Abstract
Glioblastoma Multiforme (GBM) is a highly malignant primary brain cancer that is associated with abysmal prognosis. The median survival of GBM patients is ~15 months and there have not been any significant advance in therapies in over a decade, leaving treatment options limited. There is clearly an unmet need for GBM treatment. Immunotherapies are treatments based on usurping the power of the host’s immune system to recognize and eliminate cancer cells. They have recently proven to be a successful strategy for combating a variety of cancers. Of the various types of immunotherapies, checkpoint blockade approaches have thus far produced significant clinical responses in several cancers including melanoma, non small-cell lung cancer, renal cancer and prostate cancer. This review focuses on the biological rationale for using checkpoint blockade immunotherapeutic approaches in primary brain cancer and an up-to-date summary of current and ongoing checkpoint inhibitors-based clinical trials for malignant glioma. In addition, we expand on new concepts for further improving checkpoint blockade treatments, with a particular focus on the advantages of using genetically engineered mouse models for studies of immunotherapies in GBM.
Keywords: Glioblastoma multiforme, checkpoint inhibitors, genetically engineered mouse models, cancer
Introduction
Gliomas account for the majority of malignant primary brain cancers and are considered one of the most intractable malignancies in humans. Glioblastoma Multiforme (GBM) is the most common type of malignant glioma with an incidence in the U.S. of ~13,000 new cases per year [Ostrom et al., 2015]. The median survival of GBM patients is ~15 months, which roughly translates to ~ 12,000 deaths per year [Ostrom et al., 2015]. The 5-year relative survival rate for GBM is ~3%, a statistic that has not changed in over 60 years. Clearly, there is an unmet need for a therapeutic solution.
The genomic landscape of GBM and lower grade gliomas is now well characterized due to the recent completion of The Cancer Genome Atlas [Brennan et al., 2013; Cancer Genome Atlas Research et al., 2015]. This extensive molecular characterization of gliomas has demonstrated a number of genetic mutations and signaling abnormalities that are now recognized as drivers of uncontrollable growth, invasiveness, angiogenesis and resistance to apoptosis [Brennan et al., 2013; McLendon et al., 2008; Verhaak et al., 2010]. GBMs can be stratified into Classical, Mesenchymal, Neural, and Proneural subclasses according to a well-characterized gene expression-based molecular classification [Brennan et al., 2013; McLendon et al., 2008; Phillips et al., 2006; Verhaak et al., 2010]. The Proneural subtype is further divided based on genome methylation and IDH1 mutation status. IDH1 mutant GBM tumors have hypermethylated genomic DNA (referred to as Glioma CpG Island Methylator Phenotype or G-CIMP) whereas IDH1 wild type tumors are negative for the G-CIMP phenotype. With the exception of the G-CIMP Proneural subtype, the clinical usefulness of this classification scheme has yet to be established. Brennan et al., in their seminal manuscript, demonstrated no association between the Classical, Mesenchymal, Neural and Proneural (non G-CIMP) GBM subtypes and overall survival or how patients respond to standard of care therapy [Brennan et al., 2013]. It appears that molecular typification of GBM is completely irrelevant to overall outcome and has yet to be appreciated clinically. However, the molecular classification demonstrates that defined driver mutations are associated with tumor cell wiring very specifically. For example, in the Classical subtype of GBMs, aberrant expression of EGFR is observed in 100% of the cases [Brennan et al., 2013; Verhaak et al., 2010]. Deregulated, active EGFR results in over activation of the Ras/Raf/MAPK and PI3K/Akt signal transduction pathways, which are recognized as major contributors to GBM growth and resistance to therapy. Reinforcing the Akt survival pathway in these GBMs is the observation that ~45% of these tumors exhibit deletions or mutations within the tumor suppressor gene PTEN and >90% are homozygously deleted or mutated in the INK4a/ARF (CDKN2a) locus [Brennan et al., 2013; Verhaak et al., 2010]. This triple combination of activated EGFR, loss of CDKN2a and PTEN loci is found in over a quarter of all GBM patients [Brennan et al., 2013; Verhaak et al., 2010]. Our group has demonstrated in genetically engineered mouse models (GEMMs) that these genetic events are sufficient to initiate malignant gliomagenesis and to a certain extent, sustain the growth of the resulting GBMs in mice [Acquaviva et al., 2011; Jun et al., 2012; Zhu et al., 2009]. In contrast, the non-GCIMP Proneural subclass of GBM is mostly characterized by over expression of the receptor tyrosine kinase PDGFRα and loss of p53 tumor suppressor gene function [Brennan et al., 2013; Verhaak et al., 2010]. The Proneural subclass makes upward of ~25% of all GBMs. Finally, there is a tendency for Mesenchymal GBMs to display loss of the Neurofibromatosis 1 (NF1) tumor suppressor gene along with other oncogenic events [Brennan et al., 2013].
Despite our deep understanding of molecular drivers of GBM, targeted therapies against them have remained excessively inefficient (reviewed in [Olson et al., 2014]). These clinical failures strongly support a tenet by which oncogenic drivers might be required for tumor initiation, and to a certain extent maintenance of tumor growth, but certainly do not confer oncogenic addiction in GBMs. The current standard of care for GBM patients is composed of debulking surgery followed by concomitant radio- and chemotherapy (temozolomide (TMZ)- a DNA alkylating agent with good CNS penetration) and adjuvant TMZ chemotherapy. Recurrence is the norm at which point treatment options are very limited. Despite this aggressive regimen, the prognosis of GBM patients remains abysmal, furthering the urgency for new therapies.
Characterization of the genome landscape of GBM is a Herculean accomplishment and offers an amazing source of information. It is, however, static in nature and is weakened by the lack of functionality associated with those events, especially with regards to tumor reaction from therapeutic intervention and tumor dynamics vis-à-vis its microenvironment. Furthermore, recent single GBM cell RNA-seq research exquisitely demonstrated a rather fluid transition between subtypes within the same GBM tumor, further weakening the clinical relevance of these subtypes in terms of survival and response to standard of care therapy [Patel et al., 2014]. Therefore, it is imperative that appropriate model systems based on precise molecular signatures be developed if we are to achieve significant clinical progress against GBM. This is even more important in the context of the efficacy of various immunotherapies since clinical outcomes are so intimately related to tumor cell mutational spectra. The development of advanced techniques to manipulate the mouse genome revolutionized our ability to create genetically engineering mouse models (GEMMs) of cancer, which now offer unsurpassed opportunities to develop powerful treatment paradigms.
GEMMs of GBMs
Genetically Engineered Mice: an overview
The laboratory mouse as a model system for cancer research and its use in cancer drug development is now widespread. Particularly over the past two decades, our ability to manipulate the mouse germline has allowed for the development of mouse cancer models based on the understanding of the underlying genetic processes that drive cancer initiation and maintenance. Multiple laboratories have employed techniques of transgenesis and gene targeting to create a plethora of mouse strains to study various aspects of cancer. In-depth analysis of these GEMM strains has improved our understanding of how genes are involved in tumorigenesis in humans. They also shed light on the genetic and histopathological changes associated with cancer progression, maintenance and metastatic dissemination. GEMMs have also been used to study the effects of therapeutic interventions on tumor physiology. With the recent advent of much greater insights into the molecular and cellular profiling of tumors, the long-awaited goal of creating genetically and histopathologically accurate models of cancer in the mouse is now a reality. The present era of genetically engineered mouse tumor models is very different from the earlier period of simple transgenesis and gene knock out. Currently, compound mutations are routine, constitutive transgene expression systems are being replaced by various inducible versions and conditional gene targeting strategies are feasible and now favored over germline loss-of-function mutations. More recently, the advent of CRISPR/Cas9 technologies has propelled the ease of creating precise genetic lesions homologous to those observed in human cancers. The study of various aspects of tumor biology in these refined GEM models has been enhanced by the availability of gene expression array technologies, new tools for whole genome analysis, and a variety of other powerful methods geared towards a detailed molecular and histopathologic dissection of disease progression.
With many glioma-prone strains, tissue recombination, genomic tools and pathological expertise, mouse models of human malignant glioma have made considerable contributions to cancer gene discovery and validation in addition to their usage in preclinical experimental therapeutics. There are now more than 15 published GEMMs of glioma (for a review, see [Hambardzumyan et al., 2011]) all of which are based on recapitulation of genetic lesions seen in human tumors. Early conditional transgenic-based models that are driven by activators of signal transduction pathways that are common in GBM demonstrated the efficiency of tumor formation and accuracy of tumor histopathology in animals [Charest et al., 2006]. Of the most genetically relevant, those models that use activation of EGFR or overexpression of the PDGF-B ligand or inactivation of the NF1/p53 tumor suppressor genes as etiological drivers have solidly emerged as accurate models and have gained popularity over the recent years [Hambardzumyan et al., 2011]. Note that there is currently no genetically engineered mouse model based on overexpression and activation of the PDGFRα receptor, a genomic event that is highly relevant to GBM. By using these models, several research groups, including ours, have uncovered important aspects of GBM biology, including the identity of GBM cells of origin [Alcantara Llaguno and Parada, 2016] and the role of cancer stem cell in the initiation and maintenance of GBM [Jun et al., 2014]. In addition, few GEMMs of glioma have also been utilized to study sensitivity and resistance to various treatment modalities (recently reviewed in [McNeill et al., 2015]). The importance of these models is magnified by the recognition that cancer is truly a disease of tissues and the organism as a whole rather than a collection of ill defined genetically altered tumor cells. Logic dictates that in order to properly study the intricacies of host-tumor interactions that are innate to tumor development, it is necessary to design and perform experiments under in vivo settings in which neoplastic transformation emerges in the appropriate microenvironment.
Despite these advances, the use of GEM models of GBM for preclinical research has been erroneously plagued with the common misconceptions that these models intrinsically suffer from low tumor penetrance, modest reproducibility, and a lengthy latency of tumor formation and death in addition to a need for advanced and often costly in vivo imaging techniques. Furthermore, there is a general misleading consensus among the research community regarding a perceived lack of tumor immunogenicity. These misunderstandings could not be farther from reality. Perhaps it is because of these reasons that none have been used in preclinical settings for immunotherapies thus far.
These misconceptions legitimized the use of syngeneic rodent models as the paradigms of choice for pre-clinical research on immunotherapies despite the fact that engrafted models are deficient in the stepwise genetic changes occurring during tumor initiation and progression and most of the models are devoid of parenchymal infiltration, instead growing as well circumscribed tumors. They also lack in characteristic histological features (e.g. vascularization, pseudopallisading necrosis) and they also rarely recapitulate the original tumor phenotype. Nevertheless, the perceived benefits of their robust reproducibility seemingly outweigh the many drawbacks listed above making engrafted models most often used in immunotherapeutic research. Here we review key immunotherapeutic findings in the syngeneic GL261 glioma model in the C57Bl/6 strain.
Cancer Immunotherapy
Cancer immunotherapy is an umbrella term that comprises a family of strategies, which are all designed to stimulate the immune system to promote an anti-tumor immune response. These approaches include (but are not limited to) cancer antigen immunization, antibody dependent cellular cytotoxicity (ADCC), cytokine treatment (e.g. IL-2 treatment), dendritic cell therapy, chimaeric antigen receptor T-cell therapy (CAR-T) and checkpoint blockade.
Briefly, cancer antigen immunization follow the principles of standard vaccination except that the patient is injected with a specific immunogenic peptide sequence derived from his/her tumor that is conjugated to a carrier protein. The patient’s immune system would build up a response generating humoral and cellular effects towards the peptide of interest and against the tumor. Immunizations are of interest against cancer because of the uniqueness of tumor-specific antigens while minimizing undesired systemic side effects. For glioma, Rindopepimut (Celldex) is an EGFRvIII peptide vaccine first demonstrated in syngeneic models of glioma to have efficacy in producing a specific anti-tumor humoral and cellular response. Phase I studies of rindopepimut demonstrated that it is safe and immunogenic in patients, which led to the initiation of later stage trials. The evolution and optimization of rindopepimut is well summarized and reviewed by Paff and colleagues [Paff et al., 2014].
ADCC’s basic approach is using antibodies against a particular antigen of interest with which the antibody’s Fab portion recognizes the target for cellular cytotoxicity mediated by the Fc portion. Cellular cytotoxicity is primarily mediated by NK cells; however, neutrophils and macrophages can also play a role. Currently, antibodies against HER2/neu and tumor specific gangliosides are being targeted for the treatment of gliomas [Fleurence et al., 2016; Mineo et al., 2004].
Cytokine treatment approaches are varied but all typically includes administration of specific cytokines, which are endowed with positive immune stimulating abilities. For example, IL-2 is a critical cytokine that promotes autocrine antigen specific T-cell proliferation and has been historically demonstrated by Rosenberg and colleagues to have efficacy in melanoma. IL-2 is a positively stimulating cytokine such that it stimulates certain immune cells to recognize target tumor cells. IL-2 therapy was tried in an orthotopic model of glioma [Johansson et al., 2000] and a small clinical study [Danaila et al., 1993]. Both studies however showed only modest anti-tumor benefits.
Dendritic cell therapy is based on the isolation and utilization of the patient’s dendritic cells to prime them ex vivo with tumor-derived antigens and re-implantation back into the host. Dendritic cells have the intrinsic ability to present peptide antigens on Major Histocompatability Complexes (MHC) to T-cells by interaction with the TCR. This priming induces an antigen-specific T cell proliferation and when re-introduced into patients, boost T cell mediated anti-tumor activities. This approach is the basis of clinical efforts in gliomas (recently reviewed in {Reardon, 2017 #139).
The concept of modifying immune components ex vivo and reintroduction into patients is also applied in CAR-T based therapies. Here however, the patient’s own T cells are isolated and genetically engineered to recognize a tumor antigen of interest by expressing a chimeric antigen receptor, expanded and re-implanted back into the patient. For GBM, there have been several chimeric antigen receptors directed against various tumor specific antigens (EGFRvIII, IL13Rα2, HER2) that have been tested in pre-clinical models with variable results (reviewed in {Sidaway, 2017 #162}). There have been no CART-based clinical trial for GBM, however leukemias and lymphomas seem to be responding to CART therapy [Grupp et al., 2013; Kochenderfer et al., 2015].
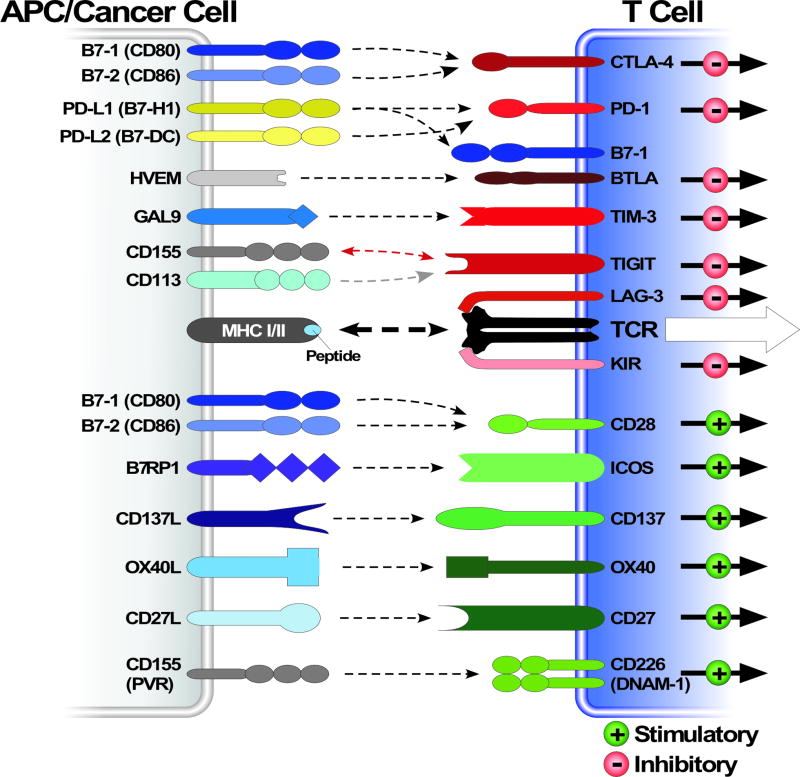
In healthy individuals, normal adaptive immunological homeostasis is regulated by a finely tuned balance of T cell co-stimulatory activating and inhibitory signals. These signals are transmitted to T cells by a series of cell surface receptors that are present on T cells called immune checkpoint receptors. The cognate ligands for these receptors are also cell surface molecules present on a variety of antigen presenting cells such as regulatory T cells (T reg), helper T cells (T helper), macrophages and dendritic cells (Figure 1). In concert with an MHC mediated activation of the T cell receptors (TCRs), these immune checkpoint receptors, when activated, elicit either negative or positive action or influence on TCR activation and T cell physiology. These immune checkpoints therefore play a critical and fine tuning role in regulating T cell activation in normalcy. Dysregulation of some of these checkpoints has been reported to be involved in autoimmune diseases and chronic infection, reinforcing their importance in T cell regulation.
Figure 1. Overview of multiple co-stimulatory and inhibitory pathways that regulate T cell responses.
Schematic depiction of various ligand-receptor interactions between antigen-presenting cells (APCs) (or cancer cells) and T cells. These ligand-receptors complexes regulate T cell activation, either negatively or positively, in response to T cell receptor stimulation through an interaction with an antigen presented as a peptide-MHC molecule complex. Some of these ligand-receptor co-stimulatory and inhibitory complexes are expressed and active during initiation of naïve T cells in lymph nodes, where dendritic cells are considered the main APCs, whereas others are expressed and active in peripheral tissues or tumor cells where they regulate the effector responses of T cells. Note that many of these ligands bind to multiple receptors with opposite effect on TCR signaling. Different ligand-receptor complexes are expressed on the surface of various APCs as well as resting, naïve and activated T cells. They have distinct kinetics of expression and affinities for their cognate binding partners. The extent of T cell activation is proportional to the strength of the TCR signaling, which is dictated by a multitude of factors that are highly spatio temporal and context dependent. Abbreviations: APC, antigen-presenting cell; TIM-3, T cell– immunoglobulin–mucin domain 3; B7RP1, B7-related protein 1; BTLA, B and T lymphocyte attenuator; GAL9, galectin 9; HVEM, herpesvirus entry mediator; ICOS, inducible T cell co-stimulator; KIR, killer cell immunoglobulin- like receptor; LAG3, lymphocyte activation gene 3; PD1, programmed cell death protein 1; PDL, PD1 ligand
In cancer, there is an obligate evasion from immunosurveillance, the biology of which is starting to emerge. Small indolent tumors are likely kept under control by a negative combination of cancer cell-centric effects and the immune system until a time at which cancer cells are either eradicated or progress. Conceptually, there appears to exist a “biological switch” that converts an otherwise controlled tumor into an unrestrained one. Molecular events responsible for this progression are likely tumor cell centric and humoral. We now know that tumor cells have evolved several strategies to overcome the negative influence of the host immune system by exploiting several aspects of the various interactions of tumor cells with the immune system.
The immune system’s efforts towards the elimination of tumor cells are through a response cycle that includes numerous steps. Briefly, the cycle begins with the production and release of tumor cell-specific antigens (during cell death). Various types of antigen presenting cells (APCs) internalize these antigens, process them, and migrate to lymph nodes to present them (through MHC loading) to resident naïve T cells in order to activate TCRs and thus prime T cells against cancer-specific antigens (see [Chen and Mellman, 2013] for a detailed review). These primed T cells (referred to as CD8+ cells), now endowed with cytotoxic capacities, migrate towards and infiltrate tumor sites, specifically recognize cancer cells, and elicit tumor-cell death, which in turn causes the release of more tumor-associated antigens, thereby continuing the cycle. This cycle between APCs and T cells and between tumor cells and T cells is intricately controlled by many ligand–receptor interactions (also known as checkpoint pathways) that are necessary to provide positive and negative signals to stimulate or inhibit T-cell activation, and to regulate the duration and intensity of the immune response mounting against tumor cells.
So far, mechanistic details are mostly known on two crucial steps that are involved in the anti tumor activation of the T-cell response. The first important interaction occurs during antigen presentation through the MHC to the TCR in the lymph node. APC’s CD80/86 ligands simultaneously interact with T cell’s CD28 co-stimulatory receptors to enhance TCR response and with T cell’s CTLA4 co-inhibitory receptors to control the TCR activity. The balance between these co-stimulatory and co-inhibitory signals can be shifted dramatically by dampening the co-inhibitory CTLA4 signal using approaches (e.g. blocking antibodies) that prevent the interaction between CD80/86 and CTLA4. This results in a more robust MHC-TCR signal and a stronger priming of T cells.
The second step occurs at the tumor site and determines the T-cell response. Primed T cells that have migrated to the tumor are now interacting with a host of cells, including tumor cells, APCs, macrophages, NK cells, etc. The strength of the MHC:TCR engagement between the above mentioned cells and T cells determine the robustness of the effector function (cytotoxicity) and it is dependent on the activation of co-stimulatory and co-inhibitory checkpoints. The co-inhibitory PDL1-PD1 complex has emerged as a crucial signal in effector T cell function and inhibition of this complex has been clinically proven to be a sound approach to boost the cytotoxic power of T cells. Not surprisingly, these immune checkpoint pathways are heavily exploited by tumor cells as a means to evade immune detection. However, they provide a plethora of potential targets for the development of anti-cancer therapeutic agents aimed at boosting the anti-cancer immune responses. Much of our knowledge on the function of these molecules in cancer has been derived from pre-clinical models of and clinical data from melanomas, lung and renal cancers. The exact participation of checkpoint pathways in primary brain tumor pathogenesis is still somewhat unknown and has just recently started to emerge. For many years, the CNS was viewed as an immune privileged organ incapable of surveillance by peripheral immunity because of the ostensible lack of a functional lymphatic system.
This view has shifted considerably in the recent years. Seminal discoveries expanded our views on the role of the peripheral immune system and the brain. We now know that the brain is drained by classical lymphatic conduits that reside within the meninges [Aspelund et al., 2015; Louveau et al., 2015]. Lymphatics are typically designed to drain interstitial fluids out of tissues for degradation and removal into the circulatory system. During infection (and in cancer), lymphatic transport is essential for supplying antigens and APCs to draining lymph nodes, a very important step in the process of establishing a proper adaptive immune response. Additionally, there is evidence of a separate process in the brain called “glymphatics” whereby CSF (carrying extracellular proteins, antigens and solutes) and interstitial fluid exchange extensively. This results in pushing ISF into the perivenous space where it can collect and drain into the cervical lymph nodes (for a review see [Jessen et al., 2015]). Together, these recent advances underscore the notion that the brain is indeed surveyed by the peripheral immune system.
The use of the GL261 GBM syngeneic preclinical model for immunotherapy
The GL261 tumor originated from a chemical carcinogenesis experiment in the late 1960s. The GL261 tumor arose from a C57BL/6 mouse that had been intracranially injected with 3-methylcholantrene. The tumor was then maintained by serial transplantations (intracranial and subcutaneous) of small tumor pieces in the syngeneic strain [Ausman et al., 1970]. After several years of serial transplantation, ex vivo single cell cultures were derived by several groups, which allowed for genetic manipulations such as ectopic expression of firefly luciferase for non invasive bioluminescence imaging monitoring of tumor growth.
GL261 tumor cells grow rapidly post-intracranial implantation conferring median survivals of 25 days (1×105 cells), 27 days (1×104 cells), 36 days (1×103 cells), and 55 days (1×102 cells) in all animals injected (100% penetrance) [Szatmari et al., 2006]. Not surprising, death is thought to arise from increased intracranial pressure [Szatmari et al., 2006]. However, the tumors derived from GL261 cells display few histopathological characteristics that are necessary to ascribe a diagnosis of GBM. First, tumors grow as well-delineated masses of cells and non-invasive imaging reveal a radial growth pattern, which is clearly contrasting the irregular and invasive growing paths of GBM. Second, tumor cells are GFAP negative and pseudopallisading necrosis is rarely observed.
Genomic analysis of GL261 revealed that these cells carry a KrasG12V mutation, a signature of chemical carcinogenesis. In addition, there are reports that the GL261 cells are also homozygously mutated on p53 at codon p53R153P [Maes and Van Gool, 2011; Szatmari et al., 2006], although others have not reported such mutation [Blaszczyk-Thurin et al., 2002], perhaps reflecting an artifact of cell culture adaptability. Mutation in Ras genes are unequivocally not considered important and significant driver gene mutations in GBM as demonstrated by the TCGA [Brennan et al., 2013; McLendon et al., 2008; Verhaak et al., 2010].
GL261 tumors are sensitive to ionizing radiation (IR), a modality that is integral to the standard of care for GBM. Treatments with single local doses of 4–10 Gy of irradiation substantially slow down tumor progression and prolong survival. However, no tumor-bearing animals are cured by radiation alone [Szatmari et al., 2006]. GL261 intracranial tumor-bearing animals are also sensitive to temozolomide (TMZ), a DNA alkylating chemotherapeutic agent that is part of GBM standard of care. Either systemic (IP injections) or local (intracranial polymer) administration of temozolomide (TMZ) alone, modestly prolong survival (~25 days control vs ~35 days IP and ~40 days local) in the GL261 model [Mathios et al., 2016]. However, the outcome of a proper standard of care regimen, which consists of concomitant IR and TMZ followed by adjuvant TMZ (2 cycles of 2 Gy q.d. 5 days on, 2 days off, with concurrent and adjuvant q.d. TMZ 67 mg/kg), remains to be determined in GL261.
Finally, GL261 intracranial tumors are considered moderately immunogenic on the basis of a few observations. Immunization of mice using GL261 cells injected subcutaneously can delay and even prevent engraftment altogether [Szatmari et al., 2006] even though lymphocyte infiltration is hardly detectable in tumors [Szatmari et al., 2006] and GL261 cells express detectable levels (albeit low) of MHC class I molecules and MHC class II molecules are virtually absent in vivo [Maes and Van Gool, 2011]. Also, co-stimulatory molecules are also present at basal levels [Maes and Van Gool, 2011]. In addition, there are a few reports on unique tumor antigens in the GL261 cell line such as the mouse homologue of AN2 (human melanoma proteoglycan) and GARC-1, which is a unique antigen for cytotoxic T cells (reviewed in [Maes and Van Gool, 2011]). These features made the GL261 a model of choice for the study of experimental immunotherapies for glioma.
The use of the GL261 model for immunotherapy studies is extensive. The GL261 model has been used in studies on adoptive transfer, serologic treatments with monoclonal antibodies and dendritic cell therapy (reviewed in details in [Maes and Van Gool, 2011]). More recently, the GL261 model has been the model of choice at the forefront on important preclinical studies on the efficacy of checkpoint inhibition for glioma therapy.
Checkpoint Molecules
CTLA-4
The inhibitory receptor Cytotoxic Lymphocyte Antigen-4 (CTLA-4) is an important negative regulatory receptor of peripheral T cell responses. CTLA-4 functions primarily in secondary lymphoid organs such as in lymph nodes. CTLA-4 counteracts the actions of the T cell co-stimulatory receptor CD28 by binding to the same ligands (CD80 and CD86) with higher affinities. CTLA-4 is not expressed on naïve T cells but is inducibly expressed upon TCR engagement. CTLA-4 activation leads to the recruitment and activation of phosphatases such as PP2A and SHP2, which counteract the activities of the TCR by dampening intracellular signaling cascades thus promoting immune tolerance. In addition, within the tumor microenvironment, CTLA-4 is highly and constitutively expressed on regulatory T cells (Tregs), mediating Tregs’ suppressive function. Several lines of evidence demonstrate a critical role for the CTLA-4 co-inhibitory signals in immune tolerance such as the lethal T cell-dependent multi-organ inflammation that develops in CTLA-4 null mice, which resemble systemic autoimmune disease. Taken together, these findings underscore the critical role for CTLA-4 in negatively controlling self-reactive T cells and T cell homeostasis in general.
Within the context of cancer treatment, one strategy is to activate anti-tumor immunity by relieving the negative feedback exerted by CTLA-4 with a blocking antibody. Several seminal studies have demonstrated that anti-CTLA-4 blocking antibodies can stimulate antitumor immune responses leading to regression of tumors and promoting long-lived immunity in mouse models of solid and hematologic cancers [Leach et al., 1996; Selby et al., 2013]. These led to the clinical development of antibodies aimed at blocking the interaction between CD80/86 and CTLA-4. Blockade of CTLA-4 has been recently FDA approved for a spectrum of malignancies including melanoma and NSCLC. Despite promising clinical responses, the mechanism of anti-CTLA-4 anti-tumor immunity is not completely understood. The therapeutic effects of anti-CTLA-4 antibodies may not only be due to blocking CTLA-4 interaction with its ligands on T cells. In fact, recent work suggests that blocking CTLA-4 may also deplete intratumoral Tregs via an Fc receptor-mediated, antibody dependent cellular cytotoxicity [Selby et al., 2013]. Further studies are necessary to fully understand the mechanism of action of anti-CTLA-4 therapy.
In glioma, single agent anti-CTLA-4 blockade has produced enhanced survival in the GL261 syngeneic mouse model. Reardon et al. has demonstrated that single agent anti-CTLA-4 treatment leads to a 25% cure rate [Reardon et al., 2015]. Although successful, responses to anti CTLA-4 alone treatments were limited and treatment efficacy was enhanced when administered in combination with another checkpoint blocking antibody such as anti-PD-1, or radiation [Belcaid et al., 2014; Reardon et al., 2015]. Reardon and colleagues demonstrated that when anti-PD-1 is combined with anti-CTLA-4, a 75% cure rate was achieved. CTLA-4 blocking therapy has also been used in combination with stimulation of 41-BB and radiation [Belcaid et al., 2014]. In this study, Belcaid et al. demonstrated a 50% cure rate. Anti-CTLA-4 is currently tested in a phase III clinical trial for patients with recurrent GBM as a monotherapy or with anti-PD-1 blockade (Table 1. Trial NCT02017717).
Table 1.
Clinical Trials of Immune Checkpoint Inhibitors in GBM
| Source | Checkpoint Target |
Antibody | Name (US) |
Phase | No. of Patients |
Title of Trial | National Clinical Trial Reg. # |
|---|---|---|---|---|---|---|---|
| BMS | PD-1 | Nivolumab | Opdivo | I | 19 | Stereotactic Radiosurgery With Nivolumab and Valproate in Patients With Recurrent Glioblastoma | NCT02648633 |
| Nivolumab | Opdivo | II | 29 | Neoadjuvant Nivolumab in Glioblastoma (Neo-nivo) | NCT02550249 | ||
| Nivolumab | Opdivo | I | 26 | Hypofractionated Stereotactic Irradiation With Nivolumab in Patients With Recurrent High Grade Gliomas | NCT02829931 | ||
| Nivolumab | Opdivo | I | 66 | Nivolumab With DC Vaccines for Recurrent Brain Tumors (AVERT) | NCT02529072 | ||
| Nivolumab | Opdivo | II | 320 | Study of Temozolomide Plus Radiation Therapy With Nivolumab or Placebo, for Newly Diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer). (CheckMate548) | NCT02667587 | ||
| Nivolumab | Opdivo | III | 550 | Study of Nivolumab Compared to Temozolomide, Given With Radiation Therapy, for Newly-diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) (CheckMate 498) | NCT02617589 | ||
| Nivolumab | Opdivo | II | 30 | Autologous Dendritic Cells Pulsed With Tumor Lysate Antigen Vaccine and Nivolumab in Treating Patients With Recurrent Glioblastoma | NCT03014804 | ||
| CTLA-4/PD-1 | Ipilimumab/Nivolumab | Yevroy/Opdivo | III | 440 | A Study of the Effectiveness and Safety of Nivolumab Compared to Bevacizumab and of Nivolumab With or Without Ipilimumab in Glioblastoma Patients (CheckMate 143) | NCT02017717 | |
| Ipilimumab/Nivolumab | Yevroy/Opdivo | I | 42 | Ipilimumab and/or Nivolumab in Combination With Temozolomide in Treating Patients With Newly Diagnosed Glioblastoma or Gliosarcoma | NCT02311920 | ||
| LAG-3/4-1BB/PD-1 | BMS-986016/Urelumab/Nivolumab | n/a, n/a, Opdivo | I | 68 | Anti-LAG-3 or Urelumab Alone and in Combination With Nivolumab in Treating Patients With Recurrent Glioblastoma | NCT02658981 | |
| Merck | PD-L1 | Pembrolizumab | Keytruda | I | 42 | Hypofractionated Stereotactic Irradiation (HFSRT)With Pembrolizumab and Bevacizumab for Recurrent HighGrade Gliomas | NCT02313272 |
| Pembrolizumab | Keytruda | 12 | Pembrolizumab (MK-3475) in Patients With RecurrentMalignant Glioma With a Hypermutator Phenotype | NCT02658279 | |||
| Pembrolizumab | Keytruda | I/II | 52 | MK-3475 in Combination With MRI-guided Laser Ablation in Recurrent Malignant Gliomas | NCT02311582 | ||
| Pembrolizumab | Keytruda | 75 | Pembrolizumab in Treating Younger Patients With Recurrent, Progressive, or Refractory High-Grade Gliomas, Diffuse Intrinsic Pontine Gliomas, or Hypermutated Brain Tumors | NCT02359565 | |||
| Pembrolizumab | Keytruda | II | 108 | Radiation Therapy Plus Temozolomide and Pembrolizumab With and Without HSPPC-96 in Newly Diagnosed Glioblastoma (GBM) | NCT03018288 | ||
| Pembrolizumab | Keytruda | II | 48 | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects (CAPTIVE) | NCT02798406 | ||
| Pembrolizumab | Keytruda | II | 20 | Pharmacodynamic Study of Pembrolizumab in Patients With Recurrent Glioblastoma | NCT02337686 | ||
| Pembrolizumab | Keytruda | II | 82 | Pembrolizumab +/− Bevacizumab for Recurrent GBM | NCT02337491 | ||
| Pembrolizumab | Keytruda | 30 | A Pilot Surgical Trial To Evaluate Early Immunologic Pharmacodynamic Parameters For The PD-1 Checkpoint Inhibitor, Pembrolizumab (MK-3475), In Patients With Surgically Accessible Recurrent/Progressive Glioblastoma | NCT02852655 | |||
| Pembrolizumab | Keytruda | I/II | 50 | Radiation Therapy With Temozolomide and Pembrolizumab in Treating Patients With Newly Diagnosed Glioblastoma | NCT02530502 | ||
| Pembrolizumab | Keytruda | I/II | 58 | Evaluation Of The Treatment Effectiveness Of Glioblastoma / Gliosarcoma Through The Suppression Of The PI3K/Akt Pathway In Compared With MK-3475 | NCT02430363 | ||
| Pfizer | PD-L1 | Avelumab | n/a | II | 43 | Avelumab With Hypofractionated Radiation Therapy in Adults With Isocitrate Dehydrogenase (IDH) Mutant Glioblastoma | NCT02968940 |
| AZ | PD-L1 | Durvalumab | n/a | II | 159 | Phase 2 Study of MEDI4736 in Patients With Glioblastoma | NCT02336165 |
| PD-L1 | Durvalumab | n/a | I/II | 62 | A Study Evaluating the Association of Hypofractionated Stereotactic Radiation Therapy and Durvalumab for Patients With Recurrent Glioblastoma (STERIMGLI) | NCT02866747 | |
| CTLA-4/PD-L1 | Tremelimumab/Durvalumab | n/a | II | 36 | Tremelimumab and Durvalumab in Combination or Alone in Treating Patients With Recurrent Malignant Glioma | NCT02794883 |
Abbreviations: BMS, Bristol Myers Squibb; AZ, Astra Zeneca; CTLA4, cytotoxic T-lymphocyte antigen-4; PD-1, programmed cell death protein 1; PD-L1, probrammed cell death 1 ligand 1; LAG-3, lymphocyte activation gene 3.
PD-1 and PDL-1
Another very important immune checkpoint is the cognate receptor ligand complex Programmed Death-1 (PD-1) receptor and its Programmed Death-Ligand 1 and 2 (PD-L1, PD-L2). PD-1 is a transmembrane receptor that exerts a major negative regulation in immune response by controlling T cell activation, T cell exhaustion and T cell tolerance. PD-1 expression is tightly regulated e.g. it appears at the surface of T cells shortly (<24 hours) after T cell activation and decreases with the elimination or clearance of antigen. Under conditions (such as chronic infection or cancer) of repetitive T cell stimulation by antigen, the levels of PD-1 expression remain high and T cells then experience multiple epigenetic modifications in addition to changes in transcription factor expression. These events result in some form of differentiation, channeling T cells into a state of exhaustion. It has been shown that exhausted T cells also can express multiple other inhibitory receptors, making them susceptible to blocking antibody inhibition of additional checkpoint pathways to recue T cells from exhaustion. Supporting this phenomenon, Kim and colleague have co-targeted TIM-3 simultaneously with PD-1 and demonstrated a much higher cure rates than with each modalities alone [Kim et al., 2017]. However, rescue of exhaustion by inhibition of alternative coinhibitory receptor(s) in a sequential manner remains to be addressed.
PD-L1 and PD-L2 are both expressed on APCs in addition to other cell types but PD-L1 appears to be more broadly expressed than PD-L2. Their expression is induced by proinflammatory cytokines. PD-L1 (or PD-L2) ligand binding to PD-1 results in tyrosine phosphorylation of the PD-1 cytoplasmic domain. PD-1 phosphotyrosine sites create SH2 domain recognition motifs and trigger the recruitment of the tyrosine phosphatase SHP-2 and its subsequent catalytic activation. This leads to a reduced tyrosine phosphorylation of TCR signaling molecules and the attenuation of signaling pathways downstream of TCR, and an overall decrease in T cell activation and cytokine production. PD-1 signaling is therefore viewed as a negative modulator of T cell function to suppress effector immune responses. In normalcy, the PD-1 pathway restrains self-reactive T cells in target organs, maintaining tolerance in tissues and protecting them from immunopathology. Mice lacking PD-1 or its ligands do not spontaneously develop autoimmune disease but rather accelerate or exacerbate autoimmunity, a phenotype that is much milder than that seen in the CTLA-4 knockout strain.
In cancer, tumor cells express PD-L1 and PD-L2 and so do other cell types (e.g., fibroblasts, endothelial cells, and other immune cells). Experimental evidence demonstrates that tumor cells have hijacked this machinery. Tumor cells express elevated PD-L1 levels, causing effector function attenuation of tumor infiltrating lymphocyte (TIL) activity [Dong et al., 2002]. In addition, several groups have shown that activation of certain oncogenes and/or loss of tumor suppressor genes can result in higher expression levels of PD-L1 in tumor cells, thus further attenuating TILs. Not surprisingly, antibody blockade of PD-1/PD-L1 axis have demonstrated positive outcomes in advanced melanoma, lung cancer and renal cell carcinoma (reviewed in [Baumeister et al., 2016]). Anti-PD-1 monoclonal antibodies have been recently FDA approved while anti-PD-L1 is in late clinical stages. Despite the promising success of anti-PD-1 blockade in the clinic, responses are varied. Several studies are aimed at determining predictors of response to anti-PD-1 blockade. To date, both the presence of TILs and high expression of tumor PD-L1 within the tumor microenvironment, are main prognostic factors of anti-PD-1 therapy.
In glioma, PD-L1 has been shown to be expressed at high levels by western blotting, flow cytometry, mRNA and IHC [Parsa et al., 2007; Wintterle et al., 2003], suggesting that anti-PD-1 therapy might be applicable. In fact, Parsa and colleagues have demonstrated that PD-L1 expressing glioma cells are susceptible to T-cell lysis, suggesting that anti-PD-1 may have clinical benefit [Parsa et al., 2007].
In the preclinical GL261 model, the success of anti PD-1 monotherapy is dependent on antibody dosage levels, with the best outcome reported being a cure rate of 50% with a systemic administration of 8 cycles (e.g. 500 ug per mouse first dose then subsequent 250ug per dose 3 days apart for the next 7 doses) [Reardon et al., 2015; Zeng et al., 2013]. Lower levels or less frequent administration of anti PD-1 antibodies appear to result in less robust effects [Zeng et al., 2013]. These experiments were conducted in two separate studies and the differences in the experimental set up were rather substantial, which limits our ability to reach comparative conclusions [Reardon et al., 2015; Zeng et al., 2013]. Nevertheless, both anti PD-1 antibody monotherapy treatments caused an increase in CD8 T cell to Treg ratio, which indicated that the successful outcome arose from the expected mechanism of action [Reardon et al., 2015; Zeng et al., 2013].
The reduced anti PD-1 posology can be compensated through the inclusion of radiation or in combination with other checkpoint blockade [Reardon et al., 2015; Zeng et al., 2013]. Stereotactic radiation in combination with anti-PD-1 showed enhanced efficacy. The median survival of untreated mice was 26 days, mice treated with anti-PD-1 and stereotactic radiation was 52 days whereas radiation alone showed 27 days and PD-1 monotherapy gave a median survival of 30 days. Note that anti-PD-1 monotherapy and can induce systemic immune memory in GL261 tumors, which was CD8+ T cell dependent [Zeng et al., 2013]. It had been demonstrated that radiation altered the immune profile of GL261 cells in vitro [Zeng et al., 2013], however the precise mechanism of synergism between radiation and anti PD-1 therapy in GL261 and in glioma in general has yet to be explored. In melanoma it has been demonstrated that radiation promotes an oligo-expansion of T-cell receptors [Twyman-Saint Victor et al., 2015].
There are currently numerous ongoing clinical trials utilizing anti-PD-1 or anti-PD-L1 for the treatment of GBM (Table 1). Anti-PD-L1 is in phase II trials for GBM patients in along with or without radiation and the VEGFR antibody Avastin (NCT02336165). There is a phase III trial of anti-PD-1 that is being conducted with temozolomide chemotherapy with radiation in patients with newly diagnosed GBM (NCT02617589) and a phase II trial of anti-PD-1 as neoadjuvant therapy (NCT02550249).
Alternative coinhibitory pathways for immunotherapy
The clinical successes of cancer immunotherapies using anti-CTLA-4 and anti-PD-1 treatments have spearheaded searches for additional coinhibitory pathways. There are now many coinhibitory molecules recognized as potential targets, including TIM-3 (T cell–immunoglobulin–mucin domain 3), LAG-3 (Lymphocyte Activation Gene-3), VISTA (V-domain immunoglobulin-containing suppressor of T cell activation), TIGIT, CD160, B7-H3, B7-H4, CD244, HHLA2, and BTNL2 (see Figure 1). Many of these coinhibitory receptors are coexpressed with PD-1 on T cells in tumors. These observations opened the possibility of combination therapy with anti-PD-1 or anti-PD-L1 and the results of coinhibitory pathway blockade synergizing with anti PD-1 pathway inhibition are reviewed extensively elsewhere [Baumeister et al., 2016].
One-two punch; combining blockade of CTLA-4 (and other coinhibitory receptors) with PD-1
As described above, the mechanisms of action of CTLA-4 and PD-1 on T cell function and activation are non-overlapping and nonredundant. CTLA-4 functions in T cell priming and maintenance of Tregs whereas PD-1 is mainly acting on T cell responses in tissues and tumors. Blockade of CTLA-4 is believed to enhance the number of tumor-specific T cells into the tumor microenvironment. Higher numbers of primed T cells translate into higher levels of T cell-mediated IFN-γ production, which upregulates PD-L1 expression in tumor microenvironment tissues and cells, making anti PD-1 blockade more effective in the setting of CTLA-4 inhibition. This suggests an elegant mechanism illustrating the synergistic benefit of combined pathway blockade observed in the clinic for melanoma [Larkin et al., 2015; Postow et al., 2015; Wolchok et al., 2013].
In glioma models, combination anti-PD-1 and anti-CTLA-4 has induced long-term survivors in GL261 tumor bearing animals [Reardon et al., 2016]. Anti PD-1 monotherapy provided a 50% survival, co-inhibition of PD-1 and CTLA-4 resulted in a 75% cure rate whereas CTLA-4 monotherapy provided a 25% cure rate. Additionally, Kim and colleagues have demonstrated that anti PD-1 treatments in combination with TIM-3 blockade can induce tumor clearance in 60% of GL261 bearing mice [Kim et al., 2017]. Although median survivals were not reported, anti PD-1 monotherapy from that study gave a 30% cure rate, while anti TIM-3 monotherapy had no effect on survival and no differences were noted between the anti TIM-3 treatment survival curve and untreated. The Lim group has demonstrated that treatment with anti-PD-1 alone or in combination with TIM-3 can influence the ratio of CD8 T cells to Tregs in the GL261 model, shifting the balance towards a CD8-like phenotype, a marker of anti-tumor immunity [Kim et al., 2017].
PD-L1 expression levels vs PD-1
Recent clinical success of anti-PD-1 therapy brings promise for the treatment of GBM. Despite this, responses to anti-PD-1 are varied, thus predictors of response is of clinical significance. Topalian et al. has shown that presence of PD-L1 ligand status best predicts response to anti-PD-1 monotherapy [Topalian et al., 2012]. In addition, Parsa et al. has shown that loss of PTEN can lead to higher levels of PD-L1 leading to decreased in vitro T-cell cytotoxicity [Parsa et al., 2007]. However, it has yet to be determined how much PD-L1 protein is required to confer sensitivity to anti PD-L1 therapy. A straightforward approach would be to exogenously manipulate the levels of PD-L1 expression in animal models that are sensitive to anti-PD-1 mono therapy. For example, a conditional PD-L1 knockout GEMM can serve as an advantageous model system to directly test and address levels of PD-L1 in a tumor cell-centric manner within the proper genetic lesions and the proper tumor microenvironment. This approach can serve as a powerful preclinical platform to directly address the role of PD-L1 ligand to response to anti-PD-1.
Resistance to Immune Checkpoint blockade
Clinical successes of mono and/or combination checkpoint blockade therapy overshadow those with poorer clinical responses. It is not surprising that resistance (up front or acquired) is observed and determining the mechanisms driving resistance must be a priority. Similar to the clinical data on other cancers, it is highly anticipated that resistance to checkpoint blockade will be witnessed in GBM. We argue that GEMMs can serve as relevant preclinical models to decipher mechanisms of resistance to checkpoint blockade due to their genetic accuracy and their natural tumor microenvironment.
Conclusion and future directions
The clinical successes of checkpoint blockade observed in melanoma, lung and renal cancers have spurred excitement towards an effective treatment for GBM. Although various clinical trials of checkpoint blockade for GBM are already underway, further work will likely be needed to develop effective combination therapies. To this date, the FDA has not yet approved checkpoint inhibitors for the treatment of GBM. Moreover, there are many questions that these trials will not address and we believe that preclinical studies in GEMM of GBM are a necessary step to achieve the ultimate goal of a durable antitumor response in patients.
For example, what are the mechanisms dictating overall response and resistance to checkpoint blockade? Is sensitivity to anti PD-1 therapy dictated by the levels of PD-L1 expression on tumor cells? If so, is there a window of expression above (of below) which anti PD-1 therapy is no longer a viable solution? What are the determining factors to a successful combination of coinhibitory pathway blockade? What mechanisms are responsible for the durability of checkpoint blockade? What are the molecules in play in determining the necessary length of therapy for achieving long-lasting effects? Are there biomarkers that can predict resistance and/or sensitivity to checkpoint inhibition therapy? Are there differences in certain subtypes of GBMs that confer sensitivity to checkpoint blockade? Insights into these questions can come from preclinical germane research using genetically relevant animal models of GBM.
The recent advances in cancer immunotherapies provide a solid foundation to develop effective therapies for GBM. It is imperative that the research community takes advantage of GEMM of GBMs to advance the field of immunotherapy for GBM and to investigate mechanisms of anti-tumor immune responses in order to fine tune relevant, newer and optimized checkpoint blockade therapies for this incurable cancer.
Acknowledgments
The authors would like to thank Bethany Delcuze and Dr. Vicky Appleman for critical review of the manuscript.
Grant sponsor: NCI; Grant number: R01 CA185137, P01 CA069246, U19 CA179563
Footnotes
The authors have no conflict of interest.
References
- Acquaviva J, Jun HJ, Lessard J, Ruiz R, Zhu H, Donovan M, Woolfenden S, Boskovitz A, Raval A, Bronson RT, Pfannl R, Whittaker CA, Housman DE, Charest A. Chronic activation of wild-type epidermal growth factor receptor and loss of Cdkn2a cause mouse glioblastoma formation. Cancer Res. 2011;71:7198–206. doi: 10.1158/0008-5472.CAN-11-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. Br J Cancer. 2016;115:1445–1450. doi: 10.1038/bjc.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–400. [PubMed] [Google Scholar]
- Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–73. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczyk-Thurin M, Ertl IO, Ertl HC. An experimental vaccine expressing wild-type p53 induces protective immunity against glioblastoma cells with high levels of endogenous p53. Scand J Immunol. 2002;56:361–75. doi: 10.1046/j.1365-3083.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O'Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372:2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest A, Wilker EW, McLaughlin ME, Lane K, Gowda R, Coven S, McMahon K, Kovach S, Feng Y, Yaffe MB, Jacks T, Housman D. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66:7473–81. doi: 10.1158/0008-5472.CAN-06-1193. [DOI] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Danaila L, Ghyka G, Ursaciuc C. Interleukin-2 (IL-2) in the treatment of malignant brain tumors (glioblastomas) Rom J Neurol Psychiatry. 1993;31:195–206. [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Fleurence J, Cochonneau D, Fougeray S, Oliver L, Geraldo F, Terme M, Dorvillius M, Loussouarn D, Vallette F, Paris F, Birkle S. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget. 2016;7:41172–41185. doi: 10.18632/oncotarget.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Parada LF, Holland EC, Charest A. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia. 2011 doi: 10.1002/glia.21142. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochem Res. 2015;40:2583–99. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Henriksson R, Bergenheim AT, Koskinen LO. Interleukin-2 and histamine in combination inhibit tumour growth and angiogenesis in malignant glioma. Br J Cancer. 2000;83:826–32. doi: 10.1054/bjoc.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HJ, Acquaviva J, Chi D, Lessard J, Zhu H, Woolfenden S, Bronson RT, Pfannl R, White F, Housman DE, Iyer L, Whittaker CA, Boskovitz A, Raval A, Charest A. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene. 2012;31:3039–50. doi: 10.1038/onc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HJ, Bronson RT, Charest A. Inhibition of EGFR induces a c-MET-driven stem cell population in glioblastoma. Stem Cells. 2014;32:338–48. doi: 10.1002/stem.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H, Mathios D, Jackson CM, Harris-Bookman S, Garzon-Muvdi T, Sheu M, Martin AM, Tyler BM, Tran PT, Ye X, Olivi A, Taube JM, Burger PC, Drake CG, Brem H, Pardoll DM, Lim M. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res. 2017;23:124–136. doi: 10.1158/1078-0432.CCR-15-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60:153–60. doi: 10.1007/s00262-010-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, Garzon-Muvdi T, Kim E, Theodros D, Polanczyk M, Martin AM, Suk I, Ye X, Tyler B, Bettegowda C, Brem H, Pardoll DM, Lim M. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180. doi: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis M, Olson JJ, Mikkelsen T, Lehman N, Aldape K, Alfred Yung WK, Bogler O, Vandenberg S, Berger M, Prados M, Muzny D, Morgan M, Scherer S, Sabo A, Nazareth L, Lewis L, Hall O, Zhu Y, Ren Y, Alvi O, Yao J, Hawes A, Jhangiani S, Fowler G, San Lucas A, Kovar C, Cree A, Dinh H, Santibanez J, Joshi V, Gonzalez-Garay ML, Miller CA, Milosavljevic A, Donehower L, Wheeler DA, Gibbs RA, Cibulskis K, Sougnez C, Fennell T, Mahan S, Wilkinson J, Ziaugra L, Onofrio R, Bloom T, Nicol R, Ardlie K, Baldwin J, Gabriel S, Lander ES, Ding L, Fulton RS, McLellan MD, Wallis J, Larson DE, Shi X, Abbott R, Fulton L, Chen K, Koboldt DC, Wendl MC, Meyer R, Tang Y, Lin L, Osborne JR, Dunford-Shore BH, Miner TL, Delehaunty K, Markovic C, Swift G, Courtney W, Pohl C, Abbott S, Hawkins A, Leong S, Haipek C, Schmidt H, Wiechert M, Vickery T, Scott S, Dooling DJ, Chinwalla A, Weinstock GM, Mardis ER, Wilson RK, Getz G, Winckler W, Verhaak RG, Lawrence MS, O'Kelly M, Robinson J, Alexe G, Beroukhim R, Carter S, Chiang D, Gould J, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008 doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill RS, Vitucci M, Wu J, Miller CR. Contemporary murine models in preclinical astrocytoma drug development. Neuro Oncol. 2015;17:12–28. doi: 10.1093/neuonc/nou288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo JF, Bordron A, Quintin-Roue I, Loisel S, Ster KL, Buhe V, Lagarde N, Berthou C. Recombinant humanised anti-HER2/neu antibody (Herceptin) induces cellular death of glioblastomas. Br J Cancer. 2004;91:1195–9. doi: 10.1038/sj.bjc.6602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN, Ryken TC Committee ACJG. The role of targeted therapies in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118:557–99. doi: 10.1007/s11060-013-1339-4. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paff M, Alexandru-Abrams D, Hsu FP, Bota DA. The evolution of the EGFRvIII (rindopepimut) immunotherapy for glioblastoma multiforme patients. Hum Vaccin Immunother. 2014;10:3322–31. doi: 10.4161/21645515.2014.983002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Gokhale PC, Klein SR, Ligon KL, Rodig SJ, Ramkissoon SH, Jones KL, Conway AS, Liao X, Zhou J, Wen PY, Van Den Abbeele AD, Hodi FS, Qin L, Kohl NE, Sharpe AH, Dranoff G, Freeman GJ. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Gokhale PC, Klein SR, Ligon KL, Rodig SJ, Ramkissoon SH, Jones KL, Conway AS, Liao X, Zhou J, Wen PY, Van Den Abbeele AD, Hodi FS, Qin L, Kohl NE, Sharpe AH, Dranoff G, Freeman GJ. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res. 2016;4:124–35. doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, Safrany G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–53. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, Bronson RT, Chen JW, Weissleder R, Housman DE, Charest A. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A. 2009;106:2712–6. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]