Abstract
Background:
Personal care products (PCPs) are exposure sources to phthalates and parabens; however, their contribution to men’s exposure is understudied.
Objectives:
We examined the association between PCP use and urinary concentrations of phthalate metabolites and parabens in men.
Methods:
In a prospective cohort, at multiple study visits, men self-reported their use of 14 PCPs and provided a urine sample (2004–2015, Boston, MA). We measured urinary concentrations of 9 phthalate metabolites and methylparaben, propylparaben, and butylparaben. We estimated the covariate-adjusted percent change in urinary concentrations associated with PCP use using linear mixed and Tobit mixed regressions. We also estimated weights for each PCP in a weighted binary score regression and modeled the resulting composite weighted PCP use.
Results:
Four hundred men contributed 1,037 urine samples (mean of 3/man). The largest percent increase in monoethyl phthalate (MEP) was associated with use of cologne/perfume (83%, ) and deodorant (74%, ). In contrast, the largest percent increase for parabens was associated with the use of suntan/sunblock lotion (66–156%) and hand/body lotion (79–147%). Increases in MEP and parabens were generally greater with PCP use within 6 h of urine collection. A subset of 10 PCPs that were used within 6 h of urine collection contributed to at least 70% of the weighted score and predicted a 254–1,333% increase in MEP and parabens concentrations. Associations between PCP use and concentrations of the other phthalate metabolites were not statistically significant.
Conclusions:
We identified 10 PCPs of relevance and demonstrated that their use within 6 h of urine collection strongly predicted MEP and paraben urinary concentrations. https://doi.org/10.1289/EHP1374
Introduction
There is ubiquitous general population exposure to ortho-phthalates (hereafter referred to as phthalates) and several parabens (CDC 2017). Phthalates are a family of chemicals commonly used as plasticizers in polyvinyl chloride plastics and in consumer products, including personal care products (PCPs), medications, and food processing and packaging materials (CDC 2017). Diethyl phthalate (DEP) is the most commonly used phthalate in PCPs (FDA 2014b). Parabens are a family of chemicals with antimicrobial preservative properties that are also widely used in PCPs, pharmaceuticals, food, and beverages to increase the shelf life of the product (Dodson et al. 2012; FDA 2014a; Guo and Kannan 2013; Moos et al. 2014). Methylparaben, propylparaben, and butylparaben are most commonly used in PCPs (Braun et al. 2014; Dodson et al. 2012; Ferguson et al. 2017).
In multiple urine samples collected from two men and three women, Koch et al. (2013) found that concentrations of the metabolites of low molecular weight phthalates such as DEP, di-n-butyl phthalate (DnBP), and di-isobutyl phthalate (DiBP) had a cyclical pattern of rise and decline suggestive of ongoing repeated nonfood exposures. Koch et al. (2013) also found that monoethyl phthalate (MEP; a metabolite of DEP), concentrations increased following showers, which suggested PCPs as a major source of DEP exposure. Koch et al. (2014) also found that urinary concentrations of the parabens were lower when the participants were given products without parabens.
Exposure to phthalates and parabens from PCP use occurs through direct dermal application (Janjua et al. 2008; Seo et al. 2016), inhalation, oral ingestion, or even transdermal exposure from air (Weschler et al. 2015). Phthalates and parabens have short biological half-lives (6–24 h) (Janjua et al. 2008; Koch et al. 2012; Moos et al. 2016), and urinary concentrations of phthalate metabolites and parabens are the preferred exposure biomarkers (Calafat et al. 2015; CDC 2017).
Certain phthalates and parabens are endocrine disruptors and have been linked to adverse health outcomes (Boberg et al. 2010; Hannas et al. 2012; Howdeshell et al. 2016; Lioy et al. 2015; Orton et al. 2014; Zoeller et al. 2014). Identifying the most important sources of phthalate and paraben exposure is of paramount importance given the widespread use and potential health effects of these chemicals. Prior epidemiological studies on the contribution of PCP use to exposure to phthalates and parabens focused primarily on women and children (Braun et al. 2014; Buckley et al. 2012; Ferguson et al. 2017; Harley et al. 2016; Just et al. 2010; Martina et al. 2012; Philippat et al. 2015; Romero-Franco et al. 2011; Sathyanarayana et al. 2008). However, there have been limited studies in men (Duty et al. 2005; Ferguson et al. 2017). Given both differences in type and frequency of PCP use across both sexes, better understanding of the specific PCPs contributing to urinary phthalate metabolite and paraben concentrations in men is necessary.
Given this gap in knowledge on the contribution of PCP use to exposure to phthalates and parabens in men, we explored the association between self-reported use of 14 PCPs and urinary concentrations of 9 urinary phthalate metabolites and 3 parabens in men recruited as part of a prospective cohort study. Because of the known use of DEP, DiBP, and parabens in PCPs (Dodson et al. 2012; Wittassek et al. 2011), we focused on MEP, mono-isobutyl phthalate (MiBP; a metabolite of DiBP), methylparaben, propylparaben, and butylparaben. We considered including mono-n-butyl phthalate (MnBP; a metabolite of DnBP), but because DnBP is infrequently used in PCPs (FDA 2014b), it was not a focus of our analysis.
Methods
Participants
The Environment And Reproductive Health (EARTH) study (2004–present), a prospective cohort, enrolled couples seeking fertility treatment at the Massachusetts General Hospital (MGH) Fertility Center to identify determinants of fertility (Braun et al. 2014; Dodge et al. 2015). Among male partners who were 18–55 y of age, approximately 50% agreed to participate. Male participants were followed from study enrollment until their partner had a live birth or the couple discontinued treatment at MGH. Approximately 30% of EARTH men had a primary diagnosis of male factor infertility (Dodge et al. 2015). In the current analysis, men were eligible if they provided at least one urine sample and completed the PCP questionnaire [see “Product Use Questionnaire in the Environment And Reproductive Health (EARTH) Study” in the Supplemental Material] between December 2004 and June 2015. All men provided informed consent. The EARTH study was approved by institutional review boards at MGH, the Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Personal Care Product (PCP) Use Questionnaire
Upon enrollment, men completed questionnaires that collected information on demographics, lifestyle, and health information, and a research nurse measured their height and weight. At recruitment and at each subsequent visit, men completed a questionnaire on PCP use within the past 24-h and at what time they last used each PCP prior to the collection of each urine sample. There were questions for the use of 16 PCPs, but in the analysis we excluded toothpaste (used in 98% of men) and nail polish ( use). Therefore, we included deodorants, shampoo, conditioner/crème rinse, hairspray/hair gel, combined other hair care products (including mousse, hair bleach, relaxer, perm, and straightener), shaving cream, aftershave, cologne/perfume, mouthwash, bar soap, liquid soap/body wash, hand sanitizer, hand/body lotion, and suntan/sunblock lotion.
Based on our previous publication that showed higher urinary concentrations of MEP following PCP use in men from the same fertility clinic population (Duty et al. 2005) and another study that showed higher urine concentrations following dermal exposure through lotion application (Janjua et al. 2007), we a priori decided to explore both a 6-h and a 24-h time window for PCP use before the urine collection. Furthermore, in women from the same fertility clinic population, we reported higher urinary biomarker concentrations when the product was used within 6 h prior to urine sample collection (Braun et al. 2014).
Urinary Measurements of Phthalate Metabolite and Paraben Concentrations
At each visit, men collected urine in a sterile polypropylene cup using standard procedures. Study staff recorded the time of collection and measured specific gravity (SG) using a handheld refractometer (National Instrument Co. Inc.). Urine samples were divided into aliquots, frozen, and stored at before shipment on dry ice to the CDC. Briefly, the analytical technique for quantification of the urinary biomarkers involved enzymatic deconjugation of the urinary metabolites, followed by solid-phase extraction, separation by high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry.
As previously described (Silva et al. 2007; Zhou et al. 2014), the CDC used solid-phase extraction–high performance liquid chromatography–isotope-dilution tandem mass spectrometry to quantify total (free plus conjugated) urinary concentrations () of three parabens (methylparaben, propylparaben, and butylparaben) and nine phthalate metabolites: MEP, MnBP, MiBP, monobenzyl phthalate [MBzP; a metabolite of butylbenzyl phthalate (BBzP)], mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) [MEHP, MEOHP, MEHHP, and MECPP are all metabolites of di(2-ethylhexyl) phthalate (DEHP)], and mono(3-carboxypropyl) phthalate [MCPP; a nonspecific metabolite of DnBP, di-n-octyl phthalate (DOP), and other high-molecular–weight phthalates]. We also calculated the molar sum of DEHP metabolites () (Braun et al. 2014). The limits of detection (LOD) ranged from 0.10 (propylparaben, butylparaben) to (MEHP). Concentrations below the LOD were replaced by the LOD divided by the square root of 2 for all biomarkers detected in at least 90% of the samples (Hornung and Reed 1990; Lubin et al. 2004).
Statistical Analysis
We calculated descriptive statistics for participants’ baseline characteristics (such as age, race, education, BMI, and smoking); time-varying characteristics (such as time of the day, season, and calendar year of the sample collection); and personal care product use patterns. Because of the relatively short half-lives of phthalates and parabens (Janjua et al. 2008; Koch et al. 2012; Moos et al. 2016) and findings of previous publications, we defined PCP use as use within the last 24 and 6 h before urine collection. We used two approaches for assessing men’s use of PCPs. The first approach (single-PCP analyses) defined use of each PCP as a binary variable (yes/no use within the 24 h preceding urine collection). Because multiple PCPs contributed to the overall body burden of phthalates and parabens, in our second approach (multi-PCP analyses) we used a composite weighted PCP score (continuous variable) as explained below.
The nine urinary concentrations of phthalate metabolites, , and three parabens were modeled as continuous variables. We examined distributions for the urinary concentrations, and because of observed skewness, we used a natural log-transformation to satisfy regression model assumptions. We examined the Spearman correlations among the concentrations of phthalate metabolites and parabens.
Covariate Adjustment
Selection of covariates was based a priori on directed acyclic graphs and previous literature (Braun et al. 2014; Duty et al. 2005; Smith et al. 2012). The final model included urine specific gravity (continuous) (O’Brien et al. 2016) to account for urine dilution, race (Caucasian or not), age (continuous), body mass index (BMI) (continuous), calendar year [continuous, because it has been shown that urinary concentrations of several phthalate metabolites have changed over time in the United States (Zota et al. 2014)], three categories for time of urine sample collection [early morning (between 0500 and 0900 hours), late morning (between 0901 and 1200 hours), or afternoon (after 1201 hours)], current smoking (yes/no), and warm season (April through September) (yes/no) to account for seasonality in Boston with higher use of certain products, specifically sunscreen, in the summer.
Multiple Imputations of the Data
Some men had incomplete PCP-use data across their repeated visits and two men were missing BMI. The missingness for each PCP question ranged from 5 (for shampoo) to 339 (for hand/body lotion). Distributions of the urinary concentrations of phthalate metabolites and parabens were not statistically different when comparing the complete and incomplete PCP-use data, suggesting that missingness was not related to our outcomes and would not likely impact our findings. We investigated missingness patterns for PCPs use and the concordance of PCP use within the same man over repeated visits. We used multiple imputation to account for the missing data, using multiple imputation by chained equations (MICE) (Sterne et al. 2009; White et al. 2011) for imputing missing PCP-use data and covariates (two men missing BMI). For any given PCP at any time point, the predictors were the same PCP used at other time points, urine specific gravity (O’Brien et al. 2016), race, age, BMI, calendar year, time of sample collection, current smoking, and season. We generated 10 imputed datasets for each analysis and used these imputed datasets in the main analysis using both of the analytical approaches described below using proc mianalyze in SAS (version 9.4; SAS Institute Inc., Cary, NC).
Regression Models Analysis
Single-PCP analyses.
We first regressed each biomarker (and ) concentration on a single-PCP and covariates in separate linear mixed effects models (LMEM) with a random intercept to account for within-person correlation among multiple longitudinal measures of a given biomarker. Because MEHP and butylparaben had nondetectable concentrations, we used mixed Tobit regression (Tobin 1958) models with random intercepts for left-censored data (Lubin et al. 2004). For MEHP and butylparaben, we took into account LODs from different analytic batches over the study period by specifying a unique LOD for each sample collection within the Tobit regression. In addition, we regressed all biomarkers on a continuous variable for total sum (0 to 14) of the PCPs used by each man at each visit.
We created heatmaps for better visualization of the estimated percent change (% change) in the urinary concentrations associated with each PCP use based on LMEM (or Tobit models for MEHP and butylparaben). We adjusted for the covariates mentioned above in all models. We considered two-sided alpha as statistically significant and indicated significance as asterisks in heatmaps.
We further explored the association between the PCP use within 6 h before urine collection (yes/no) for five of the urinary concentrations that were most likely to have PCP use as sources of their parent compound, that is, MEP (for DEP), MiBP (for DiBP), methylparaben, propylparaben, and butylparaben (Buckley et al. 2012; Dodson et al. 2012; Philippat et al. 2015; Wittassek et al. 2011).
Multi-PCP analyses.
To analyze the simultaneous impact of the 14 PCPs on a given biomarker concentration, we modified the weighted quantile score (WQS) regression model previously used for quantifying the impact of environmental mixtures on an outcome (Carrico 2013; Carrico et al. 2015; Christensen et al. 2013; Gennings et al. 2010, 2013). Given the high dimensionality and inherent correlations among the use of different PCPs as well as correlations among phthalate metabolites and parabens urinary concentrations, traditional regression is unsuitable (Carrico 2013). In addition, in previous simulation analyses, the WQS showed improvements over ordinary regression and LASSO (Carrico 2013). The WQS approach assumes that a given biomarker urinary concentration is associated with a PCP-use composite score, where this score encompasses a linear combination of PCP (0/1) use indicators. For a given urinary concentration, the approach estimates simultaneously PCP weights and an effect estimate for the resulting composite score within a regression model framework. Within each imputed data set, we used bootstrapping to empirically estimate the weights, and the data set was split (50/50) into a training set and a test set to provide valid inferences (e.g., p-values) for the estimated slope of the composite score. For biomarkers with a small percent of nondetectable concentrations (up to 10% below LOD), we constructed a weighted binary score (WBS) within the linear regression framework. For biomarkers with a moderate percent of concentrations below the LOD ( and below LOD), we performed WBS estimation within the Tobit regression model.
We present results as weighted % change in urinary concentrations of MEP, MiBP and the three parabens associated with an increase in the weighted score of the 14 PCPs, as well as the corresponding weight for each PCP (sum to 100%). Similarly, we a priori chose to present the number of PCPs (could be different depending on the chemical) that summed to at least 70% of the weighted score. The 70% of the weighted score should provide a reasonable prediction of exposure as compared with 100% of the weighted score, and may therefore be applicable for future research on exposure from PCPs. We repeated these analyses for PCPs use with 6 h of urine collection for the five urinary concentrations of higher interest.
Sensitivity analyses.
Given the low detection frequency for butylparaben (31%), we performed a secondary analysis using generalized estimating equations (GEE) with logit link after we dichotomized butylparaben urinary concentrations into detectable versus nondetectable concentrations (Dodge et al. 2015). We adjusted for the same covariates as in the primary analyses. We report adjusted odds ratios of detection associated with PCP use along with p-values). We repeated all analyses using only the complete case analyses (with no imputation). Analyses were also repeated after imputing the missing PCP data as “0,” assuming missing was “no use.”
We conducted statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC) and used the (heatmap.2) R package for heatmap generation (version 3.1.2; R Development Core Team).
Results
In the final analysis, 400 men contributed 1,037 urine samples with an average of 3 samples per man (up to 12 samples); 29% of the men provided 1 urine sample, 31% provided 2, and 40% provided at least 3. The median time between any two consecutive urine collections per man was 77 d (interquartile range: 34–126 d).
The men were mostly Caucasian (86%), nonsmokers (93%), and had a median age of 36 y, median BMI of , and a college or graduate education (84%) at the time of enrollment. Urine collection time ranged from 0600 to 1800 hours with 42% collected between 0900 and 1200 hours, and 46% of the samples collected in the warm season (Table 1).
Table 1.
Demographics for 400 men who contributed 1,037 urine samples in the Environment And Reproductive Health (EARTH) study.
| Baseline characteristics | N (%) or [range] |
|---|---|
| Age (years) | [23.9, 66.6] |
| Race | |
| Caucasian | 343 (86) |
| Black/African American | 13 (3) |
| Asian | 29 (7) |
| Other | 15 (4) |
| BMI ()a | [18.6, 50.0] |
| BMI categories | |
| Underweight: | 2 (1) |
| Normal weight: | 114 (28) |
| Overweight: | 192 (48) |
| Obese: | 92 (23) |
| Education categoriesa | |
| Less than college graduate | 50 (16) |
| College graduate | 109 (34) |
| Graduate degree | 158 (50) |
| Current smoking status | |
| Yes | 26 (7) |
| No | 374 (93) |
| Time-varying characteristics for urine sample collection | (%) |
| Warm season (April–September) | 477 (46) |
| Calendar year | |
| 2004–2009 | 522 (50) |
| 2010–2015 | 515 (50) |
| Time of the day | |
| Early morning: and | 385 (37) |
| Late morning until noon: and | 432 (42) |
| Afternoon: | 220 (21) |
Note: BMI, body mass index; N, men’s number; , urine samples’ number; SD, standard deviation. (number of men) or (number of urine samples) (%) were used for categorical/binary variables and [range] for continuous variables.
Two men missing information on BMI and 83 missing education.
Deodorants and shampoos were the most frequently () used PCPs. Suntan/sunblock lotion and other hair care products were the least frequently () used PCPs (Table 2). The concordance between specific PCP use at different study visits within the same men was relatively high, ranging from 47% for hand sanitizer to 93% for suntan/sunblock lotion.
Table 2.
Self-reported use of 14 personal care products (PCPs) within 24 and 6 h of collection of urine sample among 400 men in the Environment And Reproductive Health (EARTH) study.
| Personal care products (PCPs) | (answered yes/no) | (answered yes/no and reported time since last used) | (%) answered yes within 24 h | (%) answered yes within 6 h | % concordanta over time |
|---|---|---|---|---|---|
| Deodorant | 1,029 | 1,023 | 879 (85) | 723 (71) | 82 |
| Shampoo | 1,032 | 1,024 | 832 (81) | 584 (57) | 74 |
| Conditioner/crème rinse | 946 | 941 | 220 (23) | 149 (16) | 76 |
| Hairspray/hair gel | 952 | 948 | 291 (31) | 222 (23) | 83 |
| Other hair care productsb | 914 | 910 | 66 (7) | 46 (5) | 90 |
| Shaving cream | 993 | 993 | 390 (39) | 263 (26) | 62 |
| Aftershave | 800 | 799 | 79 (10) | 53 (7) | 87 |
| Cologne/perfume | 955 | 952 | 202 (21) | 145 (15) | 79 |
| Mouthwash | 975 | 969 | 325 (33) | 199 (21) | 72 |
| Bar soap | 1,003 | 1,001 | 755 (75) | 567 (57) | 81 |
| Liquid soap/body wash | 1,016 | 990 | 732 (72) | 508 (51) | 55 |
| Hand sanitizer | 726 | 714 | 219 (30) | 128 (18) | 47 |
| Hand/body lotion | 698 | 695 | 162 (23) | 102 (15) | 81 |
| Suntan/sunblock lotion | 863 | 862 | 25 (3) | 12 (1) | 93 |
Note: , number of visits/urine samples; PCPs, personal care products.
Concordant: same answer for product use within 24 h over different visits for the same man, only includes observations for men who completed at least two visits.
Combined other hair care products included mousse, hair bleach, relaxer, perm, and straightener.
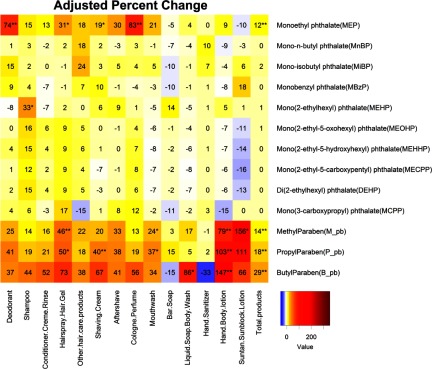
The percentage of samples (2004–2015) with detectable urinary concentrations of phthalates and parabens biomarkers ranged from 90% to 100% except for MEHP (78%) and butylparaben (31%) (Table 3). The men’s median urinary MEP and paraben concentrations were substantially lower than the medians for women from the same cohort (Braun et al. 2014) but comparable to male urinary concentrations from the National Health and Nutrition Examination Survey (NHANES) (CDC 2017) (Table 3). Spearman correlations were generally weak between phthalate metabolites and parabens (range: 0.09–0.32), weak to strong among different phthalate metabolites (range: 0.22–) with the strongest correlation occurring between metabolites from the same parent compound (e.g., DEHP), and moderate to strong among different parabens (range: 0.41–0.80) (see Figure S1). Strong correlations indicate similar sources and weak correlations indicate different sources of exposure. These correlations are in accordance with results shown in Figure 1.
Table 3.
Distributions of urinary concentrations of phthalate metabolites and parabens measured in 400 men ( urine samples)a in the Environment And Reproductive Health (EARTH) study.
| Urinary concentrationsb (c) | % (d) | GM | 10th perc | 25th perc | 50th perc | 75th perc | 90th perc | GM (NHANES)e 2005–2006 | GM (NHANES)e 2009–2010 | GM (NHANES)e 2011–2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| Monoethyl phthalate (MEP) | 100 | 48.8 | 6.86 | 16.6 | 42.0 | 145 | 458 | 107 | 61.0 | 38.1 |
| Mono-n-butyl phthalate (MnBP) | 98 | 10.6 | 2.00 | 4.90 | 12.4 | 24.7 | 43.6 | 19.8 | 14.5 | 8.14 |
| Mono-isobutyl phthalate (MiBP) | 97 | 6.44 | 1.20 | 3.20 | 7.40 | 14.9 | 27.2 | 5.65 | 7.80 | 6.54 |
| Monobenzyl phthalate (MBzP) | 95 | 3.25 | 0.50 | 1.37 | 3.60 | 8.10 | 16.2 | 9.47 | 6.93 | 4.80 |
| Mono(2-ethylhexyl) phthalate (MEHP) | 78 | 3.03 | 0.85 | 2.80 | 7.80 | 24.9 | 3.40 | 1.83 | 1.51 | |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 99 | 10.3 | 1.40 | 3.80 | 9.80 | 24.8 | 84.1 | 18.3 | 9.14 | 5.50 |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | 100 | 17.3 | 2.40 | 6.00 | 16.2 | 45.3 | 148 | 29.6 | 15.2 | 8.71 |
| Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) | 100 | 27.7 | 4.30 | 10.5 | 26.1 | 65.5 | 209 | 43.6 | 23.4 | 14.3 |
| (di(2-ethylhexyl) phthalate) metabolites (f) | — | 0.20 | 0.03 | 0.07 | 0.19 | 0.49 | 1.57 | — | — | — |
| Mono(3-carboxypropyl) phthalate (MCPP) | 97 | 3.85 | 0.60 | 1.60 | 3.70 | 9.70 | 23.2 | 2.32 | 3.37 | 3.52 |
| Methylparaben | 99 | 28.0 | 4.70 | 9.40 | 23.2 | 80.4 | 226 | 29.8 | 31.7 | 23.2 |
| Propylparaben | 90 | 2.86 | 0.14 | 0.60 | 2.30 | 12.1 | 58.6 | 2.96 | 2.77 | 2.44 |
| Butylparaben | 31 | 0.26 | 0.30 | 2.20 |
Note: GM, geometric mean; LOD, limit of detection; NHANES, National Health and Nutrition Examination Survey; perc, percentile; SD, standard deviation.
Phthalate metabolites were measured in 1,037 urine samples from 400 men and parabens in 965 samples from 383 men.
Urinary concentrations were ordered according to the molecular weights within phthalates and within parabens.
Concentrations in (except for ) were not corrected for urine dilution; concentrations were replaced by the LOD divided by the square root of 2.
LOD range (in ) for MnBP: 0.4, 0.6; MiBP: 0.2, 0.3; MBzP: 0.2, 0.3; MEHP: 0.5, 1.2; MEOHP: 0.2, 0.7; MEHHP: 0.2, 0.7; MCPP: 0.18, 0.2; M_pb: 1; B_pb: 0.1, 0.2; B_pb: 0.1, 0.2.
Concentrations from NHANES (2005–2006), (2009–2010), and (2011–2012) because our data encompassed the years 2004–2015.
metabolites sum of .
Figure 1.
Heatmap for adjusted % change in urinary phthalate metabolite and parabens concentrations associated with self-reported use of personal care products (PCPs) within 24 h of urine sample collection among 400 men who contributed 1,037 urine samples in the Environment And Reproductive Health (EARTH) study. Abbreviations: DEHP means, metabolites of of ; total products: the crude sum of PCP used within 24 h. Multiple imputation of the missing was based on concordance of product use within persons. For any given PCP at any time point, the imputation model included PCP use at other time points, urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no), and warm season (April–September) (yes/no). Analysis adjusted for urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no), warm season (April–September) (yes/no), and the product use within 24 h (yes/no). The last column for the total products represents % changes associated with each additional type of PCP used, regardless of which PCP. Urinary concentrations were ordered according to the molecular weights within phthalates and within parabens. Combined other hair care products included mousse, hair bleach, relaxer, perm, and straightener. Analysis was based on 10 imputed data using the chained equations method. *. **.
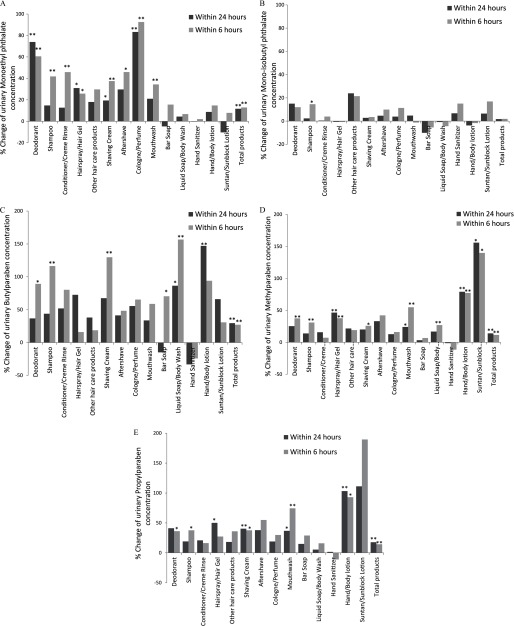
The adjusted % changes in urinary concentrations (by the single-PCP approach) were highest for MEP (e.g., 83% increase with cologne/perfume use and 74% increase with deodorant use) (Figure 1). Hairspray/hair gel, aftershave, mouthwash, shaving cream, and other hair care products predicted MEP concentrations (18–31% increase). For the three parabens, the strongest predictors were use of suntan/sunblock lotion (66–156% increase) and of hand/body lotion (79–147% increase). Hairspray/hair gel, shaving cream, aftershave, mouthwash, and deodorant were moderate predictors for parabens. Liquid soap/body wash use was a strong predictor only for butylparaben (86%). Apart from hand sanitizer and bar soap, the rest of the PCPs were moderate to weak predictors of the three parabens. Each additional type of PCP used, regardless of which PCP, was associated with a 12% increase in MEP and a 14–29% increase in parabens urinary concentrations. Although shampoo use was significantly associated with a 33% increase in the urinary concentrations of MEHP, it was not significantly associated with the other DEHP metabolites. The associations between PCP use and urinary concentrations of other phthalate metabolites were weak to null, and sometimes even negative, but none were statistically significant. The percent increase in the urinary concentrations associated with PCP use within 6 h of urine collection was generally larger and more statistically significant than for use within 24 h (Figure 2).
Figure 2.
Adjusted % change in urinary concentrations of parabens and phthalate metabolites associated with 14 personal care products used within 24 and 6 h of urine collection: (A) monoethyl phthalate (MEP) urinary concentration; (B) mono-isobutyl phthalate (MiBP) urinary concentration; (C) butylparaben urinary concentration; (D) methylparaben urinary concentration; and (E) propylparaben urinary concentration. Multiple imputation of the missing was based on concordance of product use within persons. For any given PCP at any time point, the imputation model included PCP use at other time points, urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no) and warm season (April–September) (yes/no). Analysis adjusted for urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no), warm season (April–September) (yes/no), and the product use within 24 h (yes/no). Total products: the crude sum of PCP used within 24 h. The total products’ bar graphs plotting % change associated with each additional type of PCP used, regardless of which PCP. Combined other hair care products included mousse, hair bleach, relaxer, perm, and straightener. Analysis was based on 10 imputed data using the chained equations method. *. **.
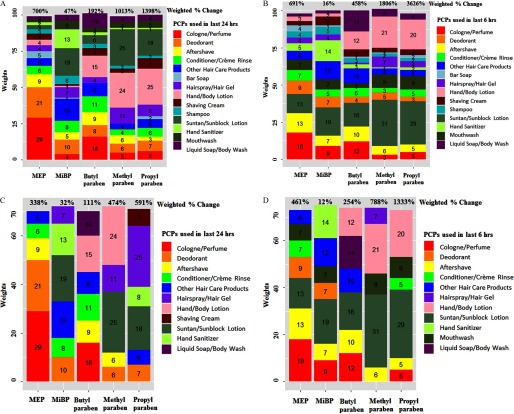
In the multi-PCP approach, we presented the different weights for each PCP depending on the association with each biomarker so that the 14 PCPs sum to 100%. When regressing these composite scores, we found that a 1-unit increase in the weighted score of the use of all 14 PCPs was associated with an increase in MEP and paraben urinary concentrations ranging from 700% to 1,398% for samples collected within 24 h of PCP use (Figure 3A; see also Table S1). When we limited PCP use to the last 6 h, a 1-unit increase in the weighted score was associated with a 458–3,626% increase in urinary concentrations of MEP and the parabens (Figure 3B; see also Table S1).
Figure 3.
Weights of personal care products (PCPs) that contribute 100% and at least 70% to the overall urinary concentrations of biomarkers associated with PCPs use within 24 h and 6 h of sample collection: A) weights of personal care products (PCPs) that contribute 100% to the overall urinary concentrations associated with PCPs use within 24 h and their overall weighted % change; B) weights of personal care products (PCPs) that contribute 100% to the overall urinary concentrations associated with PCPs use within 6 h and their overall weighted % change; C) weights of personal care products (PCPs) that contribute at least 70% to the overall urinary concentrations associated with PCPs use within 24 h and their overall weighted % change; D) weights of personal care products (PCPs) that contribute at least 70% to the overall urinary concentrations associated with PCPs use within 6 h and their overall weighted % change. Numbers presented inside the stack bars represent the weights associated with PCP use. The weights of the 14 PCPs are summed to due to approximation. The percent presented above the stacked bars represent the % change associated with PCPs. The weights presented are NOT summed to the % change, instead summed to 100% for the 14 PCPs and 70% or more for the PCPs presented above. The % changes are calculated by exponentiation of the weighted beta coefficient based on the PCPs used given the weights. Multiple imputation of the missing was based on concordance of product use within persons. For any given PCP at any time point, the imputation model included PCP use at other time points, urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no), and warm season (April–September) (yes/no). Analysis adjusted for urine specific gravity (continuous), race (Caucasian or not), age (continuous), BMI (continuous), calendar year (continuous), time of sample collection [early morning ( and ), late morning ( and ), or afternoon ()], current smoking (yes/no), warm season (April–September) (yes/no), and the product use within 24 h (yes/no). Combined other hair care products included mousse, hair bleach, relaxer, perm, and straightener. Analysis was based on 10 imputed data using the chained equations method.
We also present in Figure 3C, 3D the adjusted weighted % change in the urinary concentrations associated with the PCPs making up at least 70% of the weighted score as contributed by the highest weighted PCPs for each chemical use within 24 and 6 h of urine collection, along with the weights for each PCP (sum to at least 70%). The adjusted weighted % change in the urinary concentrations of MEP and the three parabens was associated with six or fewer PCPs (depending on the biomarker) and ranged from a 111% to a 591% increase within 24 h of urine collection and up to a 1,333% increase within 6 h of urine collection. MiBP urinary concentration was not strongly predicted by the weighted PCP score.
For MEP and the three parabens individually, seven or fewer PCPs used within 24 or 6 h explained at least 70% of the weighted PCP score. The adjusted weighted % changes within 6 h were generally higher than within 24 h of urine collection. Overall, use of 10 PCPs within 6 h prior to collection explained at least 70% of the weighted score. These included cologne/perfume, deodorant, suntan/sunblock lotion, hand/body lotion, aftershave, other hair care products, mouthwash, conditioner/crème rinse, hairspray/hair gel, and liquid soap/body wash, which explained at least a 254% and up to a 1,333% increase in MEP and the three parabens urinary concentrations (Figure 3C, 3D).
Secondary analyses performed by modeling butylparaben as a dichotomous outcome (detected vs. nondetected) provided odds ratios that were consistent with the direction of the % changes from Tobit regression (see Table S2). Sensitivity analyses using the complete case analysis (with no imputation) and after imputing the missing PCP use as no use gave consistent results with the main analysis (see Figures S2–S5 and Tables S3–S6).
Discussion
We found that self-reported PCP use among men was associated with higher urinary concentrations of MEP and three parabens (methyparaben, propylparaben, and butylparaben). As expected and consistent with a prior study of women in the same cohort (Braun et al. 2014), due to the short half-lives of phthalates and parabens (Janjua et al. 2008; Koch et al. 2012; Moos et al. 2016) and the episodic use of PCPs, observed associations were stronger for PCP use within 6 h of urine collection as compared with use 24 h prior to collection. PCP use did not predict MiBP or the other phthalate metabolite urinary concentrations, which is consistent with infrequent use of these phthalates in PCPs (Dodson et al. 2012).
To our knowledge only two large-scale studies have investigated the association between PCP use and urinary phthalate metabolites and parabens in men. Duty et al. (2005) explored associations of five PCPs used within 48 h of a single spot urine sample collection in 406 men recruited from the MGH Andrology Laboratory (2000–2003); cologne and aftershave were predictors of MEP urinary concentrations (Duty et al. 2005). More recently, using NHANES data, Ferguson et al. (2017) reported that mouthwash was a more important source of paraben exposure in men than in women. Our results were consistent with the findings from these previous studies even though both relied on only one spot urine sample per participant and had limited information about the time of PCP use relevant to the urine collection. Duty et al. (2005) assessed PCP use within 48 h before urine collection, which is likely to be too long a time window which would contribute to attenuation of associations. Ferguson et al. (2017) had no information on last time of use relative to urine sample collection. Instead, PCP use was recorded as always, sometimes, or never, introducing exposure misclassification that is likely nondifferential. Our study, with repeated urine samples from the same man and more detailed information on time of PCP use extends the literature by identifying specific predictors of phthalates and parabens exposure.
In our study, men’s self-reported information on PCP use was predictive of select phthalate (mainly DEP) and paraben exposures. Based on our results, the exposure assessment of phthalates and parabens can be improved by asking only about the most “important” PCPs used within 6 h before urine collection. We showed that 10 PCPs contributed to at least 70% of the weighted score and explained more than a 250% increase in the urinary concentrations of MEP and the three parabens. Our recommendation for refining the questionnaire includes focusing on the following 10 PCPs: cologne/perfume, deodorant, suntan/sunblock lotion, hand/body lotion, aftershave, other hair care products, mouthwash, conditioner/crème rinse, hairspray/hair gel, and liquid soap/body wash. Shortening the questionnaire would likely decrease missingness and improve participants’ response rate. In addition, restricting the period of inquiry to the 6 h prior to urine collection would provide a more precise estimate of exposure to phthalates and parabens because the urinary concentrations of the biomarkers are a better reflection of exposure in the last 6 h than in the last 24 h. It is also likely that participants will better recall their PCP use within a shorter time frame.
Our study had several potential limitations. First, our single-PCP approach may be susceptible to multiple testing statistical issues. However, we also used a multi-PCP approach using WBS regression to account for multiple testing and the resulting estimated % changes were even larger, making it unlikely that any false positives in the single-PCP analysis accounted for our findings. Butylparaben had a low detection frequency, which was consistent with findings from the NHANES (Calafat et al. 2010; CDC 2017; Ferguson et al. 2017) and a German study (Moos et al. 2015). We addressed this in two ways. First, to explicitly model nondetectable concentrations, we used Tobit regression, which accounts for missingness better than replacing those concentrations with the LOD divided by the square root of 2 and linear regression (Ferguson et al. 2017). Second, we applied a sensitivity analysis using GEE (detected vs. nondetected) as performed before (Dodge et al. 2015), and these sensitivity analyses produced results comparable to the Tobit regression.
We had no information about frequency of PCP use, amount of product used, whether it was used with hot or cold water, phthalates and parabens product content, or brand names of the PCPs. Nevertheless, the lack of this information would have likely introduced nondifferential measurement error and thus attenuated our results toward the null. In addition, knowing the brand name may not remedy this potential limitation given that even the same brand might change product formulations over time and chemicals are not always listed on product labels. Because of short half-lives and episodic use, urinary concentrations of the biomarkers will depend on the frequency of voids between PCP use and urine collection and we did not collect this information. We only focused on exposures from PCP use, not accounting for other exposure sources. However, this is less likely to affect our results for MEP and the parabens because the PCPs are considered a major source of exposure to these compounds (or their precursors) (Braun et al. 2014; Janjua et al. 2007, 2008; Just et al. 2010), whereas for other phthalates such as DEHP, food is a major source of exposure (Serrano et al. 2014). In addition, it is unlikely that other sources of exposure such as diet would meet the confounder definition because they are unlikely to be associated with PCP use, hence not affecting the study validity. Finally, most men in our study were Caucasian, overweight or obese, highly educated, and nonsmokers. Therefore, our results may be generalized only to men with similar characteristics.
Our study had several strengths, including the use of repeated collection of self-reported PCP use and urinary concentrations of phthalate metabolites and parabens for a large number of men over a 10-y period; these repeated urine samples likely increased the precision of the measurements compared with a single sample. We included in the analysis 14 PCPs, the largest number of PCPs investigated in men to date. We also included a large number of phthalate metabolites and 3 parabens. We assessed PCP use within 24 and 6 h before urine collection, more relevant exposure windows compared with PCP use within the last 48 h (Duty et al. 2005; Just et al. 2010).
Another novel aspect of our study compared with previous research was the use of a weighted PCP score to predict urinary concentrations. The WBS analyses yielded different weights for each PCP depending on the association with each biomarker. Using the single-PCP analyses for modeling, the crude sum of PCPs likely attenuated the effect due to nondifferential misclassification by giving equal weights to each PCP in association with each biomarker. In addition, the multi-PCP approach was able to adjust for the other correlated PCPs and estimate the joint effect of multiple PCP use, rather than simply using the crude sum of the PCPs or modeling each PCP separately. This approach has been used in linear regression settings (Carrico 2013; Carrico et al. 2015; Christensen et al. 2013; Gennings et al. 2010, 2013), and we extended the basic weighted quantile strategy used in earlier work to the repeated measures data and in Tobit regression framework.
Conclusions
Among a large cohort of men, we identified PCP predictors of urinary concentrations of MEP and three parabens. The results will be useful in future exposure assessment studies on PCP use as an important source of exposure to phthalates (mainly DEP) and parabens. Collecting concise PCP use information can be achieved by only asking specific questions about the use of the most relevant PCPs within a narrow exposure window (6 h) to potentially decrease missingness and improve recall decreasing misclassification while also optimizing research cost and time.
Supplemental Material
Acknowledgments
The authors gratefully acknowledge the Centers for Disease Control and Prevention (CDC) staff for measuring phthalate metabolites and paraben urinary concentrations, and M.G. Keller, R. Dadd and P. Morey (research staff), the study participants, and the clinical staff for their dedication and participation in the EARTH study. The authors also thank L. Valeri and B. Claus Henn for their useful SAS code that inspired part of this work.
This work was supported by the National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health (grants R01 ES009718, R01 ES024381, and P30 ES000002). The Leslie Silverman Industrial Hygiene Fund, the Benjamin Greely Ferris, Jr. Fellowship in Environmental Epidemiology, and the Cyprus Endowment for the Environment and Public Health at the Harvard T.H. Chan School of Public Health provided support for F.L.N. during her doctoral studies.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- Boberg J, Taxvig C, Christiansen S, Hass U. 2010. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 30(2):301–312, PMID: 20381602, 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. 2014. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol 24(5):459–466, PMID: 24149971, 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. 2012. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol 22(5):468–475, PMID: 22760436, 10.1038/jes.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, et al. 2015. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect 123(7):A166–A168, PMID: 26132373, 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. 2010. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect 118(5):679–685, PMID: 20056562, 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C. 2013. Characterization of a Weighted Quantile Score Approach for Highly Correlated Data in Risk Analysis Scenarios [PhD Dissertation]. Richmond, VA:Virginia Commonwealth University. [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120, 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. National Report on Human Exposure to Environmental Chemicals Updated Tables, January 2017. http://www.cdc.gov/exposurereport [accessed 1 February 2017].
- Christensen KLY, Carr C, Sanyal AJ, Gennings C. 2013. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health 216(6):703–709, PMID: 23491026, 10.1016/j.ijheh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge LE, Williams PL, Williams MA, Missmer SA, Toth TL, Calafat AM, et al. 2015. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect 123(7):665–671, PMID: 25767892, 10.1289/ehp.1408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect 120(7):935–943, PMID: 22398195, 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113(11):1530–1535, PMID: 16263507, 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (U.S. Food and Drug Administration). 2014a. Parabens in Cosmetics. http://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.htm [accessed 15 August 2016].
- FDA. 2014b. Phthalates. http://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128250.htm [accessed 20 June 2016].
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD. 2017. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol 27(3):326–332, PMID: 27168391, 10.1038/jes.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA. 2013. A cohort study evaluation of maternal pcb exposure related to time to pregnancy in daughters. Environ Health 12(1):66, PMID: 23962309, 10.1186/1476-069X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C, Sabo R, Carney E. 2010. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology 21 (suppl 4):S77–S84, PMID: 21422968, 10.1097/EDE.0b013e3181ce946c. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kannan K. 2013. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol 47(24):14442–14449, PMID: 24261694, 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL, et al. 2012. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci 125(2):544–557, PMID: 22112501, 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, et al. 2016. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ Health Perspect 124(10):1600–1607, PMID: 26947464, 10.1289/ehp.1510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5:46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Gray LE Jr. 2016. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int J Hyg Environ Health 220(2 pt A):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. 2008. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl 31(2):118–130, PMID: 18194284, 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. 2007. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol 41(15):5564–5570, PMID: 17822133, 10.1021/es0628755. [DOI] [PubMed] [Google Scholar]
- Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R, et al. 2010. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J Expo Sci Environ Epidemiol 20(7):625–633, PMID: 20354564, 10.1038/jes.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, et al. 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: personal care product ingredients. Toxicol Lett 231(2):261–269, PMID: 24956590, 10.1016/j.toxlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Koch HM, Christensen KL, Harth V, Lorber M, Brüning T. 2012. Di-n-butyl phthalate (DNBP) and diisobutyl phthalate (DIBP) metabolism in a human volunteer after single oral doses. Arch Toxicol 86(12):1829–1839, PMID: 22820759, 10.1007/s00204-012-0908-1. [DOI] [PubMed] [Google Scholar]
- Koch HM, Lorber M, Christensen KL, Pälmke C, Koslitz S, Brüning T. 2013. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 216(6):672–681, PMID: 23333758, 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, et al. 2015. Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol 25(4):343–353, PMID: 25944701, 10.1038/jes.2015.33. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina CA, Weiss B, Swan SH. 2012. Lifestyle behaviors associated with exposures to endocrine disruptors. Neurotoxicology 33(6):1427–1433, PMID: 22739065, 10.1016/j.neuro.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Dierkes G, Brüning T, Koch HM. 2016. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch Toxicol 90(11):2699–2709, PMID: 26608183, 10.1007/s00204-015-1636-0. [DOI] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Brüning T, Koch HM. 2014. Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int J Hyg Environ Health 217(8):845–853, PMID: 25008406, 10.1016/j.ijheh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Moos RK, Koch HM, Angerer J, Apel P, Schröter-Kermani C, Brüning T, et al. 2015. Parabens in 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. Int J Hyg Environ Health 218(7):666–674, PMID: 26253560, 10.1016/j.ijheh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227, PMID: 26219104, 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton F, Ermler S, Kugathas S, Rosivatz E, Scholze M, Kortenkamp A. 2014. Mixture effects at very low doses with combinations of anti-androgenic pesticides, antioxidants, industrial pollutant and chemicals used in personal care products. Toxicol Appl Pharmacol 278(3):201–208, PMID: 24055644, 10.1016/j.taap.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH. 2015. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ Res 140:369–376, PMID: 25929801, 10.1016/j.envres.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Franco M, Hernández-Ramírez RU, Calafat AM, Cebrián ME, Needham LL, Teitelbaum S, et al. 2011. Personal care product use and urinary levels of phthalate metabolites in mexican women. Environ Int 37(5):867–871, PMID: 21429583, 10.1016/j.envint.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, et al. 2008. Baby care products: possible sources of infant phthalate exposure. Pediatrics 121(2):e260–e268, PMID: 18245401, 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Seo JE, Kim S, Kim BH. 2016. In vitro skin absorption tests of three types of parabens using a Franz diffusion cell. J Expo Sci Environ Epidemiol 27(3):320–325, PMID: 27436697, 10.1038/jes.2016.33. [DOI] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13(1):43, PMID: 24894065, 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112, PMID: 17997365, 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. 2012. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect 120(11):1538–1543, PMID: 22721761, 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. 2009. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393, PMID: 19564179, 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. 1958. Estimation of relationships for limited dependent variables. Econometrica 26:24–36, 10.2307/1907382. [DOI] [Google Scholar]
- Weschler CJ, Bekö G, Koch HM, Salthammer T, Schripp T, Toftum J, et al. 2015. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: experimental verification. Environ Health Perspect 123(10):928–934, PMID: 25850107, 10.1289/ehp.1409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30(4):377–399, PMID: 21225900, 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Brüning T. 2011. Assessing exposure to phthalates – the human biomonitoring approach. Mol Nutr Food Res 55(1):7–31, PMID: 20564479, 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci 944:152–156, PMID: 24316527, 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bergman Å, Becher G, Bjerregaard P, Bornman R, Brandt I, et al. 2014. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ Health 13:118, PMID: 25533907, 10.1186/1476-069X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: findings from the National Health And Nutrition Examination Survey, 2001–2010. Environ Health Perspect 122(3):235–241, PMID: 24425099, 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.