Abstract
Background:
In addition to its well-established role in maintaining skeletal health, vitamin D has essential regulatory functions in female reproductive and pregnancy outcomes. Phthalates and bisphenol A (BPA) are endocrine disruptors, and previous research has suggested that these chemical agents may disrupt circulating levels of total 25(OH)D in adults.
Objectives:
We investigated the relationships between repeated measures of urinary phthalate metabolites and BPA and circulating total 25(OH)D in a prospective cohort of pregnant women.
Methods:
The present study population includes participants () in a nested case–control study of preterm birth drawn from a prospective birth cohort of pregnant women at Brigham and Women’s Hospital in Boston, Massachusetts. Urine and blood samples were collected for biomarker measurements at median 10 wk and 26 wk of gestation.
Results:
In repeated measures analysis, we observed that an interquartile range (IQR) increase in urinary mono-3-carboxypropyl phthalate (MCPP) was associated with a 4.48% decrease [95% confidence interval (CI): , ] in total 25(OH)D. We also detected inverse associations for metabolites of di(2-ethylhexyl) phthalate (DEHP) [percent difference to ]. For BPA, we observed a nonsignificant inverse association with total 25(OH)D in the overall population. Our sensitivity analysis revealed that the associations for some metabolites (e.g., MEHP) varied by race/ethnicity, which may reflect potential differences in susceptibility. In agreement with findings from repeated measures analysis, we reported that DEHP metabolites and BPA were significantly associated with an approximate 20% increase in the odds of vitamin D deficiency () [odds ratio (1.06, 1.35) for molar sum of DEHP metabolites and 1.22 (1.01, 1.47) for BPA] at median 10 wk and 26 wk, respectively.
Conclusions:
Our results provide suggestive evidence of the potential for environmental exposure to phthalates and/or BPA to disrupt circulating vitamin D levels in pregnancy. https://doi.org/10.1289/EHP1178
Introduction
Vitamin D is a prohormone that plays an integral role in the regulation of bone metabolism and calcium and phosphorous absorption (Holick 2007; Norman 2008). The major source of vitamin D in humans is exposure to ultraviolet B (UVB) radiation from sunlight, although it can also be obtained through dietary food sources or supplements (Thacher and Clarke 2011). Vitamin D from the skin and diet (vitamin and ) is biologically inactive and is transported to the liver where it is converted to 25-hydroxyvitamin D [25(OH)D], the circulating biomarker of vitamin D nutritional status (Norman 2008; Thacher and Clarke 2011). Further metabolism occurs in the kidneys, wherein 25(OH)D is hydroxylated to its biologically active metabolite, 1-25-dihydroxyvitamin (Norman 2008; Thacher and Clarke 2011); is a secosteroid hormone that initiates biological actions by interacting with its nuclear receptor at target tissues (Bikle 2014; Carlberg 2014; Haussler et al. 2013). Although it is well established that vitamin D plays an essential role in the development and maintenance of skeletal health, the presence of its nuclear receptor and metabolic enzymes in reproductive tissues, such as the placenta, uterus, and ovaries, indicates that vitamin D may also have regulatory functions in female reproductive and pregnancy outcomes (Grundmann and von Versen 2011; Luk et al. 2012; Ma et al. 2012; Pérez-López 2007).
Maintaining maternal vitamin D homeostasis in pregnancy is necessary for placentation and the maintenance of the pregnancy state as well as for normal fetal growth and development (Luk et al. 2012; Murthi et al. 2016; Ponsonby et al. 2010). Human health studies have shown that reduced levels of 25(OH)D in pregnancy are associated with various maternal and fetal complications, such as preeclampsia, spontaneous preterm birth, and restricted fetal growth (Bodnar and Simhan 2010; Bodnar et al. 2015; Murthi et al. 2016; Robinson et al. 2011). Because pregnancy represents a period of susceptibility during which slight deviations in maternal hormone levels may have detrimental maternal and fetal health consequences, pregnant women are particularly vulnerable to the effects of endocrine-disrupting chemicals.
Phthalates and bisphenol A (BPA) are industrial chemicals found in a wide range of consumer products (Meeker et al. 2009b). Exposure to these agents has been reported in pregnant women worldwide (Cantonwine et al. 2014; Casas et al. 2011; Mortensen et al. 2014; Mu et al. 2015). Both phthalates and BPA may disrupt endocrine systems, and results from epidemiological studies suggest these environmental chemicals may alter sex and thyroid hormone levels in pregnant women (Huang et al. 2007; Johns et al. 2015, 2016a; Sathyanarayana et al. 2014). Given that the active vitamin D metabolite is similar in structure to that of classic sex steroid hormones (Norman 2008), and its nuclear receptor is in the same superfamily of sex steroid and thyroid hormone receptors (Pike and Meyer 2010), it is also plausible that phthalates and/or BPA might disrupt the vitamin D endocrine axis. In our recent investigation conducted among a representative sample of U.S. adults, we reported inverse associations between urinary metabolites of di(2-ethylhexyl) phthalate (DEHP) and total 25(OH)D (Johns et al. 2016b). Urinary BPA was inversely associated with total 25(OH)D among women in our sex-stratified analyses (Johns et al. 2016b). Although our previous study showed the potential for phthalates and BPA to alter circulating levels of total 25(OH)D in adult populations, it was limited by its cross-sectional design with single biomarker measurements collected at one time point. Moreover, we are not aware of any studies that have investigated these associations in pregnant women. In the present study, we assessed the associations between environmental exposure to phthalates and BPA and plasma total 25(OH)D levels in a large, prospective cohort of pregnant women.
Methods
Study Population
The present study population includes participants in a nested case–control study of preterm birth drawn from a prospective cohort (LifeCodes) of pregnant women 18 y and older who were recruited early in gestation () at Brigham and Women’s Hospital in Boston, Massachusetts. The only exclusion criterion was higher-order multiple gestations (e.g., triplets or greater). Additional details regarding recruitment and eligibility criteria are described in detail elsewhere (Ferguson et al. 2014a, 2014b; McElrath et al. 2012). In brief, participants completed a questionnaire at the initial study visit (median: 9.7 wk of gestation; range: 4.7–19.1 wk) to collect demographic characteristics (e.g., race/ethnicity, health insurance provider, educational attainment, etc.) and relevant health information (e.g., family health history, tobacco and alcohol use). Participants were followed until delivery and provided health information [e.g., body mass index (BMI)] as well as blood and urine samples for biomarker measurements at three additional study visits: visit 2 (median: 17.9 wk of gestation; range: 14.9–32.1 wk), visit 3 (median: 26.0 wk of gestation; range: 22.9–36.2 wk), and visit 4 (median: 35.1 wk of gestation; range: 33.1–38.3 wk). The present analyses were restricted to visits 1 and 3 because plasma samples collected at only these time points were assayed for total 25(OH)D.
Of the 1,181 pregnant women included in the original birth cohort who were followed until delivery and had a singleton birth, 130 women who delivered a preterm infant ( of gestation), and 352 who delivered at or after 37 wk of gestation were included in the nested case–control population. The selection probabilities from the parent cohort population were 90.1% for cases and 33.9% for controls (Ferguson et al. 2015). In the current study, we excluded participants from this population who did not have measurements for urinary phthalate metabolites or BPA () or 25(OH)D () at either of the two study visits. The final study population () included 128 cases of preterm birth and 349 controls. The study protocols were approved by the ethics and research committees of the participating institutions, and all study participants gave written informed consent prior to participation.
Urinary Exposure Measurements
All available urine samples collected at up to two study visits during pregnancy were assayed for nine phthalate metabolites and total (free plus glucuronidated) BPA using isotope dilution-liquid chromatography-tandem mass spectrometry (ID–LC–MS/MS) at NSF International in Ann Arbor, Michigan. Additional details regarding this analytical method are described elsewhere (Lewis et al. 2013). The nine phthalate metabolites included: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MBP), monoisobutyl phthalate (MiBP), monoethyl phthalate (MEP), and mono (3-carboxypropyl) phthalate (MCPP). In addition to analyzing individual phthalate metabolites in our statistical analyses, we created a molar sum () measure of the four metabolites of DEHP (MEHP, MEHHP, MEOHP, and MECPP; ). Specifically, we divided each phthalate metabolite concentration by its molecular weight and took the sum of the individual concentrations. Urinary biomarker concentrations less than the limit of detection (LOD) were assigned a value of LOD divided by the square root of 2 (Hornung et al. 1990).
To adjust for urinary dilution in descriptive analyses, phthalate metabolites and BPA were standardized using specific gravity (SG) by the following equation (Meeker et al. 2009a): , where PSG is the specific gravity-adjusted exposure biomarker concentration (), P is the observed exposure biomarker concentration, 1.015 is the specific gravity population median, and SG is the specific gravity of the urine sample. In multivariable analyses, we used unadjusted urinary biomarker concentrations with SG added as a separate covariate because modeling corrected metabolite levels may introduce bias (Barr et al. 2005).
Plasma Vitamin D Measurements
All available plasma samples were assayed for total 25(OH)D, including plus , using a DiaSorin LIAISON® chemiluminescence immunoassay (DiaSorin Inc.) at the Laboratory for Molecular Medicine (Partners Healthcare, Boston, MA) (Ersfeld et al. 2004). The detection range of the assay is , and total coefficients of variation ranged from 9.5% to 12.6%. For quality control, the laboratory uses the U.S. National Institute of Standards and Technology (NIST) level 1 protocol (Burris et al. 2014).
Statistical Analyses
All analyses were performed using R (version 3.3.1; R Development Core Team). We conducted the present study using secondary variables measured under case–control sampling. To make our study population more representative of the original cohort from which the case–control sample arose (i.e., to correct for the over-representation of preterm-birth cases), we applied to all analyses inverse probability weights that represented the inverse sampling fractions for inclusion of controls (Richardson et al. 2007). The distributions of all urinary analytes were right-skewed so we transformed these data in statistical analyses using the natural logarithm (ln). The empirical histogram of total 25(OH)D approximated a normal distribution.
In descriptive analyses, we tabulated weighted means and standard deviations of total 25(OH)D by selected population characteristics. We used the R nlme package to fit unadjusted linear mixed models (LMMs) with a subject-specific random intercept, chosen based on Akaike’s information criterion (AIC), to account for intra-individual correlation of repeated measures over time. We used unadjusted LMMs to test the differences in mean 25(OH)D concentrations across categorical variables. To investigate the potential effects of gestational weight gain on total 25(OH)D concentrations, we calculated the absolute difference between maternal weight measured at visit 3 (median 26 wk) and prepregnancy, excluding those who lost weight between these two time points (). We regressed repeated measures of total 25(OH)D on gestational weight gain using LMMs with subject-specific random intercepts, adjusting for gestational age at time of sample collection. We tabulated weighted selected percentiles of all urinary analytes and tested the differences in mean levels between the two study visits of sample collection (visits 1 and 3) using paired t-tests of ln-transformed concentrations. To evaluate the pairwise correlations among urinary phthalate metabolites and BPA, we calculated the Spearman correlation coefficients () of specific-gravity standardized concentrations by study visit of sample collection.
In repeated measures analyses, we explored the associations between urinary biomarkers and plasma total 25(OH)D using LMMs that included subject-specific random intercepts, with 25(OH)D regressed on one analyte per model. We chose covariates based on biological and statistical considerations. We included maternal age, race/ethnicity (women who identified as white, black, or other regardless of Hispanic origin), and BMI a priori. Additional covariates—such as health insurance provider, educational attainment, season at time of sample collection, multivitamin supplement use in pregnancy, parity, fetal sex, and smoking and alcohol use in pregnancy—were added using a forward stepwise selection procedure and were retained in the final models if their inclusion resulted in change in the main effect estimates.
Crude models included fixed effects terms for gestational age at time of sample collection (continuous) and urinary SG (continuous). Full models were additionally adjusted for maternal age (continuous), BMI at time of enrollment (continuous), race/ethnicity (white, black, other/mixed race), health-insurance provider (private, public), season at time of sample collection (winter, spring, summer, fall), and multivitamin supplement use in pregnancy (yes, no). Participants missing data on key covariates were not included in the final multivariable regression analyses. Final regression models included women (). All final LMMs were repeated with an interaction term to test whether the effects of phthalates and/or BPA on circulating 25(OH)D levels varied by study visit of sample collection.
Because skin pigmentation is associated with circulating 25(OH)D concentrations (Hall et al. 2010), we performed a sensitivity analysis by stratifying LMMs by race/ethnicity to investigate whether the associations between urinary exposure biomarkers and total 25(OH)D concentrations varied by race/ethnicity. We also assessed whether these effects were modified by race/ethnicity by adding an interaction term in the LMMs for the overall study population. To improve the interpretability of results yielded from models with ln-transformed predictor variables, we presented all regression results as the percent difference () in 25(OH)D associated with an IQR (population-level) increase in urinary biomarker concentrations.
In addition to exploring associations with continuous measures of 25(OH)D, we assessed the relationships between urinary biomarkers and the odds of vitamin D deficiency, defined as total 25(OH)D concentrations (Holick et al. 2011). In this cross-sectional analysis, we stratified logistic regression models by time of sample collection in pregnancy and adjusted all models for the same covariates as those included in repeated measures analysis.
To explore potential nonlinear associations, we fitted generalized additive mixed effects models (GAMM) using the R mgcv package. For each model, we regressed repeated measures of total 25(OH)D on a penalized spline of urinary DEHP metabolites and BPA, with one urinary biomarker included per model. These multivariable GAMMs were adjusted for the same covariates as those included in LMMs, and included a random intercept for each subject. All associations were considered statistically significant at the 5% level.
Results
The population demographic characteristics of the nested case–control study population have been described in detail previously (Ferguson et al. 2014b). Briefly, the present study participants were predominately white and highly educated, and half of the women had a normal BMI (). The distributions of total 25(OH)D by population demographic characteristics are presented in Table 1. Mean 25(OH)D concentrations were significantly higher in all older age groups in comparison with women 18 to 24 y old and in participants who reported multivitamin supplement use during pregnancy in comparison with those who reported no supplement use. Women who identified as black or other race/ethnicity had significantly lower concentrations of 25(OH)D in comparison with concentrations in white women. Significantly lower concentrations were also reported in women who had public health insurance in comparison with private, in those who were overweight (BMI: ) and obese (BMI: ) in comparison with women who had a normal BMI, and in all lower educational levels in comparison with college graduates. Absolute weight gain () between measurements collected prepregnancy and at visit 3 (median 26 wk of gestation) was not associated with total 25(OH)D concentrations { ; , 0.09} in our study population.
Table 1.
Plasma 25(OH)D levels (weighted mean ± SD) by population demographic characteristics ( pregnant women).
| Population characteristics | (%)a | Total 25(OH)D (ng/mL) | p-valued |
|---|---|---|---|
| Age (years) | |||
| 18–24 | 52 (12) | 20.2 (14.9) | Ref |
| 25–29 | 95 (20) | 23.7 (15.0) | 0.01 |
| 30–34 | 188 (39) | 25.2 (13.7) | |
| 142 (29) | 26.8 (13.7) | ||
| Race/ethnicity | |||
| White | 280 (59) | 27.6 (12.6) | Ref |
| Black | 76 (16) | 19.0 (15.3) | |
| Other | 121 (25) | 21.9 (13.9) | |
| Education levelc | |||
| College graduate | 186 (41) | 26.8 (12.9) | Ref |
| Junior college or some college | 139 (30) | 25.6 (13.7) | 0.03 |
| Technical school | 76 (16) | 23.0 (16.0) | |
| High school | 66 (13) | 20.0 (14.9) | |
| Health insurance providerc | |||
| Private (ref) | 381 (81) | 25.9 (13.7) | Ref |
| Public | 84 (19) | 20.0 (15.3) | |
| BMI at initial visitc | |||
| 249 (53) | 26.8 (14.0) | Ref | |
| 125 (27) | 24.0 (14.0) | ||
| 100 (20) | 20.5 (13.7) | ||
| Fetal sex | |||
| Male | 212 (45) | 24.9 (15.5) | Ref |
| Female | 265 (55) | 24.7 (13.6) | 0.61 |
| Parity | |||
| No previous pregnancies | 214 (45) | 25.2 (13.9) | Ref |
| One previous pregnancy | 155 (34) | 25.4 (15.2) | 0.84 |
| More than one previous pregnancy | 108 (21) | 23.1 (14.0) | 0.12 |
| Tobacco usec | |||
| Smoked in pregnancy | 31 (6) | 22.1 (15.3) | Ref |
| No smoking in pregnancy | 440 (94) | 25.0 (14.3) | 0.20 |
| Alcohol usec | |||
| Alcohol use in pregnancy | 19 (5) | 25.9 (16.1) | Ref |
| No alcohol use in pregnancy | 448 (95) | 24.7 (14.3) | 0.60 |
| Multivitamin supplement usec | |||
| Supplement use in pregnancy | 324 (70) | 25.9 (13.6) | Ref |
| No supplement use in pregnancy | 147 (30) | 22.2 (15.4) | |
| Season of sample collection | |||
| Winter (ref) | 224 (27)b | 22.6 (14.2) | Ref |
| Spring | 231 (28) | 24.5 (13.9) | |
| Summer | 185 (22) | 27.8 (14.6) | |
| Fall | 197 (24) | 25.1 (14.1) |
Note: BMI, body mass index; SD, standard deviation; ref, reference category.
Proportions weighted by preterm birth case-control sampling probabilities to represent the general sampling population.
Sample size and weighted proportions refer to number of samples (not participants).
Missing observations: for education level; for insurance provider; for BMI at initial visit; for tobacco use; for alcohol use; for multivitamin supplement use.
p-Value for the difference in mean plasma total 25(OH)D concentrations in the category compared to reference (first category listed) using unadjusted linear mixed models with a random intercept for each subject.
All urinary biomarkers were highly detected in the study population, with urinary phthalate metabolites detected in at least 96% of the samples and BPA detected in 82% of the samples (Table 2). Urinary phthalate metabolites from the same parent compound were strongly correlated at both visits ( for DEHP metabolites) and were weaker among other metabolites (see Tables S1 and S2). Spearmen correlations were weak to moderate between BPA and phthalate metabolites (). Concentrations of urinary MCPP as well as DEHP metabolites, including , were significantly lower in samples collected at visit 3 (median 26 wk of gestation) in comparison with samples collected at visit 1 (median 10 wk of gestation) (Table 2). Urinary BPA did not significantly differ by study visit of sample collection. Total 25(OH)D concentrations were significantly greater in samples collected at 26 wk of gestation in comparison with those collected at 10 wk ( vs. , respectively) (Table 2).
Table 2.
Weighted median [interquartile range (IQR; 25th–75th percentiles)] of urinary and plasma biomarkers by study visit of sample collection in pregnancy.
| Biomarker | LOD | % Detectc | Visit 1 (median 10 wk) | Visit 3 (median 26 wk) | p-Valued | |||
|---|---|---|---|---|---|---|---|---|
| # Samplesb | Median (IQR) | # Samplesb | Median (IQR) | |||||
| Urinary Exposure Biomarkersa | ||||||||
| BPA () | 0.4 | 82.0 | 476 | 1.28 (0.75, 2.08) | 409 | 1.28 (0.84, 2.08) | 0.47 | |
| MEHP () | 1.0 | 96.6 | 474 | 10.1 (5.17, 24.7) | 409 | 8.10 (4.65, 16.7) | ||
| MEHHP () | 0.1 | 99.1 | 474 | 33.6 (17.4, 80.2) | 409 | 23.9 (12.3, 50.0) | ||
| MEOHP () | 0.1 | 99.2 | 474 | 16.9 (8.60, 40.3) | 409 | 14.0 (7.23, 28.7) | ||
| MECPP () | 0.2 | 99.3 | 474 | 40.6 (18.9, 107) | 409 | 30.6 (15.0, 72.8) | ||
| () | – | – | 474 | 0.37 (0.18, 0.81) | 409 | 0.28 (0.14, 0.58) | ||
| MBzP () | 0.2 | 99.4 | 474 | 6.22 (3.36, 13.4) | 409 | 5.87 (3.34, 11.8) | 0.83 | |
| MBP () | 0.5 | 99.3 | 474 | 16.1 (10.8, 26.7) | 409 | 16.1 (10.4, 25.5) | 0.37 | |
| MiBP () | 0.1 | 99.2 | 474 | 7.14 (4.51, 11.1) | 409 | 7.53 (4.61, 11.6) | 0.84 | |
| MEP () | 1.0 | 99.4 | 474 | 124 (49.0, 362) | 409 | 123 (47.2, 363) | 0.96 | |
| MCPP () | 0.2 | 97.7 | 474 | 1.68 (1.06, 3.38) | 409 | 1.57 (0.98, 3.13) | 0.01 | |
| Vitamin D | ||||||||
| 25(OH)D (ng/mL) | 4.0 | 100 | 469 | 23.8 (17.7, 30.0) | 429 | 25.6 (18.1, 31.5) | ||
Note: Analyses were weighted by preterm birth case–control sampling probabilities. LOD, limit of detection.
Urinary analyte concentrations corrected for specific gravity.
Number of plasma samples per analyte varied due to limitations in sample volume.
Percent of analyte concentrations above the detection limits.
p-Value for difference between urinary phthalate metabolite or 25(OH)D concentrations between study visits based on a paired t-test.
Results from repeated measures analysis using multivariable LMMs are reported in Table 3. Similar associations were observed between weighted and unweighted analyses (see Table S3). We detected inverse associations between DEHP metabolites and total 25(OH)D, with the strongest associations observed for MEHP (; , ), MEHHP (; , ), and MEOHP (; , ). We also found a significant inverse association between MCPP and 25(OH)D, where an IQR increase in urinary MCPP was associated with a 4.48% decrease in total 25(OH)D (, ). For BPA, we observed a nonsignificant inverse association (; , 1.45). Our interaction analysis using multivariable LMMs revealed no statistically significant interactions between any of the urinary biomarkers measured and study visit of sample collection (, 0.17; and for phthalates, ranged from 0.36 for MiBP to 0.98 for MEHP) (data not shown).
Table 3.
Repeated measures analysis: Percent difference in plasma 25(OH)D associated with an interquartile range (IQR) increase in urinary exposure biomarker concentrations.
| Urinary biomarker | IQR | (95% CI) | p-Value |
|---|---|---|---|
| BPA () | 1.94 | (, 1.45) | 0.24 |
| MEHP () | 17.6 | (, ) | 0.049 |
| MEHHP () | 60.2 | (, ) | 0.046 |
| MEOHP () | 30.0 | (, ) | 0.049 |
| MECPP () | 84.1 | (, 0.80) | 0.15 |
| () | 0.67 | (, 0.34) | 0.08 |
| MBzP () | 13.6 | 0.88 (, 5.05) | 0.68 |
| MBP () | 25.4 | (, 1.26) | 0.20 |
| MiBP () | 11.0 | (, 3.86) | 0.94 |
| MEP () | 336 | (, 3.36) | 0.94 |
| MCPP () | 3.02 | (, ) |
Note: Analyses weighted by preterm birth case–control sampling probabilities. Linear mixed models include a random intercept for each subject and are adjusted for specific gravity (continuous), maternal age (continuous), BMI at enrollment (continuous), gestational age at time of sample collection (continuous), race (black, white, other/mixed race), insurance provider (private, public), season at time of sample collection (winter, spring, summer, fall), multivitamin supplement use in pregnancy (yes, no).
In our sensitivity analysis, associations from race/ethnicity-stratified models were largely inverse (Table 4). An IQR increase in BPA was inversely associated with total 25(OH)D in white women (; , 0.20; based on data for 274 women and 506 samples) but did not appear to be associated with total 25(OH)D among women who identified as black (; , 7.51; 71 women and 121 samples) or other race/ethnicity (; , 7.59; 114 women and 210 samples). There were no significant differences in the associations for BPA by race/ethnicity, based on p-values for interaction terms (Table 4). Among women who identified as other race/ethnicity, DEHP metabolites were inversely associated with total 25(OH)D, with a significant association observed for MEHP (; , ) in comparison with a null association for white women (; , 3.31; p-interaction other race/ethnicity vs. ). A weak inverse association for MEHP was observed among black women (; , 6.60; p-interaction black vs. ). IQR increases in MCPP were associated with a percent decrease in total 25(OH)D in women of other race/ethnicity (; , ) and in black women (; , 3.86), in comparison with a weaker inverse association estimated for white women (; , 2.01; p-interaction other race/ethnicity vs. ; p-interaction black vs. ).
Table 4.
Race/ethnicity-stratified repeated measures analysis: Percent difference in total 25(OH)D associated with an interquartile range (IQR) increase in urinary exposure biomarker concentrations.
| Urinary biomarker | IQR | White women women; 506 samples | Black women women; 121 samples | Other race/ethnicity women; 210 samples | Black vs. white | Other vs. white | |||
|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | p-Value | (95% CI) | p-Value | (95% CI) | p-Value | p-Interactiona | p-Interactiona | ||
| BPA () | 1.94 | (, 0.20) | 0.06 | (, 7.51) | 0.88 | 0.99 (, 7.59) | 0.77 | 0.97 | 0.71 |
| MEHP () | 17.6 | (, 3.31) | 0.92 | (, 6.60) | 0.73 | (, ) | 0.06 | ||
| MEHHP () | 60.2 | (, 0.75) | 0.15 | 0.34 (, 7.64) | 0.93 | (, 0.50) | 0.08 | 0.27 | 0.10 |
| MEOHP () | 30.0 | (, 1.25) | 0.21 | (, 7.96) | 0.90 | (, 0.51) | 0.08 | 0.26 | 0.11 |
| MECPP () | 84.1 | (, 2.50) | 0.56 | (, 4.43) | 0.34 | (, 1.59) | 0.17 | 0.047 | 0.11 |
| () | 0.67 | (, 2.28) | 0.42 | (, 6.85) | 0.60 | (, 0.49) | 0.07 | 0.09 | 0.06 |
| MBzP () | 13.6 | 4.02 (, 9.01) | 0.12 | (,8.95) | 0.78 | (, 2.08) | 0.16 | 0.14 | |
| MBP () | 25.4 | (, 1.23) | 0.12 | 2.05 (, 15.5) | 0.77 | (, 4.37) | 0.39 | 0.97 | 0.48 |
| MiBP () | 11.0 | (, 1.77) | 0.19 | 2.08 (, 11.1) | 0.65 | 2.79 (, 10.5) | 0.48 | 0.88 | 0.96 |
| MEP () | 336 | (, 0.64) | 0.13 | 3.42 (, 9.02) | 0.24 | 1.87 (, 6.67) | 0.45 | 0.12 | 0.41 |
| MCPP () | 3.02 | (, 2.01) | 0.22 | (, 3.86) | 0.19 | (, ) | 0.02 | 0.06 | 0.08 |
Note: Analyses weighted by preterm birth case-control sampling probabilities. Linear mixed models include a random intercept for each subject and are adjusted for specific gravity (continuous), maternal age (continuous), BMI at enrollment (continuous), gestational age at time of sample collection (continuous), insurance provider (private, public), season at time of sample collection (winter, spring, summer, fall), multivitamin supplement use in pregnancy (yes, no).
p-value for the interaction between ln-transformed urinary biomarkers and race/ethnicity.
In our analysis of vitamin D deficiency by study visit of sample collection, we estimated that approximately 35% () of women were vitamin D deficient at visit 1 (median 10 wk of gestation) and 30% () at visit 3 (median 26 wk of gestation) (Table 5). We reported from our stratified logistic regression models that a unit increase in urinary DEHP metabolites was associated with a 12% to 19% increase in the odds of vitamin D deficiency at visit 1 [odds ratios ; , 1.25 for MEHP to ; , 1.34 for MEOHP]. The direction of these relationships remained at visit 3, although none of the estimates were statistically significant. We also found a significant positive association for MiBP at visit 1 (; , 1.52). For BPA, we observed a significant increase in the odds of vitamin D deficiency only at visit 3 (; , 1.47). Also at visit 3, we reported statistically significant elevated odds ratios for MBzP (; , 1.50) and MBP (; , 1.45).
Table 5.
Adjusted odds ratios (95% CI) of vitamin D deficiency () associated with a unit increase in urinary biomarkers.
| Urinary biomarkers | Odds ratio (95% CI) | p-Value |
|---|---|---|
| Visit 1: Median 10 weeks ( vitamin D deficient women, 292 controls) | ||
| BPA | 1.04 (0.87, 1.25) | 0.65 |
| MEHP | 1.12 (1.00, 1.25) | 0.06 |
| MEHHP | 1.19 (1.06, 1.33) | |
| MEOHP | 1.19 (1.07, 1.34) | |
| MECPP | 1.16 (1.03, 1.30) | 0.01 |
| 1.19 (1.06, 1.35) | ||
| MBZP | 0.95 (0.83, 1.09) | 0.49 |
| MBP | 0.96 (0.81, 1.14) | 0.62 |
| MIBP | 1.25 (1.04, 1.52) | 0.02 |
| MEP | 0.94 (0.84, 1.04) | 0.21 |
| MCPP | 1.01 (0.89, 1.14) | 0.88 |
| Visit 3: Median 26 weeks ( vitamin D deficient women, 268 controls) | ||
| BPA | 1.22 (1.01, 1.47) | 0.04 |
| MEHP | 1.12 (0.97, 1.28) | 0.11 |
| MEHHP | 1.14 (1.00, 1.30) | 0.05 |
| MEOHP | 1.13 (1.00, 1.29) | 0.06 |
| MECPP | 1.05 (0.92, 1.18) | 0.48 |
| 1.10 (0.96, 1.26) | 0.18 | |
| MBZP | 1.27 (1.08, 1.50) | |
| MBP | 1.22 (1.03, 1.45) | 0.02 |
| MIBP | 1.10 (0.91, 1.32) | 0.33 |
| MEP | 0.92 (0.83, 1.02) | 0.10 |
| MCPP | 1.05 (0.90, 1.21) | 0.54 |
Note: Analyses weighted by preterm birth case-control sampling probabilities. Logistic regression models are adjusted for specific gravity (continuous), maternal age (continuous), BMI at enrollment (continuous), gestational age at time of sample collection (continuous), insurance provider (private, public), season at time of sample collection (winter, spring, summer, fall), multivitamin supplement use in pregnancy (yes, no).
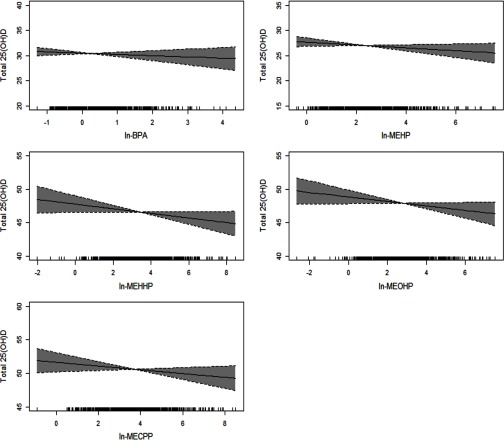
Results from our analysis in which we evaluated nonlinear associations using penalized splines for urinary biomarkers in GAMM models are presented in Figure 1. All multivariable associations were found to be linear.
Figure 1.
GAMM results for urinary DEHP metabolites and BPA () and total 25(OH)D (ng/mL), adjusted for specific gravity, maternal age, BMI at enrollment, gestational age at time of sample collection, race, insurance provider, season at time of sample collection, multivitamin supplement use in pregnancy. Analyses weighted by preterm birth case–control sampling probabilities.
Discussion
In a secondary analysis of 477 pregnant women drawn from a nested case–control study of preterm birth, we found that repeated measures of certain urinary phthalate metabolites, specifically DEHP metabolites and MCPP, were inversely associated with circulating total 25(OH)D levels. A nonsignificant inverse association between urinary BPA and total 25(OH)D was observed in the overall population analysis. Associations varied by race/ethnicity and estimates for white women were more precise than those for black or for women identifying as other race/ethnicity due to differences in the numbers of women in each group. In agreement with findings from repeated measures analysis, we reported that DEHP metabolites and BPA were significantly associated with an approximate 20% increase in the odds of vitamin D deficiency at median 10 wk (: ; , 1.35) and 26 wk (BPA: ; , 1.47), respectively.
We are aware of one previous analysis that has investigated the associations of exposure to phthalates and/or BPA on the vitamin D endocrine system in humans (Johns et al. 2016b). Our results for DEHP metabolites in the current analysis are consistent with those previously reported in a representative sample of U.S. adults 20 y and older (Johns et al. 2016b). In our earlier study utilizing data from participants in the National Health and Nutrition Examination Survey (NHANES) 2005–2010, we found significant inverse associations between urinary DEHP metabolites, including and circulating total 25(OH)D in adult men and women (Johns et al. 2016b). Furthermore, our exposure–response analysis in that previous NHANES study revealed inverse trends between quintiles of individual DEHP metabolites and total 25(OH)D (Johns et al. 2016b). GAMM model estimates for the present study population also supported linear associations between increasing exposure to DEHP metabolites and decreasing total 25(OH)D concentrations.
For BPA, we previously reported a statistically significant inverse association with total 25(OH)D when analyses were restricted to women alone (Johns et al. 2016b). The direction of this relationship is similar to those presented in the repeated measures analysis among the overall population of pregnant women in our current study. In race/ethnicity-stratified models, the magnitude of association was larger among white (; , 0.20) than among women identifying as black (; , 7.51) or other race/ethnicity (; , 7.59). These results may reflect racial differences in behaviors, lifestyle factors, and/or metabolic processes that were not captured in the present analyses, thereby potentially leading to residual confounding.
In pregnancy, the fetus relies solely on maternal levels of 25(OH)D, which in turn is converted to by a series of hydroxylation steps initiated by cytochrome P450 enzymes found in the fetal-placental unit (Bikle 2014; Rosen et al. 2012). Currently, there is a lack of a consensus on the threshold used to define optimal (or sufficient) serum 25(OH)D concentrations in pregnancy (Thorne-Lyman and Fawzi 2012; Urrutia and Thorp 2012). Furthermore, the optimal threshold may vary by gestational age as the clinical outcomes associated with reduced 25(OH)D likely differ across pregnancy (Aghajafari et al. 2013; Lucas et al. 2013). Although the data are somewhat conflicting due to the heterogeneity across human health studies, results from meta-analyses suggest that vitamin D insufficiency in pregnancy may be associated with various adverse maternal and neonatal outcomes (e.g., gestational diabetes, preeclampsia, infection, and restricted fetal growth) (Aghajafari et al. 2013; Wei 2014). Some of these effects may be explained by the regulatory role of in trophoblast function (Nguyen et al. 2015) and in responding to inflammation and infection in the placenta (Liu et al. 2011). Although the magnitude of estimated differences in 25(OH)D and odds ratios for vitamin D deficiency were relatively small in our analyses, on a population-level these decrements may have significant public health implications, especially if there is a causal association between vitamin D deficiency and adverse maternal and neonatal outcomes. Future research is required to determine the public health impact of subclinical changes in circulating 25(OH)D across diverse populations of pregnant women.
Although mechanistic studies are lacking, it is plausible that phthalates and BPA may directly and/or indirectly influence the vitamin D endocrine system at multiple points along its axis. The vitamin D endocrine system is principally regulated by: a) , which down-regulates its own production; b) parathyroid hormone, which in response to low serum calcium levels stimulates hydroxylation enzymes in the kidney to convert 25(OH)D to its active metabolite; c) serum calcium and phosphate levels; and d) fibroblast growth factor 23 (Henry 2011; Norman 2008). Several animal studies have shown that BPA may disturb calcium metabolism by inducing or inhibiting the renal expression of a vitamin D–dependent calcium-binding protein, calbindin-D9k (CaBP-9k) (Kim et al. 2013; Otsuka et al. 2012) as well as decreasing serum calcium levels (Otsuka et al. 2012) in pregnant mice. However, similar effects have not been reported for phthalates (Hong et al. 2005). These agents may also indirectly influence the vitamin D endocrine system through their effects on the metabolic enzymes involved in the conversion of cutaneous vitamin D to its active metabolite. Animal and in vitro studies have demonstrated that phthalates and BPA can alter the expression of cytochrome P450 enzymes involved in steroid and/or thyroid hormone metabolism (Liu et al. 2015; Mathieu-Denoncourt et al. 2015; Quesnot et al. 2014; Sekaran and Jagadeesan 2015). Moreover, increased messenger RNA (mRNA) expression of CYP27B1, the enzyme involved in converting 25(OH)D to its active metabolite, was observed in mice treated with BPA (Otsuka et al. 2012). Similar studies assessing the effects of phthalates on enzymes involved in the metabolism of vitamin D have not been conducted to date, and future research is required to elucidate the potential actions of these chemicals on additional components of the vitamin D endocrine system (e.g., vitamin D–binding protein, metabolic enzymes, parathyroid hormone regulation, etc.).
It is also possible that certain lifestyle or physiological factors may partially mediate the associations observed in our study. For example, although studies investigating the relationships between phthalate and BPA exposure and physical activity are lacking, recent animal studies suggest that exposure to endocrine-disrupting chemicals such as phthalates and BPA may reduce or alter voluntary physical activity in mice (Johnson et al. 2015; Schmitt et al. 2016). Because physical activity has been positively associated with 25(OH)D concentrations in pregnant women (Moon et al. 2015; Woolcott et al. 2016), physical activity may be one possible mechanism through which maternal phthalate and/or BPA exposure might contribute to decreased concentrations of 25(OH)D. In the current study, we did not collect data on physical activity from our participants. Additional analyses are required to confirm the role (if any) that physical activity plays in these relationships. It is also possible that phthalate and/or BPA exposure may influence circulating 25(OH)D levels through maternal weight gain in pregnancy. Previous research suggests that exposure to phthalates and BPA may be associated with increased weight gain in women (Song et al. 2014) and that a greater gestational weight gain may lead to a decline in 25(OH)D concentrations in pregnancy (Moon et al. 2015). However, in our study population, absolute weight gain, defined as the difference in measurements collected at visit 3 (median 26 wk of gestation) and prepregnancy, was not associated with repeated measures of 25(OH)D. Further animal and human health studies are needed to characterize the potential vitamin D–disruptive properties of phthalates and BPA and to identify their specific mechanisms of action in pregnancy.
One of the main strengths of our study included repeated measures of both the exposures and outcome of interest, which allowed for the use of statistical modeling techniques to more precisely detect the subtle associations of exposure. Nevertheless, our study has several potential limitations. Although the reference assay for measuring 25(OH)D is liquid chromatography tandem mass spectrometry (LC-MS/MS), its time-consuming and laborious procedures limit the efficiency of this method in clinical settings, in comparison with automated immunoassays (Hollis 2010; Wagner et al. 2009). The DiaSorin LIAISON® immunoassay, the assay utilized in the present study, is a widely used method in both clinical and research settings (Burris et al. 2015; Hollis 2010) and has shown excellent agreement with LC-MS/MS methods [concordance correlation coefficient ] (Farrell et al. 2012). Additionally, although we adjusted our statistical analyses for key confounding variables (e.g., season of sample collection, multivitamin supplement use, and race/ethnicity), we lacked data on dietary food intake and the frequency of use of vitamin D supplements and sunscreen. Concerning the dietary food intake, the dominant exposure pathway for phthalates such as DEHP is ingestion of contaminated food (Wormuth et al. 2006), whereas the major source of vitamin D in humans is exposure to sunlight (Hall et al. 2010; Holick 2004, 2007). Dietary sources of vitamin D are limited but necessary to maintain adequate vitamin D concentrations when sunlight-induced vitamin D synthesis is impaired or in times of insufficient sunlight (Calvo et al. 2004). Few foods naturally contain vitamin D (e.g., oily fish), although in the U.S., some dairy (e.g., milk, yogurt, and cheese), cereal, and juices are fortified with vitamin D (Holick and Chen 2008). Among these foods, dairy consumption has been associated with increased concentrations of urinary DEHP metabolites (Serrano et al. 2014). Unfortunately, we do not have dietary intake data from the women included in our study. Therefore, it is possible that our results may be affected by unmeasured confounding, particularly if specific dietary sources contributed to phthalate and BPA exposure as well as total 25(OH)D concentrations in our study population. Additionally, although sunscreen use was associated with increased concentrations of urinary phthalate metabolites, particularly MBP, in children who participated in a study of 90 adult–child pairs in California from 2007 to 2009 (Philippat et al. 2015), results from a recent NHANES analysis utilizing data from 2009 to 2012 revealed that sunscreen use was not significantly associated with urinary phthalate metabolite concentrations in adults (Ferguson et al. 2016). We did not collect data on personal care product use for the present study; therefore, we cannot determine whether sunscreen use was associated with urinary phthalate metabolite concentrations in our study participants. Our study may also be limited by our exposure assessment methods. Although we analyzed up to two repeated measures of urinary phthalate metabolite and BPA concentrations per subject, a potential for nondifferential exposure misclassification exists. Additional repeated measurements of exposure may be required to sufficiently reduce bias in our analyses involving short-lived chemicals such as BPA and phthalates (Perrier et al. 2016). Finally, we performed multiple statistical comparisons, and there is the potential that some of the detected associations may have been due to chance.
Conclusions
In conclusion, biomarkers of environmental exposure to phthalates and BPA were associated with reduced circulating total 25(OH)D levels in our study population of pregnant women. Given previous research showing the adverse effects of reduced total 25(OH)D levels in pregnancy on the mother and fetus, future studies are required to confirm these findings in additional cohorts of pregnant women and to determine the potential biological mechanisms through which these agents might influence the vitamin D endocrine system.
Supplemental Material
Acknowledgments
Subject recruitment and sample collection was originally funded by Abbott Diagnostics. Funding was also provided by the NIH, NIEHS (grants R01ES018872, P42ES017198, P01ES022844, P30ES017885, and T32ES007062). Funding support for K.K.F. was provided by the Intramural Research Program of the NIH, NIEHS.
References
- Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. 2013. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 346:f1169, PMID: 23533188. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113:192–200, PMID: 15687057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. 2014. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21:319–329, 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Platt RW, Simhan HN. 2015. Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet Gynecol 125:439–447, 10.1097/AOG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Simhan HN. 2010. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv 65:273–284, PMID: 20403218, 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Thomas A, Zera CA, McElrath TF. 2015. Prenatal vitamin use and vitamin D status during pregnancy, differences by race and overweight status. J Perinatol 35:241–245, PMID: 25357099, 10.1038/jp.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, et al. 2014. Vitamin D status among preterm and full-term infants at birth. Pediatr Res 75:75–80, PMID: 24121425, 10.1038/pr.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MS, Whiting SJ, Barton CN. 2004. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr 80:1710S–1716S. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int 62:1–11, PMID: 24161445, 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C. 2014. Genome-wide (over)view on the actions of vitamin D. Front Physiol 5:167, PMID: 24808867, 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, et al. 2011. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int 37:858–866, 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Ersfeld DL, Rao DS, Body JJ, Sackrison JL Jr., Miller AB, Parikh N, et al. 2004. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 37:867–874, 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. 2012. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem 58:531–542, PMID: 22230812, 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD. 2016. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol 27(3):326–332, PMID: 27168391, 10.1038/jes.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123:210–216, PMID: 25402001, 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. 2014a. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int 70:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014b. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M, von Versen HF. 2011. Vitamin D–roles in women's reproductive health? Reprod Biol Endocrinol 9:146, PMID: 22047005, 10.1186/1477-7827-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LM, Kimlin MG, Aronov PA, Hammock BD, Slusser JR, Woodhouse LR, et al. 2010. Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr 140:542–550, PMID: 20053937, 10.3945/jn.109.115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. 2013. Molecular mechanisms of vitamin D action. Calcif Tissue Int 92:77–98, PMID: 22782502, 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- Henry HL. 2011. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab 25:531–541, 10.1016/j.beem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Holick MF. 2004. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S. [DOI] [PubMed] [Google Scholar]
- Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281, 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930, PMID: 21646368, 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC. 2008. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S. [DOI] [PubMed] [Google Scholar]
- Hollis BW. 2010. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Endocrinol Metab Clin North Am 39:271–286, table of contents, 10.1016/j.ecl.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, Ji YK, Choi KC, Manabe N, Jeung EB. 2005. Conflict of estrogenic activity by various phthalates between in vitro and in vivo models related to the expression of Calbindin-D9k. J Reprod Dev 51:253–263, PMID: 15883486. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM, Törk I. 1990. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J Comp Neurol 297:165–181, PMID: 2115053, 10.1002/cne.902970202. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. 2007. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod 22:2715–2722, PMID: 17704099, 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. 2016a. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ Health Perspect 124(11):1808–1815, PMID: 27152641, 10.1289/EHP170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Meeker JD. 2016b. Relationships between urinary phthalate metabolite and bisphenol a concentrations and vitamin d levels in U.S. adults: National Health and Nutrition Examination Survey (NHANES), 2005-2010. J Clin Endocrinol Metab 101(11):4062–4069, PMID: 27648964, 10.1210/jc.2016-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LV, et al. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 13:4, PMID: 25596636, 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Painter MS, Javurek AB, Ellersieck MR, Wiedmeyer CE, Thyfault JP, et al. 2015. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. J Dev Orig Health Dis 6(6):539–552, PMID: 26378919, 10.1017/S2040174415001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, An BS, Yang H, Jeung EB. 2013. Effects of octylphenol and bisphenol A on the expression of calcium transport genes in the mouse duodenum and kidney during pregnancy. Toxicology 303:99–106, 10.1016/j.tox.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantaral A, et al. 2013. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 93:2390–2398, PMID: 24041567, 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhao L, Wei L, Li L. 2015. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ Sci Pollut Res Int 22:12711–12719, PMID: 25913319, 10.1007/s11356-015-4567-7. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. 2011. Vitamin D and the regulation of placental inflammation. J Immunol 186:5968–5974, PMID: 21482732, 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- Lucas R, Xiang F, Ponsonby AL. 2013. Vitamin D sufficiency in pregnancy. BMJ 346:f1675, PMID: 23533189. [DOI] [PubMed] [Google Scholar]
- Luk J, Torrealday S, Neal Perry G, Pal L. 2012. Relevance of vitamin D in reproduction. Hum Reprod 27:3015–3027, PMID: 22824625, 10.1093/humrep/des248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. 2012. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab 303:E928–E935, PMID: 22871339, 10.1152/ajpendo.00279.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS. 2015. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol 219:74–88, PMID: 25448254, 10.1016/j.ygcen.2014.11.003. [DOI] [PubMed] [Google Scholar]
- McElrath TF, Lim K-H, Pare E, Rich-Edwards J, Pucci D, Troisi R, et al. 2012. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 207:407 e1–e7, 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. 2009a. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl 30:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. 2009b. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci 364:2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HM, et al. 2015. Tracking of 25-hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. Am J Clin Nutr 102:1081–1087, 10.3945/ajcn.115.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen ME, Calafat AM, Ye X, Wong L-Y, Wright DJ, Pirkle JL, et al. 2014. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ Res 129:32–38, 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Gao F, Fan Z, Shen H, Peng H, Hu J. 2015. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ Sci Technol 49:10651–10657, PMID: 26251123, 10.1021/acs.est.5b02617. [DOI] [PubMed] [Google Scholar]
- Murthi P, Yong HE, Ngyuen TP, Ellery S, Singh H, Rahman R, et al. 2016. Role of the placental vitamin D receptor in modulating feto-placental growth in fetal growth restriction and preeclampsia-affected pregnancies. Front Physiol 7:43, PMID: 26924988, 10.3389/fphys.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Yong HE, Chollangi T, Borg AJ, Brennecke SP, Murthi P. 2015. Placental vitamin D receptor expression is decreased in human idiopathic fetal growth restriction. J Mol Med (Berl) 93:795–805, PMID: 25716068, 10.1007/s00109-015-1267-1. [DOI] [PubMed] [Google Scholar]
- Norman AW. 2008. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 88:491S–499S, PMID: 18689389. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Sugimoto M, Ikeda S, Kume S. 2012. Effects of bisphenol A administration to pregnant mice on serum Ca and intestinal Ca absorption. Anim Sci J 83:232–237, PMID: 22435627, 10.1111/j.1740-0929.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- Pérez-López FR. 2007. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol 23:13–24, 10.1080/09513590601045629. [DOI] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27:378–388, PMID: 27035688, 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH. 2015. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ Res 140:369–376, PMID: 25929801, 10.1016/j.envres.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike JW, Meyer MB. 2010. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am 39:255–269, table of contents, 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsonby AL, Lucas RM, Lewis S, Halliday J. 2010. Vitamin D status during pregnancy and aspects of offspring health. Nutrients 2:389–407, PMID: 22254029, 10.3390/nu2030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnot N, Bucher S, Fromenty B, Robin MA. 2014. Modulation of metabolizing enzymes by bisphenol A in human and animal models. Chem Res Toxicol 27:1463–1473, 10.1021/tx500087p. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, Weiland SK. 2007. Analyses of case-control data for additional outcomes. Epidemiology 18:441–445, 10.1097/EDE.0b013e318060d25c. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. 2011. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol 204:556, e551–e554, 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Adams JS, Bickle DD, Black DM, Demay MB, Manson JE, et al. 2012. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 33:456–492, 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. 2014. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction 147:401–409, PMID: 24196015, 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EE, Vellers HL, Porter WW, Lightfoot JT. 2016. Environmental endocrine disruptor affects voluntary physical activity in mice. Med Sci Sports Exerc 48:1251–1258, PMID: 26895396, 10.1249/MSS.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Jagadeesan A. 2015. In utero exposure to phthalate downregulates critical genes in Leydig cells of F1 male progeny. J Cell Biochem 116:1466–1477, PMID: 25649163, 10.1002/jcb.25108. [DOI] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13:43, PMID: 24894065, 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes Relat Metab Disord 38:1532–1537, 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher TD, Clarke BL. 2011. Vitamin D insufficiency. Mayo Clin Proc 86:50–60, PMID: 21193656, 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne-Lyman A, Fawzi WW. 2012. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 26 (suppl 1):75–90, PMID: 22742603, 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia RP, Thorp JM. 2012. Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol 24:57–64, PMID: 22327734, 10.1097/GCO.0b013e3283505ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Hanwell HE, Vieth R. 2009. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 42:1549–1556, PMID: 19631201, 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Wei SQ. 2014. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol 26:438–447, PMID: 25310531, 10.1097/GCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- Woolcott CG, Giguère Y, Weiler HA, Spencer A, Forest JC, Armson BA, et al. 2016. Determinants of vitamin D status in pregnant women and neonates. Can J Public Health 107:e410–e416, PMID: 28026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans?. Risk Anal 26:803–824, PMID: 16834635, 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.