Abstract
Introduction
The aim of this study was to compare the results of ultrasound-guided prostate biopsies (US-PB) and magnetic resonance imaging-ultrasound fusion biopsies (MRI-PB) in two contemporary cohorts and to describe the parameters orienting the choice of technique.
Methods
Two contemporary cohorts of patients undergoing US-PB or MR-PB using the Urostation® (Koelis, Grenoble, France) between November 2010 and July 2015 were analyzed retrospectively. Patients with metastatic cancer or recurrence after treatment, saturation biopsies, and US-PB performed after a negative MRI were excluded. Comparison of populations, biopsy results, and clinical and biological parameters guiding the choice of technique were studied on multivariate analysis (logistic regression) taking into account the following confounding factors: age, prostate-specific antigen (PSA) rate, prostatic volume, number of previous biopsies, and abnormal digital rectal examination.
Results
One hundred fourteen patients were included in the US-PB group and 118 in the MR-PB group. Prostate cancer was diagnosed among 65 patients in the US-PB group (detection rate 57.0%) and 70 patients in the MR-PB group (detection rate 59.3%) (odds ratio [OR] 3.00; 95% confidence interval [CI] 1.52–6.17; p=0.002). Among the cancers diagnosed in the MR-PB group, 21 were diagnosed by the two targeted biopsy cores only (15.5%). Patients undergoing MR-PB were significantly younger (p=0.0005), with a higher number of previous biopsy sessions (p<10−7) and larger prostate volume (p=0.001). PSA rate alone (p=0.23) and digital rectal examination (p=0.48) did not significantly interfere with the choice of a technique.
Conclusions
Younger patients with larger prostates and prior negative biopsy were more likely to be offered the MR-PB technique. On multivariate analysis, the detection rate was higher in the MR-PB group.
Introduction
An optimal prostate cancer screening program would detect only significant cancers (i.e., those threatening to shorten life expectancy or decrease quality of life) without over-diagnosing indolent cancers and exposing patients to unjustified treatment-induced morbidity. Such a screening strategy relies on the use of efficient diagnostic procedures.
Ultrasound-guided randomized prostate biopsies have shown their limits by exposing patients to over-diagnosis (non-significant cancer) or under-diagnosis (missed cancer due to randomized procedure).1 Prostatic multiparametric magnetic resonance imaging (mpMRI) has proven helpful in selecting patients awaiting prostate biopsies by showing high Gleason score lesions2–6 and allowing the performance of targeted biopsies using various techniques.7 MRI-ultrasound fusion platforms allow targeting without radical modifications of the surrounding environment and technique, providing precise targeting and taking into account prostatic distortion and patient movements.8–10 Although prostatic MRI before repeated biopsy is now recommended by official guidelines, its implementation before the first round of biopsies is still under evaluation, and a vast majority of patients still undergo standard transrectal ultrasound (TRUS)-guided prostate biopsy, even in centres where both techniques are available.11
Our objectives were to compare the results of these two strategies on two contemporary cohorts, and to describe the parameters orienting the choice of technique.
Methods
We performed a retrospective, monocentric study on a prospectively gathered, institutionally approved database of patients undergoing prostate biopsies between 2010 and 2015. All patients had given oral informed consent.
Inclusion and exclusion criteria – biopsy technique
All patients involved in a prostate cancer screening procedure (prostate-specific antigen [PSA]>4 ng/mL, PSA increasing rate, or pathological digital rectal examination [DRE]), undergoing 12-core ultrasound-guided prostatic biopsies (US-PB group) or 12 randomized ultrasound-guided biopsies plus two MRI-ultrasound fusion biopsies (MR-PB group) were included. Suspicious areas were defined on mpMRI interpreted using the prostate imaging reporting and data system (PI-RADS) V2 scoring system (PiRADS ≥3/5). Two targeted cores of the suspicious lesion were taken in case of a single lesion, and one core of each lesion in case of two suspicious lesions. If more than two lesions were identified on MRI, one core was taken in two lesions of highest PI-RADS score. Patients presenting with metastatic disease, symptoms related to locally advanced disease, or recurrence after treatment were excluded, as well as patients undergoing saturation biopsies or US-PB after a negative MRI.
The decision of orienting the patient towards US-PB or MR-PB was taken by the urologist in charge of the patient. US-PB was performed using a 3D transrectal ultrasound system (SonoAceX8, Medison) and targeted biopsies were performed using the Urostation® MRI-US fusion device (Koelis, Grenoble, France). All patients received preoperative prophylaxis with fluoroquinolones and rectal enema, and the procedure was conducted under pure local or neuroleptanalgesia based on patient’s preference.
Collected data
Collected data included the patient age at biopsy, MRI description, PSA rate and clinical stage at DRE, prostatic volume measured by TRUS (using the ellipsoid formula), number of prior negative biopsy sessions, and pathology results (number and location of positive biopsies, total cancer length, Gleason score of each positive biopsy).
Statistical analysis
We studied the association between the diagnosis of prostate cancer and the elected type of biopsy using a first multivariate logistic regression analysis adjusted on various identified confounding factors such as age, DRE, prostatic volume, PSA rate, and the existence of prior negative biopsies (Table 1). The distribution of Gleason scores across groups is reported in Table 2.
Table 1.
Results on multivariate analysis
| Randomized biopsies | Targeted biopsies | Adjusted OR (95% CI) (multivariate analysis) | p | |
|---|---|---|---|---|
| Prostate cancer on biopsy | 65/114 (57.0%) | 70/118 (59.3%) | 3.00 (1.52–6.17) | 0.002 |
| Explanatory variables | ||||
| Age (median), years | 70.3 | 67.5 | 1.09 (1.04–1.15) | <0.001 |
| PSA rate (median) | 9.7 | 7.9 | 1.06 (1.02–1.13) | 0.018 |
| Prostate volume (median) | 40 | 45 | 0.97 (0.95–0.99) | <0.001 |
| First round of biopsies, n (%) | 60 (92.3) | 48 (68.6) | 2.33 (1.08–5.11) | 0.032 |
| Normal DRE, n (%) | 32 (49.2) | 39 (55.7) | 0/35 (0.18–0.69) | 0.003 |
CI: confidence interval; DRE: digital rectal exam; OR: odds ratio; PSA: prostate-specific antigen.
Table 2.
Gleason score repartition in targeted and randomized biopsy groups
| Randomized biopsies | Targeted biopsies | |
|---|---|---|
| Prostate cancer on biopsy – Gleason score | 65 | 70 |
| 3+3, n (%) | 22 (34) | 23 (33) |
| 3+4, n (%) | 16 (25) | 19 (27) |
| 4+3, n (%) | 13 (20) | 15 (21) |
| ≥8, n (%) | 14 (22) | 13 (19) |
A second logistic regression model was adjusted to evaluate the impact of the parameters orienting the choice of biopsy technique (Table 3).
Table 3.
Population and parameters orienting the choice of technique
| Randomized biopsies | Targeted biopsies | Adjusted OR (95% CI) (multivariate analysis) | p | |
|---|---|---|---|---|
| Parameters orienting the choice of technique | ||||
|
| ||||
| Age, median (range), years | 68 (63–73) | 65 (62–70) | 0.917 (0.87–0.96) | 0.0005 |
| Prostate volume, median (range) | 40 (30–50) | 49 (38–66) | 1.029 (1.01–1.05) | 0.001 |
| PSA rate, median (range) | 8.2 (5.5–12.9) | 7.2 (5.6–12.0) | 0.982 (0.69–1.01) | 0.23 |
| Normal DRE | 68 | 82 | 0.791 (0.41–1.50) | 0.48 |
| First round of biopsies | 106 | 73 | 0.098 (0.04–0.22) | <0.001 |
| Total | 114 | 118 | NR | NR |
CI: confidence interval; DRE: digital rectal exam; OR: odds ratio; PSA: prostate-specific antigen.
In the two logistic regression models, explanatory variables were tested by Wald’s test. A significance threshold of 0.05 was adopted for all statistical analyses. Statistical analysis was performed using the computing environment R.
Results
Population
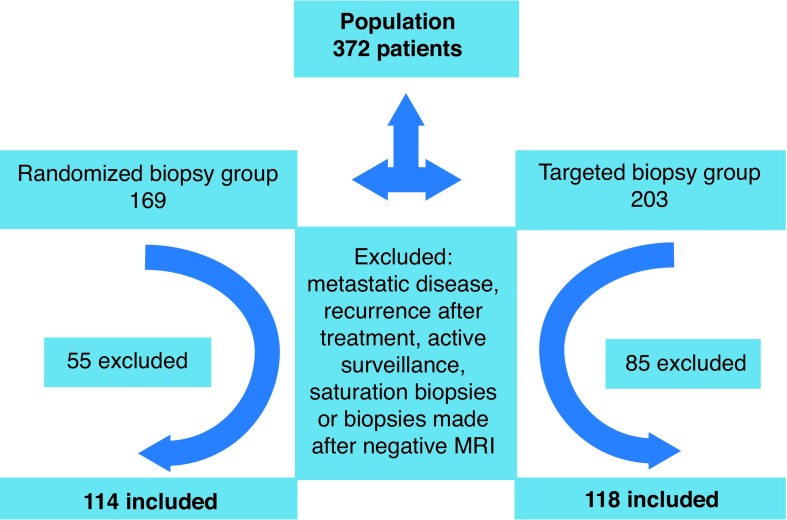
Between November 2010 and July 2015, 372 prostatic biopsies were performed, of which 169 were US-PB and 203 were MR-PB. Forty-six patients were excluded from the US-PB group and 82 from the MR-PB group. Fig. 1 summarizes the patient selection process. One hundred and fourteen patients in the US-PB group and 118 in the MR-PB group were included (Fig. 1). Patient characteristics are summarized in Table 3.
Fig. 1.
Flow chart of patient selection process. MRI: magnetic resonance imaging.
Biopsy results
Prostate cancer was detected among 70 patients in the MR-PB group (59.3%) and 65 patients (57.0%) in the US-PB group. On multivariate analysis, MR-PB allowed the detection of a significantly higher number of prostate cancer cases than US-PB (odds ratio [OR] 3.00; 95% confidence interval [CI] 1.52–6.17; p=0.002).
Among cancers diagnosed in the MR-PB group, 21 were detected by targeted biopsy cores only (15.5% of the diagnosed cancers). On average, two biopsies were positive in the MR-PB group and three in the US-PB group. The median cancer length was 10.5 mm in the US-PB group and 13 mm in the MR-PB group (Table 1). Distribution of Gleason scores across groups is presented in Table 3.
Parameters orienting the choice of technique
Younger patients (p=0.005) with a prior history of negative biopsies (p<0.001) were more likely to be offered the MRI-PB. A larger prostatic volume was also a factor predicting the choice of the targeted technique (p=0.001). PSA rate (0.23) and normal DRE (0.48) did not significantly influence the choice of technique (Table 3).
Discussion
This study confirms a higher cancer detection rate by MR-PB than US-PB after adjustment on confounding factors. To the best of our knowledge, this is the first study also reporting the parameters guiding the urologist’s choice in the diagnostic strategy. When the two techniques are available, younger patients with a past history of negative prostate biopsies presenting a larger prostate volume are more likely to be offered targeted prostate biopsies.
As expected, the two groups were not comparable and there could be some concern that both populations did not have the same risk of prostate cancer a priori. We chose to exclude patients having a negative MRI from the MR-PB group, thus allowing no targeted procedure. These patients underwent a classical randomized, echo-guided procedure and were excluded from the global analysis to prevent induced selection bias, as they were at lower risk of having significant prostate cancer. Comparing both populations after applying our inclusion criteria to the overall population that underwent prostatic biopsy between 2010 and 2015, patients in the randomized biopsy group seemed at higher risk of presenting prostate cancer based on PSA rate, DRE, age, number of previous negative biopsies, and prostate volume, therefore reducing the risk of bias when interpreting the superiority of MR-PB.
The MRI-ultrasound fusion technique is currently an interesting compromise to reduce over-diagnosis without missing a potentially aggressive cancer.3–5,12 Still, the additional cost of the technique and the extra operating time needed leave a place for conventional ultrasound-guided randomized biopsies, even in centres where both techniques are available. While some authors have suggested that all prostate cancer screening should be done by MR-PB,13 it is important to clearly define the population that will benefit most from this diagnostic technique.
The detection rates of 57.0% in the US-PB group and of 59.3% in the MR-PB group are above the usual values found in the literature (20–40% for a first round and 14–18% for a second round of biopsies).10,12,14–19 This rate reflects both the efficiency of a strategy combining pelvic MRI and targeted biopsies, especially in the case of repeated prostate biopsies, but also an institutional attitude towards prostate cancer screening — likely less aggressive than other centres.
The randomized trials published by Baco et al15 and and Tonttila et al20 failed to show a superiority of the MR-PB technique compared to standard 10–12-core randomized prostate biopsies. In our study, multivariate logistic regression analysis showed a significantly higher detection rate in the MR-PB group. These results can be explained first by the fact that we compared the association of US-PB and MR-PB to US-PB alone and not only MR-PB to US-PB. Secondly, patients in the MR-PB group were previously screened by MRI and we only included patients with a PI-RADS score ≥3/5.
Younger patients with a larger prostate were more frequently offered the MRI-PB technique, as were patients with a history of at least one prior negative prostate biopsy.1,9,21–25 Targeted biopsies were mostly dedicated to patients having at least one prior negative round of biopsies,26 whom we suspected to have an anteriorly located aggressive cancer27 or a cancer foci in a high volume of benign prostatic hyperplasia.
The limitations of this study, besides its retrospective nature, are mainly linked to the lack of systematic histological confirmation of the information obtained by prostatic biopsy (most notably in the case of negative biopsies), with false negative rate being impossible to evaluate. The choice of technique could also be considered a limitation, in that it is possible selection was cost-related.28
Conclusion
Younger patients with a larger prostatic volume and a history of prior negative biopsies were more likely to be offered the MR-PB technique. When comparing the results of both techniques on two contemporary cohorts, on multivariate analysis, the detection rate was higher in the MR-PB group.
Acknowledgements
The authors wish to thank the radiology department, and more specifically Dr. Noelie Hohn, for their help with this work.
Footnotes
Competing interests: The authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: The role of magnetic resonance imaging-targeted biopsy in detection, localization, and risk assessment. J Urol. 2014;192:648–58. doi: 10.1016/j.juro.2014.03.117. https://doi.org/10.1016/j.juro.2014.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeks CMA, Somford DM, van Oort IM, et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance-guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: A prospective, multicentre, cohort study. Invest Radiol. 2014;49:165–72. doi: 10.1097/RLI.0000000000000008. https://doi.org/10.1097/RLI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Ji A, Xie B, et al. Is magnetic resonance/ultrasound fusion prostate biopsy better than systematic prostate biopsy? An updated meta- and trial sequential analysis. Oncotarget. 2015;6:43571–80. doi: 10.18632/oncotarget.6201. https://doi.org/10.18632/oncotarget.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: A systematic review. Eur Urol. 2015;68:8–19. doi: 10.1016/j.eururo.2014.10.026. https://doi.org/10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Meng X, Rosenkrantz AB, Mendhiratta N, et al. Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion targeted prostate biopsy outcomes. Eur Urol. 2016:69512–7. doi: 10.1016/j.eururo.2015.06.005. https://doi.org/10.1016/j.eururo.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound fusion biopsy significantly upgrades prostate cancer vs. systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–9. doi: 10.1016/j.eururo.2013.05.059. https://doi.org/10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiard G, Descotes J-L, Rambeaud J-J, et al. [MRI-guided targeted prostate biopsies in the diagnosis of prostate cancer: A systematic literature review]. Prog En Urol J Assoc Fr Urol Société Fr Urol. 2012;22:903–12. doi: 10.1016/j.purol.2012.06.005. https://doi.org/10.1016/j.purol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Mozer P, Rouprêt M, Le Cossec C, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localized prostate cancer. BJU Int. 2015;115:50–7. doi: 10.1111/bju.12690. https://doi.org/10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 9.Fiard G, Hohn N, Descotes J-L, et al. Targeted MRI-guided prostate biopsies for the detection of prostate cancer: Initial clinical experience with real-time 3-dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology. 2013;81:1372–8. doi: 10.1016/j.urology.2013.02.022. https://doi.org/10.1016/j.urology.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Delongchamps NB, Portalez D, Bruguière E, et al. Are MRI-TRUS-guided targeted biopsies non-inferior to TRUS-guided systematic biopsies for the detection of prostate cancer in patients with a single suspicious focus on multiparametric prostate MRI? Results of a multicentric controlled trial. J Urol. 2016;196:1069–75. doi: 10.1016/j.juro.2016.04.003. https://doi.org/10.1016/j.juro.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Salomon L, Bastide C, Beuzeboc P, et al. Recommandations en onco-urologie 2013 du CCAFU : Cancer de la prostate. Prog En Urol. 2013;23:S69–S101. doi: 10.1016/S1166-7087(13)70048-4. https://doi.org/10.1016/S1166-7087(13)70048-4. [DOI] [PubMed] [Google Scholar]
- 12.Fourcade A, Perrouin-Verbe M, Tissot V, et al. Rôle des biopsies ciblées utilisant un système d‘enregistrement 3D avec fusion élastique écho/IRM chez les hommes ayant une suspicion de cancer prostatique. Prog En Urol. 2015;25:832. doi: 10.1016/j.purol.2015.08.230. https://doi.org/10.1016/j.purol.2015.08.230. [DOI] [PubMed] [Google Scholar]
- 13.Burruni R, Cerantola Y, Meuwly J-Y, et al. [Prostate biopsy: Which strategy for which patient?]. Rev Médicale Suisse. 2015;11:2288–90. [PubMed] [Google Scholar]
- 14.Klein J, Mayor G, De Gorski A, et al. Biopsies prostatiques ciblées par guidage IRM dans le diagnostic du cancer localisé de la prostate : taux de détection et valeur prédictive de l‘imagerie. Prog En Urol. 2014;24:812. doi: 10.1016/j.purol.2014.08.066. https://doi.org/10.1016/j.purol.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 15.Baco E, Rud E, Eri LM, et al. A randomized, controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69:149–56. doi: 10.1016/j.eururo.2015.03.041. https://doi.org/10.1016/j.eururo.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Peltier A, Aoun F, Lemort M, et al. MRI-targeted biopsies vs. systematic transrectal ultrasound guided biopsies for the diagnosis of localized prostate cancer in biopsy-naive men. BioMed Res Int. 2015;2015:571708. doi: 10.1155/2015/571708. https://doi.org/10.1155/2015/571708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. https://doi.org/10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 18.Roehl KA, Antenor JAV, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–9. https://doi.org/10.1016/S0022-5347(05)64999-3. [PubMed] [Google Scholar]
- 19.Campos-Fernandes J-L, Bastien L, Nicolaiew N, et al. Prostate cancer detection rate in patients with repeated extended 21-sample needle biopsy. Eur Urol. 2009;55:600–9. doi: 10.1016/j.eururo.2008.06.043. https://doi.org/10.1016/j.eururo.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Tonttila PP, Lantto J, Pääkkö E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: Results from a randomized, prospective, blinded, controlled trial. Eur Urol. 2016;69:419–25. doi: 10.1016/j.eururo.2015.05.024. https://doi.org/10.1016/j.eururo.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion-guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–5. doi: 10.1016/j.juro.2011.05.078. https://doi.org/10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy vs. magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. https://doi.org/10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Sonn GA, Margolis DJ, Marks LS. Target detection: Magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014;32:903–11. doi: 10.1016/j.urolonc.2013.08.006. https://doi.org/10.1016/j.urolonc.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonn GA, Natarajan S, Margolis DJA, et al. Targeted biopsy in the detection of prostate cancer using an office-based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–92. doi: 10.1016/j.juro.2012.08.095. https://doi.org/10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–15. doi: 10.1016/j.eururo.2013.03.025. https://doi.org/10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188:2152–7. doi: 10.1016/j.juro.2012.08.025. https://doi.org/10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: Magnetic resonance imaging/transrectal ultrasound fusion-guided prostate biopsy. J Urol. 2014;191:1749–54. doi: 10.1016/j.juro.2013.12.007. https://doi.org/10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Rooij M, Crienen S, Witjes JA, et al. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy vs. systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: A modelling study from a healthcare perspective. Eur Urol. 2014;66:430–6. doi: 10.1016/j.eururo.2013.12.012. https://doi.org/10.1016/j.eururo.2013.12.012. [DOI] [PubMed] [Google Scholar]