) has examined prenatal BFR levels in association with offspring autistic traits as measured by the Social Responsiveness Scale (Constantino and Gruber 2005). This cohort study including 175 women reported evidence of positive, inverse, and null associations, depending on the congener assessed. Another investigation, a population-based case–control study including 100 children, did not find significant differences in current PBDE levels between children with ASD () and those with typical development () (Hertz-Picciotto et al. 2011). However, measurements were conducted in childhood (mean age 3.7 y), after diagnosis, and thus may not represent the suspected developmentally relevant period of exposure during prenatal neurodevelopment (Rodier et al. 1996; Rice and Barone 2000; Rodier 2000).
In order to address these prior limitations and examine the potential role that prenatal exposure to BFRs plays in risk of ASD and in broader adverse neurodevelopment as defined by intellectual disability (ID), we used data from a large population-based case–control study in California. Given the suggested neurodevelopmental toxicity of BFRs, we hypothesized that higher levels of BFRs would be found in prenatal samples from mothers of children with ASD relative to mothers of control children without developmental delays, and that similar associations may be seen with ID. Given evidence for endocrine disruption activity and suggested sexually dimorphic effects of BFRs on neurobehavioral outcomes from animal work (Lilienthal et al. 2006; Faass et al. 2013), we were also interested in exploring potential sex differences in risk of ASD and ID associated with prenatal BFR exposure.
Methods
Study Population
We used data from the Early Markers for Autism (EMA) study to address these questions. Details of EMA methods have been previously described in an earlier paper (Croen et al. 2008). Briefly, EMA is a population-based case–control study designed to identify biologic markers of autism using archived prenatal and newborn blood samples from California state-wide screening programs. The EMA study sample was drawn from the population of live-born children in three counties from Southern California (Orange, San Diego, and Imperial) in 2000–2003 who participated in the newborn screening program and whose mothers enrolled in the State’s Prenatal Expanded Alpha-fetoprotein Screening Program (XAFP). Approximately 70% of women in California participate in prenatal screening, with samples stored from 7–10 diverse counties throughout the state. Newborn screening is mandatory in the state of California. Following selection (further described below), children were then linked to the prenatal specimen archive to identify those for whom prenatal specimens were available for inclusion in this study. Only mother–child pairs with live birth records linked to archived prenatal and newborn specimens were eligible for inclusion in EMA.
Diagnostic information for the study population was obtained from the California Department of Developmental Services (DDS), which provides services to individuals with substantial disabilities—including autism, intellectual disability, and other developmental disabilities. Further information on the DDS can be found on their website (http://www.dds.ca.gov). DDS data were linked to eligible live birth records for the study region to identify, based on DDS service codes: children with ASD or children with intellectual disability/developmental delay without autism (mental retardation of unknown etiology codes) (ID). GP controls were identified by randomly sampling from eligible birth certificates among strata of matching factors, excluding DDS clients. Individuals with Down syndrome were excluded from all study groups. At the time of data linkage with DDS in 2007 and 2009, children would have been between 4.5 and 9 y old; the majority of cases were between 4.5 and 7 y old when identified. GP controls were originally individually matched to ASD cases on sex, birth month, and birth year. (However, matched analyses were not used as primary analyses here in order to include those individuals originally identified as ID but whom, following clinician review, were found to meet ASD criteria; further described below in “Diagnostic Verification”). Study activities were approved by the institutional review board at Kaiser Permanente and by the California State Committee for the Protection of Human Subjects. Informed consent was not required for the EMA study, given that consent forms for the XAFP Screening program were distributed at the time of the blood collection, which stipulated that specimens and results from prenatal testing could be used for legitimate research purposes given appropriate IRB approval.
Diagnostic Verification
Final case status of children with ASD and children with ID was determined by expert clinical review of diagnostic and clinical data abstracted from DDS records, using an approach based on national surveillance methodology (Yeargin-Allsopp et al. 2003). A final diagnostic classification of ASD was given if DSM-IV-TR criteria were met; in our analytic sample with BFR measurements, 413 children originally identified as ASD in DDS met these diagnostic criteria. Additionally, a total of 132 individuals originally identified as ID in DDS were reclassified as ASD following expert review of abstracted data. ASD cases were further qualified by presence/absence of intellectual disability (defined as for ID, but in the presence of ASD). Final classification of ID (intellectual disability without autism) was based on standardized test score results found in records (composite developmental/cognitive score ) in the absence of meeting ASD criteria.
Specimens and Measurements
After screening was completed at the regional laboratory, newborn dried blood spots were routinely freezer archived, as has been done statewide since 1982, and maternal blood from Southern California was freezer archived as part of the California Department of Public Health’s Project Baby’s Breath. Maternal blood specimens were collected typically between 15 and 20 wk gestation (median: 16 wk, range 7–25 wk) in serum separator tubes by obstetrical care service providers and underwent XAFP testing within 7 d of collection at a central laboratory (). After 1–2 d of refrigeration, leftover serum and cell pellet specimens were stored at .
A total of 10 BFRs were measured in maternal serum samples (BB153, BDEs 17, 28, 47, 66, 85, 99, 100, 153, 154) at the Centers for Disease Control and Prevention. Determination of target analytes was performed by gas chromatography–isotope dilution high resolution mass spectrometry (GC-IDHRMS), employing a Double Focusing Sector (ThermoFinnigan DFS, Bremen, Germany) instrument (Jones et al. 2012). The measurements were performed in two batches March–April, 2012 () and August–December, 2013 (), that is, up to 13 y after the collection of the serum samples between year 2000 and 2003. However, serum pools have been shown to be stable over multiple years, as is expected for persistent organic pollutants such as PBDEs (Sjödin et al. 2004; Turyk et al. 2010). Hence, it can be assumed that no appreciable degradation of target analytes has occurred during the storage of the samples for close to a decade. Concentrations of the BFRs were reported as ng/g lipid weight (weight of serum lipids) after background subtraction (background refers to the contamination of blank samples in the same run as the unknowns). All concentration data were corrected for the average amount present in blank samples, and three blanks were included in every set of 30 samples. A variable amount of serum was available (median ; range: ); due to this, a variable limit of detection was applied proportional to the serum amount used, that is, detection limit (picograms) divided with the available serum amount (grams). In order to prevent biasing the data due to higher LODs at lower amount of available serum, we censored the data based on detection frequency of each individual congener. The method used to censor the data was to calculate the detection frequency for all analytes by categorized available serum amounts, that is, ; ; ; and . If the detection rate was less than 60% in a serum amount category with of serum, the data was categorized as nonreportable in that category and any lower serum amount category. One hundred five samples in the full EMA study (7.7%) had serum amounts less than .
Statistical Analyses
These analyses included singleton births with final case status (see “Diagnostic Verification”) and BFR measurements available. Individuals missing all biomarker measurements (due to insufficient sample; ), and individuals censored due to laboratory error (failure of automation system; ) were excluded, leaving a total of 1,144 individuals. This number includes 545 children with ASD (287 with intellectual disability, 221 without intellectual disability, and 37 for whom we did not have information available to make an intellectual disability classification), 181 children with ID but not autism, and 418 GP control children.
For each of the BFRs measured, the percentage of the study group with levels above the limit of detection (LOD) was calculated. We used a cutoff detection rate of at least 55% of the study population with values above the LOD for selection of congeners with sufficient number of study participants with detectable values for further analysis. Among those congeners meeting this detection rate, individual measurements were replaced with the LOD divided by the square root of 2 (Axelrad et al. 2009; Sjödin et al. 2014), although alternative methods for imputing values below the LOD were also utilized (see “Sensitivity Analyses” below). In addition to examining the individual congeners, we created a variable for combined BFR exposure by summing the individual concentrations of congeners (BB153, BDE-28, 47, 99, 100, and 153) meeting our detection rate cutoff. Other sums explored included BDE congeners only (e.g., not including BB153), including only those congeners detected in at least 60% of the study population (which removed BDE-28 from the sum), and high (BDE-100 and -153) or low (BDE-28 and -47) brominated congener sums.
Descriptive statistics were compared between the ASD, ID, and GP groups, and correlation coefficients between congeners were calculated. Geometric mean concentrations were calculated, and were also compared by diagnostic group and potential confounders. Potential covariates (listed below) were selected on the basis of a priori associations with ASD and/or the BFRs, and obtained from birth certificates or XAFP screening program records.
In order to examine the relationship between concentrations of BFRs and ASD, examine potential dose response, and account for potential nonlinearity in the association, we examined the exposure–outcome relationship using a) quartiles of chemical concentrations or their sums (based on the control distribution), b) continuous concentrations, examining odds associated with a 10-fold BFR increase, and c) cubic splines to evaluate potential nonlinearity in the association and allow for flexibility in the dose–response relationship (Durrleman and Simon 1989; Govindarajulu et al. 2007). For continuous analyses of 10-fold changes in exposure levels, continuous variables of BFR concentration (with values replaced as described) were divided by 10, so that corresponding beta coefficients would have the interpretation of a 10-unit change in exposure level. Secondary analyses also examined alternate categorizations (e.g., deciles, 95th and 5th percentiles). As stated above, matching was broken in primary analyses in order to include all final ASD cases with biomarker measurements. Logistic regression was used to obtain crude (adjusted only for matching factors) and multivariable adjusted odds ratios (OR) and 95% confidence intervals (CI) for the association between the categorized (quartiles) congeners and ASD in comparison to GP controls. All final models included (variables obtained from birth records unless noted) the study matching factors (child sex, birth month, and birth year—the latter modeled as ordinal variables), maternal age (continuous), maternal race/ethnicity (in categories), maternal weight at time of sample collection (continuous; obtained from screening program records), parity (as 0 for no prior live births or 1 for one or more prior live births), and maternal education (in categories). Categorical variables were modeled using the “class” statement in SAS (SAS Institute Inc.). Separate categories were used for missing covariate data ( or less of each study group for maternal education and race/ethnicity). Adjustment for child gestational age (obtained from screening records), and child birth weight (continuous), paternal age (continuous), and maternal birthplace (as in the United States, Mexico, or outside the United States or Mexico) did not impact results (e.g., did not change the estimate , a criterion used across analyses for deeming whether results differed; furthermore, gestational age and birthweight may be on the causal pathway); thus, these covariates were not included in final models. Secondarily, we also explored adjustment for PCBs, which were associated with ASD in a previous analysis of the same study population (Lyall et al. 2017). Tests of trend were conducted using an ordinal variable defined according to the median analyte level of each quartile, with significance determined by a Wald test () in the adjusted models. We also examined whether odds of ASD may differ according to presence of co-occurring intellectual disability by modeling ASD with and without intellectual disability separately, relative to GP controls. Separately, we also examined odds of ID relative to GP controls in parallel analyses.
Stratified analyses were conducted to examine potential effect modification of the association between BFRs and ASD by child sex, given suggestions of potentially sexually dimorphic effects from animal studies (Daniels et al. 2010; Leonetti et al. 2016; Grether et al. 2009; Shelton et al. 2010). Tests of interaction were conducted using a Wald test for the product of sex by the congener (using the median-value quartile variable; again, was used to define statistical significance).
Sensitivity Analyses
A number of sensitivity analyses were conducted to assess robustness of results. First, we examined the effect of excluding from the ASD group individuals originally identified as ID but classified as ASD following expert review (). Conditional logistic regression analyses (e.g., stratified by matching factors rather than adjusting for them as in primary logistic regression analyses) were conducted within the subset of matched ASD-GP pairs (, including 413 pairs) to examine the impact of breaking the matching and including reclassified ID cases in the ASD group in primary analyses. We also considered alternate strategies for replacing values (for congeners detected in at least 55% of samples); these included using lab-read values for low sample volumes originally censored to (uncensored data) and multiple imputation using SAS PROC MI (SAS 2015). Data used to impute missing values included all chemicals assessed, diagnostic status (ASD, ID, and GP), and covariates previously mentioned as potential confounders. We also examined whether removing those individuals with values (rather than imputing their values as in primary analysis) altered results. Finally, we tested whether adjustment for genetic ancestry of mothers, as measured by principal components (PCs) derived from genome-wide association study (GWAS) data, altered results. Details of GWAS and ancestry analyses in EMA are further described in another publication (Traglia et al. 2017); briefly, genome-wide multi-dimensional scaling (MDS) analysis was carried out on high-quality markers genotyped in maternal cell-pellet samples stored from the XAFP and in newborn samples collected through newborn screening, using a pairwise distance genomic matrix and the multi-dimensional scale functions implemented in PLINK software (Purcell et al. 2007) (–cluster and –mds plot). The resulting maternal and fetal genetic matrices included 10 PCs that summarized the genetic distance between each maternal and neonatal sample. The top two PCs, which accounted for the majority of variation, were used in ancestry-adjusted models here. PC-adjusted models included other potential confounders described above, with the exception of race/ethnicity, which would be accounted for by the PCs. Adjustment for PCs was not conducted for the ID to GP comparisons because genetic information was available only for the ASD and GP groups.
Results
Comparing basic characteristics of the study groups, the mean maternal age was higher, and there was a greater proportion of non-Hispanic whites and more highly educated mothers, in the ASD group compared with the ID or GP groups, whereas higher parity, lower maternal education, Hispanic ethnicity, maternal birth place in Mexico, use of a government insurance program, and higher proportion of preterm and low-birth-weight babies were all more common in the ID group relative to the others (Table 1). The higher proportion of males in the ASD and GP groups is a result of an original design feature of the EMA study, wherein cases and controls were matched on sex (as well as birth month and birth year). Of the 10 BFR congeners measured, 6 (BB153, BDE-28, -47, -99, -100, and -153) met our detection rate cutoff. In general, congener detection rates were similar across diagnostic groups, though rates were significantly lower in ASD cases than GP controls for BDE-85 (35% vs. 44%), -99 (92% vs. 96%), and -100 (93% vs. 96%) (Table 2). Levels of congeners measured here were in line with ranges reported for all females in the National Health and Nutrition Examination Survey (survey years 2003–2004, partially overlapping with EMA birth years), though geometric means were somewhat lower than those reported from a sample of U.S. pregnant women during the same time period (Woodruff et al. 2011) (see Table S1).
Table 1.
Characteristics of the study population by diagnostic group.
| Characteristic | ASD() | ID() | GP() |
|---|---|---|---|
| Maternal age [mean (SD)] | 29.9 (5.6) | 27.2 (6.3) | 28.7 (5.4) |
| Paternal age [mean (SD)] | 35.7 (14.6) | 36.9 (21.2) | 34.0 (14.2) |
| Total no. live births [mean (SD)] | 1.8 (0.9) | 2.3 (1.5) | 2.1 (1.0) |
| Parity [ (%)]a | |||
| Primiparous | 247 (45%) | 62 (34%) | 159 (38%) |
| Multiparous | 298 (55%) | 119 (66%) | 259 (62%) |
| Maternal age categories [ (%)] | |||
| 442 (81%) | 159 (88%) | 366 (88%) | |
| 103 (19%) | 22 (12%) | 52 (13%) | |
| Maternal weight at sample collection [ (%)] | |||
| 143 (26%) | 33 (18%) | 110 (26%) | |
| 136 (25%) | 41 (23%) | 114 (27%) | |
| 135 (25%) | 52 (29%) | 95 (23%) | |
| 131 (24%) | 55 (30%) | 99 (24%) | |
| Maternal birth place [ (%)] | |||
| United States | 272 (50%) | 79 (44%) | 202 (48%) |
| Mexico | 131 (24%) | 84 (46%) | 128 (31%) |
| Other | 142 (26%) | 18 (10%) | 88 (21%) |
| Maternal race/ethnicity [ (%)] | |||
| Non-Hispanic white | 192 (35%) | 32 (18%) | 138 (33%) |
| Asian | 82 (15%) | 9 (5%) | 45 (11%) |
| Black, Pacific Islander and other | 48 (9%) | 13 (7%) | 35 (8%) |
| Hispanic | 218 (40%) | 126 (70%) | 197 (47%) |
| Missing | 5 (1%) | 1 (0.6%) | 3 (0.7%) |
| Maternal education [ (%)] | |||
| Less than high school | 97 (18%) | 76 (42%) | 102 (24%) |
| High school | 118 (22%) | 50 (28%) | 113 (27%) |
| Some college/college degree | 222 (41%) | 42 (23%) | 143 (34%) |
| Post-graduate | 100 (18%) | 11 (6%) | 56 (13%) |
| Missing | 8 (1.5%) | 2 (1%) | 4 (1%) |
| Insurance status at delivery [ (%)] | |||
| Self and other | 19 (3%) | 7 (4%) | 18 (4%) |
| Private insurance | 289 (53%) | 49 (27%) | 207 (50%) |
| Government program | 237 (43%) | 125 (69%) | 193 (46%) |
| Child sex | |||
| Male | 446 (82%) | 104 (57%) | 345 (83%) |
| Female | 99 (18%) | 77 (43%) | 73 (17%) |
| Child preterm () [ (%)] | 66 (13%) | 37 (22%) | 44 (11%) |
| Child low birth weight () [ (%)] | 42 (8%) | 40 (22%) | 21 (5%) |
Note: ASD, autism spectrum disorder; GP, general population controls; ID, intellectual disability/developmental delay without autism.
Defined according to total live births.
Table 2.
Serum brominated flame retardant (BFR) congener limit of detection information in the Early Markers for Autism study, by case status and sex.
| BFR | Full study group () | |||||||
|---|---|---|---|---|---|---|---|---|
| LOD range | Max lipid weight (ng/g) | ASD () | ID () | GP () | Males () | Females () | ||
| BB153 | 0.3–1.5 | 62.8 | 62 | 322 (63) | 85 (53) | 241 (63) | 65 | 56 |
| BDE-17 | 0.3–1.6 | 6.2 | 2 | 8 (2) | 1 (1) | 8 (2) | 2 | 1 |
| BDE-28 | 0.3–1.5 | 24.1 | 55 | 264 (53) | 82 (53) | 220 (59) | 58 | 56 |
| BDE-47 | 0.4–8.8 | 1000 | 98 | 534 (98) | 178 (98) | 410 (99) | 98 | 99 |
| BDE-66 | 0.3–1.6 | 9.4 | 1 | 7 (1) | 0 | 8 (2) | 1 | 2 |
| BDE-85 | 0.3–1.7 | 44.3 | 39 | 177 (35)a | 65 (41) | 168 (44) | 42 | 42 |
| BDE-99 | 0.4–3.5 | 331 | 94 | 488 (92)a | 167 (95) | 390 (96) | 94 | 95 |
| BDE-100 | 0.3–2.3 | 271 | 95 | 497 (93)a | 173 (98) | 390 (96) | 96 | 95 |
| BDE-153 | 0.3–2.6 | 249 | 95 | 504 (95) | 168 (95) | 389 (96) | 95 | 96 |
| BDE-154 | 0.3–1.6 | 38.5 | 39 | 178 (35) | 62 (39) | 166 (43) | 42 | 43 |
Significant difference in detection rates according to chi-squared test comparing ASD to GP. (No significant differences in detection rates were found comparing ID with GP.)
Geometric mean concentrations of lipid-adjusted BFRs were lower in ASD cases than GP controls for five of the six of the congeners detected in of samples (all BDE congeners), as well as for the sum of these six congeners (Table 3). Levels in the ID group were lower than levels in the GP controls for all congeners detected in of samples. Comparing ID to ASD, levels in the ID group were higher for BDE-47, -99, and -100 than levels in the ASD group. With the exception of BB153, all congeners demonstrated strong and significant correlations between one another ( 0.54–0.99, p-values ; see Table S2).
Table 3.
Geometric mean brominated flame retardant congener serum concentrations (in ng/g lipid) and 95% confidence intervals from mid-pregnancy samples in the Early Markers for Autism study by diagnostic group.
| BFR | Full study group | ASD | ID | GP | ASD vs. GP | ID vs. GP |
|---|---|---|---|---|---|---|
| p-Value | p-Value | |||||
| BB153 | 1.15 (1.09, 1.22) | 1.21 (1.12, 1.31) | 1.02 (0.90, 1.17) | 1.14 (1.04, 1.25) | 0.30 | 0.20 |
| BDE-28 | 0.89 (0.85, 0.93) | 0.87 (0.82, 0.92) | 0.84 (0.77, 0.93) | 0.94 (0.88, 1.02) | 0.26 | 0.07 |
| BDE-47 | 16.0 (15.1, 17.0) | 14.7 (13.4, 16.0) | 16.3 (14.2, 18.7) | 17.9 (16.2, 19.7) | 0.002 | 0.29 |
| BDE-99 | 4.93 (4.63, 5.24) | 4.45 (4.01, 4.87) | 5.15 (4.47, 5.94) | 5.52 (4.98, 6.11) | 0.002 | 0.46 |
| BDE-100 | 3.94 (3.71, 4.19) | 3.59 (3.28, 3.93) | 3.91 (3.45, 4.44) | 4.48 (4.05, 4.95) | 0.002 | 0.10 |
| BDE-153 | 4.34 (4.08, 4.62) | 4.02 (3.67, 4.40) | 3.91 (3.41, 4.48) | 5.02 (4.51, 5.58) | 0.002 | 0.004 |
| Suma | 34.9 (32.9, 36.9) | 32.1 (29.5, 34.9) | 32.6 (28.6, 37.2) | 40.1 (36.4, 44.2) | 0.0006 | 0.02 |
Note: ASD, autism spectrum disorder; BDE, polybrominated diphenyl ether; GP, general population controls; ID, intellectual disability/developmental delay without autism. Table shows geometric means following replacement of values with LOD divided by the square root of 2 as described in the text. For descriptive data by sex, see Table S8.
Sum is the sum of all congeners shown in table (e.g., those meeting our detection rate of at least 55% of the study population with concentrations ).
When examining odds of ASD according to quartiles, individuals in higher quartiles of all BFR congeners, except BB153, had reduced odds of ASD relative to those in the lowest quartile, in analyses adjusted for the matching factors only (Model 1) and adjusted analyses (Model 2) (Table 4). In adjusted models, individuals in the third-fourth quartiles of levels of BDE-47, -99, and -100 had approximately a 30% reduction in odds of having a child with ASD; of these, only BDE-100 demonstrated a significant inverse association with ASD odds (for quartiles 2 and 3, though estimates were nearly identical across quartiles 2–4). Slightly stronger inverse associations were observed for the highest quartiles of BDE-153 (; 95% CI: 0.38, 0.84) as well as the BFR sum (; 95% CI: 0.44, 0.93). Tests of trend of BDE-153 and the combined BFR were also statistically significant, though the pattern of estimates across quartiles was not monotonic. Additional adjustment for gestational age, maternal country of birth, child birth weight, PCB levels, and other covariates available including paternal age and insurance status did not impact results (data not shown). Exploring other sums of congeners (as detailed in “Methods”) yielded similar estimates as the sum of congeners meeting our detection rate cutoff (see Table S3).
Table 4.
Odds ratios and 95% confidence intervals for associations between quartiles of prenatal brominated flame retardant serum concentrations and ASD (relative to GP controls).
| BFR/quartile | PBDE concentrationa | Caseb () | Control () | Model 1c | Model 2d | p-Trende |
|---|---|---|---|---|---|---|
| BB153 | ||||||
| Q1 | 114 | 93 | 1.0 | 1.0 | 0.86 | |
| Q2 | 0.51–1.06 | 124 | 92 | 1.10 (0.75, 1.61) | 1.14 (0.76, 1. 70) | |
| Q3 | 1.07–1.80 | 117 | 100 | 0.96 (0.65, 1.40) | 0.86 (0.56, 1.31) | |
| Q4 | 154 | 98 | 1.28 (0.88, 1.86) | 1.12 (0.73, 1.71) | ||
| BDE-28 | ||||||
| Q1 | 142 | 94 | 1.0 | 1.0 | 0.31 | |
| Q2 | 0.5–0.8 | 130 | 93 | 0.92 (0.64, 1.34) | 0.95 (0.65, 1.39) | |
| Q3 | 0.81–1.30 | 114 | 90 | 0.84 (0.57, 1.23) | 0.89 (0.60, 1.31) | |
| Q4 | 116 | 99 | 0.77 (0.53, 1.13) | 0.82 (0.56, 1.22) | ||
| BDE-47 | ||||||
| Q1 | 178 | 103 | 1.0 | 1.0 | 0.07 | |
| Q2 | 9.3–16.8 | 134 | 105 | 0.74 (0.52, 1.05) | 0.77 (0.53, 1.10) | |
| Q3 | 16.9–31.5 | 116 | 104 | 0.64 (0.45, 0.92) | 0.71 (0.49, 1.02) | |
| Q4 | 117 | 104 | 0.65 (0.45, 0.93) | 0.73 (0.50, 1.05) | ||
| BDE-99 | ||||||
| Q1 | 183 | 107 | 1.0 | 1.0 | 0.05 | |
| Q2 | 2.8–4.8 | 117 | 100 | 0.71 (0.50, 1.02) | 0.75 (0.52, 1.08) | |
| Q3 | 4.9–9.7 | 145 | 102 | 0.64 (0.44, 0.92) | 0.72 (0.49, 1.04) | |
| Q4 | 88 | 98 | 0.61 (0.42, 0.87) | 0.70 (0.48, 1.01) | ||
| BDE-100 | ||||||
| Q1 | 181 | 99 | 1.0 | 1.0 | 0.05 | |
| Q2 | 2.2–3.8 | 116 | 102 | 0.62 (0.43, 0.89) | 0.66 (0.45, 0.95) | |
| Q3 | 3.9–7.5 | 120 | 103 | 0.63 (0.44, 0.91) | 0.68 (0.47, 0.98) | |
| Q4 | 116 | 103 | 0.61 (0.43, 0.88) | 0.69 (0.47, 1.00) | ||
| BDE-153 | ||||||
| Q1 | 179 | 95 | 1.0 | 1.0 | 0.01 | |
| Q2 | 2.4–4.1 | 116 | 103 | 0.60 (0.42, 0.86) | 0.62 (0.43, 0.90) | |
| Q3 | 4.2–8.8 | 134 | 107 | 0.66 (0.46, 0.95) | 0.71 (0.49, 1.03) | |
| Q4 | 104 | 102 | 0.54 (0.37, 0.78) | 0.56 (0.38, 0.84) | ||
| Sumf | 0.01 | |||||
| Q1 | 166 | 94 | 1.0 | 1.0 | ||
| Q2 | 118 | 94 | 0.82 (0.58, 1.16) | 0.83 (0.58, 1.18) | ||
| Q3 | 35.1–70.5 | 134 | 94 | 0.93 (0.66, 1.31) | 1.01 (0.71, 1.44) | |
| Q4 | 84 | 94 | 0.58 (0.40, 0.84) | 0.64 (0.44, 0.93) |
Note: ASD, autism spectrum disorder; GP, general population controls.
Lipid-adjusted concentration in ng/g lipid, categorized into quartiles based on control distribution.
Columns show number of cases and controls, respectively, in the quartile.
Model 1: logistic regression adjusted for study matching factors (child sex, month and year of birth).
Model 2: (continuous), maternal race/ethnicity (non-Hispanic white, Asian, Black/Pacific Islander/or other, Hispanic, or Missing), maternal weight at time of sample collection (quartiles), parity (multi- vs. primiparous), and maternal education (, high school, college, graduate).
p-Trend from model with quartile median-value variable, adjusted as Model 2.
Sum of all congeners shown in this table (those with at least 55% of study population with values ).
Analyses of odds of ID produced a similar pattern of results to those seen for ASD. Given the smaller number of ID cases, these associations were not as precise as for ASD, but for all congeners higher levels were associated with reductions in odds of ID, except for BB153 and BDE-28 (Table 5).
Table 5.
Odds ratios and 95% confidence intervals for associations between quartiles of prenatal brominated flame retardant serum concentrations and ID (relative to GP controls).
| BFR/quartile | ID () | Control () | Model 1a | Model 2b | p-Trendc |
|---|---|---|---|---|---|
| BB153 | |||||
| Q1 | 41 | 93 | 1.0 | 1.0 | 0.62 |
| Q2 | 52 | 92 | 1.21 (0.72, 2.05) | 1.46 (0.84, 2.54) | |
| Q3 | 30 | 100 | 0.71 (0.40, 1.26) | 0.95 (0.51, 1.79) | |
| Q4 | 37 | 98 | 0.84 (0.48, 1.45) | 1.34 (0.72, 2.51) | |
| BDE-28 | |||||
| Q1 | 38 | 94 | 1.0 | 1.0 | 0.95 |
| Q2 | 47 | 93 | 1.24 (0.72, 2.13) | 1.30 (0.73, 2.31) | |
| Q3 | 34 | 90 | 0.97 (0.55, 1.73) | 1.00 (0.54, 1.82) | |
| Q4 | 36 | 99 | 1.06 (0.60, 1.86) | 1.12 (0.62, 2.04) | |
| BDE-47 | |||||
| Q1 | 46 | 103 | 1.0 | 1.0 | 0.30 |
| Q2 | 52 | 105 | 1.09 (0.66, 1.81) | 1.04 (0.62, 1.77) | |
| Q3 | 48 | 104 | 1.06 (0.63, 1.77) | 0.93 (0.54, 1.58) | |
| Q4 | 35 | 104 | 0.81 (0.47, 1.38) | 0.76 (0.43, 1.34) | |
| BDE-99 | |||||
| Q1 | 46 | 107 | 1.0 | 1.0 | 0.14 |
| Q2 | 46 | 100 | 0.93 (0.56, 1.55) | 0.87 (0.51, 1.49) | |
| Q3 | 48 | 102 | 1.06 (0.64, 1.78) | 0.85 (0.50, 1.46) | |
| Q4 | 36 | 98 | 0.75 (0.44, 1.29) | 0.64 (0.36, 1.13) | |
| BDE-100 | |||||
| Q1 | 46 | 99 | 1.0 | 1.0 | 0.55 |
| Q2 | 45 | 102 | 0.89 (0.53, 1.50) | 0.86 (0.50, 1.48) | |
| Q3 | 50 | 103 | 1.07 (0.65, 1.79) | 1.02 (0.60, 1.73) | |
| Q4 | 35 | 103 | 0.77 (0.45, 1.32) | 0.78 (0.44, 1.37) | |
| PBDE153 | |||||
| Q1 | 53 | 95 | 1.0 | 1.0 | 0.56 |
| Q2 | 48 | 103 | 0.93 (0.56, 1.53) | 1.01 (0.60, 1.70) | |
| Q3 | 41 | 107 | 0.74 (0.44, 1.24) | 0.83 (0.49, 1.42) | |
| Q4 | 34 | 102 | 0.71 (0.41, 1.20) | 0.90 (0.51, 1.62) | |
| Sumd | |||||
| Q1 | 45 | 94 | 1.0 | 1.0 | 0.14 |
| Q2 | 42 | 94 | 0.84 (0.52, 1.37) | 0.87 (0.52, 1.43) | |
| Q3 | 44 | 94 | 0.91 (0.56, 1.47) | 0.87 (0.53, 1.43) | |
| Q4 | 24 | 94 | 0.53 (0.31, 0.92) | 0.58 (0.33, 1.03) |
Note: GP, general population controls; ID, intellectual disability/developmental delay without autism.
Model 1: logistic regression adjusted for study matching factors (child sex, month and year of birth).
Model 2: (continuous), maternal race/ethnicity (non-Hispanic white, Asian, Black/Pacific Islander/or other, Hispanic, or Missing), maternal weight at time of sample collection (quartiles), parity (multi- vs. primiparous), and maternal education (, high school, college, graduate, missing).
p-Trend from the median value variable, adjusted as Model 2.
Sum of all congeners shown in this table (those with at least 55% of study population with values ).
Analyses parameterizing BFR concentrations continuously suggested generally null associations or modest reductions in odds of ASD associated with higher BFR levels. With the exception of BDE-153, which was associated with a 9% decrease in odds of ASD with each 10-fold increase in serum concentration (; 95% CI: 0.83, 0.99), these associations were not statistically significant (Table 6). As for quartile analyses, associations with ID were similar to those seen for ASD, with all estimates below the null.
Table 6.
Odd ratios and 95% confidence intervals for associations of ASD and ID (relative to GP controls) with 10-fold increases in brominated flame retardant concentrations from maternal serum samples (ng/g lipid).
| BFR | Adjusted Model 1a | Adjusted Model 2b | p-Valuec |
|---|---|---|---|
| ASD | |||
| BB153 | 1.00 (0.75, 1.33) | 0.97 (0.73, 1.30) | 0.85 |
| BDE-28 | 0.54 (0.25, 1.19) | 0.58 (0.26, 1.27) | 0.17 |
| BDE-47 | 0.98 (0.96, 1.00) | 0.99 (0.97, 1.01) | 0.19 |
| BDE-99 | 0.97 (0.92, 1.01) | 0.97 (0.93, 1.02) | 0.24 |
| BDE-100 | 0.94 (0.86, 1.02) | 0.95 (0.87, 1.02) | 0.17 |
| BDE-153 | 0.91 (0.83, 0.99) | 0.91 (0.83, 0.99) | 0.02 |
| Sumd | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) | 0.10 |
| ID | |||
| BB153 | 0.74 (0.37, 1.49) | 0.98 (0.55, 1.75) | 0.95 |
| BDE-28 | 0.23 (0.04, 1.24) | 0.24 (0.04, 1.25) | 0.09 |
| BDE-47 | 0.98 (0.95, 1.02) | 0.98 (0.95, 1.02) | 0.27 |
| BDE-99 | 0.95 (0.87, 1.03) | 0.94 (0.87, 1.03) | 0.17 |
| BDE-100 | 0.83 (0.68, 1.02) | 0.84 (0.68, 1.04) | 0.11 |
| BDE-153 | 0.81 (0.67, 0.99) | 0.88 (0.73, 1.07) | 0.21 |
| Sumd | 0.98 (0.95, 1.00) | 0.98 (0.95, 1.00) | 0.09 |
Model 1: logistic regression adjusted for study matching factors (child sex, month and year of birth).
Model 2: (continuous), maternal race/ethnicity (non-Hispanic white, Asian, Black/Pacific Islander/or other, Hispanic, or Missing), maternal weight at time of sample collection (quartiles), parity (multi- vs. primiparous), and maternal education (, high school, college, graduate).
p-Value for BFR coefficient from adjusted model 2.
Sum of all congeners shown in this table (e.g., those with at least 55% of study population with values ).
When examining BFRs in association with ASD with or without intellectual disability, results were generally consistent with those for ASD overall (see Table S4). Results were also stable across various sensitivity analyses performed (see Table S5), with point estimates and the pattern of results similar to primary analyses when using uncensored laboratory data (numbers shown in Table S6), multiple imputation to replace values , removing the group of individuals originally classified as ID but later determined to meet criteria for ASD, conducting matched analyses using conditional logistic regression, or adding adjustment for genetic ancestry PCs. Estimates were somewhat more variable when removing individuals with values , especially for BDE-28 (as this congener had the greatest proportion of individuals ); however, point estimates remained below the null. Due to the unexpected overall findings, we also explored alternate paramterizations and categorizations of BFRs. Cubic spline analyses suggested relationships between the congeners and ASD, if any, were linear (and tests of nonlinearity were not significant), with decreases in odds of ASD with higher BFR levels that were not statistically significant except for BDE-153 (see Figure S1). Examining high and low extremes of the distribution of BFR concentrations relative to those with levels in the middle range yielded results generally consistent with those suggested in primary analyses, with nonsignificant reductions in odds of ASD associated with BDEs in the , and increases in odds of ASD for those in the lowest 10th percentile (and particularly lowest 5th percentile) of BDEs and the sum, as compared with those with concentrations in the mid 11th–89th percentiles (see Table S7).
Results by Sex
As a consequence of original matching of ASD to GP, and including individuals reclassified from ID to ASD following expert review of records within our ASD analysis group (as described in “Methods”), there were 99, 77, and 73 girls with ASD, ID, and GP, respectively, and 446, 104, and 354 boys with these outcomes (Table 1). Detection frequencies were similar between male and female offspring (Table 2). Geometric mean concentrations were higher for BDE congeners for ASD and ID girls than for boys in these groups (see Table S8).
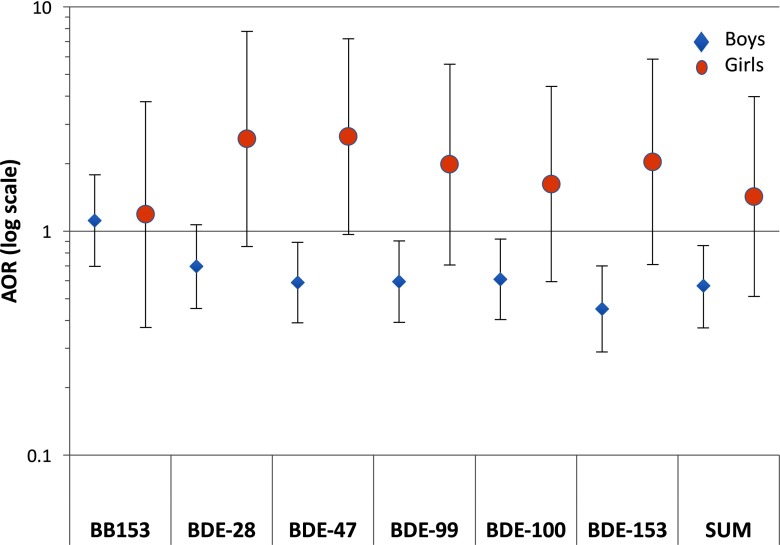
In analyses of ASD stratified by sex, ORs from quartile models of BB153 were generally close to the null and similar between boys and girls (interaction , Table 7). Estimates for all BDE congeners were below the null for boys (Table 7), and comparing the highest to the lowest quartile, were significant for all BDEs except BDE-28 (Figure 1). Overall, the estimates for boys were consistent with those for the population as a whole, as expected given that boys accounted for most of the ASD and GP observations. In contrast, ORs comparing ASD in the highest and lowest quartiles of BDE exposures in girls were above the null (Figure 1). Interaction p-values were significant for all BDE congeners, though estimates in girls were imprecise due to small numbers, and patterns for lower quartiles were inconsistent (Table 7).
Table 7.
Odds ratios and 95% confidence intervals for quartiles of prenatal brominated flame retardant congener serum concentrations in association with ASD (relative to GP controls), stratified by child sex.
| BFR/quartile | Males | Females | p-Interactiona | ||||
|---|---|---|---|---|---|---|---|
| Case/control (/) | Model 1b | Model 2c | Case/control (/) | Model 1 | Model 2 | ||
| BB153 | 0.83 | ||||||
| Q1 | 93/76 | 1.0 | 1.0 | 21/17 | 1.0 | 1.0 | |
| Q2 | 95/74 | 1.05 (0.68, 1.61) | 1.12 (0.72, 1. 75) | 29/18 | 1.32 (0.55, 3.18) | 1.27 (0.48, 3.37) | |
| Q3 | 100/85 | 0.96 (0.63, 1.46) | 0.85 (0.53, 1.36) | 17/15 | 0.93 (0.36, 2.40) | 0.99 (0.31, 3.20) | |
| Q4 | 130/81 | 1.31 (0.87, 1.99) | 1.10 (0.72, 1. 69) | 24/17 | 1.17 (0.47, 2.89) | 1.19 (0.37, 3.78) | |
| BDE-28 | 0.02 | ||||||
| Q1 | 122/76 | 1.0 | 1.0 | 20/18 | 1.0 | 1.0 | |
| Q2 | 104/74 | 0.87 (0.58, 1.32) | 0.94 (0.61, 1.44) | 26/19 | 1.23 (0.52, 2.96) | 0.92 (0.34, 2.46) | |
| Q3 | 95/72 | 0.82 (0.54, 1.25) | 0.91 (0.59, 1.40) | 19/18 | 0.96 (0.39, 2.41) | 0.70 (0.25, 2.00) | |
| Q4 | 92/89 | 0.64 (0.42, 0.97) | 0.70 (0.45, 1.07) | 24/10 | 2.20 (0.82, 5.92) | 2.58 (0.86, 7.79) | |
| BDE-47 | 0.02 | ||||||
| Q1 | 152/81 | 1.0 | 1.0* | 26/22 | 1.0 | 1.0+ | |
| Q2 | 108/89 | 0.64 (0.44, 0.95) | 0.70 (0.47, 1.04) | 26/16 | 1.37 (0.59, 3.19) | 1.31 (0.50, 3.43) | |
| Q3 | 95/82 | 0.61 (0.41, 0.92) | 0.69 (0.47, 1.00) | 21/22 | 0.78 (0.34, 1.81) | 0.89 (0.34, 2.31) | |
| Q4 | 91/91 | 0.53 (0.36, 0.79) | 0.72 (0.50, 1.05) | 26/13 | 1.66 (0.69, 4.00) | 2.64 (0.97, 7.19) | |
| BDE-99 | 0.04 | ||||||
| Q1 | 150/80 | 1.0 | 1.0* | 27/20 | 1.0 | 1.0 | |
| Q2 | 111/85 | 0.69 (0.47, 1.03) | 0.74 (0.50, 1.11) | 19/18 | 0.77 (0.32, 1.85) | 0.86 (0.32, 2.30) | |
| Q3 | 87/83 | 0.55 (0.37, 0.83) | 0.61 (0.40, 0.93) | 29/19 | 1.13 (0.50, 2.58) | 1.81 (0.70, 4.69) | |
| Q4 | 88/88 | 0.53 (0.36, 0.79) | 0.60 (0.39, 0.90) | 22/14 | 1.16 (0.48, 2.82) | 1.98 (0.71, 5.56) | |
| BDE-100 | 0.03 | ||||||
| Q1 | 151/80 | 1.0 | 1.0* | 30/19 | 1.0 | 1.0 | |
| Q2 | 101/81 | 0.66 (0.44, 0.98) | 0.72 (0.48, 1.08) | 15/21 | 0.45 (0.19, 1.08) | 0.51 (0.19, 1.38) | |
| Q3 | 92/86 | 0.57 (0.38, 0.84) | 0.61 (0.40, 0.91) | 28/17 | 1.05 (0.45, 2.44) | 1.56 (0.59, 4.17) | |
| Q4 | 92/89 | 0.55 (0.37, 0.81) | 0.61 (0.40, 0.92) | 24/14 | 1.09 (0.45, 2.62) | 1.62 (0.59, 4.42) | |
| BDE-153 | 0.02 | ||||||
| Q1 | 152/72 | 1.0 | 1.0* | 27/23 | 1.0 | 1.0 | |
| Q2 | 90/88 | 0.48 (0.32, 0.73) | 0.48 (0.32, 0.73) | 26/15 | 1.48 (0.64, 3.44) | 2.12 (0.82, 5.52) | |
| Q3 | 111/87 | 0.61 (0.41, 0.90) | 0.64 (0.43, 0.97) | 23/20 | 0.96 (0.42, 2.20) | 1.20 (0.45, 3.23) | |
| Q4 | 83/89 | 0.44 (0.29, 0.67) | 0.45 (0.29, 0.70) | 21/13 | 1.35 (0.55, 3.31) | 2.04 (0.71, 5.84) | |
| Sumd | 0.03 | ||||||
| Q1 | 143/75 | 1.0 | 1.0* | 29/20 | 1.0 | 1.0 | |
| Q2 | 90/78 | 0.79 (0.54, 1.16) | 0.82 (0.55, 1.22) | 22/17 | 0.94 (0.42, 2.09) | 1.03 (0.41, 2.59) | |
| Q3 | 117/78 | 0.92 (0.63, 1.35) | 1.02 (0.69, 1.50) | 22/17 | 0.91 (0.41, 2.05) | 1.28 (0.51, 3.20) | |
| Q4 | 68/85 | 0.52 (0.35, 0.78) | 0.57 (0.37, 0.86) | 16/11 | 1.04 (0.42, 2.57) | 1.42 (0.51, 3.98) | |
Note: GP, general population.
p-Interaction term for BFR concentration (ordinal median value variable) by sex, adjusted as in Model 2, including males and females.
Model 1: logistic regression adjusted for child month and year of birth.
Model 2 (estimates as shown in Figure 1): , maternal race/ethnicity, maternal weight at time of sample collection, parity, and maternal education.
Sum of congeners listed in table (detected in of the study population).
in test for trend across quartiles.
Figure 1.
Adjusted odds ratio (AOR) estimates from logistic regression for ASD relative to GP comparing those with in highest quartile of the BFR congener levels to the lowest (referent) quartile, adjusted for maternal age, education, race/ethnicity, weight, parity, and child year and month of birth (e.g., as in Table 3 Model 2, without adjustment for child sex). Diamonds (boys, ASD cases and 345 GP controls) and circles (girls, ASD cases and 73 GP controls). Vertical lines indicate 95% confidence intervals. Horizontal line indicates the null value of . The y axis is log-scaled for symmetry. Numbers of observations, AORs, and 95% confidence intervals for all quartiles are shown in Table 7. Sum indicates the sum of congeners shown (those with at least 55% of study population with values ).
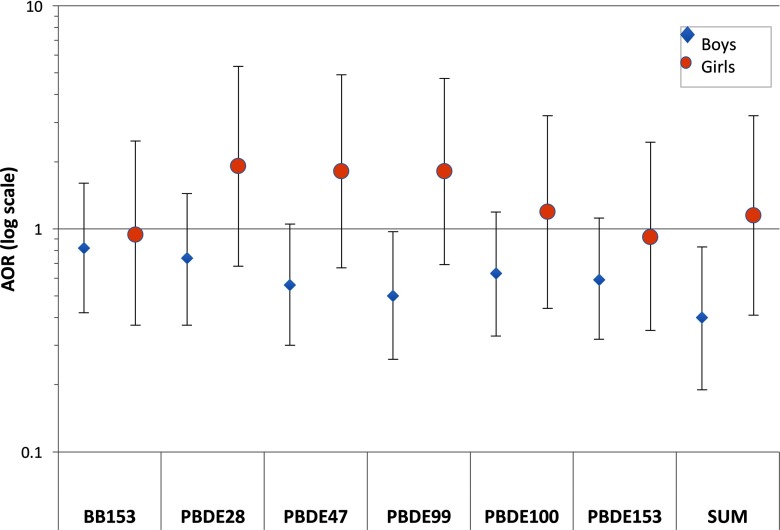
Differences in associations with ID between boys and girls were generally consistent but less pronounced than those for ASD (see Table S9). Comparing the highest to lowest quartiles, estimates were above the null for girls for BDEs 47, 99, and 100, and null for other congeners, and below the null for boys (Figure 2). Interaction terms for sex by congener levels for ID analyses were not statistically significant (see Table S9).
Figure 2.
Adjusted odds ratio (AOR) estimates for ID relative to GP, adjusted for maternal age, education, race/ethnicity, weight, parity, and child year and month of birth. Diamonds (boys, ID cases and 345 GP controls) and circles (girls, ID cases and 73 GP controls). Vertical lines indicate 95% confidence intervals. Horizontal line indicates the null value of . The y axis is log-scaled for symmetry. Numbers of observations, AORs, and 95% confidence intervals for all quartiles are shown in Table S7. Sum indicates the sum of congeners shown (those with at least 55% of study population with values ).
Discussion
To our knowledge, this is the first study to examine prenatal BFR exposure in association with ASD and ID. Contrary to expectations, ASD was inversely associated with the highest exposure levels of several congeners, with significant inverse associations estimated for BDE-100, -153, and the sum of BB153 and BDEs-28, 47, -99, -100, and -153. Odds ratios for ID showed similar patterns, but were less precise due to small numbers of cases, and generally closer to the null. However, when we stratified by sex, the inverse associations appeared to be specific to boys, whereas associations for girls (though based on small numbers and thus less precise) were more likely to be above the null.
To date, few other human studies have examined childhood neurodevelopmental effects of prenatal BFR exposure, though the majority of this literature suggests adverse impacts on child neurodevelopment (Herbstman and Mall 2014). In an analysis including 323 mother–child pairs of the CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) study (Eskenazi et al. 2013), a longitudinal birth cohort including mostly Mexican-American families from California’s Salinas Valley, individuals with the highest quartiles of the sum of 10 PBDEs measured in prenatal serum samples (including some congeners not included in our analysis) had lower attention, fine motor, and verbal comprehension scores at 5 or 7 y. Another study, conducted in Spain, reported nonsignificant inverse associations between pre- (measured in cord blood, ) and postnatal (measured in child serum at age 4, ) BDE-47 levels when comparing those above with those below the limit of detection and cognitive and motor scores as measured by the McCarthy Scales at age 4 (Gascon et al. 2011). Additional work by the same group suggested an inverse association between scores on the Bayley Scales of Infant Development and BDE-209 concentrations measured in colostrum (Gascon et al. 2012). In another study of mothers and children from New York, increases in levels of PBDEs (particularly BDE-47, -99, and -100) measured in cord blood () were associated with decreases in psychomotor and mental development scores at 12, 24, and 36 mo, and with lower IQ scores at 48 mo (Herbstman et al. 2010).
However, findings have not been consistent across all studies. In an analysis of the Pregnancy, Infection, and Nutrition study of North Carolina including 304 mothers and their children, individuals in the highest quartiles of PBDEs measured in breast milk (including the same BDE congeners included in analyses in our study) had higher scores for anxious behavior, but also higher adaptive and cognitive behavior scale scores as measured by the parent rating scale for preschoolers (age 2–5 y) of the Behavior Assessment System for Children, 2nd Edition (BASC-2), relative to those in the lowest quartile of PBDE congeners (Adgent et al. 2014). In the only study other than ours to our knowledge with prenatal PBDE measurements and ASD outcome information (Braun et al. 2014), the HOME Study (Health Outcomes and Measures of the Environment Study), which includes over 400 pregnant women and their children from the Cincinnati, Ohio, area, reported conflicting findings for different congeners measured in prenatal serum samples from 175 of its participants. Increases in BDE-28 were associated with higher SRS scores (indicating more autistic behaviors), and detectable versus nondetectable concentrations of BDE-85 were associated with fewer autistic behaviors. Six other PBDE congeners in the HOME Study had close to null, nonsignificant associations with SRS scores. In another analysis in the HOME Study including 309 participants, a 10-fold increase in BDE-47 was associated with a significantly lower full-scale IQ and significantly higher hyperactivity score at age 5 y, but there were no significant associations for younger ages examined (Chen et al. 2014a). Differences in study populations, or design (e.g., measuring PBDEs in prenatal serum vs. breast milk) may have contributed to discrepancies in prior literature. California is known to have some of the highest reported serum levels of PBDEs worldwide. Although the levels detected in our study population were lower than some other studies of pregnant women in California and elsewhere (Woodruff et al. 2011; Buttke et al. 2013; Zota et al. 2013), they were similar to those reported in CHAMACOS (also a California study of pregnant women) (Eskenazi et al. 2013), as well as the HOME Study (in Ohio) (Braun et al. 2014).
The reason for an inverse association between prenatal BFRs and odds of ASD as suggested for some congeners here is not apparent. While adjustment for a large number of factors, including demographic characteristics shown to be predictive of PBDE levels (Horton et al. 2013), as well as genetic PCs, did not materially change our results, it is possible that inverse associations may be due to unmeasured confounding by some shared influences on BFR levels and ASD, rather than a true protective association. If higher levels of flame retardants are associated with spontaneous abortion, as suggested in one study of polybrominated biphenyls (Small et al. 2011), this may have influenced the observed associations, particularly if male fetuses are more susceptible to such loss—though this is not clear (Orzack et al. 2015). It is also possible that such findings are due to chance. Results here could also be consistent with an overall lack of an association with ASD, as suggested by nonsignificant results of continuous analyses for most congeners and some of our sensitivity analyses.
Null findings in other work, along with results here, suggest that prenatal exposure to these BFRs may be unrelated to ASD risk specifically. However, prenatal exposure may influence frequently comorbid features of ASD, such as attention deficits or related impairments in cognitive scores, and these questions should be further explored. Other than examining comorbid ID, we did not have the ability to assess the influence of heterogeneity within ASD, or risk of ASD with other comorbid conditions, and associations could plausibly vary according to phenotypically distinct ASD subgroups.
When examining associations stratified by sex, our study found significant inverse associations with higher brominated PBDEs (BDE-100, -153) in boys, and nonsignificant increases in odds of ASD (particularly for BDE-28, -47) when comparing high with low quartiles of BDEs in girls. In addition, significant interactions with sex were suggested for all BDE congeners. However, patterns over all quartiles were not consistent with monotonic trends, and estimates in girls were imprecise. We cannot place too much emphasis on the results for individual congeners in our stratified analyses, given the high correlation of most PBDE congeners, as well as the smaller numbers of girls in our study as a result of the skewed sex ratio in ASD. However, sex differences in placental functioning (Rosenfeld 2015), pharmacokinetics, or methylation patterns, supported in prior work (Soldin and Mattison 2009; Huen et al. 2014) could result in sex-specific effects of BFRs on fetal development. The suggestion of sex differences seen here warrants further consideration, particularly in light of toxicologic evidence from animal models of potential sexually dimorphic effects of these chemicals (Lilienthal et al. 2006; Woods et al. 2012; Faass et al. 2013), as well as the consistent male-skewed sex ratio for ASD suggesting that risk factors that differ by sex may provide etiologic clues.
In support of such dimorphism, previous studies have suggested sex differences in the effects of prenatal BFR exposure on other outcomes. Associations with child BMI at age 7 y (Erkin-Cakmak et al. 2015), birth weight (Robledo et al. 2015), and development of sexually dimorphic brain regions in the rat (Faass et al. 2013) were all in opposite directions for males and females. In addition, some studies have suggested developmental impacts in girls, but not boys, in association with other EDCs. In the HOME Study (described above), a stronger association between prenatal bisphenol A exposure measured in maternal samples collected at 16 wk gestation and externalizing behavior at age 2 was noted for girls (Braun et al. 2009), though associations were not significant at other time points. Another analysis of the same cohort and maternal serum samples found that only in girls were there greater social deficits with higher levels of one of five prenatal organochlorine pesticides (another class of EDCs) examined (Braun et al. 2014). Of over 50 EDCs evaluated, few other sex differences, not all in the same direction, were reported in this study: a positive association with autistic traits as measured by the SRS and serum perfluorooctanesulfonic acid in boys but not girls, and opposing relationships with SRS scores in boys (negative) and girls (positive) with levels of monoethyl phthalate.
Suspected pathways through which BFRs may influence neurodevelopment include disruptions of immune markers, neurotransmitters, or calcium signaling (Hendriks et al. 2010; Grandjean and Landrigan 2014) and through impacts on thyroid hormone levels, which play a known role in neurodevelopment (de Escobar et al. 2004). There is limited animal evidence suggesting that impacts on thyroid hormone levels following exposure to other chemicals may differ between the sexes (Sulkowski et al. 2012). Some studies have reported associations between thyroid hormone (Vuong et al. 2015) or immune markers (Ashwood et al. 2009) and PBDEs only for those congeners (-28 and -47) for which we saw the strongest suggestion for an association with ASD in girls, though these findings are not uniform, with other studies reporting null or nonsignificant associations between PBDE congeners and thyroid hormones, and no differences by sex (Chevrier et al. 2011; Liu et al. 2012; Eggesbø et al. 2011; Gascon et al. 2011; Eskenazi et al. 2013). Thus, work examining mechanisms by which these chemicals may influence neurodevelopment in humans is still needed and may help elucidate associations.
This study has a number of strengths: measurement of BFR levels during gestation, a time period demonstrated to be critical to neurodevelopment (Rodier et al. 1996; Rice and Barone 2000), inclusion of over 500 cases of ASD, and ASD and ID diagnoses confirmed by expert clinician review. However, certain limitations should be noted. A number of congeners were not detected in enough of our study group in order to examine their association with the outcomes of interest, though detection rates () were similar to those reported in other studies (Chevrier et al. 2011; Adgent et al. 2014). We lacked information on exposure in other time windows, including during breastfeeding, which, although potentially highly correlated with mid-gestational exposure levels (Makey et al. 2014), could also represent critical windows of exposure for ASD and ID risk. Though our sample size was adequate in primary analyses, we had reduced power in analyses of ASD and ID in females. We did not have information on income level, though adjustment for available socioeconomic status (SES)-related variables in our study (maternal education, race/ethnicity, insurance status at delivery, and maternal birth outside the United States) did not alter our results. Concerns regarding confounding by SES may be lower in this population-based study relative to clinic-based samples. However, our study population, although comparable to its source population of California (Kharrazi et al. 2012) with a high proportion of Hispanics and individuals on government-funded insurance relative to the general U.S. population, may have reduced generalizability to some other regions. We did not have information on maternal IQ, a predictor of child IQ, though we did adjust for maternal education. We also cannot rule out potential ID misclassification, given that ID was determined only according to available standardized test scores in diagnostic records; however, such records were reviewed by a trained clinician. We did not have the ability to examine the influence of certain pregnancy complications such as gestational diabetes or changes in body mass index, factors that may influence bioavailability of these compounds and may put an individual at higher risk for ASD; however, we did adjust for maternal weight at blood sample collection. It is likely that the influence of BFR exposure could vary by genetics; thus, additional consideration of how genetics influences the impact of these exposures is warranted. Finally, though we examined adjustment for exposure to PCBs, and this did not alter findings, future work should consider employing the emerging methodologies to handle exposure to mixtures of chemicals.
Conclusions
The primary analyses of this study did not support the hypothesis that prenatal PBDEs increase risk of ASD or ID. Results overall suggested a lack of an association to reductions in odds of ASD and ID with higher levels of BFRs, particularly for ASD in children born to mothers with levels of certain BDEs in the highest quartile relative to those with levels in the lowest quartile. However, findings from stratified analyses suggested the potential for effect modification by child sex, with the inverse associations for exposure to higher levels of prenatal PBDEs observed in boys. Associations in the opposite direction were suggested for girls, but we lacked precision in these estimates owing to smaller numbers. Given limited human studies on the developmental effects of exposure to these ubiquitous chemicals, continued investigation is needed. Future work with sufficient numbers of male and female cases should confirm whether associations seen here are replicated and investigate whether PBDEs are associated with developmental disorders, and ASD in particular, through sexually dimorphic pathways.
Supplemental Material
Acknowledgments
The authors would like to thank A. Sjödin for his significant contributions to this work.
This work was funded by National Institutes of Health grant R01-ES016669. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the California Department of Public Health.
References
- Adgent MA, Hoffman K, Goldman BD, Sjödin A, Daniels JL. 2014. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol 28(1):48–57, PMID: 24313667, 10.1111/ppe.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. 2009. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol 208(1–2):130–135, PMID: 19211157, 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad DA, Goodman S, Woodruff TJ. 2009. PCB body burdens in US women of childbearing age 2001–2002: an evaluation of alternate summary metrics of NHANES data. Environ Res 109(4):368–378, PMID: 19251256, 10.1016/j.envres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. 2004. Brominated flame retardants: cause for concern? Environ Health Perspect 112(1):9–17, PMID: 14698924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, et al. 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME Study. Environ Health Perspect 122(5):513–520, PMID: 24622245, 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. 2009. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 117(12):1945–1952, PMID: 20049216, 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke DE, Wolkin A, Stapleton HM, Miranda ML. 2013. Associations between serum levels of polybrominated diphenyl ether (PBDE) flame retardants and environmental and behavioral factors in pregnant women. J Expos Sci Environ Epidemiol 23(2):176–182, PMID: 22760441, 10.1038/jes.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, et al. 2014a. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME Study. Environ Health Perspect 122(8):856–862, PMID: 24870060, 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Liu HY, Cheng Z, Man YB, Zhang KS, Wei W, et al. 2014b. Polybrominated diphenyl ethers (PBDEs) in human samples of mother–newborn pairs in South China and their placental transfer characteristics. Environ Int 73:77–84, PMID: 25090577, 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B. 2010. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect 118(10):1444–1449, PMID: 20562054, 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Sjödin A, Eskenazi B. 2011. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol 174(10):1166–1174, PMID: 21984658, 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. 2016. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 65(3):1–23, PMID: 27031587, 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. 2005. The Social Responsiveness Scale (SRS). Los Angeles, CA, Western Pyschological Services. [Google Scholar]
- Costa LG, Giordano G. 2007. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28(6):1047–1067, PMID: 17904639, 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Lederman SA, Sjödin A, Jones R, Wang S, Perera FP, et al. 2015. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7years. Neurotoxicol Teratol 52(pt B):143–150, PMID: 26344673, 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. 2008. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry 64(7):583–588, PMID: 18571628, 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerska M, Zieliński M, Kamińska J, Ligocka D. 2013. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int J Occup Med Environ Health 26(4):498–510, PMID: 24142743, 10.2478/s13382-013-0138-7. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Pan IJ, Jones R, Anderson S, Patterson DG Jr, Needham LL, et al. 2010. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect 118(1):155–160, PMID: 20056574, 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Escobar GM, Obregón MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18(2):225–248, PMID: 15157838, 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH. 2011. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect 119(7):900–907, PMID: 21245014, 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S, Simon R. 1989. Flexible regression models with cubic splines. Stat Med 8(5):551–561, PMID: 2657958. [DOI] [PubMed] [Google Scholar]
- Eggesbø M, Thomsen C, Jørgensen JV, et al. 2011. Associations between brominated flame retardants in human milk and thyroid-stimulating hormone (TSH) in neonates. Environ Res 111(6):737–743, PMID: 21601188, 10.1016/j.envres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. 2001. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect 109(9):903–908, PMID: 11673118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. 2002. A brominated flame retardant, 2,2′,4,4′,5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci 67(1):98–103, PMID: 11961221, 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Erkin-Cakmak A, Harley KG, Chevrier J, Bradman A, Kogut K, Huen K, et al. 2015. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ Health Perspect 123(6):636–642, PMID: 25738596, 10.1289/ehp.1408417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect 121(2):257–262, PMID: 23154064, 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W. 2013. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen Comp Endocrinol 188:232–241, PMID: 23619185, 10.1016/j.ygcen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. 2010. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health 9:32, PMID: 20598165, 10.1186/1476-069X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martínez D, Carsin AE, Forns J, Grimalt JO, et al. 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect 120(12):1760–1765, PMID: 23052368, 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martínez D, Forns J, Grimalt JO, Torrent M, et al. 2011. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int 37(3):605–611, PMID: 21237513, 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gómara B, Herrero L, Ramos JJ, Mateo JR, Fernández MA, García JF, et al. 2007. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol 41(20):6961–6968, PMID: 17993135, 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. 2007. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 26(20):3735–3752, PMID: 17538974, 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13(3):330–338, PMID: 24556010, 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. 2009. Risk of autism and increasing maternal and paternal age in a large North American population. Am J Epidemiol 170(9):1118–1126, PMID: 19783586, 10.1093/aje/kwp247. [DOI] [PubMed] [Google Scholar]
- Hendriks HS, Antunes Fernandes EC, Bergman A, van den Berg M, Westerink RH. 2010. PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and α4β2 nicotinic acetylcholine receptors. Toxicol Sci 118(2):635–642, 10.1093/toxsci/kfq284. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. 2014. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr Envir Health Rep 1(2):101–112, 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118(5):712–719, PMID: 20056561, 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Bergman A, Fangström B, Rose M, Krakowiak P, Pessah I, et al. 2011. Polybrominated diphenyl ethers in relation to autism and developmental delay: a case-control study. Environ Health 10(1):1, PMID: 21205326, 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. 2004. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 38(4):945–956, PMID: 14998004. [DOI] [PubMed] [Google Scholar]
- Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R, et al. 2013. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: a cross-sectional study. Environ Health 12:23, PMID: 23497089, 10.1186/1476-069X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Yousefi P, Bradman A, Yan L, Harley KG, Kogut K, et al. 2014. Effects of age, sex, and persistent organic pollutants on DNA methylation in children. Environ Mol Mutagen 55(3):209–222, PMID: 24375655, 10.1002/em.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharrazi M, Pearl M, Yang J, DeLorenze GN, Bean CJ, Callaghan WM, et al. 2012. California Very Preterm Birth Study: design and characteristics of the population- and biospecimen bank-based nested case–control study. Paediatr Perinat Epidemiol 26(3):250–263, PMID: 22471684, 10.1111/j.1365-3016.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM. 2016. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int 88:23–29, PMID: 26700418, 10.1016/j.envint.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Härer A, Grande SW, Talsness CE. 2006. Effects of developmental exposure to 2,2′,4,4′, 5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect 114(2):194–201, PMID: 16451854, 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhan H, Zeng X, Zhang C, Chen D. 2012. The PBDE-209 exposure during pregnancy and lactation impairs immune function in rats. Mediators Inflamm 2012:692467, PMID: 22619485, 10.1155/2012/692467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Sjödin A, Yoshida CK, Zerbo O, Kharrazi M, et al. 2017. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environ Health Perspect 125(3):474–480, PMID: 27548254, 10.1289/EHP277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I. 2014. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 43(2):443–464, PMID: 24518932, 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makey CM, McClean MD, Sjödin A, Weinberg J, Carignan CC, Webster TF. 2014. Temporal variability of polybrominated diphenyl ether (PBDE) serum concentrations over one year. Environ Sci Technol 48(24):14642–14649, PMID: 25383963, 10.1021/es5026118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. 2003. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect 111(9):1249–1252, PMID: 12842781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151 (suppl 3):U25–U37, PMID: 15554884. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. 2007. The epidemiology of autism spectrum disorders. Annu Rev Public Health 28:235–258, PMID: 17367287, 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munné S, Scholl T, et al. 2015. The human sex ratio from conception to birth. Proc Natl Acad Sci U S A 112(16):E2102–E2111, PMID: 25825766, 10.1073/pnas.1416546112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575, PMID: 17701901, 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr.. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(suppl 3):511–533, PMID: 10852851, 10.2307/3454543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo CA, Yeung E, Mendola P, Sundaram R, Maisog J, Sweeney AM, et al. 2015. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: the LIFE study. Environ Health Perspect 123(1):88–94, PMID: 25095280, 10.1289/ehp.1308016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. 2000. The early origins of autism. Sci Am 282(2):56–63, PMID: 10710787. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. et al. 1996. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370(2):247–261, PMID: 8808733, . [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS. 2015. Sex-specific placental responses in fetal development. Endocrinology 156(10):3422–3434, PMID: 26241064, 10.1210/en.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar F, Gunier RB, Harley KG, Parra K, et al. 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol Teratol 52(Pt B):151–161, PMID: 26271888, 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS. 2015. The MI Procedure. In: SAS/STAT 14.1 User’s Guide Cary, NC:SAS Institute, Inc, 5858–5988. [Google Scholar]
- Shelton JF, Tancredi DJ, Hertz-Picciotto I. 2010. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 3(1):30–39, PMID: 20143326, 10.1002/aur.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. 2014. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ Sci Technol 48(1):753–760, PMID: 24298999, 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE III, et al. 2004. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect 112(6):654–658, PMID: 15121506, 10.1289/ehp.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr. 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 76(7):1921–1927, 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Small CM, Murray D, Terrell ML, Marcus M. 2011. Reproductive outcomes among women exposed to a brominated flame retardant in utero. Arch Environ Occup Health 66(4):201–208, PMID: 22014192, 10.1080/19338244.2010.539640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Mattison DR. 2009. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 48(3):143–157, PMID: 19385708, 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski ZL, Chen T, Midha S, Zavacki AM, Sajdel-Sulkowska EM. 2012. Maternal thimerosal exposure results in aberrant cerebellar oxidative stress, thyroid hormone metabolism, and motor behavior in rat pups; sex- and strain-dependent effects. Cerebellum 11(2):575–586, PMID: 22015705, 10.1007/s12311-011-0319-5. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. 2009. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology 95(3):203–209, PMID: 18799892, 10.1159/000155651. [DOI] [PubMed] [Google Scholar]
- Traglia M, Croen LA, Lyall K, Windham GC, Kharrazi M, DeLorenze GN, et al. 2017. Independent maternal and fetal genetic effects on midgestational circulating levels of environmental pollutants. G3 (Bethesda) 7(4):1287–1299, PMID: 28235828, 10.1534/g3.117.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, Knobeloch L. 2010. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere 81(4):517–522, PMID: 20708772, 10.1016/j.chemosphere.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, et al. 2015. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: the HOME Study, Cincinnati, USA. Environ Health Perspect 123(10):1079–1085, PMID: 25893858, 10.1289/ehp.1408996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–885, PMID: 21233055, 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, et al. 2012. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet 21(11):2399–2411, PMID: 22343140, 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. 2003. Prevalence of autism in a US metropolitan area. JAMA 289(1):49–55, PMID: 12503976. [DOI] [PubMed] [Google Scholar]
- Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, et al. 2013. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol 47(20):11776–11784, PMID: 24066858, 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.