Abstract
Background:
Humans are commonly exposed to multiple environmental chemicals, including tetrabromobisphenol A (TBBPA; a flame retardant), triclosan (an antimicrobial agent), and bisphenol A (BPA; polycarbonate plastics). These chemicals are readily absorbed and may interact with each other.
Objectives:
We sought to determine whether TBBPA, given alone or in combination with triclosan, can modulate the concentrations of BPA and (E2).
Methods:
Female and male CF-1 mice were each given a subcutaneous injection of TBBPA, with or without concurrent triclosan, followed by dietary administration of body weight . Radioactivity was measured in blood serum and tissues through liquid scintillation counting. In subsequent experiments, female and male CF-1 mice were each given a subcutaneous injection of 0 or TBBPA and E2 was measured in urine 2–12 h after injection.
Results:
Doses as low as TBBPA significantly elevated concentrations in the uterus and ovaries of females; in the testes, epididymides, vesicular-coagulating glands, and preputial glands of males; and in blood serum, heart, lungs, and kidneys of both sexes; urinary E2 concentrations were also elevated. Lower doses of TBBPA or triclosan that had no effects on their own elevated concentrations when the two substances were given concurrently.
Conclusion:
These data indicate that TBBPA, triclosan, and BPA interact in vivo, consistent with evidence that TBBPA and triclosan inhibit enzymes that are critical for BPA and E2 metabolism. https://doi.org/10.1289/EHP1329
Introduction
Tetrabromobisphenol A (TBBPA; CAS 79-94-7) is the most-produced flame retardant, with global use over (Environment Canada and Health Canada 2013). Approximately 80% of TBBPA is used in reactive applications, where it is covalently bound to the polymer of epoxy resins for printed circuit boards in electronics equipment (Colnot et al. 2014; Shaw et al. 2014). The remaining 20% of TBBPA is used in additive applications, where it is physically blended with rather than chemically bound to the polymer, as in plastic housing for electronics equipment (Colnot et al. 2014; Shaw et al. 2014). Both reactive- and additive-treated products release TBBPA into the environment (Malkoske et al. 2016; Shaw et al. 2014). TBBPA has been detected in soil and sediment (Lee et al. 2015; Wang J et al. 2015; Zhu et al. 2014), surface and waste water (Kim et al. 2016; Xiong et al. 2015), and air and indoor dust (La Guardia and Hale 2015; Ni and Zeng 2013; Wang W et al. 2015; Wu et al. 2016b). Nonoccupational TBBPA exposure in humans occurs via inhalation and ingestion of dust, as well as through dermal contact with dust and free (unreacted) TBBPA in consumer products (Abdallah 2016; Knudsen et al. 2015). TBBPA is bioavailable in humans, as shown by its detection in human serum (Cariou et al. 2008; Fujii et al. 2014), plasma (Ho et al. 2017), breast milk (Abdallah and Harrad 2011; Fujii et al. 2014; Nakao et al. 2015), and adipose tissue (Cariou et al. 2008; Johnson-Restrepo et al. 2008).
The potential for TBBPA to act as an endocrine-disrupting chemical is not well understood. Mechanisms of endocrine disruption by TBBPA could include actions on estrogen, androgen, glucocorticoid, or thyroid hormone receptors, or combinations of any or all of these receptors (Beck et al. 2016; Hamers et al. 2006; Huang et al. 2013). Considering evidence of estrogenic actions, some studies found that TBBPA binds to estrogen receptor (ER) in in vitro assays (Li et al. 2010; Olsen et al. 2003; Suzuki et al. 2013), whereas other studies found that TBBPA failed to bind in in vitro assays (Dorosh et al. 2011; Hamers et al. 2006; Lee et al. 2012; Meerts et al. 2001; Miller et al. 2001; Molina-Molina et al. 2013; Riu et al. 2011a, 2011b) and in molecular modeling studies (Zhuang et al. 2014). More recent work has examined indirect mechanisms of estrogenicity whereby TBBPA disrupts estrogen homeostasis (Honkisz and Wójtowicz 2015; Lai et al. 2015; Sanders et al. 2016; Wikoff et al. 2016). One proposed mechanism is that TBBPA inhibits the metabolism of (E2), thus increasing its bioavailability, via interactions with conjugating enzymes (Lai et al. 2015; Sanders et al. 2016; Wikoff et al. 2016). These enzymes include estrogen sulfotransferase (SULT), UDP-glucuronosyltransferase (UGT), cytochrome p450 (CYP), and dehydrogenase () (Dumas and Diorio 2011; Wikoff et al. 2016). Another proposed mechanism is that TBBPA enhances E2 secretion via actions on aromatase (CYP19) expression (Honkisz and Wójtowicz 2015).
We previously demonstrated in vivo interactions among bisphenol A (BPA), E2, and triclosan (CAS 3,380-34-5), an antimicrobial agent found in soaps and cosmetics. Compared with vehicle-treated animals, male and female mice given a single dose of triclosan showed greater concentrations of in blood serum and in reproductive and other tissues (Pollock et al. 2014). Similarly, female mice given a single dose of triclosan showed greater concentrations of exogenous in the uterus and natural E2 in urine than did vehicle-treated animals (Pollock et al. 2016). Blastocyst implantation in inseminated female mice can be disrupted by high doses of BPA (Berger et al. 2007, 2008, 2010; Borman et al. 2015) and triclosan (Crawford and deCatanzaro 2012); lower doses of each substance that were insufficient on their own disrupted implantation when combined (Crawford and deCatanzaro 2012). Whereas triclosan alone was ineffective in a uterotrophic assay of weanling rats, elevated uterine weight occurred following concurrent triclosan and ethinyl estradiol exposure (Stoker et al. 2010). These findings are consistent with evidence that triclosan is conjugated by SULT, UGT, and CYP (Wu et al. 2016a) and that it can inhibit the activity of SULT and UGT toward other substances, including BPA and E2 (James et al. 2010, 2015; Wang et al. 2004).
Because humans are routinely exposed to multiple potential endocrine-disrupting chemicals, it is important to investigate these chemicals’ capacity to interact with each other and with endogenous steroids in vivo. Here, we undertook to measure the interactions of TBBPA, triclosan, and BPA. Whereas evidence of direct ER activation by TBBPA and triclosan is weak, BPA is a more established environmental estrogen (Rochester 2013; Seachrist et al. 2016; Ziv-Gal and Flaws 2016). Based on the proposed disruption of estrogen homeostasis via inhibitory actions of TBBPA on conjugating enzyme activity (Lai et al. 2015; Sanders et al. 2016; Wikoff et al. 2016), we hypothesized that TBBPA would elevate BPA concentrations in female and male mice and that this effect would be greatest in serum and in estrogen-binding reproductive tissues. We hypothesized that the actions of TBBPA would be additive with those of triclosan, consistent with evidence that triclosan also inhibits activity of conjugating enzymes (James et al. 2010; Wang et al. 2004). We tested these hypotheses by comparing the impact of TBBPA injection, either alone or in combination with triclosan, on concentrations of in serum and tissues. We also hypothesized that TBBPA could elevate endogenous levels of E2, the most potent natural estrogen (Kuiper et al. 1997), and tested this hypothesis by measuring the impact of TBBPA injection on urinary E2.
Methods
Animals and Housing
Female () and male () CF-1 mice aged 3–4 mo were obtained from Charles River. To standardize timing within the estrous cycle at an easily detected point where estrogen levels are moderate and relatively stable (Miller and Takahashi 2014), we selected diestrous females for use in experiments. These females were identified from a colony of mice with regular estrous cycles by vaginal cytology using published procedures (Byers et al. 2012). Animals were housed in polypropylene cages measuring () with ad libitum access to food (Teklad 8640 Certified Rodent Chow; Harlan Teklad) and water, except where otherwise stated. The colony was maintained at 21°C with a reversed 14 h light:10 h darkness cycle. All animals were treated humanely and with regard for alleviation of suffering. All procedures adhered to the standards of the Canadian Council on Animal Care and were approved by the Animal Research Ethics Board of McMaster University (Protocol 14-02-03).
Chemicals and Materials
Triclosan [5-chloro-2-(2,4-dichlorophenoxy)phenol, purity], 3,3′,5,5′-TBBPA [4,4′-isopropylidenebis(2,6-dibromophenol), purity], ( purity), and creatinine standards were obtained from Sigma-Aldrich. {, in ethanol, , } was obtained from Moravek Biochemicals. SOLVABLE solubilization cocktail, Ultima Gold scintillation cocktail, and midi-vial scintillation vials were obtained from PerkinElmer. E2 antibodies and horseradish peroxidase (HRP) conjugates were obtained from the Department of Population Health and Reproduction at the University of California, Davis, CA.
Experimental Design and Dosing
This research followed procedures previously published by this laboratory (Pollock et al. 2014, 2016). In brief, mice were weighed, individually housed, and each given a dietary supplement of peanut butter. Approximately 14–16 h later at the onset of darkness on the following day, animals were randomly assigned to treatment conditions involving a single subcutaneous (sc) injection of TBBPA and/or triclosan dissolved in peanut oil. In experiment 1, males and diestrous females received vehicle or 1, 3, 9, or TBBPA (). In experiment 2, males () and diestrous females () received a single sc injection of vehicle, TBBPA, triclosan, or . Table 1 provides TBBPA and triclosan doses in milligrams/kilogram for each treatment condition. At 30 min after injection, each animal was given a dietary supplement of in peanut butter. Food, water, and bedding were removed to prevent contamination of the treatment. At 1 h after administration, each animal was anesthetized with isoflurane, and blood was collected via cardiac puncture. Each animal was perfused with phosphate-buffered saline (PBS), and tissues were collected in preweighed scintillation vials. Tissue samples taken included the heart, lung, superficial adductor muscle from the hind leg, abdominal adipose, liver, and a cross-section of the kidney encompassing both the medulla and the cortex. Male reproductive tissues taken included one testis, one epididymis, one vesicular-coagulating (VC) gland, and one preputial gland. Female reproductive tissues taken included the whole uterus and both ovaries. Vials were reweighed following tissue collection to determine the sample wet mass; no significant changes in tissue weights were observed (data not shown).
Table 1.
Mean () TBBPA and triclosan doses in milligrams/kilogram for each treatment condition.
| BPA dose () | TBBPA dose (mg) | TBBPA dose (mg/kg) | Triclosan dose (mg) | Triclosan dose (mg/kg) | ||
|---|---|---|---|---|---|---|
| Experiment 1 | ||||||
| Females | 7 | 50 | 0 | |||
| 7 | 50 | 1 | ||||
| 7 | 50 | 3 | ||||
| 7 | 50 | 9 | ||||
| 7 | 50 | 27 | ||||
| Males | 7 | 50 | 0 | |||
| 7 | 50 | 1 | ||||
| 7 | 50 | 3 | ||||
| 7 | 50 | 9 | ||||
| 7 | 50 | 27 | ||||
| Experiment 2 | ||||||
| Females | 7 | 50 | 0 | 0 | ||
| 7 | 50 | 0 | 0.33 | |||
| 7 | 50 | 0.33 | 0 | |||
| 7 | 50 | 0.33 | 0.33 | |||
| Males | 6 | 50 | 0 | 0 | ||
| 6 | 50 | 0 | 0.33 | |||
| 6 | 50 | 0.33 | 0 | |||
| 6 | 50 | 0.33 | 0.33 |
Note: BPA, bisphenol A; , number of animals; SD, standard deviation; TBBPA, tetrabromobisphenol A.
In experiment 3, mice were weighed and were individually placed in a Plexiglas apparatus measuring () with a wire-mesh grid floor raised approximately above a Teflon-coated stainless-steel surface covered with wax paper. Animals acclimated to the novel cages for 3 d before the start of the experiment. At the onset of darkness on the fourth day, males and diestrous females received a sc injection of vehicle or TBBPA (corresponding to TBBPA/kg for females and TBBPA/kg for males) dissolved in peanut oil (). Urine was collected noninvasively at 2, 4, 6, 8, 10, and 12 h postinjection. All urine samples were placed into labeled vials and frozen at at the time of collection.
We administered triclosan and TBBPA via sc injection to mimic dermal absorption of triclosan from personal care products (Fang et al. 2016; Queckenberg et al. 2010) and free (unreacted) TBBPA from dust and consumer products (Abdallah 2016; Knudsen et al. 2015). However, percutaneous penetration is incomplete compared with sc injection; of dermally applied TBBPA is absorbed through rat skin (Knudsen et al. 2015), and of dermally applied triclosan is absorbed through mouse skin (Fang et al. 2016). We administered in a dietary supplement to mimic ingestion of BPA from dust, food, and beverages, which accounts for approximately 85–95% of total exposure in adults (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids 2015). Dietary BPA exposure leads to less-efficient first-pass hepatic metabolism and to higher serum BPA concentrations than oral bolus (Sieli et al. 2011). The 30-min latency between TBBPA and administration and the 1-h latency between treatment and tissue collection were chosen based on an effective paradigm used in previous studies (Pollock et al. 2014, 2016). We selected doses of TBBPA in experiment 1 to establish the impact of a wide range of TBBPA exposures on tissue concentrations of . To investigate potential additive effects of TBBPA and triclosan in experiment 2, we selected doses of so that the quantity of either substance was below the lowest effective dose of triclosan () required to elevate concentrations (Pollock et al. 2014). When both substances were given concurrently, the combined quantity () was greater than the lowest effective dose of triclosan used previously (Pollock et al. 2014). We selected TBBPA for experiment 3 because this dose was sufficient to modulate concentrations in experiment 1. We measured urinary E2 because there are very low concentrations of estrogen conjugates in mouse urine (Muir et al. 2001), whereas unconjugated E2 is abundant in urine and reflects systemic trends (deCatanzaro et al. 2003, 2004; Muir et al. 2001; Thorpe et al. 2014).
Blood and Tissue Processing for Liquid Scintillation Counting
Blood and tissue samples were processed for liquid scintillation counting following previously published procedures (deCatanzaro and Pollock 2016; Pollock et al. 2014, 2016; Pollock and deCatanzaro 2014). Blood samples were centrifuged at for 10 min, and serum was added to a scintillation vial containing Ultima Gold scintillation cocktail. Tissue samples were solubilized by adding SOLVABLE tissue solubilizer to each vial and placing the vials in a 50°C water bath for 4–5 h until completely dissolved. Following the addition of Ultima Gold, the vials were agitated to promote mixing of the sample with the scintillation cocktail. Each vial was stored in the darkness chamber of a Tri-Carb 2910TR Liquid Scintillation Analyzer (PerkinElmer) for 5 min to eliminate noise in the form of heat and luminescence. Radioactivity was then measured for 5 min per vial. The amount of radioactivity per sample, in disintegrations per minute (dpm), was automatically calculated via QuantaSmart software by subtracting background radiation, which is continually monitored by the scintillation counter. Frequent cleaning and monitoring of all work surfaces and equipment ensured that contamination of samples did not occur. The final dpm measures were then normalized to the weight of the sample wet mass and were reported as equivalent nanograms BPA/gram tissue or nanograms BPA/milliliter serum.
Measurement of Urinary E2
Full procedures and validations for enzyme immunoassays for mouse urine have been reported previously (Muir et al. 2001). Cross-reactivities for anti-E2 are as follows: E2, 100%; estrone, 3.3%; progesterone, 0.8%; testosterone, 1.0%; androstenedione, 1.0%, and all other measured steroids, . Urinary E2 levels were considered with and without adjustment for urinary creatinine, which corrects for differential hydration and urinary concentration among animals, and were reported as nanograms E2/milligram creatinine and nanograms E2/milliliter urine, respectively.
Statistical Analyses
All statistical analyses were performed using the R software environment (R Core Team). A comparison-wise error rate of was employed for all tests. Differences between treatments in experiment 1 were analyzed using univariate analysis of variance (ANOVA) for each tissue, with Holm-Bonferroni adjustments to correct for the number of tissues (Holm 1979). Observation of significant effects in ANOVA was followed by pairwise Newman-Keuls multiple comparisons. Differences between treatments in reproductive tissues and in serum of animals in experiment 2 were analyzed using Student’s t-test. Differences between urinary E2 concentrations of animals in experiment 3 were analyzed by factorial ANOVA comparing the effects of treatment and collection time point (repeated measures). Significant main effects or interactions in ANOVA were followed by pairwise Newman-Keuls multiple comparisons of treatment at each collection time point. Data from each experiment for individual animals are provided in Tables S1–S6.
Results
Experiment 1: Measurement of in Mice Given TBBPA
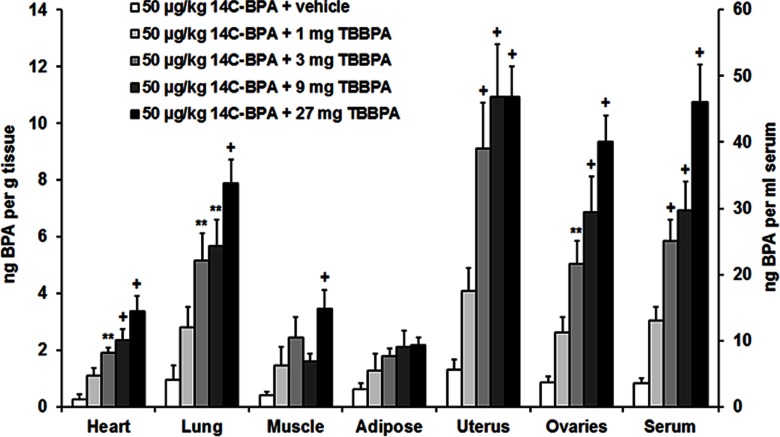
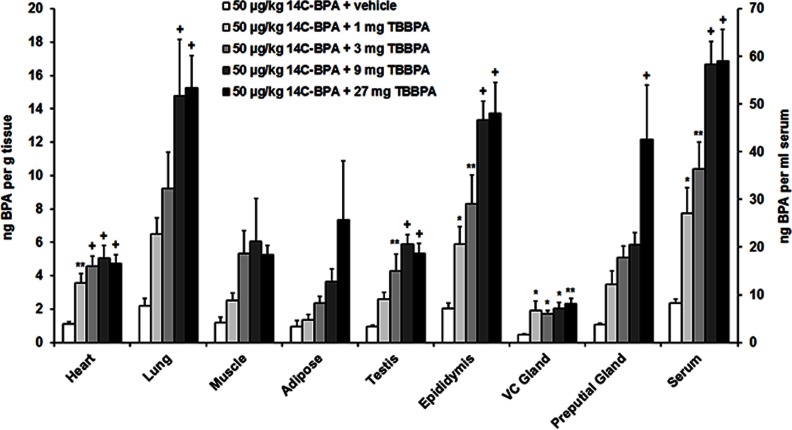
This experiment was designed to determine the impact of TBBPA on the distribution of BPA. Radioactivity was measured in tissues and serum of diestrous females (Figure 1; see also Table S1) and of males (Figure 2; see also Table S2) that received a sc injection of TBBPA followed by a dietary supplement of . Concentrations of in the liver and kidney are reported in Table 2. Pretreatment with TBBPA induced a dose-dependent increase in concentrations of in serum and in most tissues of both sexes.
Figure 1.
Mean () concentration of bisphenol A (BPA) in the heart, lung, muscle, adipose, uterus, ovaries, and serum of diestrous females following subcutaneous (sc) injection of vehicle, 1, 3, 9, or tetrabromobisphenol A (TBBPA) and subsequent dietary administration of (). Difference from vehicle treatment in the same tissue: **; +. See Table S1 for individual animal data.
Figure 2.
Mean () concentration of bisphenol A (BPA) in the heart, lung, muscle, adipose, testis, epididymis, vesicular-coagulating (VC) gland, preputial gland, and serum of males following subcutaneous (sc) injection of vehicle, 1, 3, 9, or tetrabromobisphenol A (TBBPA) and subsequent dietary administration of (). Difference from vehicle treatment in the same tissue: *; **; +. See Table S2 for individual animal data.
Table 2.
Mean () concentration of in the liver and kidney of diestrous females and males following subcutaneous injection of TBBPA and/or triclosan and subsequent dietary administration of .
| TBBPA dose (mg) | Triclosan dose (mg) | Liver (ng BPA/g) | Kidney (ng BPA/g) | |
|---|---|---|---|---|
| Experiment 1 | ||||
| Females | Vehicle | |||
| 1 | ||||
| 3 | ‡ | |||
| 9 | * | |||
| 27 | ‡ | |||
| Males | Vehicle | |||
| 1 | * | † | ||
| 3 | † | |||
| 9 | ‡ | |||
| 27 | * | |||
| Experiment 2 | ||||
| Females | Vehicle | |||
| 0 | 0.33 | |||
| 0.33 | 0 | |||
| 0.33 | 0.33 | |||
| Males | Vehicle | |||
| 0 | 0.33 | |||
| 0.33 | 0 | |||
| 0.33 | 0.33 |
Note: Significance marks indicate differences from vehicle treatment in the same tissue. BPA, bisphenol A; , number of animals; SD, standard deviation; TBBPA, tetrabromobisphenol A.
* . † . ‡.
Comparisons were made among the five treatments for each of nine tissues in females. ANOVA followed by Holm-Bonferroni correction produced significant effects of treatment for the heart, , ; lung, , ; muscle, , ; uterus, , ; ovary, , ; kidney, , ; and serum, , . Multiple comparisons revealed that the vehicle-treated group differed from the 3-, 9-, and groups for the heart, lung, uterus, ovaries, kidney, and serum. The vehicle-treated group also differed from the group for muscle.
Comparisons were made among the five treatments for each of eleven tissues in males. ANOVA followed by Holm-Bonferroni correction produced significant effects of treatment for the heart, , ; lung, , ; testis, , ; epididymis, , ; VC gland, , ; preputial gland, , ; liver, , ; kidney, , ; and serum, , . Multiple comparisons revealed that the vehicle-treated group differed from the 1-, 3-, 9-, and groups for the heart, epididymis, VC gland, kidney, and serum. The vehicle-treated group also differed from the group for the liver; the 3-, 9-, and groups for the testis; the 9- and groups for the lung; and the group for the preputial gland.
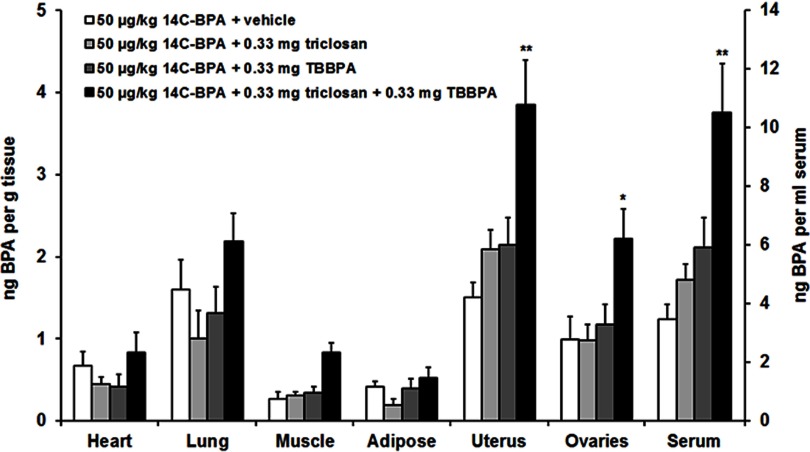
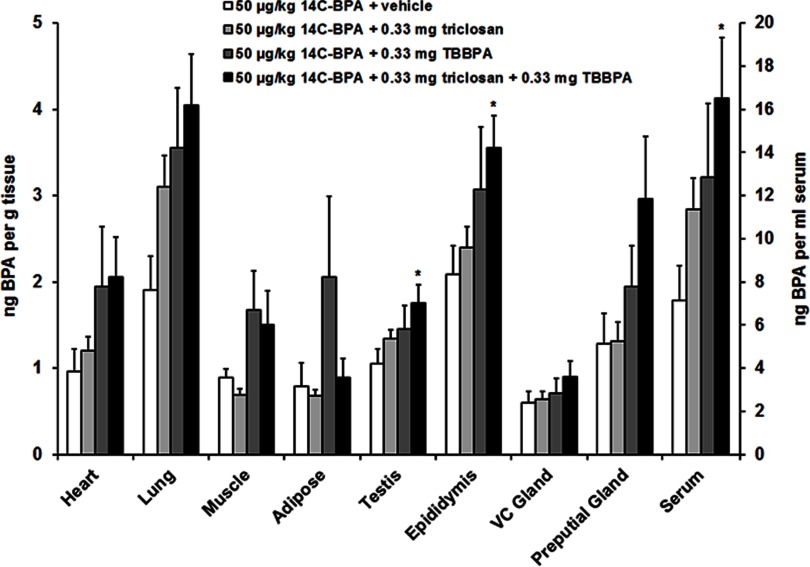
Experiment 2: Measurement of in Mice Given TBBPA and/or Triclosan
This experiment was designed to determine whether actions of TBBPA or triclosan on the distribution of BPA would be additive when the two substances were given concurrently. Radioactivity was measured in the tissues of diestrous females (Figure 3, Table 2; see also Table S3) and of males (Figure 4, Table 2; see also Table S4) that received a sc injection of TBBPA, triclosan, or both followed by a dietary supplement of . Triclosan and TBBPA showed a greater impact on concentrations in serum and reproductive tissues when administered concurrently. Tests between treatment conditions in females revealed that the vehicle-treated group differed from the group given for the uterus, , ; ovaries, , ; and serum, , . Tests between treatment conditions in males revealed that the vehicle-treated group differed from the group given for the testis, , ; epididymis, , ; and serum, , .
Figure 3.
Mean () concentration of bisphenol A (BPA) in the heart, lung, muscle, adipose, uterus, ovaries, and serum of diestrous females following subcutaneous (sc) injection of vehicle, triclosan, tetrabromobisphenol A (TBBPA), or and subsequent dietary administration of (). Difference from vehicle treatment in the same tissue: *; **. See Table S3 for individual animal data.
Figure 4.
Mean () concentration of bisphenol A (BPA) in the heart, lung, muscle, adipose, testis, epididymis, vesicular-coagulating (VC) gland, preputial gland, and serum of males following sc injection of vehicle, triclosan, tetrabromobisphenol A (TBBPA), or and subsequent dietary administration of (). Difference from vehicle treatment in the same tissue: *. See Table S4 for individual animal data.
Experiment 3: Measurement of Urinary E2 in Mice Given TBBPA
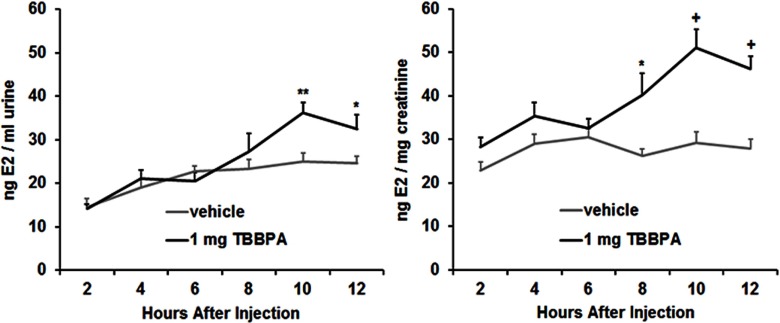
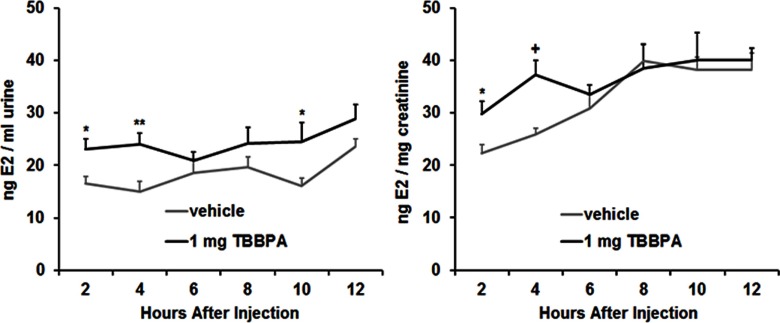
This experiment was designed to determine the impact of TBBPA on endogenous E2. Urinary E2 concentrations of diestrous females (Figure 5; see also Table S5) and of males (Figure 6; see also Table S6) were measured after a sc injection of vehicle or TBBPA. Concentrations of E2 are reported for uncorrected (nanograms E2/milliliter urine) and corrected (nanograms E2/milligram creatinine) measures. In females, ANOVA on uncorrected measures showed a significant main effect of collection time point, , , and a significant interaction, , . ANOVA on creatinine-corrected measures showed significant main effects of treatment, , , and collection time point, , , and a significant interaction, , . Multiple comparisons revealed that the vehicle-treated females differed from the TBBPA-treated females at 8 h after injection for the corrected measures, as well as at 10 and 12 h after injection for both the uncorrected and corrected measures. In males, ANOVA on uncorrected measures showed significant main effects of treatment, , , and collection time point, , , but no significant interaction. ANOVA on corrected measures showed only a significant main effect of collection time point, , . Multiple comparisons revealed that the vehicle-treated males differed from the TBBPA-treated males at 2 and 4 h after injection for both the uncorrected and corrected measures, as well as at 10 h after injection for the uncorrected measures.
Figure 5.
Mean () concentration of urinary E2, expressed as nanograms (E2)/milliliter urine and nanograms E2/milligram creatinine, following subcutaneous (sc) injection of vehicle or tetrabromobisphenol A (TBBPA) in diestrous females (). Significant difference from vehicle treatment at the same time point: *; **; +. See Table S5 for individual animal data.
Figure 6.
Mean () concentration of urinary (E2), expressed as nanograms E2/milliliter urine and nanograms E2/milligram creatinine, following subcutaneous (sc) injection of vehicle or tetrabromobisphenol A (TBBPA) in males (). Significant difference from vehicle treatment at the same time point: *; **; +. See Table S6 for individual animal data.
Discussion
These data show that TBBPA greatly magnifies concentrations of BPA in serum and tissues and that it elevates urinary concentrations of E2. When animals received an oral dose of , radioactivity was greater in serum, reproductive tissues, and elsewhere in female and male mice that were pretreated with TBBPA. Radioactivity was also greater in serum and reproductive tissues of mice given . Urinary E2 concentrations were elevated in animals given TBBPA. Our novel findings that TBBPA modulates E2 and BPA concentrations underscore a concern (Osimitz et al. 2014) that molecular modeling and in vitro studies may not address biological activity in vivo.
There are several potential mechanisms through which TBBPA, triclosan, BPA, and E2 could interact. These include direct actions at ER, transport proteins in blood, and enzymes involved in steroid synthesis and metabolism. There is conflicting evidence regarding direct binding to ER of TBBPA (Lee et al. 2012; Li et al. 2010; Molina-Molina et al. 2013; Suzuki et al. 2013) and triclosan (Gee et al. 2008; Henry and Fair 2013; Stoker et al. 2010). Insofar as there is such binding, actions of TBBPA or triclosan would be competitive with binding of , producing an opposing effect to that observed in experiments 1 and 2. Similarly, competition for transport proteins in blood would presumably reduce concentrations in serum and tissues. Our findings in experiments 1 and 2 are much more consistent with competition among TBBPA, triclosan, and for metabolic enzymes. Enzymes of particular interest are those involved in phase II metabolism, including UGT and SULT (Dumas and Diorio 2011; Wikoff et al. 2016). The major metabolite of BPA in rodents is the monoglucuronide conjugate resulting from interaction with hepatic UGT 2B1 and potentially other isoforms (Inoue et al. 2001; Kurebayashi et al. 2010; Yokota et al. 1999; Zalko et al. 2003). Other metabolites of BPA in rodents include the monosulfate conjugate resulting from interaction with SULT 1A1 (Yalcin et al. 2016; Zalko et al. 2003), as well as the diglucuronide (Zalko et al. 2003), disulfate (Yalcin et al. 2016), and glucuronide/sulfate (Inoue et al. 2016) diconjugates.
Sulfate and glucuronide conjugates of TBBPA (Borghoff et al. 2016) and triclosan (Fang et al. 2016) have also been observed in rodents. TBBPA can inhibit the activity of SULT 1E1 and SULT 1A1 (Gosavi et al. 2013; Hamers et al. 2006; Harju et al. 2007; Kester et al. 2002) and can reduce the expression of genes in the liver that encode SULT 1E1 and SULT 2A1 (Sanders et al. 2016). Triclosan can inhibit sulfonation and glucuronidation of BPA in human liver fractions (Wang et al. 2004).
Our findings in experiment 3 can be explained by competition between TBBPA and E2 for metabolic enzymes. In addition to phase II metabolism (described above), phase I metabolism involving CYP and is important for estrogen metabolism (Dumas and Diorio 2011; Wikoff et al. 2016). TBBPA can inhibit CYP 2C9 and CYP 3A4 activity (Ames 2013) and activity (NIH/NCBI) in human liver fractions. TBBPA can reduce expression of genes in the liver that encode , but it can increase expression of genes that encode certain CYP isoforms (Sanders et al. 2016). Our findings in experiment 3 can also be explained by actions of TBBPA on enzymes involved in steroid synthesis. One study found that TBBPA up-regulates aromatase expression and activity in human choriocarcinoma cells for up to 72 h in vitro (Honkisz and Wójtowicz 2015). The latency of TBBPA action on E2, up to 8–12 h in females, could be attributed to increased E2 biosynthesis.
We found that TBBPA magnified concentrations of in the heart, lung, kidney, and blood serum of mice, as well as in the uterus and ovary of females and in the testis, epididymis, VC gland, and preputial gland of males. The impact of TBBPA on concentrations in tissues and in serum is consistent with that previously shown for triclosan (Pollock et al. 2014), except TBBPA appears to have larger effects. This is particularly evident in males because pretreatment with triclosan elevated concentrations in only the epididymis and blood serum (Pollock et al. 2014). The greatest impact of TBBPA on concentrations was in the lung, reproductive tissues, kidney, and blood serum. The localization of to the lung and reproductive tissues is consistent with the high expression of and in these tissues (Couse et al. 1997; Kuiper et al. 1997). Of the tissue samples collected, the highest concentrations of were in the liver and in the kidney. This observation is consistent with findings from previous studies of the distribution of BPA at doses ranging from 0.5 to (Kim et al. 2004; Kurebayashi et al. 2005; Pollock and deCatanzaro 2014). These organs are involved in the metabolism and excretion of ingested BPA, and radioactivity in these tissues does not necessarily reflect tissue deposition of . Concentrations of were greater in males than in females in most nonreproductive tissues and in blood serum. In vehicle-treated animals in experiment 1, average concentrations in males were greater than those in females for the heart (394%), lung (230%), muscle (294%), adipose tissue (152%), serum (228%), liver (167%), and kidney (433%). These findings are consistent with the distribution of BPA in certain tissues of male and female rats (Kurebayashi et al. 2005) and may be explained by differences in BPA metabolism, as shown by sex- and tissue-specific expression of numerous UGT isoforms (Buckley and Klaassen 2007).
Greater concentrations of urinary E2 following TBBPA administration were most evident in females approximately 8–12 h postinjection but were also observed in males approximately 2–4 h postinjection. This discrepancy in latency between males and females may be influenced by differences in the metabolism of TBBPA, differences in estrogen synthesis, or both. The rate of TBBPA glucuronide production is faster in male rat liver fractions (Zalko et al. 2006), whereas aromatase expression is greater in the ovaries than in the testes (Golovine et al. 2003). Taken together, these processes may hasten the influence of TBBPA on E2 concentrations in males but result in greater effects of TBBPA on E2 concentrations in females. Slight but persistent elevations in E2 can lead to adverse reproductive and health outcomes in mammals. In mice, heightened E2 levels can prevent intrauterine blastocyst implantation and cause pregnancy failure (Thorpe et al. 2013). In humans, elevated E2 from hormone-replacement therapy correlates with increased risk of breast, endometrial, and ovarian cancer (Million Women Study Collaborators 2003, 2005, Beral and Million Women Study Collaborators 2007).
Data from the 2011–2012 U.S. National Health and Nutrition Examination Survey (NHANES) indicated that 72% of human urine samples contain detectable triclosan concentrations ranging from 2.3 to (Han et al. 2016). Although NHANES did not report TBBPA concentrations in urine, TBBPA was detected in 93% of plasma samples and in 89% of urine samples in a study of 140 healthy adults in China (Ho et al. 2017). Some published reports have estimated daily TBBPA exposure levels in the range of to (Environment Canada and Health Canada 2013; NTP 2014; Wikoff et al. 2015). However, these exposure estimates are derived from concentrations of TBBPA in environmental media and may not account for all exposure pathways. Furthermore, interactions among chemicals may influence their distribution, metabolism, and excretion, as indicated by our data. One study suggested that disruption of homeostatic control of TBBPA and estrogen conjugation is unlikely in humans because the doses required to produce uterine tumors in rodents are orders of magnitude greater than exposure estimates in humans (Borghoff et al. 2016). However, our data show clear in vivo interaction between TBBPA and triclosan, indicating that it is not appropriate to consider only one chemical in isolation. Inhibition of enzymes involved in estrogen metabolism has been shown for a number of environmental chemicals and their metabolites, including parabens (Ozaki et al. 2016; Prusakiewicz et al. 2007), phthalates (Ozaki et al. 2016), polychlorinated biphenyls (Kester et al. 2000; Wang and James 2007), and polyhalogenated aromatic hydrocarbons (Kester et al. 2002). Given the potential adverse reproductive and carcinogenic outcomes of persistently elevated estrogenic activity, these findings demonstrate the importance of considering multiple toxicants when determining regulatory exposure limits.
Conclusion
These data demonstrate that concurrent exposure to TBBPA elevates concentrations of dietary BPA in reproductive and other tissues. TBBPA and triclosan have additive effects in their capacity to modulate concentrations of BPA. TBBPA also elevates measures of urinary E2 in mice. These effects are consistent with competition among these synthetic chemicals and endogenous steroids for conjugating enzymes. These results indicate that TBBPA and triclosan, both of which have negligible direct effects on ER, can have indirect estrogenic effects.
Supplemental Material
Acknowledgements
We greatly appreciate the assistance of N. Vecchi, R. Ghasemi, and R. Weaver with experimental procedures.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada awarded to D.deC. (grant numbers RGPIN/03649-2015, EQPEQ/390407-2010).
References
- Abdallah MA-E. 2016. Environmental occurrence, analysis and human exposure to the flame retardant tetrabromobisphenol-A (TBBP-A)-A review. Environ Int 94:235–250, PMID: 27266836, 10.1016/j.envint.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Abdallah MA-E, Harrad S. 2011. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environ Int 37(2):443–448, PMID: 21167604, 10.1016/j.envint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Ames B. 2013. Tetrabrominated Bisphenol A Inhibition Studies on Six Cytochrome P450 Enzymes in Human Liver Microsomes [Dissertation]. Hayward, CA:California State University. [Google Scholar]
- Beck KR, Sommer TJ, Schuster D, Odermatt A. 2016. Evaluation of tetrabromobisphenol A effects on human glucocorticoid and androgen receptors: A comparison of results from human- with yeast-based in vitro assays. Toxicology 370:70–77, PMID: 27693315, 10.1016/j.tox.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V, Million Women Study Collaborators. 2007. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 369(9574):1703–1710, PMID: 17512855, 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- Berger RG, Foster WG, deCatanzaro D. 2010. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol 30(3):393–400, PMID: 20599497, 10.1016/j.reprotox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Berger RG, Hancock T, deCatanzaro D. 2007. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol 23(2):138–144, PMID: 17070006, 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. 2008. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17β-estradiol. Reprod Toxicol 26(2):94–99, PMID: 18638542, 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Borghoff SJ, Wikoff D, Harvey S, Haws L. 2016. Dose- and time-dependent changes in tissue levels of tetrabromobisphenol A (TBBPA) and its sulfate and glucuronide conjugates following repeated administration to female Wistar Han Rats. Toxicol Reports 3:190–201, 10.1016/j.toxrep.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman ED, Foster WG, Greenacre MKE, Muir CC, deCatanzaro D. 2015. Stress lowers the threshold dose at which bisphenol A disrupts blastocyst implantation, in conjunction with decreased uterine closure and e-cadherin. Chem Biol Interact 237:87–95, PMID: 26026914, 10.1016/j.cbi.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. 2007. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35(1):121–127, PMID: 17050650, 10.1124/dmd.106.012070. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS One 7:e35538, PMID: 22514749, 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R, Antignac J-P, Zalko D, Berrebi A, Cravedi J-P, Maume D, et al. 2008. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 73(7):1036–1041, PMID: 18790516, 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Colnot T, Kacew S, Dekant W. 2014. Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): Implications for risk assessment. Arch Toxicol 88(3):553–573, PMID: 24352537, 10.1007/s00204-013-1180-8. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson J, Korach KS. 1997. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138(11):4613–4621, 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Crawford BR, deCatanzaro D. 2012. Disruption of blastocyst implantation by triclosan in mice: Impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol 34(4):607–613, PMID: 23059059, 10.1016/j.reprotox.2012.09.008. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Muir C, Beaton EA, Jetha M. 2004. Non-invasive repeated measurement of urinary progesterone, 17β-estradiol, and testosterone in developing, cycling, pregnant, and postpartum female mice. Steroids 69(10):687–696, PMID: 15465115, 10.1016/j.steroids.2004.07.002. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Muir C, Beaton E, Jetha M, Nadella K. 2003. Enzymeimmunoassay of oestradiol, testosterone and progesterone in urine samples from female mice before and after insemination. Reproduction 126(3):407–414, PMID: 12968948, 10.1530/reprod/126.3.407. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Pollock T. 2016. Absorption and distribution of estradiol from male seminal emissions during mating. J Endocrinol 231(3):245–257, PMID: 27758953, 10.1530/JOE-16-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosh A, Dĕd L, Elzeinová F, Pĕknicová J. 2011. Assessing oestrogenic effects of brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on MCF-7 cells. Folia Biol (Praha) 57(1):35–39, PMID: 21457653. [PubMed] [Google Scholar]
- Dumas I, Diorio C. 2011. Estrogen pathway polymorphisms and mammographic density. Anticancer Res 31(12):4369–4386, PMID: 22199302. [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). 2015. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 13:3978, 10.2903/j.efsa.2015.3978. [DOI] [Google Scholar]
- Environment Canada, Health Canada. 2013. Screening assessment report: Phenol, 4,4'-(1-methylethylidene) bis[2,6-dibromo, Chemical Abstracts Service Registry Number 79-94-7; Ethanol, 2,2'-[(1-methylethylidene)bis[(2,6-dibromo-4,1-phenylene)oxy]]bis, Chemical Abstracts Service Registry Number 4162-45-2; Benzene, 1,1'-(1-methylethylidene)bis[3,5-dibromo-4-(2-propenyloxy)-Chemical Abstracts Service Registry Number 25327-89-3. http://www.ec.gc.ca/ese-ees/BEE093E4-8387-4790-A9CD-C753B3E5BFAD/FSAR_TBBPA_EN.pdf [accessed 10 December 2016].
- Fang J-L, Vanlandingham M, da Costa GG, Beland FA. 2016. Absorption and metabolism of triclosan after application to the skin of B6C3F1 mice. Environ Toxicol 31(5):609–623, PMID: 25410937, 10.1002/tox.22074. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Nishimura E, Kato Y, Harada KH, Koizumi A, Haraguchi K. 2014. Dietary exposure to phenolic and methoxylated organohalogen contaminants in relation to their concentrations in breast milk and serum in Japan. Environ Int 63:19–25, PMID: 24263137, 10.1016/j.envint.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Gee RH, Charles A, Taylor N, Darbre PD. 2008. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol 28(1):78–91, PMID: 17992702, 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- Golovine K, Schwerin M, Vanselow J. 2003. Three different promoters control expression of the aromatase cytochrome P450 gene (Cyp19) in mouse gonads and brain. Biol Reprod 68(3):978–984, PMID: 12604651, 10.1095/biolreprod.102.008037. [DOI] [PubMed] [Google Scholar]
- Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. 2013. Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environ Health Perspect 121(10):1194–1199, PMID: 23959441, 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, et al. 2006. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92(1):157–173, PMID: 16601080, 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Han C, Lim YH, Hong YC. 2016. Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general U.S. population from 2003 to 2012. Environ Pollut 208(Pt B):803–810, PMID: 26602792, 10.1016/j.envpol.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Harju M, Hamers T, Kamstra JH, Sonneveld E, Boon JP, Tysklind M, et al. 2007. Quantitative structure–activity relationship modeling on in vitro endocrine effects and metabolic stability involving 26 selected brominated flame retardants. Environ Toxicol Chem 26(4):816–826, PMID: 17447568, 10.1897/06-308R.1. [DOI] [PubMed] [Google Scholar]
- Henry ND, Fair PA. 2013. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J Appl Toxicol 33(4):265–272, PMID: 21935973, 10.1002/jat.1736. [DOI] [PubMed] [Google Scholar]
- Ho KL, Yuen KK, Yau MS, Murphy MB, Wan Y, Fong BMW, et al. 2017. Glucuronide and sulfate conjugates of tetrabromobisphenol A (TBBPA): Chemical synthesis and correlation between their urinary levels and plasma TBBPA content in voluntary human donors. Environ Int 98:46–53, PMID: 27717582, 10.1016/j.envint.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat 6(2):65–70, 10.2307/4615733. [DOI] [Google Scholar]
- Honkisz E, Wójtowicz AK. 2015. Modulation of estradiol synthesis and aromatase activity in human choriocarcinoma JEG-3 cells exposed to tetrabromobisphenol A. Toxicol Vitr 29(1):44–50, PMID: 25223798, 10.1016/j.tiv.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Huang GY, Ying GG, Liang YQ, Zhao JL, Yang B, Liu S, et al. 2013. Hormonal effects of tetrabromobisphenol A using a combination of in vitro and in vivo assays. Comp Biochem Physiol C Toxicol Pharmacol 157(4):344–351, PMID: 23501287, 10.1016/j.cbpc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kemanai S, Sano C, Kato S, Yokota H, Iwano H. 2016. Bisphenol A glucuronide/sulfate diconjugate in perfused liver of rats. The Journal of Veterinary Medical Science 78(5):733–737, PMID: 26782136, 10.1292/jvms.15-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yokota H, Makino T, Yuasa A, Kato S. 2001. Bisphenol A glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab Dispos 29(8):1084–1087, PMID: 11454725. [PubMed] [Google Scholar]
- James MO, Ambadapadi S, Falany C. 2015. Triclosan inhibits the activity of expressed human sulfotransferases (SULTs) towards their diagnostic substrates [Abstract]. FASEB J 29:622. [Google Scholar]
- James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE. 2010. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int 36(8):942–949, PMID: 19299018, 10.1016/j.envint.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Adams DH, Kannan K. 2008. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere 70(11):1935–1944, PMID: 18037156, 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kester MHA, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, et al. 2000. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: A novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141(5):1897–1900, PMID: 10803601, 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Kester MHA, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, et al. 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J Clin Endocrinol Metab 87(3):1142–1150, PMID: 11889178, 10.1210/jcem.87.3.8311. [DOI] [PubMed] [Google Scholar]
- Kim CS, Sapienza PP, Ross IA, Johnson W, Luu HMD, Hutter JC. 2004. Distribution of bisphenol A in the neuroendocrine organs of female rats. Toxicol Ind Health 20(1–5):41–50, PMID: 15807407, 10.1191/0748233704th186oa. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Lee IS, Oh JE. 2016. Occurrence, removal and release characteristics of dissolved brominated flame retardants and their potential metabolites in various kinds of wastewater. Environ Pollut 218:551–557, PMID: 27524250, 10.1016/j.envpol.2016.07.037. [DOI] [PubMed] [Google Scholar]
- Knudsen GA, Hughes MF, McIntosh KL, Sanders JM, Birnbaum LS. 2015. Estimation of tetrabromobisphenol A (TBBPA) percutaneous uptake in humans using the parallelogram method. Toxicol Appl Pharmacol 289(2):323–329, PMID: 26387765, 10.1016/j.taap.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138(3):863–870, PMID: 9048584, 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kurebayashi H, Nagatsuka SI, Nemoto H, Noguchi H, Ohno Y. 2005. Disposition of low doses of 14C-bisphenol A in male, female, pregnant, fetal, and neonatal rats. Arch Toxicol 79(5):243–252, PMID: 15902421, 10.1007/s00204-004-0628-2. [DOI] [PubMed] [Google Scholar]
- Kurebayashi H, Okudaira K, Ohno Y. 2010. Species difference of metabolic clearance of bisphenol A using cryopreserved hepatocytes from rats, monkeys and humans. Toxicol Lett 198(2):210–215, PMID: 20599483, 10.1016/j.toxlet.2010.06.017. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC. 2015. Halogenated flame-retardant concentrations in settled dust, respirable and inhalable particulates and polyurethane foam at gymnastic training facilities and residences. Environ Int 79:106–114, PMID: 25812808, 10.1016/j.envint.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Lai DY, Kacew S, Dekant W. 2015. Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents. Food Chem Toxicol 80:206–214, PMID: 25818463, 10.1016/j.fct.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kim TS, Kim CY, Kang IH, Kim MG, Jung KK, et al. 2012. Evaluation of in vitro screening system for estrogenicity: Comparison of stably transfected human estrogen receptor-α transcriptional activation (OECD TG455) assay and estrogen receptor (ER) binding assay. J Toxicol Sci 37(2):431–437, PMID: 22467034, 10.2131/jts.37.431. [DOI] [PubMed] [Google Scholar]
- Lee IS, Kang HH, Kim UJ, Oh JE. 2015. Brominated flame retardants in Korean river sediments, including changes in polybrominated diphenyl ether concentrations between 2006 and 2009. Chemosphere 126:18–24, PMID: 25655576, 10.1016/j.chemosphere.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Li J, Ma M, Wang Z. 2010. In vitro profiling of endocrine disrupting effects of phenols. Toxicol In Vitro 24(1):201–207, PMID: 19765641, 10.1016/j.tiv.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Malkoske T, Tang Y, Xu W, Yu S, Wang H. 2016. A review of the environmental distribution, fate, and control of tetrabromobisphenol A released from sources. Sci Total Environ 569–570:1608–1617, PMID: 27325014, 10.1016/j.scitotenv.2016.06.062. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, et al. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109(4):399–407, PMID: 11335189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Takahashi JS. 2014. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 4:195, PMID: 24478756, 10.3389/fendo.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Wheals BB, Beresford N, Sumpter JP. 2001. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect 109(2):133–138, PMID: 11266322, 10.2307/3434765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million Women Study Collaborators. 2003. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362(9382):419–427, PMID: 12927427, 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Million Women Study Collaborators. 2005. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 365(9470):1543–1551, PMID: 15866308, 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- Molina-Molina JM, Amaya E, Grimaldi M, Sáenz JM, Real M, Fernández MF, et al. 2013. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–136, PMID: 23714657, 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Muir C, Spironello-Vella E, Pisani N, deCatanzaro D. 2001. Enzyme immunoassay of 17β-estradiol, estrone conjugates, and testosterone in urinary and fecal samples from male and female mice. Horm Metab Res 33(11):653–658, PMID: 11733867, 10.1055/s-2001-18692. [DOI] [PubMed] [Google Scholar]
- Nakao T, Akiyama E, Kakutani H, Mizuno A, Aozasa O, Akai Y, et al. 2015. Levels of tetrabromobisphenol A, tribromobisphenol A, dibromobisphenol A, monobromobisphenol A, and bisphenol A in Japanese breast milk. Chem Res Toxicol 28(4):722–728, PMID: 25719948, 10.1021/tx500495j. [DOI] [PubMed] [Google Scholar]
- Ni HG, Zeng H. 2013. HBCD and TBBPA in particulate phase of indoor air in Shenzhen, China. Sci Total Environ 458–460:15–19, PMID: 23639907, 10.1016/j.scitotenv.2013.04.003. [DOI] [PubMed] [Google Scholar]
- NIH/NCBI (National Institutes of Health, National Center for Biotechnology Information). PubChem BioAssay Database; AID=893; version 1.2. https://pubchem.ncbi.nlm.nih.gov/bioassay/893 [accessed 14 January 2017].
- NTP (National Toxicology Program). 2014. “Technical Report on the Toxicology Studies of Tetrabromobisphenol A (CAS NO. 79-94-7) in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/N Mice (Gavage Studies).” https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr587_508.pdf [accessed 15 October 2016].
- Olsen CM, Meussen-Elholm ETM, Samuelsen M, Holme JA, Hongslo JK. 2003. Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4,4’-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins. Pharmacol Toxicol 92(4):180–188, PMID: 12753421. [DOI] [PubMed] [Google Scholar]
- Osimitz TG, Dourson ML, Hayes AW, Kacew S. 2014. Crystallographic analysis and mimicking of estradiol binding: Interpretation and speculation. Environ Health Perspect 122(4):A91, PMID: 24691106, 10.1289/ehp.1307987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Sugihara K, Watanabe Y, Ohta S, Kitamura S. 2016. Cytochrome P450-inhibitory activity of parabens and phthalates used in consumer products. J Toxicol Sci 41(4):551–560, PMID: 27432241, 10.2131/jts.41.551. [DOI] [PubMed] [Google Scholar]
- Pollock T, deCatanzaro D. 2014. Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses. Reprod Toxicol 49:145–154, PMID: 25181699, 10.1016/j.reprotox.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Pollock T, Greville LJ, Tang B, deCatanzaro D. 2016. Triclosan elevates estradiol levels in serum and tissues of cycling and peri-implantation female mice. Reprod Toxicol 65:394–401, 10.1016/j.reprotox.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Pollock T, Tang B, deCatanzaro D. 2014. Triclosan exacerbates the presence of 14C-bisphenol A in tissues of female and male mice. Toxicol Appl Pharmacol 278(2):116–123, PMID: 24784443, 10.1016/j.taap.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Harville HM, Zhang Y, Ackermann C, Voorman RL. 2007. Parabens inhibit human skin estrogen sulfotransferase activity: Possible link to paraben estrogenic effects. Toxicology 232(3):248–256, PMID: 17306434, 10.1016/j.tox.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, Bastian B, et al. 2010. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother 54(1):570–572, PMID: 19822703, 10.1128/AAC.00615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, et al. 2011a. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119(9):1227–1232, PMID: 21561829, 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, et al. 2011b. Characterization of novel ligands of ERα, ERβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci 122(2):372–382, PMID: 21622942, 10.1093/toxsci/kfr132. [DOI] [PubMed] [Google Scholar]
- Rochester JR. 2013. Bisphenol A and human health: A review of the literature. Reprod Toxicol 42:132–155, PMID: 23994667, 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Coulter SJ, Knudsen GA, Dunnick JK, Kissling GE, Birnbaum LS. 2016. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol Appl Pharmacol 298:31–39, PMID: 26988606, 10.1016/j.taap.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. 2016. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol 59:167–182, PMID: 26493093, 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SD, Harris JH, Berger ML, Subedi B, Kannan K. 2014. Brominated flame retardants and their replacements in food packaging and household products: Uses, human exposure, and health effects. In: Toxicants in Food Packaging and Household Plastics: Exposure and Health Risks to Consumers. Snedeker S, ed. London, UK:Springer, 61–93. [Google Scholar]
- Sieli PT, Jašarevic E, Warzak DA, Mao J, Ellersieck MR, Liao C, et al. 2011. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect 119(9):1260–1265, PMID: 21642047, 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM. 2010. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol Sci 117(1):45–53, PMID: 20562219, 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Tue NM, Malarvannan G, Sudaryanto A, Takahashi S, Tanabe S, et al. 2013. Similarities in the endocrine-disrupting potencies of indoor dust and flame retardants by using human osteosarcoma (U2OS) cell-based reporter gene assays. Environ Sci Technol 47(6):2898–2908, PMID: 23398518, 10.1021/es304691a. [DOI] [PubMed] [Google Scholar]
- Thorpe JB, Burgess PS, Sadkowski M, deCatanzaro D. 2013. Estrogen-progesterone balance in the context of blastocyst implantation failure induced by predator stress. Psychoneuroendocrinology 38(12):3048–3056, PMID: 24090584, 10.1016/j.psyneuen.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Thorpe JB, Gould KE, Borman ED, deCatanzaro D. 2014. Circulating and urinary adrenal corticosterone, progesterone, and estradiol in response to acute stress in female mice (Mus musculus). Horm Metab Res 46(3):211–218, PMID: 24446162, 10.1055/s-0033-1363958. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu L, Wang J, Pan B, Fu X, Zhang G, et al. 2015. Distribution of metals and brominated flame retardants (BFRs) in sediments, soils and plants from an informal e-waste dismantling site, South China. Environ Sci Pollut Res 22(2):1020–1033, PMID: 25106518, 10.1007/s11356-014-3399-1. [DOI] [PubMed] [Google Scholar]
- Wang L-Q, Falany CN, James MO. 2004. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos 32(10):1162–1169, PMID: 15269185, 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- Wang L-Q, James MO. 2007. Sulfonation of 17β-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus. Aquat Toxicol 81(3):286–292, PMID: 17239972, 10.1016/j.aquatox.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Abualnaja KO, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, et al. 2015. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ Int 83:183–191, PMID: 26177148, 10.1016/j.envint.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Wikoff D, Thompson C, Perry C, White M, Borghoff S, Fitzgerald L, et al. 2015. Development of toxicity values and exposure estimates for tetrabromobisphenol A: Application in a margin of exposure assessment. J Appl Toxicol 35(11):1292–1308, PMID: 25825072, 10.1002/jat.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff DS, Rager JE, Haws LC, Borghoff SJ. 2016. A high dose mode of action for tetrabromobisphenol A-induced uterine adenocarcinomas in Wistar Han rats: A critical evaluation of key events in an adverse outcome pathway framework. Regul Toxicol Pharmacol 77:143–159, PMID: 26828025, 10.1016/j.yrtph.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chitranshi P, Loukotková L, Gamboa da Costa G, Beland FA, Zhang J, et al. 2016a. Cytochrome P450-mediated metabolism of triclosan attenuates its cytotoxicity in hepatic cells. Arch Toxicol 91(6):2405–2423, PMID: 27896399, 10.1007/s00204-016-1893-6. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li Y, Kang D, Wang J, Zhang Y, Du D, et al. 2016b. Tetrabromobisphenol A and heavy metal exposure via dust ingestion in an e-waste recycling region in Southeast China. Sci Total Environ 541:356–364, PMID: 26410710, 10.1016/j.scitotenv.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Xiong J, An T, Zhang C, Li G. 2015. Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ Geochem Health 37(3):457–473, PMID: 25503846, 10.1007/s10653-014-9658-8. [DOI] [PubMed] [Google Scholar]
- Yalcin EB, Kulkarni SR, Slitt AL, King R. 2016. Bisphenol A sulfonation is impaired in metabolic and liver disease. Toxicol Appl Pharmacol 292:75–84, PMID: 26712468, 10.1016/j.taap.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S, et al. 1999. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J 340 (Pt 2):405–409, PMID: 10333482, 10.1042/0264-6021:3400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalko D, Prouillac C, Riu A, Perdu E, Dolo L, Jouanin I, et al. 2006. Biotransformation of the flame retardant tetrabromo-bisphenol A by human and rat sub-cellular liver fractions. Chemosphere 64(2):318–327, PMID: 16473389, 10.1016/j.chemosphere.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, et al. 2003. Biotransformations of bisphenol A in a mammalian model: Answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111(3):309–319, PMID: 12611660, 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZC, Chen SJ, Zheng J, Tian M, Feng AH, Luo XJ, et al. 2014. Occurrence of brominated flame retardants (BFRs), organochlorine pesticides (OCPs), and polychlorinated biphenyls (PCBs) in agricultural soils in a BFR-manufacturing region of North China. Sci Total Environ 481:47–54, PMID: 24576782, 10.1016/j.scitotenv.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Zhang C, Liu W. 2014. Atomic insights into distinct hormonal activities of bisphenol A analogues toward PPARγ and ERα receptors. Chem Res Toxicol 27(10):1769–1779, PMID: 25233466, 10.1021/tx500232b. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A, Flaws JA. 2016. Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertil Steril 106:827–856, PMID: 27417731, 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.