Abstract
Background:
Evidence for an association between transportation noise and cardiovascular disease has increased; however, few studies have examined metabolic outcomes such as diabetes or accounted for environmental coexposures such as air pollution, greenness, or walkability.
Objectives:
Because diabetes prevalence is increasing and may be on the causal pathway between noise and cardiovascular disease, we examined the influence of long-term residential transportation noise exposure and traffic-related air pollution on the incidence of diabetes using a population-based cohort in British Columbia, Canada.
Methods:
We examined the influence of transportation noise exposure over a 5-y period (1994–1998) on incident diabetes cases in a population-based prospective cohort study () of metropolitan Vancouver (BC) residents who were 45–85 y old, with 4-y of follow-up (1999–2002). Annual average transportation noise (Lden), air pollution [black carbon, particulate matter with aerodynamic diameter (PM2.5), nitrogen oxides], greenness [Normalized Difference Vegetation Index (NDVI)], and neighborhood walkability at each participant’s residence were modeled. Incident diabetes cases were identified using administrative health records.
Results:
Transportation noise was associated with the incidence of diabetes [interquartile range (IQR) increase, 6.8 A-weighted decibels (dBA); (95% CI: 1.05, 1.10)]. This association remained after adjustment for environmental coexposures including traffic-related air pollutants, greenness, and neighborhood walkability. After adjustment for coexposure to noise, traffic-related air pollutants were not associated with the incidence of diabetes, whereas greenness was protective.
Conclusion:
We found a positive association between residential transportation noise and diabetes, adding to the growing body of evidence that noise pollution exposure may be independently linked to metabolic health and should be considered when developing public health interventions. https://doi.org/10.1289/EHP1279
Introduction
Over the past decade, there has been increasing evidence that transportation noise exposure, such as road traffic noise, leads to poorer cardiovascular health. A recent review suggested that risk for adverse cardiovascular health outcomes, such as heart attacks and stroke, increased by 7–17% for a 10-dB increase in road traffic noise exposure (Basner et al. 2014). This increase is biologically plausible (Babisch 2014; Recio et al. 2016): Noise exposure is hypothesized to cause physiological stress reactions in individuals (Recio et al. 2016), which in turn lead to increases in cardiovascular disease risk factors such as blood pressure, blood fats, and blood glucose concentrations. These risk factors lead to increased risk of high blood pressure and arteriosclerosis (e.g., narrowing of arteries because of fat deposits) and are related to serious events such as heart attacks and strokes (Babisch 2014; Basner et al. 2014). Noise exposure at night may also interfere with sleep, which may also affect diabetes via effects on glucose regulation, appetite, and energy expenditure (Eriksson et al. 2014).
Given the widely accepted causal pathways for noise and cardiovascular health (Babisch 2014; Recio et al. 2016), we would expect to observe associations between noise exposure and metabolic risk factors. To date, relatively few studies have examined the influence of transportation noise exposure on metabolic risk factors for cardiovascular health such as body mass index (BMI), waist circumference, central obesity (Oftedal et al. 2015; Pyko et al. 2015), and blood fats (Sørensen et al. 2015), with studies suggesting small effects on these outcomes. Diabetes is another metabolic risk factor that places an enormous burden on the Canadian population (PHAC 2011) but has received only limited study. A cohort study of 57,053 Danish adults, 50–64 y old, who were exposed to annual average road traffic noise ranging from 48–70 dB found that a 10-dB higher level of road traffic noise during the 5 y preceding diagnosis was associated with an increased risk of incident diabetes identified from registry data {incidence rate ratio of 1.11 [95% confidence interval (CI) 1.05, 1.18]} after adjusting for age, sex, body mass index, waist circumference, education, air pollution (nitrogen oxides), and lifestyle characteristics (Sørensen et al. 2013).
One potential confounding factor in studies examining associations of environmental noise on diabetes is air pollution (Eze et al. 2015; Thiering and Heinrich 2015). There is robust evidence for a prospective association between air pollution and cardiovascular health, and emerging evidence suggests an association between air pollution and the incidence of type 2 diabetes (Balti et al. 2014; Thiering and Heinrich 2015; Wang et al. 2014) and diabetes-associated mortality (Li et al. 2014). The hypothesized pathological mechanism between air pollution and cardiovascular disease differs from that proposed for noise. Air pollution is thought to provoke inflammatory and oxidative stress responses, which promote a variety of pathological processes related to cardiovascular disease including thrombosis, hypercoagulability, atherosclerosis, endothelial dysfunction, mitochondrial dysfunction, and insulin resistance (Chin 2015; Thiering and Heinrich 2015). However, few studies of air pollution and diabetes have taken into account coexisting noise exposure or other potential environmental confounders such as neighborhood walkability (Creatore et al. 2016; Paquet et al. 2014; Sundquist et al. 2015) or greenness (Thiering and Heinrich 2015), which have also been found to have associations with diabetic risk factors, incidence of diabetes, and cardiovascular disease and mortality.
This study examined the influence of long-term residential exposure to transportation noise and traffic-related air pollution on the incidence of diabetes using a population-based cohort drawn from linked health administration databases in British Columbia (Canada). We have previously reported the joint influences of air pollution (Henderson et al. 2007) and noise (Gan et al. 2012b) on cardiovascular mortality (Gan et al. 2012a) in this cohort. In the present study, we also examined the impacts of exposure to neighborhood greenness and neighborhood walkability on the association of residential transportation noise and air pollution exposure with the incidence of diabetes.
Methods
Study Population
Cohort data were accessed through Population Data BC (www.popdata.bc.ca/data). British Columbia has a mandatory health insurance program that covers nearly all of the residents in the province (Chamberlayne et al. 1998). We used the Central Registry data, Physician Visit, and Hospital Discharge data sets of the BC Medical Services Plan (MSP) provided by the BC Ministry of Health (British Columbia Ministry of Health; British Columbia Ministry of Health) and vital statistics data provided by the British Columbia Vital Statistics Agency. The cohort was enumerated from the MSP central registry and comprised all metropolitan Vancouver adult residents 45–84 y old who were registered with the provincial health insurance plan and who had lived in the study region during the 5-y exposure period (January 1994–December 1998) and during a 4-y follow-up period (January 1999–December 2002). Persons missing data for more than a total of 15 mo or in more than 3 consecutive months during the exposure period were also excluded to reduce misclassification of exposures. We excluded individuals who had a diagnosis of diabetes before or during the 5-y exposure period (1994–1998) (). The study was approved by the Behavioral Research Ethics Board of the University of British Columbia (certificate # H08-00185). Informed consent was not sought or required: Anonymized data were provided by Population Data BC, and no contact was made with individuals in the cohort.
Air Pollution and Noise Exposure Estimation
Individual-level residential exposures to transportation noise (predominantly road traffic noise but including aircraft and rail noise) and to traffic-related air pollutants were estimated using noise propagation and land-use regression models, respectively. A detailed methodology for noise exposure is described elsewhere (Gan et al. 2012b). Briefly, we used CadnaA, a model-based computer program developed by DataKustik (Greifenberg, Germany), with the following inputs. Traffic volumes were obtained from a 2003 transportation planning model, road widths were estimated as the distance between the center lines of the outermost lanes, and road type was based on the provincial Digital Road Atlas (Setton et al. 2005); each road type was automatically assigned a specific percentage of truck traffic. The model also took into account the influence of road speed limits, traffic lights at intersections, road gradients (changes in elevation along a given road), road surface (paved or loose surface), bridges (heights of the road segments above ground), buildings (height, footprint, and reflection/absorption characteristics), and topography. Aircraft noise was estimated from the Airport Authority aircraft noise exposure forecast contours for 2003 (Transport Canada 2005). Railway noise exposure assessment was based on railway operation data including length of trains, velocity, percentage of disc brakes, and number of each type of train by day, evening, and night.
Based on the data above, annual day–evening–night A-weighted equivalent continuous noise levels (Lden dbA) were calculated for a grid. The Lden metric integrates noise levels during the day (Lday, 0600 hours–1800 hours), the evening (1800 hours–2200 hours), and the night (Lnight, 2200 hours–0600 hours); it reflects increased sensitivity of residents to community noise during the evening and the night by adding a 5-dBA weight to evening noise levels and a 10-dBA weight to nighttime noise levels (WHO 2011).
Based on the estimated noise levels, we calculated an annual average noise level for each 6-character postal code area by geometrically averaging the noise levels of all grid cell values contained in a postal code area; this geometric mean was assigned to all subjects in the postal code. Noise levels were calculated from road traffic and aircraft separately and from all sources combined.
In the study region, postal code areas varied greatly in size depending on the population density: in urban areas, a postal code typically represents one high-rise building or one side of a city block; however, in rural areas, a postal code may represent a larger area. Because metropolitan Vancouver is a highly urbanized region, most postal codes represent small geographical areas: on average, a residential postal code included individuals. In larger postal code areas, the use of the geometric mean noise level reduces bias casued by heterogeneous exposure areas because it gives heavier weighting to higher noise estimates, which are grid points closer to roads where residences are most likely situated.
The noise level (Lden dBA) was analyzed for interquartile increases in exposure (). Noise level was also analyzed categorically, following the method described by Gan et al. (2012a). We compared cohort members in the 10th decile of exposure (), the sixth through ninth deciles of exposure (62–69 dBA), and the second through fifth deciles of exposure (58–61 dBA) with a reference group comprising cohort members in the lowest (first) decile of exposure () (Gan et al. 2012a).
High-spatial-resolution land-use regression models were used to estimate residential exposures to air pollutants including nitrogen dioxide (), nitric oxide (NO), particulate matter with aerodnamic diameter (), and black carbon in 2003. The models were built at a resolution of and then smoothed for a final resolution of . Land-use regression models can be used to assign household-level exposures in community health studies by combining information about land use (e.g., traffic indicators, population density), with air monitoring data of the urban airshed (Bertazzon et al. 2015). We have previously demonstrated the stability of the spatial component of these exposure estimates, supporting their application to the time period of interest (Wang et al. 2012). In this airshed, black carbon, based on the particle light absorption coefficient, was highly correlated with the concentration of elemental carbon measured by traditional thermal/optical reflectance (); Black carbon is approximately equivalent to elemental carbon (Rich 2002). As in previous analyses of this cohort, these estimates were then temporally adjusted with regulatory air quality monitoring data to calculate monthly concentrations and average concentrations during the 5-y exposure period for each postal code area (Gan et al. 2012a; Henderson et al. 2007). IQR measures were calculated for each air pollutant. Both the noise and the air pollution estimates took residential changes of address within the exposure period into account, resulting in 5-y time-weighted exposure averages.
Greenness and Walkability
Residential greenness was measured using the satellite-derived Normalized Difference Vegetation Index (NDVI) of greenness (Hystad et al. 2014). The average greenness values were extracted for buffers around residential postal code centroids, and both yearly (1992–2002) and seasonal greenness values were calculated. Neighborhood walkability (2001) (Frank et al. 2010) is a composite index of built-environment characteristics around residential postal codes that may influence opportunities for physical activity (Frank et al. 2005; Hystad et al. 2014), including net residential density, retail floor space-to-land area ratio, land use mix, and street connectivity or intersection density, within a road network distance around each postal code centroid. High index levels indicate an environment that encourages walking, whereas low index levels represent environmental features that inhibit walking and promote driving and obesity (Frank et al. 2004).
Diabetes Case Definition
International Statistical Classification of Diseases and Related Health Problems, 9th Revision (ICD-9; WHO 1977) and International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10; WHO 2010) codes for diabetes were used to identify incident diabetes cases in the follow-up period (ICD-9 code 250, with ICD-10 coding back-translated to ICD-9 coding). We excluded subjects who had prior hospital or doctor visits for diabetes during the exposure period to identify new (incident) cases. The administrative records did not enable type 1 diabetes to be distinguished from type 2 diabetes.
Standardized Canadian definitions for identifying diabetes using administrative data sets were used in this study (Lix et al. 2008); these case definitions have been evaluated and were found to have good agreement with self-reporting of disease in the Canadian Community Health Survey (Lix et al. 2008). The diabetes case definition used was any one hospitalization for diabetes, or two physician or health care provider visits for diabetes within a 1-y period.
Covariates
Individual-level age and gender data were available from the MSP administrative health database. Neighborhood-level data from the 2001 Statistics Canada census were linked to the database to account for variance in socioeconomic status and ethnicity, both of which are potential confounders for associations of transportation noise exposure and traffic-related air pollution with diabetes. These data were available at the census dissemination area level (400–700 persons) and were assigned based on residential postal codes. Socioeconomic status was measured using neighborhood income quintiles. South Asians in Canada have a higher prevalence of diabetes than the general population (Rana et al. 2014), and residences of individuals of South Asian ethnicity may be spatially clustered. We therefore conducted sensitivity analyses with the inclusion of a census measure of neighborhoods with of the population being of South Asian ethnicity. A similar analysis was conducted for Chinese ethnicity, given the high prevalence of this ethnicity in the study area.
Statistical Analyses
All analyses were performed using Stata (v.13; StataCorp LLC). Initial descriptive statistics reported the prevalence and range of exposures and outcomes. Correlations examined the associations between the noise exposure, air pollution, walkability, and greenness estimates. Initial single-exposure logistic regression analyses examined the crude univariate association for an IQR increase of each exposure (noise, , NO, black carbon, , greenness, and walkability) with diabetes and adjusted for age, gender, and area-level household income. Smoothing splines (gam function, R v.2.15.0, The R Project for Statistical Computing) were used to investigate the functional relationship between incident diabetes cases and Lden noise exposure. We examined unadjusted values and adjusted for age, gender, and area-level household income.
To assess whether the associations of noise exposure with diabetes were independent of spatially covarying air pollution, greenness, and walkability, incremental coexposure regression models were run. We first adjusted individually for each air pollutant measure, greenness, and walkability; then, we adjusted for each air pollutant that was significant in the first stage along with greenness and walkability. All models used IQR measures of the environmental exposures to enable comparison between observed associations for the different pollutants. To test the sensitivity of these final coexposure regression models for ethnicity, models were then rerun with additional adjustment for South Asian and Chinese ethnicity.
Results
The cohort comprised 380,738 individuals. Of these, 3.4% were identified as incident diabetes cases during the follow-up period (Table 1). The average transportation noise exposure (Lden) for the cohort was 63 dBA. For air pollution, the average exposures were as follows: , ; NO, ; , , and particle light absorbance, . The average subject age was 58 y, and 46% were male. One-fifth of the sample lived in neighborhoods where of the population was of South Asian ethnicity, and nearly one-half of the sample lived in neighborhoods where of the population was of Chinese ethnicity. All covariates were significantly associated with all of the exposures and outcomes ().
Table 1.
Descriptive statistics showing the exposures, outcomes, and covariates: Metropolitan Vancouver resident cohort, 45–85 y old (1994–1998), .
| Category | Mean or % | IQR | Range | ||
|---|---|---|---|---|---|
| Exposures | |||||
| Transportation noise Lden (dBA) | 63.4 | 6.8 | |||
| Transportation noise Lden (dBA) lowest decile | 36,685 | 9.6% | - | ||
| Transportation noise Lden (dBA) 2nd–5th deciles | 154,796 | 40.6% | - | 58–61 | |
| Transportation noise Lden (dBA) 6th–9th deciles | 151,989 | 39.9% | - | 62–69 | |
| Transportation noise Lden (dBA) 10th decile | 37,268 | 9.9% | - | ||
| () | 32.1 | 8.4 | 14.4 – 57.8 | ||
| NO () | 32.0 | 13.13 | 8.8–126.0 | ||
| () | 4.1 | 1.6 | 0 – 10.2 | ||
| Black carbon () | 1.5 | 0.9 | 0 – 5.0 | ||
| Greenness (NDVI Index) | 0.32 | 0.12 | |||
| Neighborhood Walkability Index | 0.31 | 4.3 | |||
| Outcomes | |||||
| Incident diabetes cases over 4 years | 12,941 | 3.4% | |||
| Covariates | |||||
| Age (years) | 58 | 17 | 45–83 | ||
| Male | 175,219 | 46.0% | |||
| Quintiles of area level household income from census | |||||
| Income: 0 | 55,626 | 14.6% | |||
| Income: 1 | 65,567 | 17.2% | |||
| Income: 2 | 73,602 | 19.3% | |||
| Income: 3 | 84,426 | 22.2% | |||
| Income: 4 | 101,517 | 26.7% | |||
| South Asian population in neighborhood | 74,911 | 19.6% | |||
| Chinese population in neighborhood | 183,044 | 48.1% | |||
Note: dBA, A-weighted decibels; IQR, interquartile range; Lden, annual average noise exposure; NDVI, Normalized Difference Vegetation Index; NO, nitric oxide; , nitrogen dioxide; , particulate matter with aerodynamic diameter .
Table 2 shows correlations between the environmental exposures in the cohort. Transportation noise exposure was most strongly correlated with black carbon () and NO () but was weakly correlated with () and (). Greenness showed a strong negative correlation with the walkability index (), but both greenness and walkability showed only weak correlations with noise and with the air pollutants.
Table 2.
Correlations between environmental exposures: Metropolitan Vancouver resident cohort, 45–85 y old (1994–1998), .
| Category | Lden | NO | Black carbon | Greenness (NDVI) | Walkability index | ||
|---|---|---|---|---|---|---|---|
| Lden (dBA) | 1.00 | ||||||
| () | 0.24 | 1.00 | |||||
| NO () | 0.42 | 0.47 | 1.00 | ||||
| () | 0.14 | 0.52 | 0.29 | 1.00 | |||
| Black carbon () | 0.47 | 0.25 | 0.52 | 0.11 | 1.00 | ||
| Greenness (NDVI) | 1.00 | ||||||
| Walkability Index | 0.16 | 0.38 | 0.38 | 0.28 | 0.14 | 1.00 |
Notes: dBA, A-weighted decibels; Lden, annual average noise exposure; NDVI, Normalized Difference Vegetation Index; NO, nitric oxide; , nitrogen dioxide.
Noise exposure was associated with incidence of diabetes after adjustment for age, gender, and area-level household income; there was an 8% increase in the incidence of diabetes with an IQR increase in noise exposure (Table 3). Because there was no a priori understanding of the shape of the relationship between exposure and outcome, we also examined noise exposure as a categorical variable (Gan et al. 2012a). Odds ratios for diabetes were 32% higher for those in the 10th decile of exposure [ (95% CI: 1.22, 1.43)] compared with those in the first decile. Compared with the first decile, odds ratios were 18% higher for those in the sixth–ninth deciles of noise exposure [ (95% CI: 1.11, 1.26)] and 9% higher for those in the second–fifth deciles of noise exposure [ (95% CI: 1.02, 1.06)].
Table 3.
Adjusted associations of transportation noise exposures with incident diabetes cases per one interquartile range increase in exposure (single-exposure models).
| Exposure | Incident diabetes | |
|---|---|---|
| Crude OR (95% CI) | AOR (95% CI) | |
| Transportation noise exposure | ||
| Lden (dBA) | 1.10 (1.08, 1.13) | 1.08 (1.05, 1.10) |
| Traffic-related air pollution | ||
| ( ) | 1.05 (1.03, 1.07) | 1.00 (0.98, 1.02) |
| NO () | 1.06 (1.05, 1.09) | 1.04 (1.01, 1.05) |
| () | 1.06 (1.05, 1.08) | 1.03 (1.01, 1.05) |
| Black carbon () | 1.05 (1.03, 1.06) | 1.03 (1.01, 1.04) |
| Greenness | ||
| Greenness (NDVI) | 0.83 (0.81, 0.85) | 0.90 (0.87, 0.92) |
| Walkability index | ||
| Walkability | 1.09 (1.06, 1.11) | 1.01 (0.98, 1.04) |
Note: Each row is a separate model. AOR adjusted for gender, age, area-level household income. AOR, adjusted odds ratio; CI, confidence interval; dBA, A-weighted decibels; Lden, annual average noise exposure; NDVI, Normalized Difference Vegetation Index; NO, nitric oxide; , nitrogen dioxide; OR, odds ratio; , particulate matter with aerodynamic diameter .
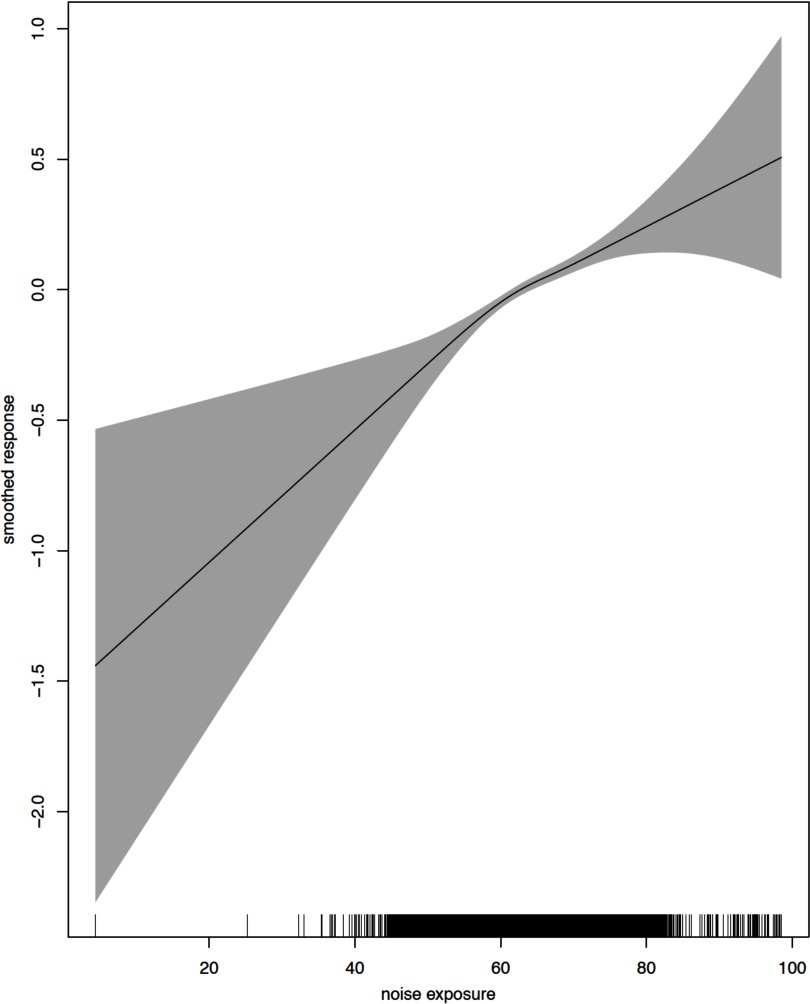
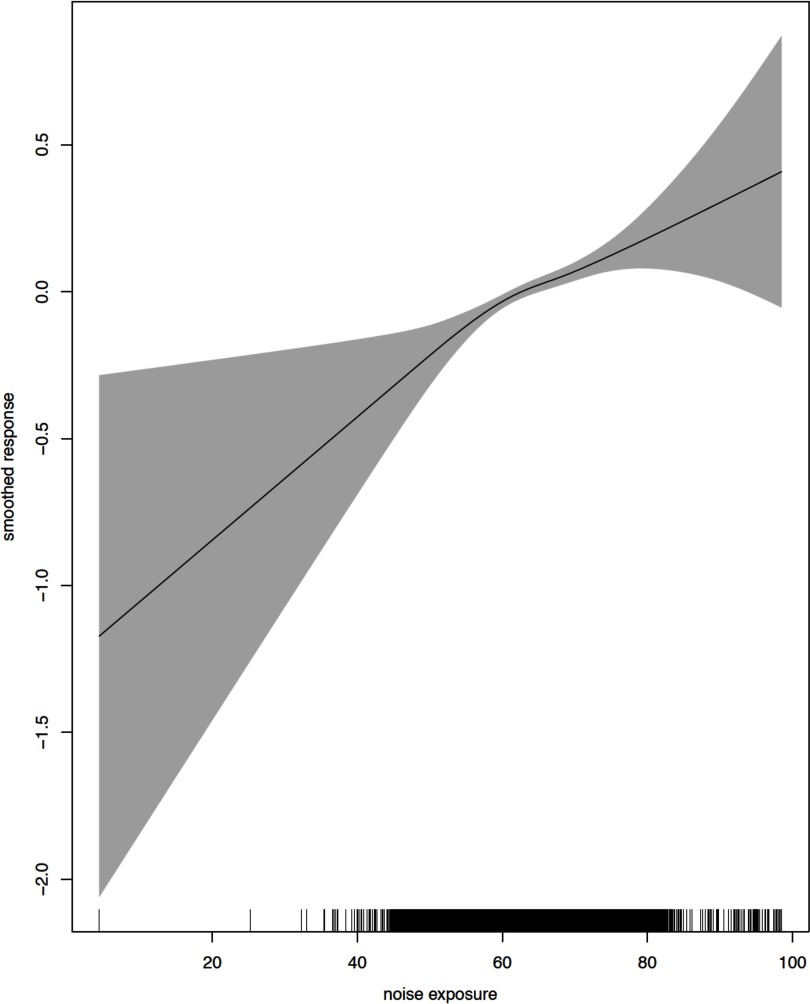
Figures 1 and 2 show the crude and adjusted dose–response relationships between Lden and incident diabetes cases. The relationships were found to be linear for a wide range of the data with the exception of extreme exposure values. Departure from linearity with wider confidence limits was observed close to the maximum and minimum exposure estimates, where the number of events was substantially lower.
Figure 1.
Functional relationship between noise exposure (Lden) and incident diabetes cases: unadjusted. Line is the log odds of diabetes incidence (log base 10); gray area is the 95% confidence interval.
Figure 2.
Functional relationship between noise exposure (Lden) and incident diabetes cases: adjusted for age, gender, and area-level household income. Line is the log odds of diabetes incidence (log base 10); gray area is the 95% confidence interval.
After adjustment for gender, age, and area-level household income, and black carbon were significantly associated with diabetes (Table 3): An IQR increase was associated with a 3% increase in odds for the incidence of diabetes [ (95% CI: 1.01, 1.05); (95% CI: 1.01, 1.05) for and black carbon, respectively]. Similarly, an IQR increase in NO exposure increased the risk of diabetes by 4% [ (95% CI: 1.01, 1.05)]. and walkability were not associated with diabetes after adjustment for gender, age, and area-level household income, whereas increasing residential greenness showed a protective association [ (95% CI: 0.87, 0.92).
The associations between transportation noise exposure and incidence of diabetes were independent of spatially covarying air pollution, greenness, and walkability. In the models adjusted for environmental coexposures (Table 4, Model 1) an interquartile dBA increase in transportation noise exposure remained associated with a 6% increase in the odds for incidence of diabetes [ (95% CI: 1.03, 1.09). This association was robust to further adjustment for South Asian ethnicity (Table 4, Model 2) and Chinese ethnicity (Table 4, Model 3).
Table 4.
Associations of transportation noise exposure (IQR) with incident diabetes cases, further adjusted for environmental coexposures; AOR (95% CI).
| Exposures | Environmental coexposures added to the model containing noise exposure (Lden) | |||||||
|---|---|---|---|---|---|---|---|---|
| PM2.5 only | NO only | Black carbon only | Greenness only | Walkability only | PM2.5, greenness, walkability | NO, greenness, walkability | Black carbon, greenness, walkability | |
| Model 1: Environmental coexposures | ||||||||
| Lden (dBA) | 1.06 (1.06, 1.07)* | 1.07 (1.04, 1.10)* | 1.07 (1.04, 1.10)* | 1.05 (1.03, 1.06)* | 1.07 (1.06, 1.08)* | 1.06 (1.03, 1.08)* | 1.06 (1.03, 1.09)* | 1.06 (1.03, 1.09)* |
| NO () | 1.01 (1.00, 1.04) | 1.00 (0.97, 1.03) | ||||||
| () | 1.03 (1.02, 1.05)* | 1.01 (1.00, 1.03) | ||||||
| Black carbon () | 1.01 (0.99, 1.03) | 1.00 (0.98, 1.02) | ||||||
| Greenness (NDVI) | 0.91 (0.89, 0.94)* | 0.89 (0.87, 0.92)* | 0.90 (0.87, 0.92)* | 0.90 (0.87, 0.93)* | ||||
| Walkability | 1.01 (1.00, 1.04) | 0.95 (0.91, 0.99)* | 0.95 (0.92, 0.98)* | 0.96 (0.95, 1.00)* | ||||
| Model 2: | ||||||||
| Lden (dBA) | 1.06 (1.01, 1.07)* | 1.06 (1.03, 1.09)* | 1.06 (1.01, 1.07)* | 1.05 (1.01-1.06)* | 1.06 (1.01, 1.07)* | 1.04 (1.00, 1.06)* | 1.06 (1.03, 1.09)* | 1.05 (1.02, 1.08)* |
| NO () | 1.01 (1.00, 1.04) | 1.00 (0.99, 1.03) | ||||||
| () | 1.03 (1.01, 1.04)* | 1.01 (1.00, 1.03) | ||||||
| Black carbon ) | 1.01 (0.99, 1.03) | 1.00 (0.99, 1.02) | ||||||
| Greenness (NDVI) | 0.91 (0.89-0.94)* | 0.89 (0.86, 0.92)* | 0.90 (0.86, 0.93)* | 0.90 (0.87, 0.93)* | ||||
| Walkability | 1.02 (0.99, 1.04) | 0.97 (0.92, 0.99)* | 0.97 (0.94, 1.00) | 0.97 (0.95, 0.99)* | ||||
| Model 3: | ||||||||
| Lden (dBA) | 1.06 (1.04, 1.09)* | 1.07 (1.04, 1.09)* | 1.06 (1.03, 1.09)* | 1.05 (1.02-1.06)* | 1.05 (1.01, 1.06)* | 1.05 (1.03-1.08)* | 1.05 (1.03, 1.08)* | 1.05 (1.02, 1.08)* |
| NO () | 1.01 (1.00, 1.03) | 1.00 (0.97, 1.02) | ||||||
| () | 1.02 (1.00, 1.04)* | 1.01 (1.00-1.03) | ||||||
| Black carbon ) | 1.02 (1.00, 1.05) | 1.00 (0.98, 1.04) | ||||||
| Greenness (NDVI) | 0.93 (0.91, 0.96)* | 0.90 (0.87, 0.93)* | 0.90 (0.86, 0.93)* | 0.90 (0.87, 0.93)* | ||||
| Walkability | 0.99 (0.91, 1.00) | 0.96 (0.92, 0.99)* | 0.94 (0.91, 0.97) | 0.96 (0.92, 0.99)* | ||||
Note: All models are adjusted for age, gender, and area-level household income. Models are presented within columns and use interquartile range (IQR) increments for every environmental exposure metric. AOR, adjusted odds ratio; CI, confidence interval; dBA, A-weighted decibels; Lden, annual average noise exposure; NDVI, Normalized Difference Vegetation Index; NO, nitric oxide; , nitrogen dioxide; , particulate matter with aerodynamic diameter .
Statistically significant ().
Odds ratios for diabetes were 32% higher for those in the 10th (highest) decile of noise exposure (95% CI: 22%, 43%) and 18% higher for those in the sixth–ninth decile of noise exposure (95% CI: 11%, 26%) compared with those in the first decile after adjustment for age, gender, and income. These estimates were reduced in models that included other environmental exposures: Odds ratios in the highest noise decile were between 1.16 and 1.20 (Table 5). Again, associations were robust to further adjustment for South Asian ethnicity and Chinese ethnicity.
Table 5.
Associations of transportation noise exposure (percentiles) with incident diabetes cases, further adjusted for environmental coexposures; AOR (95% CI).
| Exposures | Environmental coexposures added to the model containing noise exposure (noise percentiles) | |||
|---|---|---|---|---|
| , greenness, walkability | Black carbon, greenness, walkability | NO, greenness, walkability | , greenness, walkability | |
| Model 1: Environmental coexposures | ||||
| Noise: 2nd-5th percentile | 1.04 (0.98, 1.12) | 1.05 (0.98, 1.12) | 1.05 (0.98, 1.13) | 1.05 (0.98, 1.13) |
| Noise: 6th-9th percentile | 1.10 (1.02, 1.17)* | 1.10 (1.02, 1.16)* | 1.11 (1.03, 1.19)* | 1.11 (1.04, 1.19)* |
| Noise: 10th percentile | 1.16 (1.07, 1.26)* | 1.20 (1.11, 1.30)* | 1.17 (1.08, 1.28)* | 1.17 (1.08, 1.27)* |
| Model 2: | ||||
| Noise: 2nd-5th percentile | 1.03 (0.96, 1.09) | 1.03 (0.96, 1.11) | 1.03 (0.96, 1.11) | 1.03 (0.97, 1.11) |
| Noise: 6th-9th percentile | 1.07 (1.00, 1.14)* | 1.07 (1.00, 1.14)* | 1.07 (1.00, 1.14)* | 1.08 (1.01, 1.15)* |
| Noise: 10th percentile | 1.13 (1.04, 1.23)* | 1.13 (1.03, 1.22)* | 1.13 (1.03, 1.22)* | 1.14 (1.05, 1.25)* |
| Model 3: | ||||
| Noise: 2nd-5th percentile | 1.03 (0.97, 1.10) | 1.03 (0.96, 1.11) | 1.03 (0.97, 1.11) | 1.04 (0.97, 1.11) |
| Noise: 6th-9th percentile | 1.07 (1.00, 1.14)* | 1.07 (1.00, 1.14)* | 1.07 (1.00, 1.15)* | 1.08 (1.01, 1.16)* |
| Noise: 10th percentile | 1.13 (1.04, 1.23)* | 1.13 (1.03, 1.22)* | 1.13 (1.04, 1.23)* | 1.15 (1.06, 1.25)* |
Note: All models are adjusted for age, gender, and area-level household income. Reference group is “Noise: 1st percentile.” AOR, adjusted odds ratio; CI, confidence interval; NO, nitric oxide; , nitrogen dioxide.
Statistically significant ().
The associations of the traffic-related air pollutants with diabetes were not robust to adjustment for environmental coexposures. The association of with diabetes was independent of spatially covarying noise exposure but became borderline significant after further adjustment for greenness and walkability (Table 4). NO and black carbon were not associated with diabetes after adjustment for covarying noise exposure.
The protective associations of residential greenness and walkability with diabetes remained in the models adjusting for environmental coexposures. An IQR increase in greenness remained associated with a 10% reduction in odds for diabetes, and an IQR increase in walkability remained associated with a 5% reduction in odds for diabetes.
Discussion
This large-scale population-based cohort study found robust associations between residential transportation noise exposure and the incidence of diabetes. These associations were not explained by spatially varying environmental coexposures (a range of traffic-related air pollutants, greenness, walkability) or by ethnicity. Traffic-related air pollutants were not independently associated with the incidence of diabetes after adjustment for environmental coexposures. Neighborhood greenness and walkability showed protective associations with the incidence of diabetes in fully adjusted models.
Transportation Traffic Noise and the Incidence of Diabetes
This study found that an IQR increase in transportation noise exposure in the Vancouver cohort ( dBA Lden increase) in the preceding 5 y was associated with a 6% increase in odds for incidence of diabetes over a 4-y period. This finding is similar to that of a previous Danish cohort study of 57,053 50–64-y-olds exposed to road traffic noise ranging from 48–70 dB Lden that found that a 10-dBA higher level of road traffic noise during the 5 y preceding diagnosis was associated with an increased risk for the incidence of diabetes [ (95% CI: 1.05, 1.18)] after adjusting for age, gender, BMI, waist circumference, education, air pollution (nitrogen oxides), and lifestyle characteristics (Sørensen et al. 2013). Both studies had large sample sizes, and both found a prospective association of transportation noise and incident diabetes cases after taking nitrogen oxides into account. Our study extends the knowledge base by including adjustments for other environmental exposures including walkability, greenness, and a range of traffic-related air pollutants.
Our finding of a relationship between transportation noise and the incidence of diabetes is compatible with Babisch’s hypothesized pathway (Babisch 2014) in which chronic transportation noise exposure can lead to physiological stress reactions in the endocrine system (e.g., the hypothalamic–pituitary–adrenal axis) and the sympathetic nervous system that result in hormonal changes (to, e.g., cortisol, norepinephrine, epinephrine), which in turn lead to increases in cardiovascular disease risk factors including blood pressure, blood fats, and blood glucose concentrations. Alternatively, residential transportation noise can cause sleep loss, which has metabolic consequences in terms of glucose regulation, appetite, and energy expenditure (Eriksson et al. 2014). Our findings support the hypothesized role of sleep loss and its metabolic impact as a potential explanation for the association between transportation noise and the incidence of diabetes because noise exposure was estimated over a 24-h period. However, we were unable to examine sleep loss as a moderator of our associations. Studies exploring physiological stress and sleep loss as explanations for associations of noise with diabetic (Eriksson et al. 2014) and cardiovascular outcomes remain a research priority.
Traffic-Related Air Pollutants and the Incidence of Diabetes
This study found a modest association of with the incidence of diabetes that was attenuated by covarying noise exposure. NO and black carbon were not associated with the incidence of diabetes after adjustment for environmental coexposures. was not associated with the incidence of diabetes. Recent meta-analyses concluded that there was emerging evidence for an association between air pollution and the incidence of type 2 diabetes (Balti et al. 2014; Thiering and Heinrich 2015; Wang et al. 2014), but these studies did not take coexisting noise exposure or other environmental confounders into account. Our findings contrast with those of previous studies that suggested robust associations between air pollutants and diabetes (Balti et al. 2014; Chen et al. 2013; Krämer et al. 2010; Thiering and Heinrich 2015; Wang et al. 2014). We examined NO in addition to because NO has a different spatial distribution that is more indicative of primary traffic pollutant emissions (Henderson et al. 2007; Wang et al. 2012). Our findings suggest that and NO did not increase risk for the incidence of diabetes after taking environmental coexposures into account. Previous studies of and diabetes provided inconclusive evidence (Andersen et al. 2012; Balti et al. 2014; Krämer et al. 2010; Wang et al. 2014).
Greenness and the Incidence of Diabetes
Greenness showed a protective association with the incidence of diabetes, with an IQR increase in greenness being associated with a 10% decrease in odds for the incidence of diabetes in the fully adjusted models. Our findings are consistent with those of previous studies that suggest short-term associations between greenness and improved diabetic outcomes (Astell-Burt et al. 2014; Thiering et al. 2016). However, our study is the first to confirm the association using prospective data, and it is the first to show that the associations for greenness were independent of air pollution, in contrast with a smaller-scale study of adolescents that examined insulin resistance (Thiering et al. 2016).
Walkability and the Incidence of Diabetes
Neighborhood walkability showed a protective association with the incidence of diabetes, with an interquartile increase in walkability score being associated with a 5% decrease in odds for the incidence of diabetes in the fully adjusted models. A previous study found a protective association between neighborhood walkability and 4-y incidence of diabetes; however, this association was attenuated by adjustment for individual sociodemographic factors (Sundquist et al. 2015) and did not take environmental coexposures, including noise, air pollution, and greenness, into account (Sundquist et al. 2015). A recent Canadian time-series study found that the incidence of diabetes was lowest in neighborhoods with the highest walkability scores compared with less-walkable neighborhoods (Creatore et al. 2016), taking sociodemographic factors and distance to the nearest park into account. Our analyses suggest that associations of neighborhood walkability with the incidence of diabetes were independent of noise and air pollution.
Comparing the increased risk for the incidence of diabetes with an interquartile increase among the different environmental exposures in the fully adjusted models, the largest change in risk was observed for greenness, followed by transportation noise exposure and neighborhood walkability. Traffic-related air pollutants showed smaller influences on the incidence of diabetes than the other environmental exposures examined.
Limitations and Strengths
Individual-level environmental exposures were linked using postal codes for the residential addresses. No method can measure true exposure, and sources of error in estimated exposure include not modeling all salient features of the local environment and not taking individual factors, such as room orientation and time spent at home, into account (Gan et al. 2012a). Further limitations of the noise modeling include potential measurement error caused by the lack of data for some environmental aspects; there are no transport prediction guidelines for Canada, so we used noise prediction guidelines from Germany for road and railway noise. The use of administrative health databases meant that we were unable to take individual-level socioeconomic and diabetic risk factors (BMI, family history of diabetes, smoking history, diet, and local food environment) into account. Such factors may confound findings observed between noise and the incidence of diabetes. However, we were able to partially adjust for some of these factors by using neighborhood-level socioeconomic status (SES) measures, and the risk estimates found in the present study are similar to those found in a similar Danish study that did adjust for individual-level socioeconomic and diabetic risk factors (Sørensen et al. 2013). We have attempted to account for certain diabetic risk factors such as physical activity and obesity via adjustment for neighborhood-level walkability and greenness, but residual confounding remains a possibility. Census-based income measures used in this study do not fully capture variations in accumulated wealth. Wealthier people live in neighborhoods that are more conducive to being physically active and maintaining healthier diets. Affluent communities have higher-quality pedestrian environments and tend to be safer and to promote active living. This study was reliant on administrative records for diabetes diagnosis, which will have missed undiagnosed cases and residents who do not attend health care providers, although registration in the BC universal health care system is very high (nearly 100%). These administrative records do not distinguish between type 1 and type 2 diabetes; environmental exposures may be more important for type 2 diabetes given the importance of disease-conducive environmental factors for type 2 versus type 1 diabetes. However, when we compared a case definition of one hospitalization or two physician or health care provider visits within a 2-y period with our case definition based on a 1-y period, similar results were obtained (results not shown). In the Canadian population, the age-standardized incident rate for diabetes cases in 1999–2000 was 5.3 individuals per 1,000 population (PHAC 2011). In our sample of 380,738 individuals over 4 y, we would therefore have expected an incident case rate of 8,071 if there were no age restrictions on our sample. We actually observed 12,941 incident diabetes cases; this higher rate reflected the higher prevalence in incidence for South Asian and Chinese populations residing in the Vancouver area as well as for our older population, which was focused on the key age group for the incidence of type 2 diabetes. Therefore, our prevalence rate seems much as would be expected.
Further strengths of the study include the estimation of environmental exposures over a 5-y period (taking into account residential mobility), with follow-up for the incidence of diabetes over a 4-y period. We believe that the conclusions could be generalizable to other North American cities because similar correlations have been observed between these environmental exposures in other North American cities: for example, road traffic noise and air pollution (Allen et al. 2009); air pollution and walkability (James et al. 2015); and greenness and air pollution (Rao et al. 2014; Su et al. 2011).
Conclusion
Our study found an increasing risk of diabetes with increasing exposure to transportation noise, but not with increasing exposure to traffic-related air pollutants. The results highlight the importance of taking noise into account when planning interventions to reduce the health impact of transportation. Noise pollution was independently associated with the incidence of diabetes in adult residents of metropolitan Vancouver, British Columbia. Further studies of environmental coexposures and individual-level potential confounders are needed.
Acknowledgements
This work was funded by sabbatical leave supported by Queen Mary University of London to C.C. C.C. would also like to thank the School of Population and Public Health, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia for supporting a visiting professorship during which this work was undertaken. The Border Air Quality Study was supported in part by Health Canada via an agreement with the British Columbia Centre for Disease Control. Additional support was provided by the Centre for Health and Environment Research at the University of British Columbia, funded by the Michael Smith Foundation for Health Research.
All inferences, opinions, and conclusions drawn in this research article are those of the authors, and do not reflect the opinions or policies of the Data Steward(s).
References
- Allen RW, Davies H, Cohen MA, Mallach G, Kaufman JD, Adar SD. 2009. The spatial relationship between traffic-generated air pollution and noise in 2 US cities. Environ Res 109(3):334–342, PMID: 19193368, 10.1016/j.envres.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ, Raascou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, et al. 2012. Diabetes incidence and long-term exposure to air pollution: A cohort study. Diabetes Care 35(1):92–98, PMID: 22074722, 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell-Burt T, Feng X, Kolt GS. 2014. Is neighborhood green space associated with a lower risk of type 2 diabetes? Evidence from 267,072 Australians. Diabetes Care 37(1):197–201, PMID: 24026544, 10.2337/dc13-1325. [DOI] [PubMed] [Google Scholar]
- Babisch W. 2014. Updated exposure-response relationship between road traffic noise and coronary heart diseases: a meta-analysis. Noise Health 16(68):1–9, PMID: 24583674, 10.4103/1463-1741.127847. [DOI] [PubMed] [Google Scholar]
- Balti EV, Echouffo-Tcheugui JB, Yako YY, Kengne AP. 2014. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 106(2):161–172, PMID: 25262110, 10.1016/j.diabres.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, et al. 2014. Auditory and non-auditory effects of noise on health. Lancet 383(9925):1325–1332, PMID: 24183105, 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzon S, Johnson M, Eccles K, Kaplan GG. 2015. Accounting for spatial effects in land use regression for urban air pollution modeling. Spat Spatiotemporal Epidemiol 14-15:9–21, PMID: 26530819, 10.1016/j.sste.2015.06.002. [DOI] [PubMed] [Google Scholar]
- British Columbia Ministry of Health. Discharge abstract database (hospital separations). V2. Population data BC, data extract. https://www.popdata.bc.ca/data/ [accessed 15 October 2014].
- British Columbia Ministry of Health. Medical services plan (MSP) payment information file. V2. Population data BC. https://www.popdata.bc.ca/data/ [accessed 15 October 2014].
- British Columbia Vital Statistics Agency. Vital statistics deaths. V2. Population data BC. Vital statistics agency. 2009. http://www.popdata.bc.ca/data [accessed 15 October 2014].
- Chamberlayne R, Green B, Barer ML, Hertzman C, Lawrence WJ, Sheps SB. 1998. Creating a population-based linked health database: a new resource for health services research. Can J Public Health 89(4):270–273, PMID: 9735524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. 2013. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 121(7):804–810, PMID: 23632126, 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MT. 2015. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 101(4):253–256, PMID: 25552258, 10.1136/heartjnl-2014-306379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, et al. 2016. Association of neighborhood walkability with change in overweight, obesity and diabetes. JAMA 315(20):2211–2220, PMID: 27218630, 10.1001/jama.2016.5898. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Hilding A, Pyko A, Bluhm G, Pershagen G, Östenson CG. 2014. Long-term aircraft noise exposure and body mass index, waist circumference, and type 2 diabetes: a prospective study. Environ Health Perspect 122(7):687–694, PMID: 24800763, 10.1289/ehp.1307115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, et al. 2015. Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta-analysis. Environ Health Perspect 123(5):381–389, PMID: 25625876, 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LD, Andresen M, Schmid TL. 2004. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med 27(2):87–96, PMID: 15261894, 10.1016/j.amepre.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Frank LD, Schmid TL, Sallis JF, Chapman JE, Saelens BE. 2005. Linking objectively measured physical activity with objectively measured urban form: Findings from SMARTRAQ. Am J Prev Med 28(2 suppl 2):117–125, PMID: 15694519, 10.1016/j.amepre.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Frank LD, Sallis J, Saelens B, Leary L, Cain K, Conway T, et al. 2010. The development of a walkability index: Application to the neighborhood quality of life study. Br J Sports Med 44(13):924–933, PMID: 19406732, 10.1136/bjsm.2009.058701. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Davies HW, Koehoorn M, Brauer M. 2012a. Association of long-term exposure to community noise and traffic-related air pollution with coronary heart disease mortality. Am J Epidemiol 175(9):898–906, PMID: 22491084, 10.1093/aje/kwr424. [DOI] [PubMed] [Google Scholar]
- Gan WQ, McLean K, Brauer M, Chiarello SA, Davies HW. 2012b. Modeling population exposure to community noise and air pollution in a large metropolitan area. Environ Res 116:11–16, PMID: 22520824, 10.1016/j.envres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Henderson SB, Beckerman B, Jerrett M, Brauer M. 2007. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ Sci Technol 41(7):2422–2428, PMID: 17438795, 10.1021/es0606780. [DOI] [PubMed] [Google Scholar]
- Hystad P, Davies HW, Frank L, Van Loon J, Gehring U, Tamburic L, et al. 2014. Residential greenness and birth outcomes: evaluating the influence of spatially correlated built-environment factors. Environ Health Perspect 122(10):1095–1102, PMID: 25014041, 10.1289/ehp.1308049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Hart JE, Laden F. 2015. Neighborhood walkability and particulate air pollution in a nationwide cohort of women. Environ Res 142:703–711, PMID: 26397775, 10.1016/j.envres.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, et al. 2010. Traffic-related air pollution and incident type 2 diabetes: Results from the SALIA cohort study. Environ Health Perspect 118(9):1273–1279, PMID: 20504758, 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Fang D, Xu D, Wang B, Zhao S, Yan S, et al. 2014. Main air pollutants and diabetes-associated mortality: A systematic review and meta-analysis. Eur J Endocrinol 171(5):R183–R190, PMID: 25298377, 10.1530/EJE-14-0287. [DOI] [PubMed] [Google Scholar]
- Lix LM, Yogendran MS, Shaw SY, Burchill C, Metge C, Bond R. 2008. Population-based data sources for chronic disease surveillance. Chronic Dis Can 29(1):31–38, PMID: 19036221. [PubMed] [Google Scholar]
- Oftedal B, Krog NH, Pyko A, Eriksson C, Graff-Iversen S, Haugen M, et al. 2015. Road traffic noise and markers of obesity - a population-based study. Environ Res 138:144–153, PMID: 25710788, 10.1016/j.envres.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Paquet C, Coffee NT, Haren MT, Howard NJ, Adams RJ, Taylor AW, et al. 2014. Food environment, walkability, and public open spaces are associated with incident development of cardio-metabolic risk factors in a biomedical cohort. Health Place 28:173–176, PMID: 24880234, 10.1016/j.healthplace.2014.05.001. [DOI] [PubMed] [Google Scholar]
- PHAC (Public Health Agency of Canada). 2011. Diabetes in Canada: Facts and figures from a Public Health Perspective. Ottawa, Canada:Public Health Agency of Canada. [Google Scholar]
- Pyko A, Eriksson C, Oftendal B, Hilding A, Ostenson CG, Krog NH, et al. 2015. Exposure to traffic noise and markers of obesity. Occup Environ Med 72(8):594–601, PMID: 26009579, 10.1136/oemed-2014-102516. [DOI] [PubMed] [Google Scholar]
- Rana A, de Souza RJ, Kandasamy S, Lear SA, Anand SS. 2014. Cardiovascular risk among South Asians living in Canada: a systematic review and meta-analysis. CMAJ Open 2(3):E183–E191, PMID: 25295238, 10.9778/cmajo.20130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, George LA, Rosenstiel TN, Shandas VAD. 2014. Assessing the relationship among urban trees, nitrogen dioxide, and respiratory health. Environ Pollut 194:96–104, 10.1016/j.envpol.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Recio A, Linares C, Ramón Banegas J, Díaz J. 2016. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: an integrative model of biological mechanisms. Environ Res 146:359–350, 10.1016/j.envres.2015.12.036. [DOI] [PubMed] [Google Scholar]
- Rich K. 2002. Air Pollution and Patients with Implanted Cardiac Defibrillators: an Epidemiological Analysis and Assessment of Exposure [Master’s Thesis]. Vancouver, BC:University of British Columbia. [Google Scholar]
- Setton EM, Hystad PW, Keller CP. 2005. Road classification schemes - good indicators of traffic volume? http://web.uvic.ca/∼ssrl01/SSRLtemp/SSL05-014-TRAFFIC.pdf [accessed 11 July 2001].
- Sørensen M, Andersen ZJ, Nordsborg RB, Becker T, Tjonneland A, Overvad K, et al. 2013. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ Health Perspect 121(2):217–222, PMID: 23229017, 10.1289/ehp.1205503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M, Hjorteberg D, Eriksen KT, Ketzel M, Tjonneland A, Overvad K, et al. 2015. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: a cross-sectional study. Environ Int 85:238–243, PMID: 26425807, 10.1016/j.envint.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Su JG, Jerrett M, de Nazelle A, Wolch J. 2011. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ Res 111(3):319–328, 10.1016/j.envres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Eriksson U, Mezuk B, Ohlsson H. 2015. Neighborhood walkability, deprivation and incidence of type 2 diabetes: a population-based study on 512,061 Swedish adults. Health Place 31:24–30, PMID: 25463914, 10.1016/j.healthplace.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Heinrich J. 2015. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab 26(7):384–394, PMID: 26068457, 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Thiering E, Markevych I, Brüske I, Fuertes E, Kratzsch J, Sugiri D, et al. 2016. Associations of residential long-term air pollution exposures and satellite-derived greenness with insulin resistance in German adolescents. Environ Health Perspect 124(8):1291–1298, PMID: 26863688, 10.1289/ehp.1509967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transport Canada. 2005. Tp 1247 - aviation - land use in the vicinity of airports. https://www.tc.gc.ca/eng/civilaviation/publications/tp1247-menu-1418.htm [accessed 11 July 2001].
- Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. 2014. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol 171(5):R173–R182, PMID: 25298376, 10.1530/EJE-14-0365. [DOI] [PubMed] [Google Scholar]
- Wang R, Henderson SB, Sbihi H, Allen RW, Brauer M. 2012. Temporal stability of land use regression models for traffic-related air pollution. Atmos Environ 34:312–319, 10.1016/j.atmosenv.2012.09.056. [DOI] [Google Scholar]
- WHO (World Health Organization). 1977. International Statistical Classification of Diseases 9th Revision. Geneva, Switzerland:World Health Organization; http://apps.who.int/iris/handle/10665/40492 [accessed 1 August 2017]. [Google Scholar]
- WHO. 2010. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Geneva, Switzerland:World Health Organization; http://apps.who.int/classifications/icd10/browse/2010/en [accessed 1 August 2017]. [Google Scholar]
- WHO. 2011. Burden of Disease from Environmental Noise: Quantification of Healthy Life Years Lost in Europe. Geneva, Switzerland:World Health Organization; http://www.who.int/quantifying_ehimpacts/publications/e94888/en/ [accessed 1 August 2017]. [Google Scholar]