Abstract
Background:
The European Food Safety Authority recently concluded that the exposure of small children (1–3 y old) to brominated diphenyl ether (BDE)-99 may exceed acceptable levels defined in relation to neurodevelopmental toxicity in rodents. The flame retardant BDE-209 may release BDE-99 and other lower brominated BDEs through biotic and abiotic degradation, and all age groups are exposed not only to BDE-209 and -99 but also to a cocktail of BDE congeners with evidence of neurodevelopmental toxicity. The possible risks from combined exposures to these substances have not been evaluated.
Objectives:
We performed a congener-specific mixture risk assessment (MRA) of human exposure to combinations of BDE-209 and other BDEs based on estimated exposures via diet and dust intake and on measured levels in biologic samples.
Methods:
We employed the Hazard Index (HI) method by using BDE congener-specific reference doses for neurodevelopmental toxicity.
Results:
Our HI analysis suggests that combined exposures to polybrominated diphenyl ethers (PBDEs) may exceed acceptable levels in breastfeeding infants (0–3 mo old) and in small children (1–3 y old), even for moderate (vs. high) exposure scenarios. Our estimates also suggest that acceptable levels of combined PBDEs may be exceeded in adults whose diets are high in fish. Small children had the highest combined exposures, with some estimated body burdens that were similar to body burdens associated with developmental neurotoxicity in rodents.
Conclusions:
Our estimates corroborate reports from several recent epidemiological studies of associations between PBDE exposures and neurobehavioral outcomes, and they support the inclusion of BDE-209 in the persistent organic pollutant (POP) convention as well as the need for strategies to reduce exposures to PBDE mixtures, including maximum residue limits for PBDEs in food and measures for limiting the release of PBDEs from consumer waste. https://doi.org/10.1289/EHP826
Introduction
Owing to a legacy of pollution related to the past use of commercial polybrominated diphenyl ethers (PBDEs) as flame retardants, humans today are exposed to combinations of multiple PBDE congeners. Although other commercial PBDE mixtures are listed as persistent organic pollutants (POPs) under the Stockholm Convention (UNEP 2013), commercial decabromodiphenyl ether (c-decaBDE) is not yet included. There are, however, voluntary agreements to phase out its use. C-deca-BDE is widely used in plastics and textiles and consists mainly of decabromodiphenyl ether (BDE-209) () (ECHA 2012). Lower-brominated, more toxic PBDEs are liberated through the abiotic and biotic debromination of c-decaBDE in the environment and within organisms (see the review by Kortenkamp et al. 2013), and these processes may contribute to the generation of PBDE mixtures.
The potential for combined effects of BDE-209 and lower-brominated BDE congeners led Norway, in 2013, to nominate c-decaBDE for inclusion in the Stockholm convention. The basis of Norway’s proposal was a congener-specific human health risk assessment of mixtures of decaBDE and other PBDE congeners that was commissioned by the Norwegian Environmental Protection Agency (Kortenkamp et al. 2013), which we summarize and expand upon in this paper.
The need for conducting mixture risk assessment (MRA) derives from evidence that humans, at all life stages, come into contact with PBDE mixtures. Furthermore, several congeners, including BDE-209, -47, -99, -153, -183, -203, and -206 are capable of inducing neurodevelopmental effects in rodents (Viberg et al. 2006, 2007; Viberg 2009; EFSA 2011). In these studies, alterations in spontaneous behavior, indicating impaired habituation in new environments, and hyperactivity were consistently found in young animals after PBDE exposure. Impaired learning occurred in adult animals exposed prenatally to BDE congeners. Although complex measures of developmental neurotoxicity in rodent models cannot be equated directly with outcomes in humans, experimental findings have raised concerns about the potential for neurodevelopmental effects of PBDE exposures in babies and young children, potentially resulting in IQ loss and other consequences (EFSA 2011). Accordingly, neurodevelopmental toxicity in rodents has been the basis for reference doses used in human risk assessments for BDE-47, -99, -153, and -209 (EFSA 2011). The precise mechanisms by which PBDEs exert neurodevelopmental toxicity have not been fully established. Some PBDEs have been shown to cause pregnane X receptor (PXR)- and constitutive androstane receptor (CAR)-mediated induction of liver enzymes responsible for thyroid hormone clearance, which might compromise brain development by causing thyroid hormone insufficiency (EFSA 2011; Costa and Giordano 2011). Another possible mode of action is via direct toxicity to developing neurons (Costa and Giordano 2011). Given the structural similarity of PBDE congeners, the same potential modes of action might also be expected to operate when PBDEs are present as mixtures. Accordingly, the European Union Scientific Committee SCHER (European Commission 2011) proposed that PBDEs should be subjected to MRA using dose addition as the evaluation concept, with the assumption that all PBDEs exhibit the same mode of action and toxicity.
Meek et al. (2011) performed an MRA for PBDEs to illustrate the tiered mixture assessment framework developed under the auspices of the World Health Organization International Programme on Chemical Safety (WHO/IPCS). In this analysis, they assigned toxicity values to groups of PBDE congeners (e.g., a value for all 42 tetra-brominated PBDEs) instead of using congener-specific data. Their evaluation suggested large margins of exposure (MOEs), such that estimated human exposures were well below minimum exposure levels associated with neurobehavioral effects in mice. Consequently, the authors concluded that an in-depth evaluation of combined exposures to PBDEs from a human health perspective was a low priority. In contrast, the European Food Safety Authority (IEFSA) (EFSA 2011) performed a congener-specific assessment based only on estimated dietary intakes and concluded that the MOE for a single congener, BDE-99, was insufficient for toddlers (1–3 y old). Exposure via other routes was not considered by EFSA.
For the present analysis, we performed a congener-specific MRA for PBDEs that accounted for multiple life stages (fetal life, infancy, early childhood, and adulthood) and exposures via inhalation or ingestion of dust as well as dietary exposures. In addition, we addressed uncertainty about the potential role of BDE-209 and its degradation products by including higher-brominated BDEs in our evaluation. To our knowledge, this is the first time that this approach has been used for PBDEs.
Scenario-based exposure assessments of PBDEs estimate intake doses, and this process has to rely on several assumptions about food consumption, dust inhalation, body weight, and other factors. To achieve a degree of verification of these assumptions, we also performed MRAs based on PBDE body burdens, which we estimated from human biomonitoring studies of PBDE serum levels.
In conducting our MRA, we chose the hazard index (HI) approach (Teuschler and Hertzberg 1995). The HI is the sum of hazard quotients (the ratio of the estimated intake dose to the reference dose) of all chemicals in the mixture. In single-chemical risk assessments, exposures are deemed acceptable if they do not exceed reference values, such that the hazard quotient (HQ) is . The HI extends this concept to include all components of the mixture under consideration such that estimated exposures are acceptable only if the sum of the individual intake-to-reference value ratios for all of the components is . The approach can also be applied to concentrations in tissues, provided that reference concentrations are available.
We conducted our analysis with an orientation towards the tiered MRA approach developed by WHO/IPCS (Meek et al. 2011). The lowest tier uses conservative assumptions about exposures and toxicities. If these “worst case” estimates suggest acceptable risks, no further assessment is needed; otherwise, the assessment proceeds to the next higher tier where less conservative (more “realistic”) assumptions are used for both the exposure assessment and the hazard assessment components of the MRA. This process continues, using more refined data and increasingly less conservative assumptions, until no further refinements are possible based on available information.
To make the assessment relevant to BDE-209 and its degradation products, we had to retain higher-brominated BDEs in our evaluation. However, reference doses needed for hazard characterizations are currently available only for BDE-47, -99, -153, and -209 (EFSA 2011) (Table 1). Therefore, for our most conservative assessment, we used the reference dose for BDE-99 as the reference dose (critical value) for all congeners with missing information, thus assuming that all are as potent as BDE-99. For our next level of assessment (moderately conservative), we adopted a read-across approach, whereby the reference dose of the nearest neighboring congener was assigned to each congener with missing information. For our least-conservative assessment, we omitted from the analysis all BDE congeners for which hazard information was not available, which is somewhat contradictory to the idea of refining an analysis by introducing less conservative but more realistic assumptions. Consequently, we refer to our highly conservative, moderately conservative, and least conservative assessments as Levels 1, 2, and 3, respectively, rather than referring to them as “tiers,” as in Meek et al. (2011). In addition to using more or less conservative assumptions about reference doses, we applied 3–4 different exposure scenarios within Levels 2 and 3.
Table 1.
Derivation of reference doses from single administration studies in rodents.
| Congener | Critical end point | () | Critical body burden at ()a | Critical daily intake for humans associated with a body burden in rodents at (ng/kg bw/d) (EFSA 2011)b | Reference dose (ng/kg bw/d)c |
|---|---|---|---|---|---|
| BDE-47 | Mice, locomotion | 309 | 232 | 172 | 68.8 |
| BDE-99 | Mice, total activity | 12 | 9 | 4.2 | 1.68 |
| BDE-153 | Mice, total activity | 83 | 62 | 9.6 | 3.84 |
| BDE-209 | Mice, total activity | 1,700 | 425d | 1,700,000 | 17,000 |
Note: , benchmark dose for a 10% neurodevelopmental toxicity effect; bw, body weight; PBDE, polybrominated diphenyl ether.
The PBDE body burden in rodents at a dose equivalent to the , calculated by using a one-compartment toxicokinetic model. Taken from EFSA (2011), p 157, Table 40.
These values were obtained by EFSA by reverse toxicokinetic modeling using the critical rodent body burdens at . See EFSA (2011), p 158 ff, Chapter 9; this applies to BDE-47, -99 and -153. For BDE-209, the critical intake equals .
We derived these values by dividing the critical daily intake for humans associated with a body burden in rodents at for BDE-47, -99 and -153 by a factor of 2.5; for BDE-209, a factor of 100 was applied (EFSA 2011).
Our calculation, assuming 25% absorption (EFSA 2011).
Methods
Choice of Mixture Risk Assessment Method
We used the HI method (Teuschler and Hertzberg 1995) and attempted a stepwise approach related to the WHO/IPCS framework (Meek et al. 2011). The HI method can be defined by the following formula:
| [1] |
where is the exposure level of the component, is the acceptable level of the component, and is the number of components in the mixture. An HI is interpreted as exceedance of acceptable combined exposures.
Systematic Literature Search for Exposure Data
To compile exposure data, including levels in dust, fetal livers, mother’s milk, and serum concentrations in children and adults, we used the following search terms: 2,3,4,5,6-pentabromo-1-(2,3,4,5,6-pentabromophenoxy)benzene, decabromodiphenylether, decabrominated diphenyl ether, decabrominated diphenyl ethers, decabromodiphenyl oxide, decaBDE, deca-BDE, BDE-209, BDE209, BDE 209, PBDE-209, PBDE209, 1163-19-5, deca-BDEs, decaBDEs, deca BDEs. All terms were joined with “OR” and covered all years, with English as the search language. We used Web of Knowledge (including Web of Science and MEDLINE/PubMed). This search yielded 983 results in October 2013, with 21 results added in June 2016. We applied the selection criteria detailed below to build the data sets for our MRA. A literature database was built to contain all the unique results from the defined search (see Tables S1, S4, and S5).
Study Selection for Evidence of Coexposure to PBDE Congeners
For dietary exposures, we used the extensive dietary survey by EFSA (2011) which describes intake levels of BDE-28, -47, -99, -100, -153, -154, -183, and -209. This is by far the most extensive survey worldwide of PBDE exposures via food. The dietary intake estimates were based on measured values of these 8 congeners in 3,933 food samples representing different food categories, with a total of 25,824 observations. These data were combined with survey data used to estimate daily intakes of the different foods by adults and by subgroups of children in European Union member states. For intake estimates via breast milk and dust, we considered all studies that in addition to the eight congeners analyzed by EFSA (2011) also identified at least one of the following congeners that are relevant as BDE-209 debromination products: BDE-196, -197/204, -198/203, -202, -206, and -207. Studies that did not contain data about at least one of these congeners were excluded from consideration, as were studies that did not contain congener-specific data (i.e., literature that reported only summed values, “Sum PBDE,” “octa-BDE,” and so forth).
Life Stages
We conducted exposure estimates for four life stages: the developing fetus, breastfed infants (0–3 mo of age), toddlers (1–3 y of age), and adults.
Estimating Dietary Intakes of PBDE Congeners from Food Consumption Data for Specific Age and Consumer Groups
We estimated the PBDE intake of breastfed infants based on our review of studies of PBDE levels in breast milk (see Table S1), with the assumption of a mean intake of breast milk per day (average milk consumption), a high intake of per day (high milk consumption), a milk fat content of 3.5%, and an average body weight of (EFSA 2011). Four levels with decreasing conservatism were used for the MRA (see Table S6): one based on the highest PBDE breast milk levels reported from studies all over the world, one based on the highest levels reported in European studies, and two using the data reported by Jakobsson et al. (2012). We selected the Jakobsson study because of its well-documented sampling protocol and its description of study subjects that followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. Another advantage of the Jakobsson et al. (2012) study was that median values (and not means) were reported. Only three levels of this analysis are shown in Table 2, omitting the most conservative MRA. We used three dietary exposure scenarios for young children (1–3 y old) (see Table S2). The most conservative (highest exposure) scenario used intakes estimated by EFSA (2011) assuming high food consumption (at the 95th percentile for the EFSA study population) and using the upper bound value for the median concentration of each congener in food samples. Here, the upper bound is the value obtained when the numeric value of the limit of detection (LOD) (reported by each laboratory) is assigned as the concentration for all samples reported as , and the numeric value of the limit of quantitation (LOQ) (reported by each laboratory) is assigned as the concentration for all samples reported as (EFSA 2011). The intermediate scenario used the same individual congener concentrations but assumed the average level of food consumption, and the least conservative scenario assumed average food consumption and used the lower bound value for the median concentration of each congener in food samples, where the lower bound value is the value obtained when all samples reported as or are assigned a concentration of 0 (EFSA 2011).
Table 2.
Compilation of hazard indices calculated for various exposure scenarios in the level 2 (intermediate conservatism) and 3 (low conservatism) PBDE mixture risk assessment.
| Life stage | Exposure scenario | Level 2 hazard indexa | Level 3 hazard indexb | |||
|---|---|---|---|---|---|---|
| No. | Description, location | PBDE levels, statistic | Reference | |||
| Infants (0–3 months) | 1 | High exposure through high breast milk consumption, Europe | Highest means | Table S1 | 11 | 8 |
| 2 | Moderate to low exposure via average breast milk consumption, Sweden | Median | Jakobsson et al. (2012) | 5.4 | 3.6 | |
| 3 | Low exposure, Sweden | Minimum | Jakobsson et al. (2012) | 2.4 | 1.6 | |
| Small children (1–3 years) | 1 | High exposure via high food consumption (95th percentile), high exposure via dust, Europe | Food: Upper bound median; Dust: Highest means in homes, Europec | Food: Table S2 Dust: Table S4 | 5.6 | 3.4 |
| 2 | Moderate exposure via food, moderate exposure via dust in homes, Sweden | Food: Upper bound median; Dust: Means in Swedish homesd | Food: Table S2, Dust: Björklund et al. (2012) | 2.7 | 1.5 | |
| 3 | Lower exposure via food, low exposure via dust | Food: Lower bound median; Dust: Minima in Swedish homesd | Food: Table S2, Dust: Björklund et al. (2012) | 1.1 | 0.8 | |
| Adults | 1 | High exposure via high food consumption (95th percentile), additional fish consumption, high exposure via dust | Food and additional fish intake: Upper bound median; Dust: Highest means any location | Food: Table S3, Dust: Table S4 | 6.8 | 4.0 |
| 2 | As 1, but average food consumption and moderate exposure via dust | Food and additional fish intake: Upper bound median; Dust: Highest means Europe | Food: Table S3, Dust: Table S4 | 2.8 | 0.98 | |
| 3 | High exposure via high food consumption (95th percentile), but no additional fish consumption, high exposure via dust, Europe | Food: Upper bound median; Dust: Highest means Europe | Food: Table S3, Dust: Suppl Mat Table S4 | 1.25 | 0.6 | |
| 4 | Moderate to low exposure via food, no additional fish consumption, moderate exposure via dust in homes, Europe | Food: Upper bound median; Dust: Highest means Europe | Food: Table S3, Dust: Table S4 | 0.66 | 0.33 | |
Note: PBDE, polybrominated diphenyl ether. Details of all calculations are shown in Tables S6 (infants), S7 (small children) and S8 (adults).
Corresponding to intermediate conservatism and calculated using the read-across approach to bridge missing reference doses.
Corresponding to low conservatism and calculated based on BDE-47, -99, -153 and -209 only.
Calculated assuming dust intake at the 95th percentile ( for a child), Trudel et al. (2011).
Calculated assuming moderate ingestion of dust ( for a child).
For adults, we considered four dietary exposure scenarios, all of which used intakes estimated by EFSA (2011) based on the upper bound of the median concentration of each congener in food samples (see Table S3). The most conservative scenario also assumed high food consumption (at the 95th percentile for the EFSA study population) plus high consumption of fish with individual congener concentrations based on the upper-bound average values measured in fish with fat. We also used two intermediate scenarios: one assumed high food intake without any fish intake; the other assumed average food intake with high fish intake. The least conservative scenario for adult dietary intake assumed average food intake with no fish consumption.
Estimating PBDE Congener Intakes via Dust Ingestion
In addition to the eight congeners considered by EFSA (2011) for dietary intake, we selected studies that permitted assessments of PBDE exposures via dust for BDE-196, -197, -203, -206, -207, and -208, based on the PBDE dust levels measured in various environments (see Table S4). For Level 1 assessments, we used the highest mean value reported for each congener by any study that measured concentrations in dust samples collected from any location (see Table S4). For Level 2 or Level 3 MRAs in children, we used the highest mean value of each congener reported by any European study for home dust samples, the geometric mean concentration of each congener in 18 Swedish home dust samples reported by Björklund et al. (2012), or the lowest measured concentration of each congener reported for the same samples. Concentrations from the Björklund et al. study were those measured in dust samples collected by researchers rather than in samples collected by study participants (Swedish women who delivered singleton healthy children at Uppsala University Hospital). In addition, we assumed either a high level of dust ingestion [, the 95th percentile reported by Trudel et al. (2011)] or a moderate level of dust ingestion [, corresponding to a value between the 50th and 95th percentiles reported by Trudel et al. (2011)]. For Level 2 or Level 3 MRAs of adults, we used the highest mean value of each congener reported by any European study for home dust samples only. We selected the study by Björklund et al. (2012) for two reasons: First, considering that the dietary intake estimates of PBDE in our analysis were of European origin, we chose a European study of dust levels in the interest of maintaining consistency. Second, of all European studies, Björklund et al. (2012) reported the most complete set of highly brominated PBDEs. On the basis of these data, PBDE intake via dust for children and adults was estimated using , which is the 95th percentile value for ingestion of dust for children who were 3 to 6 y old (Calabrese et al. 1996), and which lies between the 95th percentile and the 50th percentile (). For adults, we applied , the 95th percentile (Stanek et al. 1997), and , which is the central tendency value from the U.S. Environmental Protection Agency (U.S. EPA 2011). To estimate the contribution of dust ingestion in units of nanograms PBDE per kilogram body weight per day, the body weight was assumed to be equal to the default values recommended by EFSA (2011) for toddlers (1–3 y old) and adults: and , respectively.
Estimating Body Burdens for PBDE Congeners
We estimated PBDE body burdens on the basis of measured congener-specific PBDE levels (lipid weight basis) from biomonitoring studies using serum or adipose tissue by multiplication with the fraction of body lipids appropriate for each age group: 0.07 for fetuses at gestational weeks 10–13 (Zhao et al. 2013); 0.09 (median) or 0.14 (95th percentile) for stillborns (Hawkes et al. 2011); 0.2 for breastfeeding infants; and 0.29 for children 1–3 y old and for adults (Carberry et al. 2010; Jackson et al. 2002). The purpose of this analysis was to evaluate whether combined PBDE body burdens in humans reach rodent critical body burdens associated with neurodevelopmental toxicity.
Our literature search failed to locate relevant studies with breastfeeding infants. For fetal life, only one study was available that met our selection criteria, the one by Zhao et al. (2013), but we also considered Schecter et al. (2007). The measurements in the Zhao et al. (2013) study are from 65 elected abortions. The results from the Schecter et al. (2007) study are based on measurements in liver tissue from 5 stillborn infants and 6 live-born infants who died within 7 d of birth, most of whom had serious abnormalities.
We identified three studies among children (Lunder et al. 2010; Sahlström et al. 2014; Wu et al. 2015) and 10 studies among adults (see Table S5, which also gives information about the size of the study populations, their gender, and their location) that satisfied our selection criteria. We selected all three studies for our MRA in children. For MRA in adults, we chose Antignac et al. (2009) because this was the largest study (91 subjects) that reported on the most complete set of higher-brominated PBDEs. All of the chosen studies reported PBDE serum levels as maxima, means (Antignac et al. 2009), medians (Lunder et al. 2010; Sahlström et al. 2014), or geometric means (Sahlström et al. 2014; Wu et al. 2015). We used two exposure scenarios for our MRA. The most conservative (highest exposure) was based on maximum PBDE levels. The less conservative scenario used mean levels (Antignac et al. 2009), medians (Lunder et al. 2010; Sahlström et al. 2014), or geometric means (Wu et al. 2015).
Selection of Reference Doses for PBDE Congeners
We utilized the points of departure for BDE-47, -99, -153, and -209 derived by EFSA (2011), which are based on the lower 95% confidence limits for benchmark doses for a 10% neurodevelopmental toxicity effect in the mouse, . EFSA used the rodent doses to estimate the corresponding rodent “critical” body burdens of BDE-47, -99, and -153 (Table 1) and then derived the human intakes of BDE-47, -99, and -153 that lead to the same critical body burdens in humans using toxicokinetic modeling under steady-state conditions. This body burden approach was chosen by EFSA (2011) to correct for differences between rodents and humans in the elimination kinetics of most PBDEs. As a result of such differences, exposure to similar external doses of PBDEs will produce higher concentrations in humans than in rodents. For this reason, body burdens provide a better basis for comparisons between humans and rodents. For the risk-characterization step, EFSA considered a margin of exposure (MOE) of 2.5 to be adequate for the BDE congeners where body burden considerations were applied (BDE-47, -99, -153) (EFSA 2011). For BDE-209, the toxicokinetics in animals and humans are assumed to be similar; therefore, EFSA (2011) applied the external dose level () obtained from animal studies directly for the human health risk characterization instead of using a body-burden approach, and an MOE of 100 was judged to be sufficient. Because the HI approach is incompatible with the application of an MOE and requires reference doses as input values, we used the minimum MOEs judged by EFSA (2011) to be acceptable as uncertainty factors to obtain reference doses (a factor of 2.5 for BDE-47, -99, and -153 and a factor of 100 for BDE-209) (Table 1). To conduct an MRA for fetuses, we estimated a critical body burden for BDE-209 based on the BDE-209 reference dose and assuming an absorption fraction of 25% (Table 1).
Scenarios Considered in the Exposure Assessment Component of MRA
We analyzed various exposure scenarios organized in terms of different exposure levels, from high to moderate to low. Scenarios for different levels of dietary exposures were combined with scenarios for different levels of exposure to dust to complete the MRAs for each age group (Table 2). As for the specific values used for dietary and dust exposures individually, diet and dust exposures were combined to create scenarios for exposures through both routes that represented high, moderate, or low levels of conservatism.
Dealing with Data Gaps in the Hazard Assessment Component of MRA
Only 4 congeners (BDE-47, -99, -153, and -209) have been evaluated to a level of detail that permits hazard characterization (EFSA 2011); data suitable for hazard characterization are not available for the other 12 congeners considered in our analysis. To deal with this difficulty, we made various assumptions in the hazard assessment arm of our MRA that substituted for missing data. Accordingly, in Level 1 of our assessment (high conservatism), we filled the gaps left by missing reference doses for congeners by assuming that they are as potent as BDE-99. In Level 2 (intermediate conservatism), we adopted a read-across approach whereby missing reference doses were bridged by using the reference dose of the neighboring congener as follows: BDE-28 was assigned the reference dose for BDE-47, BDE-100 was assigned that for BDE-99, BDE-154 and -183 were assigned that for BDE-153, and the remaining highly brominated congeners (BDE-196 and above) were assigned the reference dose for BDE-209. Finally, in Level 3 of the assessment (low conservatism), we only used reference doses for BDE-47, -99, -153, and -209, and we disregarded the remainder of the congeners. Details of this procedure can be found in Tables S6–S8. Because this process cannot be equated with the refinements at higher tiers intended by Meek et al. (2011), we used the term “levels” rather than “tiers.”
Results
For all life stages except fetal life (see below), Level 1 assessments produced very high HIs. These HIs were derived from calculations assuming that all BDE congeners not assigned a reference dose are as toxic as the most toxic congener, BDE-99. However, because other BDEs have been shown to be less potent than BDE-99 (EFSA 2011; see also Table 1), these HIs have limited risk assessment relevance other than to signal the need for refined analyses. For this reason, we have not considered them further in the main text of this paper. The corresponding calculations can be found in Tables S6–S8.
MRA for Breastfeeding Infants
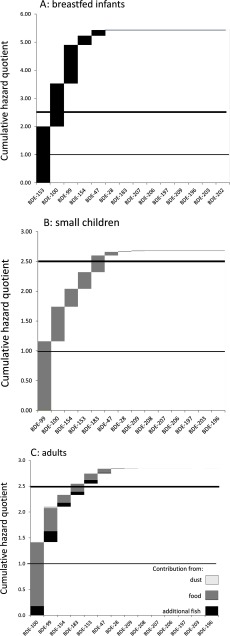
In Level 2 (intermediate conservatism), we adopted a read-across approach to fill the gaps left by missing reference doses for BDE congeners and began by considering the highest reported mean PBDE levels in breast milk from European studies assuming high milk consumption (Scenario 1). This scenario produced an HI of 11, driven by BDE-99, -100, and -153 (Table 2; see also Table S6). With the median PBDE levels in breast milk measured by Jakobsson et al. (2012) in Sweden and the assumption of average milk intake, an HI of 5.4 was obtained (Scenario 2; Table 2, Figure 1A). The minimum PBDE levels reported by Jakobsson et al. (2012) still resulted in an HI of 2.4 (Scenario 3) (Table 2).
Figure 1.
Cumulative hazard quotient plots for selected Level 2 scenarios of polybrominated diphenyl ether (PBDE) mixture risk assessment. (A) Breastfeeding infants (0–3 mo), assuming average milk consumption and median PBDE breast milk levels in Sweden (Jakobsson et al. 2012), scenario 2 (Table 2; see also Table S6). (B) Small children (1–3 y old), moderate exposure via food, with average food consumption, upper bound median PBDE levels in food (EFSA 2011) and mean PBDE dust levels in Swedish homes Björklund et al. (2012), scenario 2 (Table 2; see also Table S7). (C) Adults, assuming moderate exposure via food, with average food consumption, upper bound median PBDE levels, and additional fish consumption (EFSA 2011), highest mean PBDE dust levels in Europe, scenario 2 (Table 2; see also Table S8). Details of all exposure scenarios are described in Table 2 and in Tables S6–S8. Horizontal lines show hazard indexes (HIs) of 1, above which a margin of exposure (MOE) of 2.5 cannot be maintained, and 2.5, above which exposures are equivalent to the body burdens in rodents associated with neurodevelopmental effects at the benchmark dose for a 10% neurodevelopmental toxicity effect ().
When we restricted the analysis to the four congeners evaluated by EFSA (2011) (Level 3), the scenarios above yielded HI values ranging from 8 (Scenario 1) to 1.6 (Scenario 3) (Table 2). In all of these cases, BDE-209 and other highly brominated congeners (BDE-197 and higher) did not contribute to the HI.
MRA for Small Children (Toddlers, 1–3 y Old)
Estimated PBDE exposures for small children were influenced not only by assumptions about food intake but also by assumptions about exposure via dust inhalation and ingestion (see Table S7). In Level 1 of the analysis (high conservatism), we assumed high PBDE intake via food and high levels of dust ingestion ( for a child), and we used the highest PBDE dust levels measured in any location in the world. This analysis produced an HI of 23. In the Level 2 analysis (moderately conservative), we considered exposure scenarios that assumed high or moderate PBDE intake via food; high or moderate levels of dust ingestion [ or for a child, respectively, based on data reported by Trudel et al. (2011)]; and high, moderate, or low congener concentrations in dust.
At one extreme of the Level 2 analyses, we assumed high PBDE intake via food [toddlers at the 95th percentile of food consumption, with upper-bound median PBDE levels (EFSA 2011)] and exposure via dust based on the highest mean PBDE dust levels measured in any European home. This scenario gave an HI of 5.6 (Scenario 1; Table 2).
Based on moderate exposure via food, with average food consumption and upper bound median PBDE food levels (EFSA 2011), and with the geometric mean PBDE dust levels in Swedish homes reported by Björklund et al. (2012), an HI of 2.7 was obtained (Scenario 2; Table 2, Figure 1B; see also Table S7). This scenario assumed moderate dust ingestion of for a child weighing . Björklund et al. (2012) measured PBDE levels in dust samples collected by researchers in 2008 in the homes of 18 primiparous Swedish women who each delivered a healthy child at Uppsala University Hospital. The HI values were driven by BDE-99, -100, -154, -153, and -183. A calculation based on lower bound median dietary PBDE intakes (EFSA 2011) and the minimum PBDE dust levels in Swedish home environments (Björklund et al. 2012) yielded an HI of 1.1 (Scenario 3). In this scenario, BDE-99 and -100 were drivers of the HI.
When only congeners with EFSA reference doses were considered (BDE-47, -99, -153, and -209) (Level 3, low conservatism), two of the three scenarios analyzed still yielded HI values , ranging from 3.4 with high exposure via food combined with the highest mean PBDE dust levels in Europe (Scenario 1) to 0.8 (Table 2; see also Table S7). The contribution of BDE-209 to the HI was small for all scenarios.
MRA for Adults
The most conservative Level 2 MRA produced an HI of 6.8 (Scenario 1; Table 2). This scenario assumed the 95th percentile of food consumption with individual congeners based on the upper bound value of median concentrations (EFSA 2011), high consumption of fish () with individual congeners at the upper bound value of mean concentrations in fish with fat (EFSA 2011), and moderate consumption of dust () (U.S. EPA 2011) with individual congener concentrations at the highest mean values reported for dust samples from any location in the world (Table 2; see also Table S8). The HI was driven by BDE-100, -99, -154, -183, and -153. Scenario 2, based on the same exposures via fish and dust and the same individual congener concentrations in food (upper bound value of median concentrations) used for Scenario 1 but with only average (vs. high) food consumption, produced an HI of 2.8 (Table 2; see also Table S8.)
With food consumption at the 95th percentile in Europe but without taking account of exposure via fish, we observed an HI of 1.25 (Scenario 3). Only under the assumption of average food consumption with PBDE congener levels based on the upper bound value of the medians (EFSA 2011), with dust exposure as before, but with no fish consumption, could we achieve an HI below 1 (0.66; Scenario 4). Our assumptions regarding the presence or absence of exposure via additional fish consumption strongly influenced our HI estimates for adults. The proportional contribution of estimated PBDE exposure via dust varied between 5% and 20% depending on the estimated contribution of PBDE exposure via food and additional fish intake.
HI estimates for Level 3 MRAs, which assumed that exposures were limited to the four BDE congeners with reference doses only, ranged from 4.0 to 0.33 (Table 2; see also Table S8). The contribution of BDE-209 to the HI was very small for all scenarios evaluated.
MRA Based on Body Burden Estimations
To investigate whether combined estimated human body burdens would reach the critical body burdens associated with neurodevelopmental effects in rodents, we conducted MRAs using PBDE body burden estimations derived from human biomonitoring studies of PBDE levels in serum lipids. This analysis could only be conducted for fetuses, small children, and adults, but not for breastfeeding infants because, to our knowledge, data on PBDE serum levels for this age group are not available. To derive HQs, we divided congener-specific body burden estimates by the critical body burden values in rodents for neurodevelopmental effects that were estimated by EFSA (2011) to arrive at with an uncertainty factor of 2.5.
The only study of PBDE levels in fetuses that measured BDE-209 and at least one other highly brominated congener (and thus met our selection criteria) was an analysis of 65 Chinese fetuses aborted during the first trimester of pregnancy (10–13 wk) (Zhao et al. 2013). A study from the United States based on measurements in liver tissue from 5 stillborn infants and 6 live-born infants who died within 7 d of birth measured only BDE-209 but no other highly brominated congeners (Schecter et al. 2007). Using the mean and maximum congener levels determined by Zhao et al. (2013), we estimated the corresponding mean and maximum body burdens for each measured congener (EL in Equation 1) and derived congener-specific HQs by using the rodent critical body burden values for neurodevelopmental effects from EFSA (2011) (Table 1), together with an uncertainty factor of 2.5 (AL in Equation 1). The resulting HQs in Level 1 of the analysis (high conservatism), where congeners with missing toxicity data were assumed to be as toxic as BDE-99, summed to small HI values (0.027) when based on the maximum PBDE levels from Zhao et al. (2013), indicating that unacceptable exposures are not likely to arise. Because historical PBDE exposure patterns in China differ from those elsewhere, we extended our evaluation to estimated body burdens derived from the PBDE congener levels reported by Schecter et al. (2007). This analysis yielded higher HIs than those based on the levels reported by Zhao et al. (2013), but there was still no need to refine the analysis further because the values remained well below 1 (Table 3; see also Table S9).
Table 3.
Hazard indices for PBDE body burdens estimated from tissue levels and compared to critical body burdens associated with neurodevelopmental toxicity in rodents, assuming intermediate (level 2) and low conservatism (level 3).
| Life stage | Exposure scenarios used as inputs for body burden estimations | Level 2 hazard indexa | Level 3 hazard indexb | |||
|---|---|---|---|---|---|---|
| Country | Tissue | Reference | Metric for PBDE levels | |||
| Fetal life | USA | Fetal liver | Schecter et al. 2007 | Medianc | 0.29 | ND |
| Mediand | 0.18 | ND | ||||
| Small children (1–4 years) | Sweden | Serum | Sahlström et al. 2014 | Maximum | 0.8 | 0.5 |
| Median | 0.1 | 0.038 | ||||
| USA | Lunder et al. 2010 | Maximum | 3.4 | 1.8 | ||
| Median | 1.3 | 0.8 | ||||
| Children (2–8 years) | USA | Serum | Wu et al. 2015 | 95th percentile | 13.7 | 8.4 |
| Geometric mean | 2.5 | 1.4 | ||||
| Adults | France | Serum | Antignac et al. 2009 | Maximum | 2.1 | 1.64 |
| Mean | 0.38 | 0.31 | ||||
| USA | Lunder et al. 2010 | Maximum | 1.5 | 0.92 | ||
| Mean | 0.41 | 0.27 | ||||
Note: Detailed calculations are shown in Tables S10–S15. ND, not determined; PBDE, polybrominated diphenyl ether.
Corresponding to intermediate conservatism and calculated using the read-across approach to bridge missing reference doses and the critical body burden in rodents at the benchmark dose for a 10% neurodevelopmental toxicity effect () (Table 1); see Tables S9, S10, and S12.
Corresponding to low conservatism and calculated based on BDE-47, -99, -153, and -209 only; see Tables S11, S13, and S15.
Median PBDE levels, body burden calculated assuming the 95th percentile of body fat proportion (Hawkes et al. 2011).
Median PBDE levels, body burden calculated assuming median body fat proportion (Hawkes et al. 2011).
We performed three sets of MRAs based on body burden data for children using data on congener concentrations in serum samples reported by three studies. For the first study of twenty-four 11–15-mo-old Swedish children (Sahlström et al. 2014), we estimated individual congener concentrations as either the maximum concentration reported for each congener in any of the study samples or the median concentration over all study samples (see Table S5). For the Level 2 MRA (using reference values for neighboring congeners as the values for congeners without established values), this estimation resulted in HIs of 0.8 and 0.1, respectively (Table 3; see also Table S10), and the corresponding HIs for the Level 3 MRA (assuming exposures were to BDE-47, -99, -153, and -209, i.e., to the four congeners with reference doses only) were 0.5 and 0.038, respectively (Table 3; see also Table S11).
The second study was based on serum congener concentrations reported by Lunder et al. (2010) for 20 children 1.5–4 y of age living in 11 U.S. states. As for Sahlström et al. (2014), we used either the maximum concentration reported for each congener in any study sample or the median concentration over all study samples (see Table S5), which resulted in Level 2 HIs of 3.4 and 1.3, respectively (Table 3; see also Table S10), and in Level 3 HIs of 1.8 and 0.8, respectively (Table 3; see also Table S11).
The third study was based on serum congener concentrations reported by Wu et al. (2015) for a study population of 67 California children 2–8 y of age. For these MRAs, we assumed individual congener concentrations corresponding to the 95th percentile for the study population or concentrations corresponding to the geometric mean value for the population (see Table S5), which resulted in Level 2 HIs of 13.7 and 2.5, respectively (Table 3; see also Table S10), and in Level 3 HIs of 8.4 and 1.4, respectively (Table 3; see also Table S11).
We performed two sets of MRAs using body burden data for adults based on serum concentrations for individual congeners reported by two studies. The first study measured PBDEs in serum samples collected from 93 French women who underwent Caesarian sections at a single hospital (Antignac et al. 2009); the second measured PBDEs in serum samples provided by 20 women living in 11 U.S. states who had at least one child, including 3 women who were pregnant when the samples were collected (Lunder et al. 2010). For both studies, we performed Level 2 and Level 3 MRAs using the maximum concentration of each congener in any study sample and using the mean concentration of each congener over all samples. The Level 2 analysis of the French data yielded HIs of 2.1 and 0.38, respectively, and the Level 3 analysis produced HIs of 1.64 and 0.31, respectively (Table 3; see also Tables S12 and S13).
The Level 2 analysis of the U.S. data yielded corresponding HIs of 1.5 and 0.41, respectively, and the Level 3 analysis produced HIs of 0.92 and 0.27, respectively (Table 3; see also Tables S14 and S15).
Discussion
The HI estimates based on most of our congener-specific MRAs for PBDEs suggest that tolerable combined exposures may be exceeded () in breastfeeding infants, young children, and adults, even with exposure scenarios that assumed average congener concentrations, average food or dust intakes, or a combination of these factors. Our assumptions and the resulting estimates require confirmation, but they support concerns about potential consequences of PBDE exposures for neurodevelopment in babies and in children.
Our estimates and their interpretation are based on several assumptions, including the assumption that developmental neurotoxicity of BDE-47, -99, -153, and -209 in rodents has relevance to humans, in terms of both quality and potency, and the assumption that PBDEs act together in a dose-additive manner. The first of our assumptions is a central tenet of established single-BDE congener risk assessment (EFSA 2011), and we see no reason to doubt its applicability to joint exposures to several BDEs.
Regarding the second of our assumptions, there is scant empirical evidence to support the idea that PBDEs act in a dose-additive manner. To our knowledge, only one in vitro study has evaluated the effects of exposure to multiple PBDEs (Tagliaferri et al. 2010). Specifically, combined exposures to BDE-47 and -99 were reported to have synergistic cytotoxic effects on neuronal cells. More recently, Pellacani et al. (2014) reported evidence of both synergistic and dose-additive effects on in vitro cytotoxicity in neuronal cells when either BDE-47 or BDE-99 was combined with individual polychlorobiphenyl (PCB) congeners. Considering their similar toxicity profiles (Costa et al. 2014), it is plausible that several PBDEs are capable of producing joint neurodevelopmental toxicity (European Commission 2011). There is evidence of combined effects between BDE congeners and PCBs in vitro (He et al. 2009, 2010) and in vivo with neurotoxicity in mice as the end point of investigation (Eriksson et al. 2006; He et al. 2011). Coexposure of BDE-99 and methyl mercury exacerbated the neurotoxicity of the former in mice (Fischer et al. 2008). In these studies, the combined effects of PBDEs and PCBs or methyl mercury were evaluated in relation to a common adverse effect (e.g., cytotoxicity in neuronal cells) irrespective of the molecular mechanisms that led to these adverse outcomes. It is likely that PBDEs trigger a multitude of different pathways, all leading to developmental neurotoxicity, for example, by producing thyroid hormone insufficiency via induction of thyroid hormone–metabolizing liver enzymes or by direct cytotoxicity on neurons, or both. There is considerable potential for the induction of combined effects (synergistic, additive) when pollutants other than PBDEs are present (e.g., other heavy metals, perchlorate), but this has not been investigated systematically. In the absence of such information, our analysis, with its assumption of additive combination effects, may well turn out to be insufficiently conservative because we could only consider PBDEs.
Our HI estimates were influenced by our assumptions about the potency of 12 BDE congeners that do not have established reference doses. The read-across approach, which we used in Level 2 analyses, has the advantage of making good use of available chemical analytical data, but it rests on the untested assumption that the toxicities of BDE-47, -99, -153, and -209 can be extrapolated quantitatively from those of their nearest neighbors. Although there is already evidence that BDE-203, and -206 produce the same neurodevelopmental effects in rodents (Viberg 2009), the available data do not permit dose–response analyses and are therefore insufficient to derive reference doses as was possible for BDE-47, -99, -153, and -209. With our read-across method (Level 2), the combined HI was more strongly influenced by BDE-100 (assumed to be as potent as BDE-99, with a critical body burden of ) than by BDE-154 and -183 (assigned the reference value of BDE-153, ), and it was least influenced by the higher-brominated congeners that were assigned the reference value of BDE-209 () (Figure 1). If BDE-206 or -208 is more toxic than BDE-209, our Level 2 MRAs may have underestimated the risks of combined exposures.
It is not possible to determine how well our estimates approximate the true extent of combined risks given multiple sources of uncertainty. However, our least conservative Level 3 assessments are likely to have underestimated risks because they made the tacit assumption that only the four toxicologically evaluated congeners contributed to risk, and the remaining 12 congeners were assumed to be toxicologically inert. Nevertheless, the Level 3 HIs are useful as lower-bound estimates of the true risks of cumulative PBDE exposures, which (if all other assumptions about exposures, dose additivity, and the relevance of rodent data are valid) are likely to lie somewhere between the Level 2 and Level 3 HI estimates (Table 2).
For a quantitative interpretation of these HI values, it is helpful to consider that values suggest that the MOE of 2.5 utilized by EFSA (2011) in their risk assessments of BDE-47, -99, and -153 is no longer maintained. Values suggest intakes that under steady-state conditions will result in body burdens comparable to those in rodents at the borderline of observable neurodevelopmental toxicity (EFSA 2011).
A case could be made for viewing the EFSA (2011) MOE of 2.5 as insufficiently protective, with an MOE of 7.5 judged as more appropriate for the three lower-brominated BDE congeners (Martin et al. 2013). However, in view of the already large HI values produced by application of the 2.5 MOE, this argument would not materially change the outcome of our assessment. Adoption of an MOE of 7.5 for defining reference doses would have led to 3-fold larger HIs.
Nearly all Level 2 and Level 3 scenarios for breastfed infants produced estimated HIs (Table 2). The Level 2 HI values were driven by BDE-153, -100, and -99, all of which had congener-specific HQs based on all but the lowest exposure scenario (see Table S13), consistent with inadequate MOEs for the individual congeners. EFSA (2011) also estimated single-congener HQs for BDE-99 and -153 in infants but dismissed concerns about insufficient MOEs with the assumption that critical body burdens could not be attained during infancy because it takes approximately 10 y (3–4 half-lives) for BDEs to reach a toxicokinetic steady state. However, our HI estimates for combined exposures raise concerns that highly exposed breastfed infants might reach a combined PBDE body burden close to that associated with neurodevelopmental toxicity in rodents, even before a steady state is established. Future studies should compare PBDE body burdens estimated based on serum levels in breastfed infants with body burdens associated with developmental neurotoxicity in rodents. To our knowledge, however, such data are currently not available.
For small children, HI values of approximately 1 were estimated only for exposure scenarios that assumed lower-bound median PBDE intakes via food (EFSA 2011) and the minimum PBDE levels reported for dust samples collected by researchers from 18 homes in Sweden (Björklund et al. 2012).
When body burdens for children were estimated based on serum PBDE levels reported by two U.S. studies (one of 20 children, 1.5–4 y old, living in 11 U.S. states, and one of 67 California children 2–8 y old) (Lunder et al. 2010; Wu et al. 2015), the HI values were , suggesting exposures within the range associated with neurodevelopmental effects in rodents (Table 3). In contrast, HIs were when estimated using serum PBDE levels reported for 24 Swedish children who were 11–15 mo old, even when maximum concentrations were assumed (Sahlström et al. 2014).
Ranges of HI estimates based on serum PBDE concentrations in children (Table 3) were broadly consistent with HIs based on PBDE intakes via food and dust (Table 2), which provides some additional support for the assumptions used to estimate PBDE intakes.
Level 2 HI estimates for adults that assumed upper-bound median intakes in food and fish, high concentrations in dust, high fish intake, and either average or high food consumption, exceeded values for acceptable combined PBDE exposures (HIs of 2.8 and 6.8, respectively) (Table 2). In contrast, most Level 3 HIs for the same exposure scenarios that excluded all congeners other than BDE-47, -99, -153, and -209 were close to, or below, 1.
The body burden HIs derived from maximum PBDE levels in serum lipids in Level 2 of the MRA (Antignac et al. 2009, Lunder et al. 2010) were also relatively close to the critical burdens in rodents (Table 3), whereas those estimated from median PBDE levels were considerably lower.
Large estimated HI values for infants (0–3 mo) and toddlers (1–3 y old), which suggest that MOEs may be insufficient relative to critical body burdens associated with observable neurodevelopmental toxicity in mice, support concerns about the potential for neurodevelopmental effects in humans. Two recent systematic reviews (Kim et al. 2014, Roth and Wilks 2014) noted that current epidemiological evidence is limited but concluded that findings support potential links between PBDE exposures and neurodevelopment in infants and in children. Recent epidemiological studies have reported that pre- and perinatal PBDE exposures (based on levels in maternal serum, cord blood, and colostrum) are adversely associated with measures of neurodevelopment in toddlers and children (1–7 y old) (Herbstman et al. 2010, Gascon et al. 2012, Eskenazi et al. 2013, Chen et al. 2014).
Our analysis provides additional perspectives for future epidemiological studies. In addition to evaluating associations between neurodevelopmental outcomes and pre- or perinatal PBDE exposures, it would be helpful to estimate associations with PBDE exposures during infancy (based on concentrations in breast milk) and early childhood. To our knowledge, associations between childhood PBDE exposures and neurodevelopment have been evaluated by only one study, which reported that PBDE concentrations in serum samples from 272 seven-year-old children living in California were associated with lower IQ scores at the same age (Eskenazi et al. 2013). Some epidemiological studies (e.g., Eskenazi et al. 2013) have used the simple, unweighted sum of BDE-47, -99, -100, and -153 as the primary exposure measure, but future studies should consider using summed concentrations that are weighted according to the potency of individual congeners in rodents. This approach would be analogous to the HQs used in our analysis, which indicated that BDE-99, -100, -153, -154, and -183 made the greatest contributions to HI estimates for all life stages (Figure 1), thus supporting a greater emphasis on these congeners in future observational and experimental studies.
Conclusion
The results of our congener-specific MRA add to concerns about the potential consequences of combined PBDE exposures for the mental development of babies and young children and strongly support further risk-management measures aimed at limiting PBDE exposures. Although BDE-209 itself contributed little to our estimates of combined risk, it serves as a potential source of more toxic congeners that may be released through degradation (Kortenkamp et al. 2013). In addition, although exposures will continue to decline because of existing bans on lower-brominated congeners as well as new efforts to phase out BDE-209, environmental exposures will persist as PBDEs are released from manufactured products (including furniture and other consumer goods) as they break down over time (Stubbings and Harrad 2014). Therefore, there is an ongoing need to define maximum residue limits for regulating PBDE contamination of food and to expand existing measures to manage the release of PBDEs from electronic waste to include other consumer products as well.
Supplemental Material
Acknowledgments
Portions of the analysis were originally presented in a report commissioned by the Norwegian Environmental Protection Agency (Kortenkamp et al. 2013). Risk of combination effects between decabromodiphenyl ether and other polybrominated diphenyl ethers. Norwegian Environment Agency, Report M223-2014 (http://www.miljodirektoratet.no/Documents/publikasjoner/M223/M223.pdf). The analysis was extended and finalized with support from the Oak Foundation, Geneva, Switzerland, and from the SOLUTIONS project, funded by the European Union 7th Frame Work Programme, grant agreement no. 603437, all of which is gratefully acknowledged.
References
- Antignac JP, Cariou R, Zalko D, Berrebi A, Cravedi JP, Maume D, et al. 2009. Exposure assessment of French women and their newborn to brominated flame retardants: Determination of tri- to deca-polybromodiphenylethers (PBDE) in maternal adipose tissue, serum, breast milk and cord serum. Environ Pollut 157(1):164–173, PMID: 18804904, 10.1016/j.envpol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Björklund J, Sellström U, de Wit C, Aune M, Lignell S, Darnerud P. 2012. Comparisons of polybrominated diphenyl ether and hexabromocyclododecane concentrations in dust collected with two sampling methods and matched breast milk samples. Indoor Air 22(4):279–288, PMID: 22212125, 10.1111/j.1600-0668.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Stanek EJ, Barnes R. 1996. Methodology to estimate the amount and particle size of soil ingested by children. Implications for exposure assessment at waste sites. Regul Toxicol Phar 24(3):264–268, PMID: 8975756, 10.1006/rtph.1996.0139. [DOI] [PubMed] [Google Scholar]
- Carberry AE, Colditz PB, Lingwood BE. 2010. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatr Res 68(1):84–88, PMID: 20351656, 10.1203/PDR.0b013e3181df5421. [DOI] [PubMed] [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, et al. 2014. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME study. Environ Health Perspect 122(8):856–862, PMID: 24870060, 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de LR, Tagliaferri S, Pellacani C. 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230(2):282–294, PMID: 24270005, 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. 2011. Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant?. Neurotoxicology 32(1):9–24, PMID: 21182867, 10.1016/j.neuro.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency). 2012. “Support Document for Identification of Bis(pentabromophenyl) ether as a Substance of Very High Concern Because of Its PBT/vPvB Properties.” Adopted 29 November 2012, Helsinki, Finland:ECHA. 188 pp. [Google Scholar]
- EFSA (European Food Safety Authority). 2011. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on Polybrominated Diphenyl Ethers (PBDEs) in Food. EFSA J 9(5):2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Fischer C, Fredriksson A. 2006. Polybrominated diphenyl ethers, a group of brominated flame retardants, can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral defects. Toxicol Sci 94(2):302–309, PMID: 16980691, 10.1093/toxsci/kfl109. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. 2013. In Utero and childhood polybrominated diphenyl ether (PBDE) Exposures and neurodevelopment in the CHAMACOS Study. Environ Health Perspect 121(2):257–262, PMID: 23154064, 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. 2011. Opinion on “Chemicals and the Water Framework Directive: Draft Environmental Quality Standards” (PBDE). Luxembourg:European Commission; 10.2772/95861 [DOI] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. 2008. Coexposure of neonatal mice to a flame retardant PBDE 99 (2,2',4,4',5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol Sci 101(2):275–285, PMID: 17982161, 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martínez D, Carsin A, Forns J, Grimalt JO, et al. 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect 120(12):1760–1765, PMID: 23052368, 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. 2011. Gender- and gestational age-specific body fat percentage at birth. Pediatrics 128(3):e645–e651, PMID: 21824882, 10.1542/peds.2010-3856. [DOI] [PubMed] [Google Scholar]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, et al. 2009. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology 30(1):10–15, PMID: 19022289, 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- He P, Wang A, Niu Q, Guo L, Xia T, Chen X. 2011. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol Ind Health 27(3):279–288, PMID: 20947653, 10.1177/0748233710387002. [DOI] [PubMed] [Google Scholar]
- He W, Wang A, Xia T, Gao P, Xu B, Xu Z, et al. 2010. Cytogenotoxicity induced by PBDE-47 combined with PCB153 treatment in SH-SY5Y cells. Environ Toxicol 25(6):564–572, PMID: 19562743, 10.1002/tox.20517. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118(5):712–719, PMID: 20056561, 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. 2002. The effect of sex, age and race on estimating percentage fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord 26(6):789–796, PMID: 12037649, 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- Jakobsson K, Fång J, Athanasiadou M, Rignell-Hydbom A, Bergman A. 2012. Polybrominated diphenyl ethers in maternal serum, umbilical cord serum, colostrum and mature breast milk. Insights from a pilot study and the literature. Environ Int 47:121–130, PMID: 22819984, 10.1016/j.envint.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Kim YR, Harden FA, Toms L-ML, Norman RE. 2014. Health consequences of exposure to brominated flame retardants: a systematic review. Chemosphere 106:1–19, PMID: 24529398, 10.1016/j.chemosphere.2013.12.064. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Evans RM, Martin OV, Faust M, Backhaus T. 2013. “Risk of Combination Effects between Decabromodiphenyl Ether And Other Polybrominated Diphenyl Ethers.” London, UK:Norwegian Environment Agency, Report M223-2014; http://www.miljodirektoratet.no/Documents/publikasjoner/M223/M223.pdf [accessed 5 July 2017]. [Google Scholar]
- Lunder S, Hovander L, Athanassiadis I, Bergman A. 2010. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol 44(13):5256–5262, PMID: 20540541, 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- Martin OV, Martin S, Kortenkamp A. 2013. Dispelling urban myths about default uncertainty factors in chemical risk assessment–sufficient protection against mixture effects? Environ Health 12(1):53, PMID: 23816180, 10.1186/1476-069X-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek ME, Boobis AR, Crofton KM, Heinemeyer G, Van Raaij M, Vickers C. 2011. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul Toxicol Pharmacol 60:S1–S14, PMID: 21466831, 10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Pellacani C, Tagliaferri S, Caglieri A, Goldoni M, Giordano G, Mutti A, et al. 2014. Synergistic interactions between PBDEs and PCBs in human neuroblastoma cells. Environ Toxicol 29(4):418–427, PMID: 22434561, 10.1002/tox.21768. [DOI] [PubMed] [Google Scholar]
- Roth N, Wilks MF. 2014. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: a systematic review of the epidemiological literature using a quality assessment scheme. Toxicol Lett 230(2):271–281, PMID: 24583043, 10.1016/j.toxlet.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Sahlström LM, Sellström U, De Wit CA, Lignell S, Darnerud PO. 2014. Brominated flame retardants in matched serum samples from Swedish first-time mothers and their toddlers. Environ Sci Technol 48(13):7584–7592, PMID: 24927135, 10.1021/es501139d. [DOI] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Chi Tung K, Harris TR, Päpke O, Rosen R. 2007. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health A 70(1):1–6, PMID: 17162494, 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- Stanek EJ, Calabrese EJ, Barnes R, Pekow P. 1997. Soil ingestion in adults - results of a second pilot study. Ecotoxicol Environ Saf 36:249–257, 10.1006/eesa.1996.1510. [DOI] [PubMed] [Google Scholar]
- Stubbings WA, Harrad S. 2014. Extent and mechanisms of brominated flame retardant emissions from waste soft furnishings and fabrics: A critical review. Environ Int 71:164–175, PMID: 25042535, 10.1016/j.envint.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, et al. 2010. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro 24(1):116–122, PMID: 19720130, 10.1016/j.tiv.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Teuschler LK, Hertzberg RC. 1995. Current and future risk assessment guidelines, policy, and methods development for chemical mixtures. Toxicology 105(2–3):137–144, PMID: 8571352, 10.1016/0300-483X(95)03207-V. [DOI] [PubMed] [Google Scholar]
- Trudel D, Scheringer M, von Goetz N, Hungerbühler K. 2011. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol 45(6):2391–2397, PMID: 21348481, 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme). 2013. Proposal to list decabromodiphenyl ether (commercial mixture, c-BDE-209) in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. Persistent Organic Pollutants Review Committee. Ninth meeting. Rome, 14–18 October 2013 chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.9-2.English.pdf [accessed 5 July 2017].
- U.S. EPA (U.S. Environmental Protection Agency). 2011. Exposure Factors Handbook (2011 Edition). Washington, DC:National Center for Environmental Assessment. [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. 2007. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209). Neurotoxicology 28(1):136–142, PMID: 17030062, 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. 2006. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci 92(1):211–218, PMID: 16611620, 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viberg H. 2009. Exposure to polybrominated diphenyl ethers 203 and 206 during the neonatal brain growth spurt affects proteins important for normal neurodevelopment in mice. Toxicol Sci 109(2):306–311, PMID: 19380496, 10.1093/toxsci/kfp074. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Moran RE, Sjodin A, Jones RS, Tancredi DJ, et al. 2015. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ Health 14:23, PMID: 25884939, 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ruan X, Li Y, Yan M, Qin Z. 2013. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol 47(11):5939–5946, PMID: 23621775, 10.1021/es305349x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.