Abstract
Background:
Quaternary ammonium salts (QUATS), such as cetylpyridinium chloride (CPC) and benzalkonium chloride (BAK), are frequently used in antiseptic formulations, including toothpastes, mouthwashes, lozenges, throat and nasal sprays, and as biocides. Although in a recent ruling, the U.S. Food and Drug Administration (FDA) banned CPC from certain products and requested more data on BAK’s efficacy and safety profile, QUATS, in general, and CPC and BAK, in particular, continue to be used in personal health care, food, and pharmaceutical and cleaning industries.
Objectives:
We aimed to assess CPC's effects on mitochondrial toxicity and endocrine disruption in vitro.
Method:
Mitochondrial consumption and adenosine triphosphate (ATP) synthesis rates of osteosarcoma cybrid cells were measured before and after CPC and BAK treatment. Antiestrogenic effects of the compounds were measured by a luciferase-based assay using recombinant human breast carcinoma cells (VM7Luc4E2, ).
Results:
CPC inhibited both mitochondrial consumption [ ] and ATP synthesis ( ), and additional findings supported inhibition of mitochondrial complex 1 as the underlying mechanism for these effects. In addition, CPC showed concentration-dependent antiestrogenic activity half maximal effective concentration [(): )]. BAK, another antimicrobial QUATS that is structurally similar to CPC, and the pesticide rotenone, a known complex 1 inhibitor, also showed mitochondrial inhibitory and antiestrogenic effects. In all three cases, there was overlap of the antiestrogenic activity with the mitochondrial inhibitory activity.
Conclusions:
Mitochondrial inhibition in vitro occurred at a CPC concentration that may be relevant to human exposures. The antiestrogenic activity of CPC, BAK, rotenone, and triclosan may be related to their mitochondrial inhibitory activity. Our findings support the need for additional research on the mitochondrial inhibitory and antiestrogenic effects of QUATS, including CPC and BAK. https://doi.org/10.1289/EHP1404
Introduction
Antimicrobial quaternary ammonium salts (QUATS) compounds, such as cetylpyridinium chloride (CPC) and benzalkonium chloride (BAK), have been used in personal care products, such as soaps and body washes, until the recent U.S. Food and Drug Administration (FDA) ruling (FDA 2016), and are currently being used in hand lotions, toothpastes, mouthwashes, nasal sprays, lozenges, deodorants, intravaginal sponges, and in multidose pharmaceutical formulations, such as eye drops (Lang et al. 2013; Tan et al. 2002). The antimicrobial properties of QUATS were first discovered in the 1930s, and since then, they have been widely used as topical antiseptics and disinfectants (Tischer et al. 2012). In general, the QUATS are a group of compounds that contains a positively charged nitrogen atom in their otherwise lipophilic chemical structures, and these structural characteristics (lipophilic cations) make them favorable to be taken up by mitochondria (Murphy and Smith 2007). Some of the QUATS, such as dequalinium chloride (Gamboa-Vujicic et al. 1993) and n-decyl trimethylammonium bromide (Inácio et al. 2013), have been previously reported to inhibit mitochondrial oxidative phosphorylation at low concentrations. Although the recent FDA ruling (FDA 2016) revoked the generally recognized as safe (GRAS) status of CPC in certain products and requested more evidence supporting GRAS status for BAK due to concerns about their potential to promote antimicrobial resistance and other potential safety issues, including possible hormonal effects (FDA 2016), their usage in numerous other formulations in health care, food, and pharmaceutical and cleaning industries are being continued.
Mitochondria are the critical cellular organelles responsible for energy generation and cellular homeostasis. In the last decade, mitochondrial dysfunction has emerged as a potential contributing pathologic mechanism for several health problems, including cardiac diseases (Schwarz et al. 2014), diabetes (Szendroedi et al. 2011), obesity (Heinonen et al. 2015), Alzheimer’s disease, Parkinson’s disease (Yan et al. 2013), and cancer (Rogalinska 2016). Since initial steps of testosterone, progesterone, and estrogen biosynthesis take place in the mitochondrial matrix (Felty and Roy 2005; Ramalho-Santos and Amaral 2013), a disruption in mitochondrial integrity or function could possibly lead to endocrine disruption. In addition, several studies have shown that mitochondrial gene expression (Chen et al. 2009; Sanchez et al. 2015), structure, function (Arnold et al. 2012), and morphology (Hara et al. 2014) can be regulated by estrogen, and mitochondrial effects of estrogen are thought to work through the mitochondrially localized estrogen receptor () (Liao et al. 2015). The mitochondrial regulation of estrogen signaling at the cellular level, however, has not been well studied. Several endocrine disrupting chemicals, such as triclosan (TCS) (Newton et al. 2005; Weatherly et al. 2016), bisphenol A (BPA) (Jiang et al. 2014; Xia et al. 2014) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Aly and Domènech 2009; Chen et al. 2010; Shertzer et al. 2006), have also been shown to cause mitochondrial dysfunction. A mechanism through which mitochondrial dysfunction might cause endocrine disruption is not known.
For the present study, we evaluated the in vitro effects of CPC as a representative QUATS compound on mitochondrial function and mitochondrial complex 1, and evaluated its antiestrogenic activity.
Materials and Methods
Cell Lines and Cell Culture
The Leber's Hereditary Optic Neuropathy (LHON) osteosarcoma cytoplasmic hybrids (cybrids) were kindly gifted from Drs. Valerio Carelli and Andrea Martinuzzi, and the retinal ganglion cell line (RGC-5) was purchased from American Type Culture Collection. The RGC-5 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing L-glutamine and of sodium pyruvate (DMEM; Corning) and 10% fetal bovine serum (FBS; Corning). For the osteosarcoma cells, the DMEM was further supplemented with uridine (Sigma), and antibiotics () of of streptomycin (Gibco). The cells were maintained under 5% carbon dioxide at 37°C.
The human breast carcinoma MCF-7-derived VM7Luc4E2, cells were grown and maintained in alpha Minimum Essential Medium (Gibco-BRL) containing 10% fetal bovine serum and maintained at 37°C under 5% and 85% humidity.
Chemicals and Chemical Library
The Pharmakon collection containing 1,600 FDA-approved/clinically evaluated drugs [ dimethylsulfoxide (DMSO)] was purchased from Microsource Discovery Systems Inc. CPC (Cat# C0732; CAS No. 6,004-24-6) and all other chemicals were purchased from Sigma-Aldrich unless otherwise specified. The adenosine Triphosphate (ATP) Bioluminescence Assay Kit CLS II was purchased from Roche Life Science. The ATP-free Adenosine diphosphate (ADP) was purchased from Cell Technology. BAK (Cat# B6295; CAS No. 63,449-41-2) stock solutions were prepared assuming a molecular weight of 375 as determined by perchloric acid titration by the manufacturer. Rotenone (Cat# 45,656; CAS No. 83-79-4) and DMSO (Cat# D8418; CAS No. 67-68-5) were obtained from Sigma-Aldrich and used as a positive control and vehicle control, respectively.
Mitochondrial Complex I–Driven ATP Synthesis Measurement Assay
Mitochondrial complex 1-driven ATP synthesis assays (mtCIDAS) of vehicle- and CPC-treated LHON mutation (11,778) carrying osteosarcoma cybrid cells were performed as previously described with slight modification (Datta et al. 2016). In the previous high-throughput screen, 2 h of rotenone treatment was done after 22 h of drug treatment; however, in the current study, the cells were treated for 24 h with either vehicle or CPC at the specified concentrations (without any rotenone treatment). Subsequently, the conditioned media was removed, and the cells were permeabilized with streptolysin O. Permeabilized cells were incubated with a buffer containing complex I substrates for 30 min, and the mitochondrial ATP production was measured by using the ATP Bioluminescence Assay Kit CLS II following manufacturer’s instruction.
Oxygen Consumption Assay by BD Biosensor Plates
Oxygen consumption was measured after 2 h of incubation with the compounds of interest or controls during the high-throughput screening in RGC-5 cells, as previously described (Sahdeo et al. 2014). Briefly, RGC-5 cells were grown in the media specified above and were aliquoted (70,000 cells, media/well) into 384 well oxygen biosensor plates (BD Biosciences) and allowed to equilibrate for 20–30 min. The compounds were diluted in phosphate-buffered saline (PBS) (). The compounds () DMSO or [2-([4-(trifluoromethoxy)phenyl] hydrazinylidene)propanedinitrile (FCCP) were added in their respective wells, and fluorescence was monitored using a POLARstar Omega Plate Reader (BMG Labtech) set at 37°C. Fluorescence was monitored at 0 and 2 h postaddition, and plates were incubated at 37°C under 5% between readings. The final concentration of DMSO was 0.1%, and DMSO and FCCP () were used as negative and positive controls, respectively (Sahdeo et al. 2014). Each compound was evaluated in triplicate ().
Oxygen Consumption Assay by Clarke Electrode and Seahorse XF24 Flux Analyzer
Mitochondrial consumption rates of osteosarcoma cybrid cells carrying healthy (control) or 11,778 LHON mutant mitochondrial DNA were measured with a Seahorse XF-24 system (Seahorse Biosciences, currently Agilent Inc.) (Danielson et al. 2002; Tomilov et al. 2014) and an Oxytherm Clark electrode system (Hansatech) (Liu et al. 2009). For oxygen consumption assay by Seahorse XF24 flux analyzer, 50,000 control osteosarcoma cybrids cells in medium/well was plated on 24-well plates and incubated overnight. Media was changed to unbuffered DMEM, 20% FBS, Glutomax (Thermofisher Scientific Cat# 35050061), 100 mm sodium pyruvate, 25 mm glucose, and pH 7.4. Cells were pre-equilibrated for 20 min; oxygen consumption rates (OCR) were recorded with Seahorse XF-24 before and after addition of CPC.
Cell-Based ER-Mediated Bioassay
Recombinant human breast carcinoma cells (VM7Luc4E2, ) were grown and maintained as previously described (Rogers and Denison 2000). These cells contain a stably integrated, ER-responsive firefly luciferase reporter plasmid, pGudLuc7ERE. Cells were maintained in estrogen-stripped media for 5 d before they were plated into white, clear-bottomed 96-well tissue culture dishes at 75,000 cells/well and allowed to attach for 24 h. Cells were then incubated with carrier solvent (DMSO; 1% final solvent concentration), (, ), the indicated concentration of compound (for measurement of agonist activity), or the indicated concentration of compound plus (for measurement of antagonist activity) for 24 h at 37°C with triplicate wells per chemical or control. After incubation, cells were rinsed twice with PBS, lysed with Promega cell lysis buffer, and shaken for 20 min at room temperature to allow complete cell lysis. Luciferase activity in each well was measured using an Orion microplate luminometer as previously described (Baston and Denison 2011).
Assessment of Cytotoxicity
Cytotoxicity of CPC- and BAK-treated cells were assessed after 24 h of incubation under a brightfield microscope. The cells were examined for any gross morphological changes (such as rounding or detachment), and the concentrations of CPC and BAK at which cells did not show any gross morphological changes were considered as nontoxic concentrations.
Data Analysis and Statistics
Fluorescence readings representing oxygen consumption were recorded at 2 h post-chemical library treatment, and luminescence readings for ATP content were collected after 24 h of chemical treatment. The fold change from baseline (FCB) was calculated as previously reported (Datta et al. 2016; Sahdeo et al. 2014). Briefly, for oxygen consumption, fluorescence was measured immediately after drug addition (t0) and after 2 h incubation. The FCB was calculated by normalizing postincubation readings to the t0 reading. FCB responses for drug-treated wells were then normalized to the average FCB for the 16 vehicle-treated wells producing the fold change from vehicle value for each well. For ATP synthesis, cells were drug treated for 22 h and then treated with rotenone () for 2 h in 96-well plates. The plate median was determined and fold change of ATP synthesis rate over the plate median for each drug-treated well was calculated. The concentration–response curves were generated, and the half maximal inhibitory concentration values were determined by nonlinear regression curve fit analysis using Graphpad Prism 5.0 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com).
Results
Evaluation of Mitochondrial Function of Cells Exposed to Quaternary Ammonium Salts
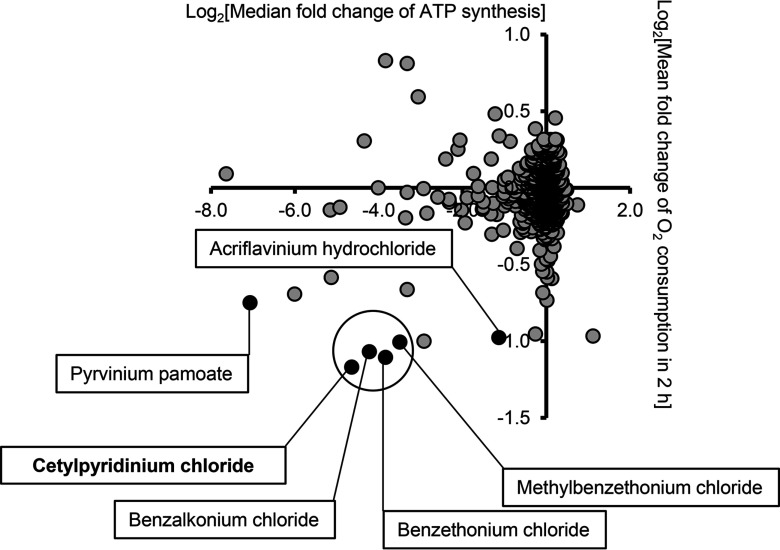
We previously reported two high-throughput screens of a library of clinically evaluated additives, disinfectants, and drugs to identify their effects on two of the mitochondrial functional parameters, i.e., mitochondrial consumption (Sahdeo et al. 2014) and complex 1-driven ATP synthesis (CIDAS) (Datta et al. 2016). Mitochondrial consumption was measured using BD biosensor plates in RGC-5 cells after 2 h of compound treatment. MtCIDAS was measured in permeabilized compound and rotenone-treated () cybrid cells containing LHON mutation (11,778) using a bioluminescence method. In order to identify the compounds that affect both mitochondrial consumption and mtCIDAS, we plotted the results of the consumption screen (Sahdeo et al. 2014) against the mtCIDAS screen (Datta et al. 2016) (Figure 1). Of the 11 compounds in the bottom left quadrant (indicative of simultaneous inhibition of mitochondrial consumption and mtCIDAS), 6 were found to be QUATS, making QUATS the single largest mitochondrial inhibitory structural class. QUATS are lipophilic cations, which is a structure that has previously been shown to be preferentially taken up into mitochondria (Murphy and Smith 2007). There were 10 QUATS compounds common in both the screens, and 6 out of 10 QUATS compounds showed inhibition of mtCIDAS (24 h) and mitochondrial consumption (2 h) when tested at concentration (Figure 1). The QUATS compounds that did not show mitochondrial inhibition are: clidinium bromide, hexamethonium bromide, tolonium chloride, and cefalonium. In the high-throughput screen, CPC was identified as the most potent of the QUATS tested with regard to mitochondrial consumption and mtCIDAS (Figure 1).
Figure 1.
Effects of quaternary ammonium salts on mitochondrial consumption and complex 1-driven adenosine triphosphate (ATP) synthesis (CIDAS) in vitro. We evaluated 1,600 drugs and preservatives from the Pharmakon collection for mitochondrial activity. For CIDAS, 11,778 mutant osteosarcoma cybrids were treated with the drugs () for 22 h followed by 2 h incubation with rotenone (). Subsequently, the cells were permeabilized, and mitochondrial CIDAS (mtCIDAS) was measured. For oxygen consumption assay, the RGC-5 cells were incubated with the compounds for 2 h, and the fluorescence was measured. The data are presented as vs from three independent observations. Six quaternary ammonium salts (QUATS) that inhibit mitochondrial consumption and mtCIDAS are highlighted here.
In Vitro Effects of CPC on Mitochondrial Consumption and Complex 1-Driven ATP Synthesis
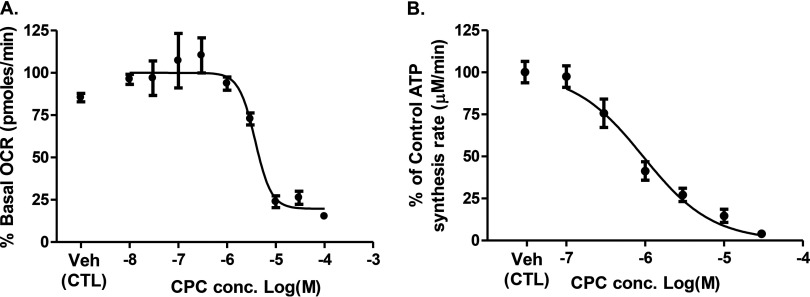
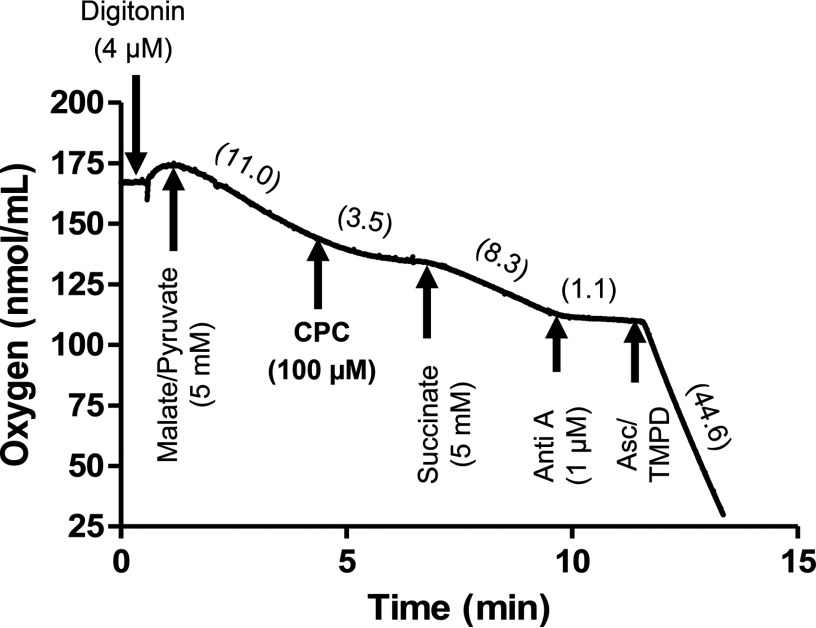
Mitochondrial consumption was inhibited after 30 min of incubation with CPC ( , Figure 2A). MtCIDAS, which was measured after 24 h (to allow more complete penetration of the chemical into mitochondria) also decreased in a concentration-dependent manner ( , Figure 2B). QUATS, such as dequalinium chloride (Gamboa-Vujicic et al. 1993) and n-decyl-N,N,N-trimethylammonium () (Inácio et al. 2013), are already known to inhibit complex 1 reduced nicotinamide adenine dinucleotide [(NADH)-ubiquinone oxidoreductase]; hence, we hypothesized that CPC might inhibit mitochondrial function by targeting complex 1 (NADH-ubiquinone oxidoreductase) in the mitochondrial electron transport chain. The mitochondrial electron carrier ubiquinone carries electrons deposited at mitochondrial complex 2 (succinate dehydrogenase) to complex 3 (ubiquinol-cytochrome c oxidoreductase), independent of complex 1 inhibition (Nicholls and Ferguson 2013). Therefore, addition of a complex 2 substrate (succinate) subsequent to a complex 1 inhibitor in permeabilized cells should allow mitochondrial consumption to resume. Hence, rescue of CPC-induced mitochondrial consumption inhibition by a complex 2 substrate (succinate) suggests that the target of CPC is complex 1. CPC () was added to digitonin-permeabilized osteosarcoma cells respiring on complex 1 substrates malate () and pyruvate (). Within 3 min of CPC addition, the mitochondrial consumption rate decreased from the baseline rate of to , an decrease (Figure 3). After addition of succinate (), the mitochondrial consumption rate increased to , which is consistent with a direct effect of CPC on complex 1 that inhibited mitochondrial consumption.
Figure 2.
Mitochondriotoxic effects of cetylpyridinium chloride (CPC). (A) Inhibition of mitochondrial consumption by CPC. The osteosarcoma cells were treated with CPC at specified concentrations (), and cellular oxygen consumption was measured after 10 min of initial addition and for 4 times total at 10-min intervals. The data are presented as average percentage of basal from three independent observations. (B) Inhibition of mitochondrial complex 1-driven adenosine triphosphate (ATP) synthesis (mtCIDAS) by CPC. The osteosarcoma cells were treated with CPC at specified concentrations () for 22 h followed by 2-h incubation with rotenone (). The mtCIDAS was measured in permeabilized cells. Data are presented as average fold change of ATP synthesis from three independent observations. The values for mitochondrial consumption and mtCIDAS inhibition are and , respectively. The values were determined by nonlinear regression curve fit analysis using Graphpad Prism 5.0.
Figure 3.
The cetylpyridinium chloride (CPC)–dependent respiration defect was overcome by complex II substrate succinate, but not complex I substrate. Osteosarcoma cybrids were permeabilized with digitonin (), and respiration was initiated with complex I substrate (malate/pyruvate, ). Then the cells were treated with CPC (), followed by the complex II substrate (succinate, ), a complex III inhibitor, antimycin A (Anti A, ), and the complex IV substrate ascorbate (Asc, ) and N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) () mixture. Oxygen consumption rates (OCR, nmol/ml/min) of the cybrids were measured for 1 min after each addition. The OCRs after each addition are indicated in the parentheses from one representative experiment repeated two times. The CPC-dependent respiration defect was overcome by complex II substrate succinate, but not complex I substrate.
In Vitro Effects of CPC and BAK on Estrogenic Signaling
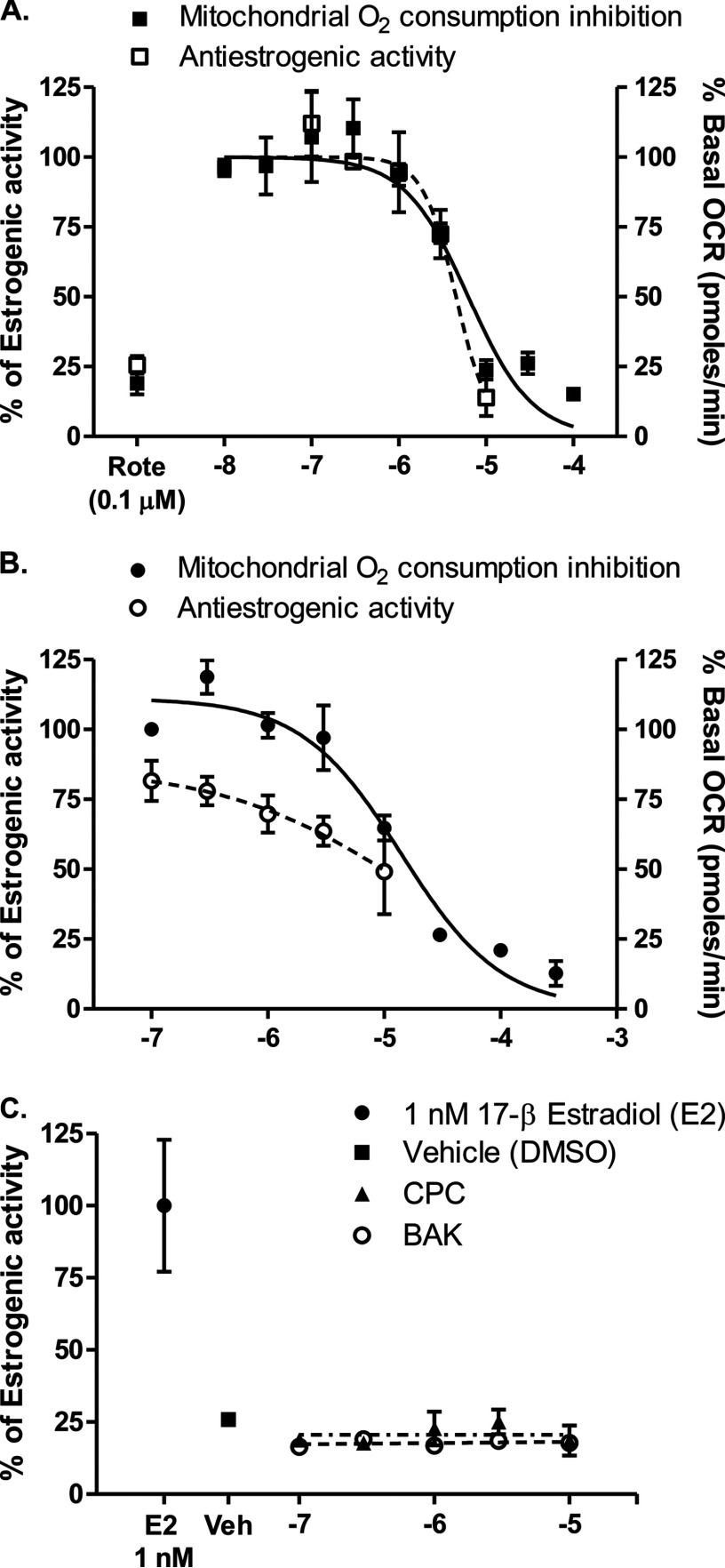
Mitochondria are essential for proper steroidogenesis (Ramalho-Santos and Amaral 2013). Multiple endocrine disruptors, such as TCS (Weatherly et al. 2016), BPA (Kaur et al. 2014; Lin et al. 2013), and TCDD (Chen et al. 2010), have shown to adversely affect mitochondrial function, suggesting a possible correlation between mitochondrial function and endocrine disruption. We hypothesized that mitochondrial inhibitors might disrupt endocrine signaling, and tested QUATS for antiestrogenic and proestrogenic effects. At its highest nontoxic concentration (), CPC showed 86% inhibition of estrogen activity in VM7Luc4E2, human breast carcinoma cells after 24 h of incubation (Figure 4A). The concentration–response curve for antiestrogenic activity overlapped with the concentration–response curve for mitochondrial consumption inhibition in osteosarcoma cybrids after 10 min of incubation with CPC (Figure 4A). The similarity in the dose–response curves for the two assays suggests that CPC’s antiestrogenic activity might be a consequence of CPC-mediated inhibition of the mitochondrial electron transport chain. In previous studies, triclosan was shown to disrupt mitochondrial function (Weatherly et al. 2016) and inhibit estrogen signaling ( ) (Ahn et al. 2008), similar to CPC ( ). In addition, we also tested another QUATS, BAK, for its mitoinhibitory and antiestrogenic effect. BAK was a comparatively weaker mitochondrial complex 1 inhibitor and showed weaker antiestrogenic effects (Figure 4B). CPC and BAK did not show any estrogen-stimulating effects (up to ) in VM7Luc4E2, human breast carcinoma cells after 24 h of incubation (Figure 4C).
Figure 4.
Comparison of cetylpyridinium chloride (CPC)’s and benzalkonium chloride (BAK)’s antiestrogenic activities with mitochondrial consumption inhibitory activities. The cells (osteosarcoma cybrids for mitochondrial consumption experiment and VM7Luc4E2, cells for estrogenic activity measurement experiments) were treated with (A) CPC and (B) BAK at specified concentrations for 10 min for the consumption experiment and 24 h (in the presence of ) for the antiestrogenic effect. (C) VM7Luc4E2, cells were treated with CPC or BAK at the specified concentrations or estradiol (, ) for 24 h, and the estrogenic activity was measured. Rotenone (Rote), a standard complex 1 inhibitor, was used as a positive control at a single concentration (). The values for CPC are for mitochondrial consumption inhibition and for its antiestrogenic activity. The values for BAK are for mitochondrial consumption and for its antiestrogenic activity. The value for estrogenic activity of CPC could not be determined. The solid lines represent the nonlinear regression curve fit for mitochondrial inhibitory activity, and the dotted lines () represent the nonlinear regression curve fit for estrogenic activity of the compounds. The data are presented as percentage of estrogen and percentage of basal from three independent observations. The values were determined by nonlinear regression curve fit analysis using Graphpad Prism 5.0.
Mitochondrial Disruption as Basis of in Vitro Antiestrogenic Activity: CPC and Rotenone
From the above data, we hypothesized that the antiestrogenic activity of the QUATS was dependent on their mitochondrial complex 1 inhibitory activity. To test this hypothesis, we evaluated rotenone (), a standard mitochondrial complex 1 inhibitor and a known environmental pollutant, for its antiestrogenic activity. Rotenone () showed 75% inhibition of estrogen activity in VM7Luc4E2 human breast carcinoma cells after 24 h, and reduced mitochondrial oxygen consumption in osteosarcoma cybrids by 81% after 10 min (Figure 4A). Rotenone does not interfere with estrogen binding to estrogen receptors (Olson and Sheehan 1979); therefore, we hypothesize, as we do for CPC and BAK, that its antiestrogenic effects may be related to mitochondrial inhibition. It seems possible that mitochondrial disruption underlies the inhibition of estrogenic signaling observed in response to CPC, BAK, and rotenone, but further investigation is necessary to fully substantiate this potential mechanism.
Discussion and Conclusion
QUATS, such as CPC and BAK, are antimicrobial agents occur in toothpaste, mouthwash, lozenges, throat and nasal sprays, shampoos, hand lotions, creams, eye drops, biocides, intravaginal sponges, consumer antiseptic rubs, and other products that come into contact with epithelial cells. In 2016, the FDA ruled that some ingredients used in consumer antiseptic wash products, including CPC as well as TCS and other antiseptics, were not GRAS or generally recognized as effective (GRAE), and that wash products including these ingredients could not be sold after September 2017 (FDA 2016). Additional data on BAK are being requested by the FDA to establish its GRAS/GRAE status. However, CPC, BAK, and other antiseptics continue to be used in other products in consumer first-aid, food, personal hygiene product, and cleaning industries.
We performed a high-throughput screen of 1,600 antiseptics, additives, and drugs, and found that, of the numerous structural classes of compounds included in the screen, the QUATS were the most mitochondrially toxic class, both in terms of inhibition of ATP synthesis and mitochondrial consumption. QUATS have the structure of lipophilic cations, which are known to be preferentially taken up by mitochondria (Murphy and Smith 2007). Lipophilic cations, including tetramethylrhodamine methyl ester (Floryk and Houštêk 1999), 1-methyl-4-phenylpyridinium (Davey et al. 1992), and triphenylphosphonium ions (Ross et al. 2005) are known to dose-dependently accumulate in the mitochondrial matrix. Six out of ten QUATS showed inhibition of mitochondrial consumption as well as mtCIDAS. This indicates a possible structure activity relationship in context to the mitochondrial effects of QUATS. A detailed comparison of the antimicrobial efficacy and mitochondrial inhibitory effects of the QUATS needs to be performed in order to identify the QUATS with high antimicrobial efficacy with minimum mitochondrial effects. Among the mitochondrial inhibitory QUATS, CPC was the most potent in the preliminary screen and was used as the representative of the mitochondrial inhibitory QUATS for further studies. Mechanistic investigation of CPC, the representative QUATS, established that CPC inhibits mitochondrial complex 1 (Figure 3) and therefore impedes mitochondrial consumption and CIDAS in a concentration-dependent manner. Furthermore, we recently demonstrated that the QUATS BAK is also a mitochondrial complex 1 inhibitor (Datta et al. 2017). Although antimicrobial agents, including antibiotics and antiseptics, are generally conceived as nontoxic to human mitochondria, in a recent study, antibiotic-induced functional impairment of host mitochondria have shown to cause serious adverse effects in eukaryotic model systems (Moullan et al. 2015). In addition, induction of mitochondrial dysfunction has been proposed as potential mechanisms for adverse effects observed in clinical settings during therapeutic use of antibiotics (Kalghatgi et al. 2013) and antiretrovirals (Kohler and Lewis 2007).
We hypothesize that CPC’s antiestrogenic effects may be mediated through effects on mitochondrial complex 1 inhibition. Rotenone, an established mitochondrial complex 1 inhibitor (Heinz et al. 2017), was used as a positive control and also showed antiestrogenic effects at the single dose tested. However, additional research is needed to confirm whether effects on mitochondrial function may contribute to effects on estrogen signaling.
Although CPC continues to be used in mouthwash, toothpaste, lozenges, throat sprays, and nasal sprays, and BAK is used in intravaginal spermicidal sponges, body and hand washes, and eyedrops, pharmacokinetic studies on these QUATS are scarce, and tissue-level exposures resulting from the use of personal care products that contain these compounds are unknown. A pharmacokinetic study performed in rats indicated that BAK is absorbed after single oral administration and distributed in tissues at low micromolar concentrations () (Xue et al. 2004). In the same study, aspiration of BAK through the lungs markedly increased absorption and tissue distribution of BAK (Xue et al. 2004). This is particularly important in the context of BAK and other QUATS exposure. BAK is often used as a household disinfectant and biocide in the form of sprays and aerosols. From the above-mentioned study, it seems that inhalation of aerosols containing BAK could be a potential route for tissue exposure. According to the report by European Union’s Scientific Committee on Consumer Safety (SCCS 2015), predicted aggregate absorption of CPC through various cosmetic formulations, such as mouth rinse, toothpaste, denture adhesive, denture cleaner, body lotion, face cream, hand cream, and deodorant spray, for adult humans is approximately body weight/day, with an estimated/day, with an estimated absorption of 50% from oral administration and 10% from dermal administration. Due to the scarcity of pharmacokinetic studies on long-term or short-term exposure of CPC and BAK by various routes, exact determination of physiologically relevant tissue concentrations of these compounds is not possible at this time.
A recent study demonstrated that exposure to QUATS mixture, including benzalkonium chloride, caused reproductive toxicity and reduced fertility in mice (Melin et al. 2014; Melin et al. 2016). It is our hypothesis that inhibition of mitochondrial function and subsequent disruption of estrogenic signaling is possibly the mechanistic basis of the toxicity observed in these studies. Further mechanistic investigation is needed to conclusively prove if that is indeed true.
In summary, our findings suggest that the QUATS CPC and BAK, which are used as disinfectants in consumer products, inhibit mitochondrial complex 1 and show antiestrogenic activity in vitro at low (micromolar) concentrations that may be physiologically relevant. We hypothesize a mechanistic relationship between these outcomes, whereby the antiestrogenic activity of these compounds is mediated by mitochondrial inhibition. Our observations that some QUATS do not inhibit mitochondria suggest structure-activity relationship (SAR) to explore antimicrobial QUATS without antimitochondrial activity. Overall, our findings strongly support the need for further investigation of the underlying mechanisms and potential consequences of chronic exposure to CPC and BAK in consumer products.
Acknowledgment
The following NIH awards supported this work: R01 NS077777, R01 EY012245, and PO1 AG025532 to G.A.C.
References
- Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, et al. 2008. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ Health Perspect 116(9):1203–1210, PMID: 18795164, 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly HA, Domènech O. 2009. Cytotoxicity and mitochondrial dysfunction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in isolated rat hepatocytes. Toxicol Lett 191(1):79–87, PMID: 19686823, 10.1016/j.toxlet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Arnold S, Victor MB, Beyer C. 2012. Estrogen and the regulation of mitochondrial structure and function in the brain. J Steroid Biochem Mol Biol 131(1–2):2–9, PMID: 22326731, 10.1016/j.jsbmb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Baston DS, Denison MS. 2011. Considerations for potency equivalent calculations in the Ah receptor-based CALUX bioassay: Normalization of superinduction results for improved sample potency estimation. Talanta 83(5):1415–1421, 10.1016/j.talanta.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JQ, Cammarata PR, Baines CP, Yager JD. 2009. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 1793(10):1540–1570, PMID: 19559056, 10.1016/j.bbamcr.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Liao TL, Wei YH, Tzeng CR, Kao SH. 2010. Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Mol Hum Reprod 16(5):361–372, PMID: 20083559, 10.1093/molehr/gaq004. [DOI] [PubMed] [Google Scholar]
- Danielson SR, Wong A, Carelli V, Martinuzzi A, Schapira AH, Cortopassi GA. 2002. Cells bearing mutations causing Leber's hereditary optic neuropathy are sensitized to Fas-Induced apoptosis. J Biol Chem 277(8):5810–5815, PMID: 11741983, 10.1074/jbc.M110119200. [DOI] [PubMed] [Google Scholar]
- Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA. 2017. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci 58(4):2406–2412, PMID: 28444329, 10.1167/iovs.16-20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Tomilov A, Cortopassi G. 2016. Identification of small molecules that improve ATP synthesis defects conferred by Leber's hereditary optic neuropathy mutations. Mitochondrion 30:177–186, PMID: 27497748, 10.1016/j.mito.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GP, Tipton KF, Murphy MP. 1992. Uptake and accumulation of 1-methyl-4-phenylpyridinium by rat liver mitochondria measured using an ion-selective electrode. Biochem J 288(2):439–443, 10.1042/bj2880439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-The-Counter Human Use. https://www.gpo.gov/fdsys/pkg/FR-2016-09-06/pdf/2016-21337.pdf [accessed 3 November 2016]. [PubMed]
- Felty Q, Roy D. 2005. Estrogen, mitochondria, and growth of cancer and non-cancer cells. J Carcinog 4:1, PMID: 15651993, 10.1186/1477-3163-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floryk D, Houštêk J. 1999. Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Biosci Rep 19(1):27–34, 10.1023/A:1020193906974. [DOI] [PubMed] [Google Scholar]
- Gamboa-Vujicic G, Emma DA, Liao SY, Fuchtner C, Manetta A. 1993. Toxicity of the mitochondrial poison dequalinium chloride in a murine model system. J Pharm Sci 82(3):231–235, 10.1002/jps.2600820302. [DOI] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. 2014. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA 111(1):486–491, PMID: 24297907, 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. 2015. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64(9):3135–3145, PMID: 25972572, 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. 2017. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci Rep 7:45465, PMID: 28374803, 10.1038/srep45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio AS, Costa GN, Domingues NS, Santos MS, Moreno AJ, Vaz WL, et al. 2013. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity to mammalian epithelial cells. Antimicrobial agents and chemotherapy 57:2631–2639, PMID: 23529737, 10.1128/AAC.02437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu J, Li Y, Chang H, Li G, Xu B, et al. 2014. Prenatal exposure to bisphenol A at the reference dose impairs mitochondria in the heart of neonatal rats. J Appl Toxicol 34(9):1012–1022, PMID: 24105817, 10.1002/jat.2924. [DOI] [PubMed] [Google Scholar]
- Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, et al. 2013. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci Transl Med 5(192):192ra185, 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Chauhan V, Gu F, Chauhan A. 2014. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic Biol Med 76:25–33, PMID: 25101517, 10.1016/j.freeradbiomed.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Kohler JJ, Lewis W. 2007. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen 48(3–4):166–172, PMID: 16758472, 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- Lang JC, Roehrs RE, Jani R. 2013. Ophthalmic Preparations in Remington: The Science and Practice of Pharmacy. 21st Edition Troy DB, ed Philadelphia, PA:Lippincott Williams & Wilkins. [Google Scholar]
- Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH. 2015. Estrogen receptor-β in mitochondria: Implications for mitochondrial bioenergetics and tumorigenesis. Ann N Y Acad Sci 1350:52–60, PMID: 26301952, 10.1111/nyas.12872. [DOI] [PubMed] [Google Scholar]
- Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, et al. 2013. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 4:e460, PMID: 23328667, 10.1038/cddis.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Veena CK, Morgan JB, Mohammed KA, Jekabsons MB, Nagle DG, et al. 2009. Methylalpinumisoflavone inhibits hypoxia-inducible factor-1 (HIF-1) activation by simultaneously targeting multiple pathways. J Biol Chem 284(9):5859–5868, PMID: 19091749, 10.1074/jbc.M806744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin VE, Melin TE, Dessify BJ, Nguyen CT, Shea CS, Hrubec TC, et al. 2014. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol 50:163–170, 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin VE, Melin TE, Dessify BJ, Nguyen CT, Shea CS, Hrubec TC. 2016. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod Toxicol 59:159–166, 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, et al. 2015. Tetracyclines disturb mitochondrial function across eukaryotic models: A call for caution in biomedical research. Cell Rep S2211-1247(15):00180–00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. 2007. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47:629–656, PMID: 17014364, 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Newton AP, Cadena SM, Rocha ME, Carnieri EG, Martinelli de Oliveira MB. 2005. Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett 160(1):49–59, PMID: 16023799, 10.1016/j.toxlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. 2013. Bioenergetics 4. 4th Edition. Burlington, MA:Elsevier Academic Press. [Google Scholar]
- Olson ME, Sheehan DM. 1979. Failure of rotenone to interfere with 17 beta-estradiol action in the rat uterus. Cancer Res 39(11):4438–4440, PMID: 498075. [PubMed] [Google Scholar]
- Ramalho-Santos J, Amaral S. 2013. Mitochondria and mammalian reproduction. Mol Cell Endocrinol 379(1-2):74–84, PMID: 23769709, 10.1016/j.mce.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Rogalinska M. 2016. The role of mitochondria in cancer induction, progression and changes in metabolism. Mini Rev Med Chem 16(7):524–530, PMID: 26471969. [DOI] [PubMed] [Google Scholar]
- Rogers JM, Denison MS. 2000. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In Vitr Mol Toxicol 13(1):67–82, PMID: 10900408. [PubMed] [Google Scholar]
- Ross MF, Kelso GF, Blaikie FH, James AM, Cochemé HM, Filipovska A, et al. 2005. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 70(2):222–230, PMID: 15807662. [DOI] [PubMed] [Google Scholar]
- SCCS (European Union Scientific Committee on Consumer Safety). 2015. Revised Opinion on Cetylpyridinium Chloride. Luxembourg:European Commission Secretariat of the Scientific Committee, https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_171.pdf [accessed 10 July 2017].
- Sahdeo S, Tomilov A, Komachi K, Iwahashi C, Datta S, Hughes O, et al. 2014. High-throughput screening of FDA-approved drugs using oxygen biosensor plates reveals secondary mitofunctional effects. Mitochondrion 17:116–125, PMID: 25034306, 10.1016/j.mito.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MI, Shearwood AM, Chia T, Davies SM, Rackham O, Filipovska A. 2015. Estrogen-mediated regulation of mitochondrial gene expression. Mol Endocrinol 29(1):14–27, 10.1210/me.2014-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Siddiqi N, Singh S, Neil CJ, Dawson DK, Frenneaux MP. 2014. The breathing heart - mitochondrial respiratory chain dysfunction in cardiac disease. Int J Cardiol 171(2):134–143, PMID: 24377708, 10.1016/j.ijcard.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Shertzer HG, Genter MB, Shen D, Nebert DW, Chen Y, Dalton TP. 2006. TCDD decreases ATP levels and increases reactive oxygen production through changes in mitochondrial F0F1-ATP synthase and ubiquinone. Toxicol Applied Pharmacol 217(3):363–374, 10.1016/j.taap.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Phielix E, Roden M. 2011. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 8(2):92–103, PMID: 21912398, 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- Tan L, Nielsen NH, Young DC, Trizna Z. 2002. Use of antimicrobial agents in consumer products. Arch Dermatol 138(8):1082–1086, PMID: 12164747. [DOI] [PubMed] [Google Scholar]
- Tischer M, Pradel G, Ohlsen K, Holzgrabe U. 2012. Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions? ChemMedChem 7(1):22–31, PMID: 22113995, 10.1002/cmdc.201100404. [DOI] [PubMed] [Google Scholar]
- Tomilov A, Bettaieb A, Kim K, Sahdeo S, Tomilova N, Lam A, et al. 2014. Shc depletion stimulates brown fat activity in vivo and in vitro. Aging cell 13(6):1049–1058, PMID: 25257068, 10.1111/acel.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shim J, Hashmi HN, Kennedy RH, Hess ST, Gosse JA. 2016. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol 36(6):777–789, PMID: 26204821, 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y, et al. 2014. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PloS one 9(2):e90443, PMID: 24587367, 10.1371/journal.pone.0090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Hieda Y, Saito Y, Nomura T, Fujihara J, Takayama K, et al. 2004. Distribution and disposition of benzalkonium chloride following various routes of administration in rats. Toxicol Lett 148(1–2):113–123, PMID: 15019095, 10.1016/j.toxlet.2003.12.068. [DOI] [PubMed] [Google Scholar]
- Yan MH, Wang X, Zhu X. 2013. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62:90–101, PMID: 23200807, 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]