Abstract
Background:
India is undergoing rapid urbanization with simultaneous increases in the prevalence of cardiovascular disease (CVD). As urban areas become home to an increasing share of the world’s population, it is important to understand relationships between the built environment and progression towards CVD.
Objective:
We assessed associations between multiple measures of the built environment and biomarkers of early vascular aging (EVA) in the Population Study of Urban, Rural and Semiurban Regions for the Detection of Endovascular Disease and Prevalence of Risk Factors and Holistic Intervention Study (PURSE-HIS) in Chennai, India.
Methods:
We performed a cross-sectional analysis of 3,150 study participants. EVA biomarkers included systolic and diastolic blood pressure (SBP and DBP), central pulse pressure (cPP) and flow-mediated dilatation (FMD). Multiple approaches were used to assign residential exposure to factors of the built environment: Moderate Resolution Imaging Spectroradiometer (MODIS)-derived normalized difference vegetation index (NDVI), a measure of vegetation health and greenness; Landsat-derived impervious surface area (ISA); and Visible Infrared Imaging Radiometer Suite (VIIRS)-derived nighttime lights (NTL). Multivariable regression models were used to assess associations between each built environment measure and biomarkers of EVA, adjusting for age, body mass index (BMI), cooking fuel type, energy intake, sex, physical activity, smoking, socioeconomic status, and stress.
Results:
Residing in areas with higher ISA or NTL, or lower greenness, was significantly associated with elevated SBP, DBP, and cPP, and with lower FMD, adjusting for age, BMI, sex, smoking status, and other CVD risk factors. An interquartile range decrease in greenness had the largest increase in SBP [ (95% CI: 2.9, 5.6)], DBP [ (95% CI: 0.4, 2.0)] and cPP [ (95% CI: 2.0, 4.1)], and the largest decrease in FMD [ (95%CI: , ].
Conclusion:
Greenness, ISA, and NTL were associated with increased SBP, DBP, and cPP, and with reduced FMD, suggesting a possible additional EVA pathway for the relationship between urbanization and increased CVD prevalence in urban India. https://doi.org/10.1289/EHP541
Introduction
Projections on population indicate that by 2050, people will reside in urban areas [United Nations, Department of Economic and Social Affairs, Population Division (UNDESAPD) 2015]. India had the world’s second-largest urban population at people in 2014, and its urban population is expected to double by 2050, with four large cities (Ahmadabad, Bangalore, Chennai, and Hyderabad) projected to become megacities (population ) by 2030 (UNDESAPD 2015). Rapid urbanization is occurring in India and in other Asian countries with corresponding increases in the prevalence of cardiovascular disease (CVD) and cardiac-related mortality (Allender et al. 2010; Reddy and Yusuf 1998; Yusuf et al. 2001). Because urban areas are increasingly becoming home to larger populations, it is important to understand the intraurban variability of the built environment and progression towards cardiovascular mortality.
CVD is the leading cause of death worldwide, and hypertension is the single largest risk factor for noncommunicable disease mortality (Lim et al. 2012; Lozano et al. 2012). Low- and middle-income countries (LMIC) are disproportionately affected, and ischemic heart disease is the number one cause for years of life lost in India (GBD 2013 Mortality and Causes of Death Collaborators 2015; Lozano et al. 2012). Early vascular aging (EVA), irrespective of chronological age, is an accelerated process involving biochemical, enzymatic, and cellular changes in artery walls and is characterized by tissue biomarkers of endothelial dysfunction and arterial wall stiffening and thickening, with an increase in blood pressure (BP) (Kotsis et al. 2013). EVA is an independent predictor of CVD mortality (Kotsis et al. 2011). In susceptible individuals, EVA could occur because of inflammation and oxidative stress, particularly in the presence of other cardiovascular risk factors (Kotsis et al. 2011). EVA can be investigated noninvasively through measurement of arterial stiffness, BP, carotid intima-media thickness, aortic and carotid and luminal dilation, and endothelial dysfunction (Laurent 2012; Nilsson et al. 2009). A limited but growing body of literature has found urbanization and associated cardiovascular risk factors to be associated with some of the biomarkers of EVA such as increased BP (Allender et al. 2010; Attard et al. 2015).
Community- and individual-level data from censuses, questionnaires, and photos have been used to create multifactor urbanization scales that have included data on a wide range of contextual areas (e.g., population density, access to transportation, economic activity, education, health services) (Cyril et al. 2013). Urbanization has been associated with increases in chronic disease risk factors in LMIC (Cyril et al. 2013; Van de Poel et al. 2012): a growing number of studies have reported an inverse association with physical activity (Allender et al. 2010; Allender et al. 2011; Assah et al. 2011; Attard et al. 2015; Sullivan et al. 2011), whereas there is a positive relationship in high-income countries (Fan et al. 2014; Parks et al. 2003). Urbanization through expansion of cities and rural-urban migration has been accompanied by nutritional transition, which when coupled with reduced physical activity has led to an energy intake–physical activity mismatch that is becoming increasingly well documented in many developing countries (Reddy 2002) including India (Allender et al. 2010; Patel et al. 2015; Sullivan et al. 2011).
Urban environments can expose residents to other cardiovascular risk factors such as stress and loss of social cohesion through reduced access to open greenspace (de Vries et al. 2013; Nielsen and Hansen 2007; Stigsdotter et al. 2010). Although not fully understood, there are a number of potential mechanisms by which exposure to greenness can influence health. Access to nature through greenspace can be a restorative mechanism to reduce stress (Morita et al. 2007) while simultaneously promoting social interaction, which has been reported to improve physical and mental health (de Vries et al. 2013; Maas et al. 2009; Sugiyama et al. 2008). Residential access to greenspace has been associated with improved cardiovascular and respiratory health through increased physical activity (Dadvand et al. 2014; James et al. 2015). Additionally, there is a limited body of research that has shown potential cobenefits between increased greenness and adjacent trees and a reduction in air pollution (Dadvand et al. 2012; Dadvand et al. 2015; Hagler et al. 2012). Limited research has been conducted on circadian misalignment caused by chronic exposure to artificial light at night, which is hypothesized to suppress melatonin production and to disrupt daily rhythms, resulting in negative effects on psychological, cardiovascular, and metabolic functions (Cho et al. 2015; Fonken et al. 2013; Rybnikova et al. 2016; Scheer et al. 2009). However, no observational studies have examined the relationship between artificial light at night and subclinical markers of CVD.
To date, studies have assessed the impact of urbanization on cardiovascular health in Asia by utilizing aggregate population health data and multicomponent scales that included demographic factors, infrastructure, and economic and social services to delineate urban versus rural or less-urban areas (Allender et al. 2010; Attard et al. 2015; Cyril et al. 2013). As such, our understanding of aspects of the built environment that may individually contribute to progression toward CVD have not been fully elucidated on an intraurban scale. Rapid urbanization in India has led to land-use changes and urban activity that have been detected by satellites, such as decreases in overall vegetation cover or greenness and increases in nighttime lights in urban areas such as Chennai (Pandey and Seto 2015; Paul and Nagendra 2015). A growing body of literature supports an association between greenness and cardiovascular mortality (Hu et al. 2008; Lachowycz and Jones 2014; Mitchell and Popham 2008; Richardson and Mitchell 2010; Villeneuve et al. 2012) and morbidity (Pereira et al. 2012), but there have been fewer studies of associations with subclinical indicators of EVA (James et al. 2015; Markevych et al. 2014). Nighttime lights (NTL) are another factor that has recently been examined in relation to body mass index (BMI) and breast cancer (Hurley et al. 2014; Rybnikova et al. 2016) but has not been examined for a relationship with CVD.
The Population Study of Urban, Rural and Semiurban regions for the Detection of Endovascular Disease and Prevalence of Risk Factors and Holistic Intervention Study (PURSE-HIS) was designed to examine the prevalence of CVD and risk factors in Tamil Nadu, India. Here, we present a cross-sectional examination of the association between satellite measures of the built environment and biomarkers of EVA while adjusting for traditional CVD risk factors in PURSE-HIS participants residing in the city of Chennai. Specifically, we used multiple approaches to estimate factors of the built environment such as greenness, NTL, and impervious surface area (ISA) ratio to examine associations with various indicators of EVA: systolic and diastolic blood pressure (SBP and DBP), central pulse pressure (cPP), and endothelial dysfunction [measured as flow-mediated dilatation (FMD)].
Methods
Study Population
Details on the PURSE-HIS study design and population have been published elsewhere (Thanikachalam et al. 2015); here, we provide a brief overview of the PURSE-HIS Chennai population. The urban-area subjects were drawn from Chennai, the capital city, and the semiurban- and rural-area subjects were selected from the Thiruvallur and Kanchipuram districts, respectively, in the state of Tamil Nadu, India. A two-stage random cluster sampling method was used to recruit participants from 2009–2011. The primary sampling unit was that of administrative divisions to provide a geographical distribution of residential areas across Chennai. The second stage of address selection within divisions used a random sampling to select streets and addresses. An individual house was the sampling unit. Members of the household old without documented evidence of carcinoma, severe psychiatric illness, stage IV cardiac failure, or HIV infection were eligible to be recruited into the study. The analysis presented here was restricted to all participants who consented to participate in the PURSE-HIS study with a residence within the Chennai city boundary (). The study was approved by the Institutional Ethics Committee (IEC-06/53/47) at Sri Ramachandra University, Chennai, India, and was registered with Clinical Trials Registry, India (CTRI/2011/04/001,677). Participation of human subjects in the study did not occur until after informed written consent was obtained.
Questionnaire and Clinical Data
All study participants completed an informed consent enrollment process at their place of residence along with an interviewer-administered questionnaire that collected individual demographic, medical history, and additional CVD risk factor data. Study clinic visits were scheduled at the time of enrollment. Using an automated oscillometric BP device (Omron Sem-1; Omron Healthcare), BP was measured three times from the participant’s dominant arm while the participant was at rest and seated; the average SBP and DBP were calculated from the three measures and used for analysis. Radial artery waveforms were recorded from the wrist of the dominant arm with a high-fidelity micromanometer (SPC-301; Millar Instruments). Pulse wave analysis (SphygmoCor; AtCor Medical) was then used to estimate a corresponding central (ascending aortic) waveform cPP using a generalized transfer function (Karamanoglu et al. 1993), which has been prospectively validated for the assessment of ascending aortic BP (Pauca et al. 2001). The FMD of the left brachial artery was measured using a high-resolution B mode ultrasonography system (Envisor Philip) with a linear transducer frequency of and an oscillometric sphygmomanometer (WelchAllyn) while the participant was in supine position. The brachial artery flow and diameter were measured at rest, and after 5 min ischemia, the maximum peak flow velocity was measured within 15 s, and arterial diameter was measured at 45, 90, 180, and 300 s. Percentage of FMD (peak diameter–baseline diameter/baseline diameter) was used for analysis. Validation procedures for each biomarker have been published in detail (Thanikachalam et al. 2015).
Fasting blood samples were collected to assess various biomarkers, which included low-density lipoproteins (LDL) and fasting blood sugar (FBS) levels. Height and weight were measured to calculate BMI, which was categorized using recommended BMI cut points for Asian adults (World Health Organization Expert Consultation 2004; Misra et al. 2009). Nutritional status was assessed using a 24-h recall of food intake and a food frequency questionnaire, which was used to determine an energy intake score (Schatzkin et al. 2003). Physical activity was measured by a physiotherapist using the global physical activity questionnaire and the three-level scoring protocol categorization of low ( metabolic equivalent of task(MET)-min/wk), moderate (600–2,999 MET-min/week), and high ( MET-min/week) activity and was used to calculate the sedentary score (Bull et al. 2009). Anxiety was assessed by a clinical psychologist using the Hamilton Anxiety Rating Scale (Shear et al. 2001. Individual-level socioeconomic status (SES) scores were based on the Kuppuswamy classification scale, which combines education, occupation, and income (Kumar et al. 2007).
Exposure Assessment
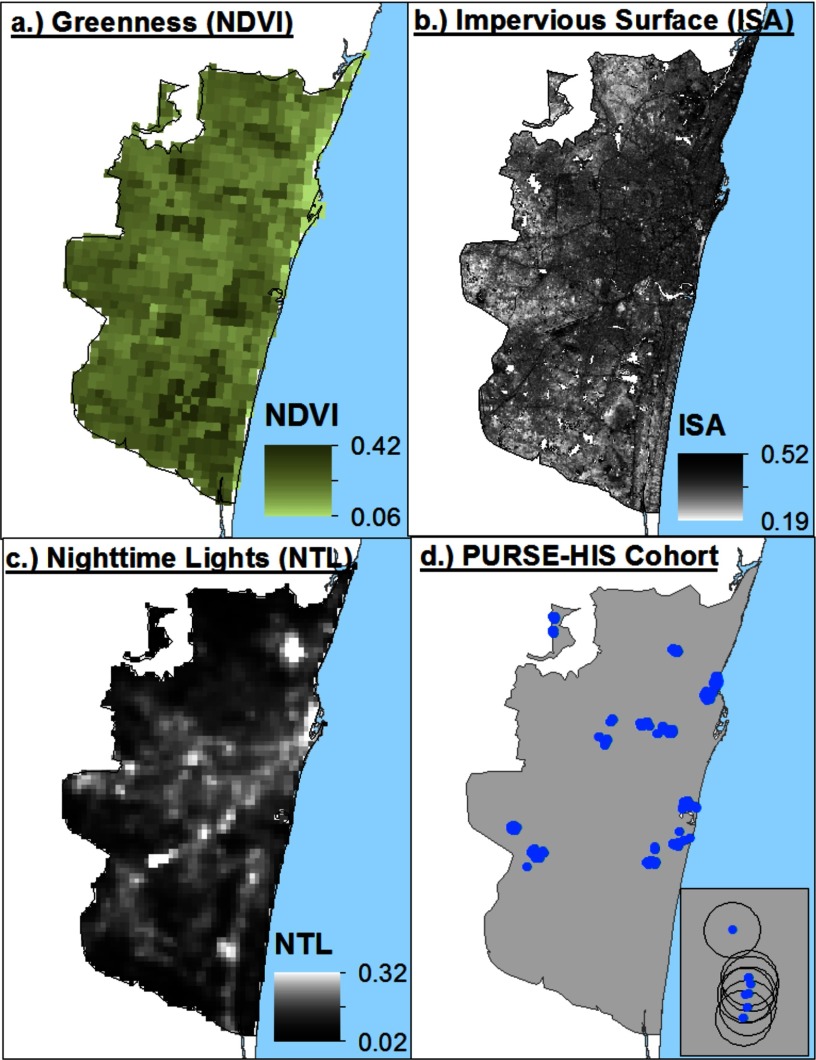
We characterized the built environment using multiple methods: a satellite-derived measure of vegetation health and greenness (normalized difference vegetation index, NDVI), NTL, and ISA. Residential exposure to ISA and NDVI was calculated for each participant based upon the intersecting grid value assigned to the geolocated address overlaid with the gridded satellite data (Figure 1D). We used a radius and a radius as a measure of residential exposure to greenness and as an approximate measure of residential walking exposure, respectively. Similar methods have previously been used to characterize greenness exposure (Casey et al., 2016, James et al., 2014; James et al. 2016).
Figure 1.
Satellite-derived measures of greenness, impervious surface area (ISA), nighttime lights (NTL) and Population study of Urban, Rural, Semiurban Endovascular Disease and Holistic Intervention Study (PURSE-HIS) residential locations in Chennai, India.
Greenness was measured using the NDVI, an index that indicates photosynthetic activity in plants. We used two 16-d NDVI composites (February and June 2009) derived from the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor at resolution onboard the Terra satellite. The MODIS NDVI product used is the MOD13Q1 [National Aeronautics and Space Administration (NASA)/U.S. Geological Survey (USGS) 2015] Version 5 data, which have been corrected for atmospheric contamination from water, clouds, and aerosols, and will hereafter be referred to as greenness.
Along with greenness, we used NTL generated from the day/night band (DNB) from the Visible Infrared Imaging Radiometer Suite (VIIRS) onboard NASA’s Suomi National Polar-Orbiting Partnership (NPP) satellite. NPP-VIIRS NTL have a spatial resolution of and are very sensitive to low levels of visible light. NTL from Defense Meteorological Satellite Program (DMSP) images are measured as 6-bit digital numbers, ranging from 0 to 64, related to radiance at the sensor (). NTL data from an older instrument, the DMSP–Operational Linescan System (OLS), have been shown to be strongly correlated with many social and economic characteristics that are difficult to measure at a fine spatial scale, including urban population and population density (Sutton et al. 1997; Sutton et al. 2001, Zhuo et al. 2009) and economic activity (Doll et al. 2000; Sutton and Costanza 2002; Ghosh et al. 2013). Because NPP-VIIRS NTL data have higher spatial and radiometric resolution than DMSP-OLS, we expect them to be able to measure finer variations of NTL within cities. We selected a cloud-free composite of NTL data for January 2013 (Baugh et al. 2013), the coldest month in Chennai.
The third characteristic we examined was impervious surface, the urban land features—roads, rooftops, parking lots, sidewalks, and so on—that water cannot infiltrate. ISA ratios were extracted from two Landsat 5 Thematic Mapper (TM) images (from 6 May 2009 and 15 February 2009) using linear spectral mixing analysis (LSMA) (Wu and Murray 2003) at a resolution. LSMA enables quantification of the percentage of each land cover component (end member) within a pixel. To perform the LSMA, image data were converted to normalized reflectance, and water was masked. Shade effects were ignored because they were not significant in the image. A maximum noise fraction (MNF) transformation was used to minimize the influence of band correlation and noise, and the first three components were plotted on scatterplots as a guide for pure end member selection. End members for vegetation, soil, low albedo, and high albedo were selected from the image. Finally, a fully constrained linear spectral unmixing model was applied to unmix mixed pixels into the four end members. The impervious surface fraction was calculated as the sum of the low albedo and high albedo fractional components.
Statistical Analyses
Student’s t-tests and analyses of variance (ANOVAs) were used to assess univariate associations between each EVA biomarker (SBP, DBP, cPP, and FMD) and CVD risk factors such as age, BMI, FBS, sex, LDL, physical activity, smoking status (smoker/nonsmoker), anxiety score, and SES. Bivariate models were used to assess the association between CVD risk factors and EVA measures and greenness, NTL, and ISA.
We conducted univariate and multivariable linear regression to examine the independent associations between greenness, ISA, and NTL and each biomarker of EVA, adjusting for age, sex, BMI, and smoking status in a parsimonious model. We also developed a second multivariable regression model that adjusted for additional covariates associated with the biomarkers of EVA such as sedentary score, Kupusswamy socioeconomic score, anxiety score, smoking status, energy intake, and household cooking fuel type [liquid petroleum gas (LPG), kerosene, wood, and LPG with kerosene and/or wood (LPG mixed)]. We utilized cooking fuel type as a proxy measure for indoor exposures and classified participants into indoor fine particulate matter () exposure gradients of low (LPG), medium (LPG mixed), and high (no LPG), similar to solid versus clean fuel categorizations that have been used as indicators in previous global burden of disease models to assess the effects of household air pollution (Balakrishnan et al. 2013). Income, education, and SES were not significantly associated with EVA in univariate and bivariate analysis, but SES was retained in all models. All models were checked for influential cases and collinearity. All statistical analyses were performed using SAS v.10.1 (SAS Institute Inc.). Sensitivity analyses were also performed to examine whether physical activity was a modifer of the association between greenness and biomarkers of EVA by stratifying the parsimonious model by physical activity levels (low, moderate, and high). We also developed a hierarchical model adjusting for random effects by administrative area.
Results
The majority of the PURSE-HIS Chennai population was female, old, and nonsmokers. Most of the participants had not completed secondary education, had an annual income below 6,000 INR, had a nutritional deficit, performed a moderate level of physical activity, used LPG for cooking fuel, and had a (Table 1). Being older, male, a current smoker, obese, or having an excess energy intake, higher anxiety, or a low physical activity level was associated with significantly higher mean SBP and DBP (Table 2). Being older, male, a current smoker, obese, or having a low physical activity level was associated with significantly higher cPP and lower FMD. A comparison of SES quartiles did not indicate a significant mean difference for SBP, DBP, and cPP.
Table 1.
Demographic characteristics of the PURSE-HIS population.
| Characteristic | % () or mean (SD; minimum–maximum) |
|---|---|
| Sex | |
| Female | 56.5 (1,779) |
| Male | 43.5 (1,371) |
| Age (y) | |
| 38.1 (1,201) | |
| 40–50 | 33.0 (1,038) |
| 51–60 | 20.5 (645) |
| 8.4 (266) | |
| BMI () | |
| Underweight () | 6.1 (193) |
| Normal weight (18.5–22.9) | 24.4 (769) |
| Overweight (23–24.9) | 17.5 (550) |
| Obese () | 52.0 (1,638) |
| Energy intake | |
| Deficit | 42.7 (1,346) |
| Adequate | 16.0 (504) |
| Excess | 41.3 (1,304) |
| Smoker | |
| Ever | 12.5 (395) |
| Never | 87.5 (2,755) |
| Physical activity | |
| Low | 22.3 (702) |
| Moderate | 73.7 (2,322) |
| High | 4.0 (126) |
| Educational attainment | |
| None | 21.9 (690) |
| Primary and middle | 41.7 (1,314) |
| Secondary | 27.0 (850) |
| Higher education | 9.4 (296) |
| Annual income (INR) | |
| 27.9 (879) | |
| 4001–6000 | 26.5 (835) |
| 6001–10000 | 26.6 (838) |
| 19.0 (599) | |
| High LDL () | |
| Yes | 34.1 (1,074) |
| No | 65.9 (2,076) |
| Cooking fuel type | |
| LPG | 60.0 (1,891) |
| LPG mixed | 24.6 (775) |
| No LPG | 11.7 (369) |
| Missing | 3.7 (115) |
| Socioeconomic score | 8.2 (3.5; 3–18) |
| Anxiety score | 7.3 (6.5; 0–43) |
| Sedentary score | 5.2 (1.9; 0.2–10.6) |
Note: BMI, body mass index; INR, Indian rupees; LDL, low-density lipoprotein; LPG, liquid petroleum gas; PURSE-HIS, Population Study of Urban, Rural, Semiurban Endovascular Disease and Holistic Intervention Study; SD, standard deviation.
Table 2.
Mean and standard deviation of biomarkers of vascular aging stratified by demographic variables.
| Characteristic | Biomarkers of EVA | %FMD | ||
|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | cPP (mmHg) | ||
| Sex | ||||
| Female | 125.5 (20.3) | 77.3 (10.9) | 48.2 (15.4) | 19.1 (8.2) |
| Male | 132.0 (20.1) | 80.9 (11.8) | 51.1 (14.9) | 15.8 (7.7) |
| Age (y) | ||||
| 120.6 (15.1) | 76.6 (10.5) | 44.0 (10.2) | 18.9 (8.2) | |
| 40–50 | 128.8 (20.3) | 80.2 (11.9) | 48.6 (13.9) | 17.6 (7.9) |
| 51–60 | 135.9 (22.5) | 80.4 (11.5) | 55.5 (17.6) | 16.5 (8.0) |
| 142.7 (22.0) | 79.7 (11.8) | 63.1 (18.9) | 15.1 (8.0) | |
| BMI () | ||||
| Underweight () | 122.7 (20.7) | 74.6 (12.2) | 48.2 (14.6) | 18.4 (8.6) |
| Normal weight (18.5–22.9) | 125.8 (20.4) | 76.9 (11.2) | 48.9 (15.1) | 17.9 (7.8) |
| Overweight (23–24.9) | 128.3 (21.2) | 79.1 (12.0) | 49.2 (14.8) | 17.4 (7.8) |
| Obese () | 130.2 (20.0) | 80.2 (11.0) | 50.0 (15.5) | 17.7 (8.4) |
| Socioeconomic score (quartiles) | ||||
| 1st | 127.9 (20.6) | 78.4 (11.5) | 49.4 (15.5) | 18.1 (8.1) |
| 2nd | 127.2 (20.5) | 78.5 (11.7) | 48.6 (14.9) | 17.9 (8.1) |
| 3rd | 129.3 (21.3) | 79.2 (11.3) | 50.1 (15.3) | 17.2 (8.0) |
| 4th | 129.7 (19.1) | 79.7 (11.1) | 50.1 (15.0) | 17.2 (8.7) |
| Anxiety score (quartiles) | ||||
| 1st | 126.8 (20.6) | 78.5 (11.1) | 48.3 (15,4) | 18.0 (7.6) |
| 2nd | 127.6 (19.8) | 78.5 (11.3) | 49.0 (14.9) | 17.1 (8.0) |
| 3rd | 129.8 (20.9) | 79.7 (12.2) | 50.0 (14.4) | 18.1 (8.7) |
| 4th | 129.4 (20.5) | 78.8 (11.3) | 50.7 (15.8) | 17.7 (8.4) |
| Physical activity | ||||
| Low | 132.0 (22.0) | 80.9 (11.6) | 51.0 (16.7) | 16.5 (8.0) |
| Moderate | 127.5 (19.9) | 78.3 (11.2) | 49.2 (14.9) | 18.1 (8.1) |
| High | 123.5 (19.5) | 77.2 (13.4) | 46.4 (11.6) | 17.7 (8.0) |
| Smoking status | ||||
| Smoker | 131.3 (19.4) | 80.8 (11.3) | 49.3 (15.4) | 14.7 (7.0) |
| Nonsmoker | 127.9 (20.6) | 78.6 (11.4) | 50.4 (14.2) | 18.1 (8.2) |
| Energy intake | ||||
| Excess | 129.9 (20.5) | 80.1 (11.3) | 49.4 (15.4) | 17.6 (8.3) |
| Adequate | 127.9 (19.8) | 78.5 (10.8) | 49.8 (15.4) | 17.7 (8.1) |
| Deficit | 126.9 (20.5) | 77.7 (11.7) | 49.1 (15.5) | 17.8 (8.0) |
Note: BMI, body mass index; cPP, central pulse pressure; DBP, diastolic blood pressure; EVA, early vascular aging; %FMD, percent flow mediated dilation; SBP, systolic blood pressure.
Participants residing in the northeast areas of the city had higher exposures to ISA and NTL but lower exposures to greenness, whereas participants residing in the southwest had an inverse relationship (Figure 1). As expected, exposure to ISA and NTL had a moderate positive correlation (), whereas greenness had a strong inverse correlation with ISA () and a moderate correlation with NTL ( (see Table S1). We compared exposure to greenness, ISA, and NTL by demographic variables (see Table S2). Participants with higher exposure to greenness and lower exposure to ISA and NTL reported having high physical activity compared with participants with moderate and low physical activity. Additionally, participants in the lowest quartile for anxiety score had higher exposure to greenness and lower exposures to ISA and NTL. Those in the lowest SES quartile had significantly lower mean exposure to ISA and NTL and higher exposure to greenness compared with the third and fourth SES quartiles.
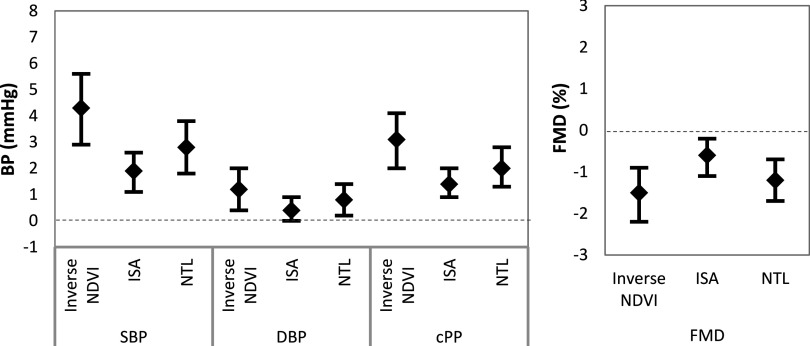
In univariate analysis, ISA, NTL, and greenness were significantly associated with all four biomarkers (SBP, DBP, cPP, and FMD) (see Table S3). Figure 2 presents associations for interquartile range (IQR) increases in exposure for greenness (), ISA (), and NTL () adjusted for age, anxiety score, BMI, energy intake, household cooking fuel type (LPG, LPG mixed, and no LPG), Kupusswamy socioeconomic score, sedentary score, sex, and smoking status. We report the associations with greenness as the inverse of NDVI to directionally align with ISA and NTL. An IQR decrease in residential exposure to greenness was associated with , , and increases in SBP, DBP, and cPP, respectively. An IQR increase in NTL was associated with , , and increases in SBP, DBP, and cPP, respectively. ISA had lower-magnitude and lower-strength associations, with an IQR increase associated with , , and increases in SBP, DBP, and cPP, respectively. An IQR decrease in residential exposure to greenness, an IQR increase in ISA, and an IQR increase in NTL were associated with a , and decrease in FMD, respectively.
Figure 2.
Multivariable regression model for the effect of an interquartile range (IQR) increase in residential exposure to greenness (), impervious surface area (ISA) (), and nighttime lights (NTL) () measures on biomarkers of vascular aging. NDVI, Normalized Difference Vegetation Index.
Additional models were developed to test for associations in a parsimonious model adjustment including only age, sex, BMI, and smoking status (see Figure S1) and stratified by physical activity on the relationship between greenness and each biomarker of EVA (see Figure S2). The parsimonious model adjustment resulted in slightly larger magnitudes and strengths of association in all models. Models stratified by physical activity did not identify this variable to be a modifier of the relationship between greenness and biomarkers of EVA (see Figure S2).
Analyses were performed to examine the associations between radius exposures to greenness, ISA, and NTL and measures of EVA (see Table S4), which were lower per IQR than the residential exposure for all built environment measures.
Discussion
We used high-spatial-resolution () satellite imagery to represent different aspects of the urban built environment and found significant associations between greenness, ISA, and NTL and biomarkers of EVA (SBP, DBP, cPP, and FMD) in a cross-sectional study. Participants residing in areas with higher residential exposure to greenness had significantly lower SBP, DBP, and cPP, and higher FMD. Conversely, participants residing in areas with higher residential exposure to ISA and NTL had significantly higher SBP, DBP, and cPP, and lower FMD.
Associations between residential exposure to greenness and our biomarkers of EVA support the growing body of research examining the association between greenness and cardiovascular outcomes (James et al. 2015) as well as the hypothesis that environmental factors can accelerate vascular aging (Koivistoinen et al. 2007). However, there are other behavioral and environmental exposures that may have similar spatial patterns to greenness that could be associated with increased CVD in urban populations. These exposures include decreased physical activity, poor nutrition, loss of social cohesion, and increased noise and air pollution (Allender et al. 2011; de Vries et al. 2013; Attard et al. 2015; James et al. 2015). We were able to partially control for some of these factors at the individual level by adjusting for SES, energy intake, sedentary score, and anxiety, and we did not find moderate or strong correlations between these terms and our built environment measures (see Table S1).
Greenness has been shown to be associated with physical activity and cardiovascular health and is hypothesized to reduce exposure to air pollution, noise, and temperature while increasing physical activity and lowering stress (James et al. 2015). Stratification was performed to examine physical activity as a potential modifier of the association between greenness and each biomarker of EVA, but it produced mixed results in our study (see Figure S2). Participants with high physical activity exhibited a nonsignificant association between greenness and biomarkers of EVA. In a recent study, James et al. (2016) also reported nonsignificant results in the relationship between greenness and mortality with stratification by physical activity. James et al. (2016) did observe that physical activity was a significant mediator of the relationship between greenness and mortality, but physical activity explained only a small proportion (4%) of the greenness–mortality association. Although we did not conduct mediation analysis, we did observe that our measure of physical activity (sedentary score) was poorly correlated with greenness (see Table S1). However, our results may not be generalizable to studies in North America, which have focused on capturing recreational physical activity. Additionally, previous research within India has reported that physical activity predominantly occurs through nonrecreational activities such as occupation for males and residential chores for females (Rao et al. 2008; Sullivan et al. 2011; Vaz and Bharathi 2004; Yadav and Krishnan 2008). Therefore, our reliance on residential exposure assignment could introduce misclassification, particularly among males. In addition, our results may be more generalizable to other large cities within LMIC with similar occupational physical activity patterns.
Greenness is a measure of vegetation and could be spatially correlated with air and noise pollution because vegetation can remove air pollutants associated with CVD (Nowak et al. 2006) and can reduce exposure to noise pollution (Gidlöf-Gunnarsson and Öhrström 2007). Although we were unable to account for air and noise pollution directly in our study owing to a lack of measurements in this region, previous research has observed significant associations between CVD risk and BP and greenness after adjusting for air pollutants, noise, or both. A prospective survival study that adjusted for air pollution observed that increased exposure to greenness was significantly associated with decreased CVD risk (Villeneuve et al. 2012). Adjustment for air pollution and noise pollution in separate models produced similar results with a slight reduction in the size of the effect of greenness on CVD risk. Another analysis, from a German birth cohort study, found that residential greenness was significantly associated with lower SBP and DBP in children after adjusting for air pollution and noise (Markevych et al. 2014). Although we did not directly adjust for air and noise pollution, we tried to adjust in part for air pollution by including cooking fuel as a proxy exposure. Our observed effect of greenness on biomarkers of EVA adds support to the relationship between greenness and subclinical measures of CVD. In addition, we hypothesize that NTL and ISA might be spatially correlated with higher air and noise pollution because light and ISA typically come from increased human activity and urban elements related to the sources of these pollutants.
Urbanization is rapidly affecting the developing world. However, little is known about how different aspects of the built environment affect chronic diseases and risk factors for CVD in rapidly urbanizing areas because most studies have taken place in developed countries (Allender et al. 2011). The multiple measures we examined potentially capture different aspects of the built environment in Chennai, which is currently undergoing rapid urbanization. NTL could be associated with CVD through disruption of circadian rhythms (Scheer et al. 2009) and potentially through biomarkers of EVA, but we have limited ability to examine this directly in the PURSE-HIS study. Both NTL and ISA could also serve as proxy measures for urban density, but we could not disentangle the specific pathway contributions. Future studies of greenness and urbanization should consider including ISA and NTL to better understand how additional aspects of the built environment could be associated with CVD.
EVA is a complex process that can be characterized by aortic and carotid dilation and stiffening, endothelial dysfunction, and intimal thickening (Laurent 2011). Our analysis focused on only a select number of EVA measures, and future work could investigate additional measures of EVA such as pulse wave velocity and carotid intima-media thickness to characterize a broader scope of EVA metrics. Additionally, we were unable to adjust for potentially relevant factors such as inadequate sewage disposal, water- and food-borne illnesses and infectious diseases (e.g., malaria), and increased air pollution (e.g., fine particulate matter and nitrogen dioxide), which are more prevalent or in higher exposure concentrations in LMIC; this may limit the generalizability of our findings to cities in high-income countries because EVA could occur as a result of chronic inflammation and oxidative stress (Kotsis et al. 2011). Because urban areas in high-income countries have a more stable built environment, have lower air pollution exposure levels, and are rarely affected by the same additional factors as those in developing countries, our findings may not be generalizable to existing major urban areas in more developed countries and may be more comparable to developing areas of the world that are undergoing rapid urbanization. However, with the majority of urban transition taking place in developing countries, our results are highly relevant and can inform on health benefits (increased greenness) and detriments (increased ISA and NTL) of unplanned urbanization in similar locales.
In rapidly urbanizing areas, bare soil from construction sites and areas in transition is easily misassigned as impervious surface area, leading to overestimation of the exposure to ISA, particularly in the urban outskirts. Therefore, our observed associations with ISA may overestimate this aspect of the built environment and may not be generalizable to urban areas in developed countries. Another limitation of our study was that our exposure was assigned at the residence level and not at the individual level, which could introduce exposure misclassification into our model for participants who spend large amounts of time away from their residence. Integration of microenvironment time–activity models could reduce this error.
There is a growing body of evidence that supports an association between residential exposure to greenness and CVD (James et al. 2015), but there is less evidence for an association with pathways leading to CVD risk such as blood pressure (Markevych et al. 2014). The significant associations we observed between residential greenness and biomarkers of EVA add support to this hypothesis in an area undergoing rapid urbanization. Additionally, to the best of our knowledge, we are the first to investigate associations between ISA and NTL and biomarkers of EVA using an observational study design. However, our study was cross-sectional, and longitudinal cohort studies with multiple environmental stressor models will be needed to strengthen causal interpretation. Our findings are relevant for policy makers and urban planners regarding the potential health benefits and adverse impacts from multiple aspects of the urban environment when designing cities in rapidly urbanizing areas.
Conclusions
Participants residing in areas with higher ISA and NTL and lower greenness had significantly higher SBP, DBP and cPP and lower FMD. Our findings indicate that higher residential exposure to ISA and NTL could increase biomarkers of EVA and that greenness could have a protective effect, and they suggest a possible additional pathway or modify existing risk factors to explain the increased prevalence of CVD in rapidly urbanizing populations in India. Further studies are needed to fully elucidate the relationship between greenness, ISA, and NTL and additional biomarkers of EVA, particularly longitudinal analyses that would be able to delineate changes in built environment factors and progression to CVD.
Supplemental Material
Acknowledgments
The Population Study of Urban, Rural and Semiurban Regions for the Detection of Endovascular Disease and Prevalence of Risk Factors and Holistic Intervention Study (PURSE-HIS) is supported by a grant from the Drugs and Pharmaceutical Research Program under the Technology Development and Transfer Division, Department of Science and Technology, Government of India (Project no. VI-D&P/151/06-07/TDT) and is supported by Sri Ramachandra University, Porur, India. K.J.L. was supported by a Yale Climate and Energy Institute Postdoctoral Fellowship Award. E.C.S. was supported by a National Aeronautics and Space Administration (NASA) Harriett G. Jenkins Predoctoral Fellowship. NASA’s Terra/ Moderate Resolution Imaging Spectroradiometer (MODIS), Suomi National Polar-Orbiting Partnership satellite, and Landsat 5 Thematic Mapper (TM) datasets were acquired from the Level-1 Land and Atmosphere Archive and Distribution System (LAADS) Distributed Active Archive Center (DAAC), which is located in the Goddard Space Flight Center in Greenbelt, MD (https://ladsweb.nascom.nasa.gov/).
References
- Allender S, Wickramasinghe K, Goldacre M, Matthews D, Katulanda P. 2011. Quantifying urbanization as a risk factor for noncommunicable disease. J Urban Health 88(5):906–918, PMID: 21638117, 10.1007/s11524-011-9586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allender S, Lacey B, Webster P, Rayner M, Deepa M, Scarborough P, et al. 2010. Level of urbanization and noncommunicable disease risk factors in Tamil Nadu, India. Bull World Health Organ 88(4):297–304, PMID: 20431794, 10.2471/BLT.09.065847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assah FK, Ekelund U, Brage S, Mbanya JC, Wareham NJ. 2011. Urbanization, physical activity, and metabolic health in Sub-Saharan Africa. Diabetes Care 34(2):491–496, PMID: 21270205, 10.2337/dc10-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard SM, Howard AG, Herring AH, Zhang B, Du S, Aiello AE, et al. 2015. Differential associations of urbanicity and income with physical activity in adults in urbanizing China: findings from the population-based China Health and Nutrition Survey 1991-2009. Int J Behav Nutr Phys Act 12:152, PMID: 26653097, 10.1186/s12966-015-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Ghosh S, Ganguli B, Sambandam S, Bruce N, Barnes DF, et al. 2013. State and national household concentrations of PM2.5 from solid cookfuel use: results from measurements and modeling in India for estimation of the global burden of disease. Environ Health. 11 12(1):77, PMID: 24020494, 10.1186/1476-069X-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh K, Hsu FC, Elvidge CD, Zhizhin M. 2013. Nighttime lights compositing using the VIIRS day-night band: Preliminary results. APAN Proceedings 35:70–86, 10.7125/APAN.35.8. [DOI] [Google Scholar]
- Bull FC, Maslin TS, Armstrong T. 2009. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6(6):790–804, PMID: 20101923, 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- Casey JA, James P, Rudolph KE, Wu CD, Schwartz BS. 2016. Greenness and birth outcomes in a range of Pennsylvania communities. Int J Environ Res Public Health 13(3):pii: E311, PMID: 26978381, 10.3390/ijerph13030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. 2015. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 32(9):1294–1310, PMID: 26375320, 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- Cyril S, Oldroyd JC, Renzaho A. 2013. Urbanisation, urbanicity, and health: a systematic review of the reliability and validity of urbanicity scales. BMC Public Health 13:513, PMID: 23714282, 10.1186/1471-2458-13-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, de Nazelle M, Triguero-Mas A, Schembari M, Cirach E, Amoly, et al. 2012. Surrounding greenness and exposure to air pollution during pregnancy: an analysis of personal monitoring data. Environ Health Perspect 120(9):1286–1290, PMID: 22647671, 10.1289/ehp.1104609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Rivas I, Basagaña X, Alvarez-Pedrerol M, Su J, De Castro Pascual M, et al. 2015. The association between greenness and traffic-related air pollution at schools. Sci Total Environ 523:59–63, PMID: 25862991, 10.1016/j.scitotenv.2015.03.103. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Villanueva CM, Font-Ribera L, Martinez D, Basagaña X, Belmonte J, et al. 2014. Risks and benefits of green spaces for children: A cross-sectional study of associations with sedentary behavior, obesity, asthma, and allergy. Environ Health Perspect 122(12):1329–1335, PMID: 25157960, 10.1289/ehp.1308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S, van Dillen SM, Groenewegen PP, Spreeuwenberg P. 2013. Streetscape greenery and health: stress, social cohesion and physical activity as mediators. Soc Sci Med 94:26–33, PMID: 23931942, 10.1016/j.socscimed.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Doll CH, Muller JP, Elvidge CD. 2000. Night-time imagery as a tool for global mapping of socioeconomic parameters and greenhouse gas emissions. AMBIO: A Journal of the Human Environment 29(3):157, 10.1579/0044-7447-29.3.157. [DOI] [Google Scholar]
- Fan JX, Wen M, Kowaleski-Jones L. 2014. Rural-urban differences in objective and subjective measures of physical activity: findings from the National Health and Nutrition Examination Survey (NHANES) 2003-2006. Prev Chronic Dis 11:E141, PMID: 25144676, 10.5888/pcd11.140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. 2013. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms 28(4):262–271, PMID: 23929553, 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171, 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T, Anderson S, Elvidge C, Sutton P. 2013. Using nighttime satellite imagery as a proxy measure of human well-being. Sustainability 5(12):4988–5019, 10.3390/su5124988. [DOI] [Google Scholar]
- Gidlöf-Gunnarsson A, Öhrström E. 2007. Noise and well-being in urban residential environments: the potential role of perceived availability to nearby green areas. Landsc Urban Plan 83(1–2):115–126, 10.1016/j.landurbplan.2007.03.003. [DOI] [Google Scholar]
- Hagler GSW, Lin MY, Khlystov A, Baldauf RW, Isakov V, Faircloth J, et al. 2012. Field investigation of roadside vegetative and structural barrier impact on near-road ultrafine particle concentrations under a variety of wind conditions. Sci. Total Environ 419:7–15, PMID: 22281040, 10.1016/j.scitotenv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Hu Z, Liebens J, Rao KR. 2008. Linking stroke mortality with air pollution, income, and greenness in northwest Florida: an ecological geographical study. Int J Health Geogr 7:20, PMID: 18452609, 10.1186/1476-072X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, et al. 2014. Light at night and breast cancer risk among California teachers. Epidemiology 25(5):697–706, PMID: 25061924, 10.1097/EDE.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Banay RF, Hart JE, Laden F. 2015. A Review of the Health Benefits of Greenness. Curr Epidemiol Rep 2(2):131–142, PMID: 26185745, 10.1007/s40471-015-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Hart JE, Banay RF, Laden F. 2016. Exposure to Greenness and Mortality in a Nationwide Prospective Cohort Study of Women. Environ Health Perspect 124(9):1344–1352, PMID: 27074702, 10.1289/ehp.1510363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Berrigan D, Hart JE, Hipp JA, Hoehner CM, Kerr J, et al. 2014. Effects of buffer size and shape on associations between the built environment and energy balance. Health Place 27:162–170, PMID: 24607875, 10.1016/j.healthplace.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. 1993. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J 14(2):160–167, PMID: 8449191, 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- Koivistoinen T, Kööbi T, Jula A, Hutri-Kähönen N, Raitakari OT, Majahalme S, et al. 2007. Pulse wave velocity reference values in healthy adults aged 26–75 years. Clin Physiol Funct Imaging 27:191–196, PMID: 17445071, 10.1111/j.1475-097X.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Antza C, Stabouli S. 2013. Pathophysiology of early vascular ageing-Opportunities for treatment. Open Hypertens J 5:58–62, 10.2174/1876526201305010058. [DOI] [Google Scholar]
- Kotsis V, Stabouli S, Karafillis I, Nilsson P. 2011. Early vascular aging and the role of central blood pressure. J Hypertens 29:1847–1853, PMID: 21799443, 10.1097/HJH.0b013e32834a4d9f. [DOI] [PubMed] [Google Scholar]
- Kumar N, Shekhar C, Kumar P, Kundu AS. 2007. Kuppuswamy’s socioeconomic status scale-updating for 2007. Indian J Pediatr 74:1131–1132, PMID: 18174655. [PubMed] [Google Scholar]
- Lachowycz K, Jones AP. 2014. Does walking explain associations between access to greenspace and lower mortality? Soc Sci Med 107(100):9–17, PMID: 24602966, 10.1016/j.socscimed.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S. 2012. Defining vascular aging and cardiovascular risk. J Hypertens 30 Suppl:S3–S8, PMID: 23124102, 10.1097/HJH.0b013e328353e501. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260, 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380:2095–2128, 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevych I, Thiering E, Fuertes E, Sugiri D, Berdel D, Koletzko S, et al. 2014. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: results from the GINIplus and LISAplus studies. BMC Public Health 14:477, PMID: 24886243, 10.1186/1471-2458-14-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, et al. 2009. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 57:163–170, PMID: 19582986. [PubMed] [Google Scholar]
- Mitchell R, Popham F. 2008. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet 372(9650):1655–1660, PMID: 18994663, 10.1016/S0140-6736(08)61689-X. [DOI] [PubMed] [Google Scholar]
- Maas J, van Dillen SME, Verheij RA, Groenewegen PP. 2009. Social contacts as a possible mechanism behind the relation between green space and health. Health Place 15:586–592, PMID: 19022699, 10.1016/j.healthplace.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Morita E, Fukuda S, Nagano J, Hamajima N, Yamamoto H, Iwai Y, et al. 2007. Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as possible method of stress reduction. Public Health 121:54–63, PMID: 17055544, 10.1016/j.puhe.2006.05.024. [DOI] [PubMed] [Google Scholar]
- NASA/USGS (National Aeronautics and Space Administration/U.S. Geological Survey). 2015. LP DAAC: Land Processes Distributed Active Archives Center. Vegetation Indices 16-Day L3 Global 250m. https://lpdaac.usgs.gov/dataset_discovery/modis/modis_products_table/mod13q1 [accessed 4 March 2015].
- Nielsen TS, Hansen KB. 2007. Do green areas affect health? Results from a Danish survey on the use of green areas and health indicators. Health Place 13:839–850, PMID: 17392016, 10.1016/j.healthplace.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Boutouyrie P, Laurent S. 2009. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 54:3–10, PMID: 19487587, 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- Nowak DJ, Crane DE, Stevens JC. 2006. Air pollution removal by urban trees and shrubs in the United States. Urban For Urban Green 4:115–123, 10.1016/j.ufug.2006.01.007. [DOI] [Google Scholar]
- Pandey B, Seto KC. 2015. Urbanization and agricultural land loss in India: comparing satellite estimates with census data. J Environ Manage 148:53–66, 10.1016/j.jenvman.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Parks SE, Housemann RA, Brownson RC. 2003. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J Epidemiol Community Health 57(1):29–35, PMID: 12490645, 10.1136/jech.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Narayan KM, Cunningham SA. 2015. Unhealthy weight among children and adults in India: urbanicity and the crossover in underweight and overweight. Ann Epidemiol 25(5):336–341.e2, PMID: 25795227, 10.1016/j.annepidem.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Pauca AL, O'Rourke MF, Kon ND. 2001. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38(4):932–937, PMID: 11641312, 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- Paul S, Nagendra H. 2015. Vegetation change and fragmentation in the mega city of delhi: mapping 25 years of change. Appl Geogr 58:153–166, 10.1016/j.apgeog.2015.02.001. [DOI] [Google Scholar]
- Pereira G, Foster S, Martin K, Christian H, Boruff BJ, Knuiman M, et al. 2012. The association between neighborhood greenness and cardiovascular disease: an observational study. BMC Public Health 12:466, PMID: 22720780, 10.1186/1471-2458-12-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Gokhale M, Kanade A. 2008. Energy costs of daily activities for women in rural India. Public Health Nutr 11:142–150, PMID: 17565761, 10.1017/S1368980007000055. [DOI] [PubMed] [Google Scholar]
- Reddy KS, Yusuf S. 1998. Emerging epidemic of cardiovascular disease in developing countries. Circulation 97:596–601, PMID: 9494031, 10.1161/01.CIR.97.6.596. [DOI] [PubMed] [Google Scholar]
- Reddy KS. 2002. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr 5(1A):231–237, PMID: 12027289, 10.1079/PHN2001298. [DOI] [PubMed] [Google Scholar]
- Richardson EA, Mitchell R. 2010. Gender differences in relationships between urban green space and health in the United Kingdom. Soc Sci Med 71(3):568–575, PMID: 20621750, 10.1016/j.socscimed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Rybnikova NA, Haim A, Portnov BA. 2016. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int J Obes 40(5):815–823, PMID: 26795746, 10.1038/ijo.2015.255. [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, et al. 2003. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: Results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol 2003 32:1054–1062, 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106:4453–4458, PMID: 19255424, 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, et al. 2001. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety 13:166–178, PMID: 11413563, 10.1002/da.1033. [DOI] [PubMed] [Google Scholar]
- Stigsdotter UK, Ekholm O, Schipperijn J, Toftager M, Kamper-Jørgensen F, Randrup TB. 2010. Health promoting outdoor environments–Associations between green space, and health, health-related quality of life and stress based on a Danish national representative survey. Scand J Public Health 38:411–417, PMID: 20413584, 10.1177/1403494810367468. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Leslie E, Giles-Corti B, Owen N. 2008. Associations of neighbourhood greenness with physical and mental health: Do walking, social coherence and local social interaction explain the relationships? J Epidemiol Community Health 62(5):e9, PMID: 18431834, 10.1136/jech.2007.064287. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Kinra S, Ekelund U, Bharathi AV, Vaz M, Kurpad A, et al. 2011. Socio-demographic patterning of physical activity across migrant groups in India: results from the Indian Migration Study. PLoS One 6:, 10.1371/journal.pone.0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton P, Roberts C, Elvidge C, Meij H. 1997. A comparison of nighttime satellite imagery and population density for the continental United States. Photogramm Eng Remote Sensing 63(11):1303–1313. [Google Scholar]
- Sutton PC, Costanza R. 2002. Global estimates of market and non-market values derived from nighttime satellite imagery, land cover, and ecosystem service valuation. Ecological Economics 41(3):509–527, 10.1016/S0921-8009(02)00097-6. [DOI] [Google Scholar]
- Sutton P, Roberts D, Elvidge C, Baugh K. 2001. Census from Heaven: an estimate of the global human population using night-time satellite imagery. Int J Remote Sens 22(16):3061–3076, 10.1080/01431160010007015. [DOI] [Google Scholar]
- Thanikachalam S, Harivanzan V, Mahadevan MV, Murthy JS, Anbarasi C, Saravanababu CS, et al. 2015. Population Study of Urban, Rural, and Semiurban Regions for the Detection of Endovascular Disease and Prevalence of Risk Factors and Holistic Intervention Study: Rationale, Study Design, and Baseline Characteristics of PURSE-HIS. Glob Heart 10(4):281–289, PMID: 26014656, 10.1016/j.gheart.2014.11.002. [DOI] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division (UNDESAPD). 2015. World Urbanization Prospects: The 2014 Revision, (ST/ESA/SER.A/366). http://esa.un.org/unpd/wup/Publications/Files/WUP2014-Report.pdf [accessed 19 January 2016].
- Van de Poel E, O'Donnell O, Van Doorslaer E. 2012. Is there a health penalty of China’s rapid urbanization? Health Econ 21 (4):367–385, 10.1002/hec.1717. [DOI] [PubMed] [Google Scholar]
- Vaz M, Bharathi AV. 2004. Perceptions of the intensity of specific physical activities in Bangalore, South India: implications for exercise prescription. J Assoc Physicians India 52:541–544, PMID: 15645977. [PubMed] [Google Scholar]
- Villeneuve PJ, Jerrett M, Su JG, Burnett RT, Chen H, Wheeler AJ, et al. 2012. A cohort study relating urban green space with mortality in Ontario, Canada. Environ Res 115:51–58, PMID: 22483437, 10.1016/j.envres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- World Health Organization Expert Consultation, 2004. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163, PMID: 14726171, 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Wu C, Murray AT. 2003. Estimating impervious surface distribution by spectral mixture analysis. Remote sensing of Environment 84(4):493–505, 10.1016/S0034-4257(02)00136-0. [DOI] [Google Scholar]
- Yadav K, Krishnan A. 2008. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in north India. Obes Rev 9:400–408, PMID: 18627500, 10.1111/j.1467-789X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ôunpuu S, Anand S. 2001. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104:2746–2753, PMID: 11723030, 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Ichinose T, Zheng J, Chen J, Shi PJ, Li X. 2009. Modelling the population density of China at the pixel level based on DMSP/OLS non‐radiance‐calibrated night‐time light images. Int J Remote Sensing 30(4):1003–1018, 10.1080/01431160802430693. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.