Abstract
Osteoblast commitment and differentiation are controlled by multiple growth factors including members of the Notch signaling pathway. JAGGED1 is a cell surface ligand of the Notch pathway that is necessary for murine bone formation. The delivery of JAGGED1 to induce bone formation is complicated by its need to be presented in a bound form to allow for proper Notch receptor signaling. In this study, we investigate whether the sustained release of JAGGED1 stimulates human mesenchymal cells to commit to osteoblast cell fate using poly-ethylene glycol malemeide (PEG-MAL) hydrogel delivery system. Our data demonstrated that PEG-MAL hydrogel constructs are stable in culture for at least three weeks and maintain human mesenchymal cell viability with little cytotoxicity in vitro. JAGGED1 loaded on PEG-MAL hydrogel (JAGGED1-PEG-MAL) showed continuous release from the gel for up to three weeks, with induction of Notch signaling using a CHO cell line with a Notch1 reporter construct, and qPCR gene expression analysis in vitro. Importantly, JAGGED1-PEG-MAL hydrogel induced mesenchymal cells towards osteogenic differentiation based on increased Alkaline phosphatase activity and osteoblast genes expression including RUNX2, ALP, COL1, and BSP. These results thus indicated that JAGGED1 delivery in vitro using PEG-MAL hydrogel induced osteoblast commitment, suggesting that this may be a viable in vivo approach to bone regeneration.
Keywords: JAGGED1, notch, bone, hydrogel
INTRODUCTION
Growth factor delivery to induce bone formation is a well-established regenerative approach to bone loss. Osteoblast commitment and differentiation are controlled by multiple signaling pathways including Notch, Wnt, TGF superfamily.1–3 TGFβ/BMP signaling involves multiple ligands and receptors, with the TGFβ pathway involved in cellular proliferation and extracellular matrix deposition and inhibition of osteoblasts differentiation and mineralization, whereas BMP signaling promotes osteoblast differentiation.2,4–6 Bone regenerative approaches involving scaffolding carriers for stem cells and growth factor delivery have been well described in multiple animal models.7–11 BMP2 delivery via a collagen sponge scaffold in humans is primarily used, and is only FDA approved for patients over the age of 18 for use during spinal fusion and maxillary sinus lifts to treat maxillary bone deficiency. Limitations with BMP2 exist due to a heterogeneous response to BMP2 therapy and there are no other available commercial bone regenerative therapies.12,13 Unfortunately, BMP2 delivery can be associated with significant local inflammation due to the supratherapeutic doses delivered, that can be life threatening when used in the head and neck area.14,15 Alternative regenerative approaches to bone loss have been investigated. These include autogenous bone marrow with platelet rich plasma, bioactive glass, hydrogel delivery of vascular endothelial growth factor (VEGF), collagen with BMP7; however, these strategies have not demonstrated in vivo efficacy.16
Multiple cell types, including bovine bone cells and mes-enchymal stem cells (MSC) in combination with or without scaffolding have been used in skeletal tissue engineering and regeneration approaches.17–20 Localized cell delivery to treat bone loss has included bovine bone cells, demineralized bone allograft, and MSC using scaffolding constructs of polylactic-coglycolide copolympers (PLGA), fibronectin, hydroxyapatite, and other inorganic and ceramic constructs.20 The source of MSC can be from the bone marrow, periosteum, trabecular bone, adipose, and dental tissues.21 Each of these approaches has demonstrated promising results in vitro, but had mixed success in bone regeneration in animal models.21
In this study, we evaluate the osteogenic potential of the Notch pathway in vitro by delivering JAGGED1 using polyethylene glycol maleimide (PEG-MAL) hydrogels to humane embryonic mesenchymal cells. Targeting the Notch signaling pathway, we demonstrate that controlled delivery of JAGGED1, a cell surface ligand member of the Notch pathway, induces osteoblast commitment of human palate mesenchymal stem cells.22,23 JAGGED1 plays an important role in bone development, and interruption of its function has been associated with obvious clinical manifestations of bony loss correlated with a higher incidence of bone fracture.24 Global loss of JAGGED1 signaling leads to early embryonic death due to severe vascular anomalies.25 Conditional deletion of JAGGED1 in the cranial neural crest, using Wnt1-Cre, phenocopies the craniofacial phenotype of human Alagille syndrome with severe maxillary bone deficiency.26 Overexpression of JAGGED1 in previous studies demonstrated the osteoninductive properties of JAGGED1 in vitro.27 The delivery of JAGGED1 to induce bone formation is complicated by its need to be presented in a bound form, to allow for proper Notch receptor signaling.28 The success of the delivery method is dependent on the choice of the scaffold being used. We describe the use of hydrogel scaffolding for the delivery JAGGED1 using PEG-MAL, as this approach has been successfully used with other soluble growth factors in vitro and in vivo.29 PEG-MAL hydrogel adheres to the surrounding tissues, induces minimal amounts of inflammation, is enzyme-degraded and safely excreted in the urine, allows easy cell delivery, and the maleimide groups react with available thiols in the target biomolecules to incorporate them into the hydrogel.30 In this study, we demonstrate the osteoinductive effects of JAGGED1-PEG-MAL and its degradation characteristics during in vitro embryonic maxillary mesenchymal cell commitment into osteoblasts.
MATERIALS AND METHOD
Cell culture
Human Embryonic Palatal Mesenchyme cells (HEPM cells) were obtained from ATCC (Manassas, VA) and Notch reporter cells (Chinese Hamster Ovary Cells, CHO cells) that support Notch1 signaling, but do not express endogenously Notch receptors (a kind gift of the Elowitz lab).31
Cells were maintained in alpha MEM supplemented with 10% FBS, 100 U.mL−1 penicillin, 0.1 mg.mL−1 streptomycin at 37°C in a 5% CO2-humidified atmosphere. Osteoblast differentiation was induced by culturing HEPM in osteogenic media by the addition of 50 μg/mL ascorbic acid to growth media and the media was refreshed every 2–3 days. Alkaline Phosphatase activity was quantitated after 7 days of culture. To determine the difference in alkaline phosphatase production, we will dissect out maxillary mesenchymal cells from the palates. The cells’ alkaline phosphatase activity will be measured using p-nitrophenyl phosphatase release measured at 410 nm absorbance compared to total protein using the Bradford protein assay
PEG-MAL hydrogel preparation and cell encapsulation
Four-arm maleimide end-functionalized PEG macromer (20 kDA, Laysan Bio) was pre-functionalized by reacting PEG-MAL with 2.0 mM RGD peptide (GRGDSPC, New England Peptide, NEP) at 37°C for 1 hour (Supporting Information Fig. S1). 5 × 105 cells were mixed with the pre-functionalized PEG-MAL macromer and polymerized by addition of GPQ crosslinker peptide (GCRDQGWIGQPGDRCG, New England Peptide, NEP) and maintained in Alpha-MEM supplemented with 10% FBS, penicillin and streptomycin and cultured in an incubator at 37°C at 5% CO2.
JAGGED1 indirect immobilization and notch signaling activation
Culture well chambered coverglass were pre-coated with rabbit anti-human IgG (10 μg/mL) in phosphate saline buffer (PBS) for 30 min at 37°C and subsequently blocked with cell culture growth medium for 30 min. Chambered coverglass were then coated with 5 μg/mL JAGGED1/Fc (JAG1–3138 H, Creative BioMart) diluted in growth media for 2 hours at 37°C. As control for JAGGED1, human IgG (5 μg/mL) was used. Chambers coated with JAGGED1/Fc or IgG were washed with growth medium and 5 ×105 cells CHO cells were seeded. Cells were maintained in Alpha-MEM supplemented with 10% FBS, penicillin and streptomycin and stored in an incubator at 37°C, 5% CO2 for 72 hours. Cells were analyzed on a fluorescent microscopy for Notch signaling.
JAGGED1/fc-PEG-MAL hydrogel preparation and notch signaling activation
Human JAGGED1/Fc or Human IgG were mixed with Protein G Dynabeads (Fisher Scientific) in 100 μL phosphate buffered saline (PBS) for 10 min at room temperature under rotation. After 3 times wash with PBS, JAGGED1-immobilized or IgG-immobilized on Protein G Dynabeads were washed four times with PBS and mixed with pre-functionalized 5% PEG-MAL with 2.0 mM RGD peptide in 10 mM HEPES buffer for 1 hour. 5 ×105 HEPM cells were encapsulated and the mixture of cells-hydrogel was cast in the bottom of a 24 well cell culture plate on a chambered cell culture coverglass and cross-linked with addition of GPQ at 1:2 molar ratio of GPQ peptide to available MAL groups.30 Then, cells were maintained in Alpha-MEM supplemented with 10% FBS, 50 μg/mL ascorbic acid, penicillin and streptomycin and cultured in an incubator at 37°C, 5% CO2. Total RNA was isolated using TRIzol after 2 days in culture for Notch signaling activation and 7 days for osteoblast differentiation.
JAGGED1 release profile
JAGGED1/Fc (10 μg/mL) coupled to Protein G Dynabeads was prefunctionalized with 5%PEG-MAL, 2.0 mM RGD and cross-linked with addition of GPQ. JAGGED1/Fc-PEG-MAL hydrogel was cast in the bottom of a 24-well tissue culture plate and placed in an incubator at 37°C at 5% CO2 with 250 μL of PBS. PBS was refreshed and collected every 2–3 days for up to 20 days and Jagged/Fc release was quantified using Human JAGGED1 ELISA Kit (R&D Systems).
PEG-MAL stability in vitro
PEG-MAL degradation was analyzed by staining the remaining hydrogel in culture using crystal violet, which stains cells and extracellular matrix in purple. 1 ×105 HEPM cells were encapsulated in PEG-MAL at various concentration of PEG-MAL (0.625%–20%) pre-functionalized with RGD. The mixture of cells and hydrogels was cast in a corner of a 24 well cell culture plate and placed in an incubator at 37°C, 5% CO2. The medium was refreshed every 2–3 days for up to 20 days. After 20 days, cells were removed by RIPA buffer treatment and the remaining PEG-MAL hydrogel were stained for 4 hours with crystal violet dye.
PEG-MAL hydrogel degradation was analyzed using an NIR fluorescent dye. 5%PEG-MAL was labeled with VivoTag-S 645-MAL (Perkin Elmer), a thiol (-SH, sulfhydryl) reactive maleimide-containing a red fluorochrome. Labeled PEG-MAL was functionalized with 2.0 mM RGD, cast in a corner of a 24-well tissue culture plate, cross-linked with addition of GPQ and placed in an incubator at 37°C, 5% CO2 with 250 μL of PBS. PBS was refreshed and collected every 2–3 days for up to 20 days. PEG-MAL degradation products released in PBS were imaged on a BRUKER infrared imaging system (Bruker Imaging, Billerica, MA).
RT-PCR
Total RNA was extracted using TRIzol (Invitrogen, Grand Island, NY, USA), and cDNAs were synthesized following DNase I treatment using the high-capacity cDNA reverse-transcription kit (Applied Biosystems, USA). Quantitative PCR (qPCR) were performed by using SYBR green qPCR. The primer sets used for qPCR analysis are listed in Supporting Information Table S1. Specificity of amplification was verified by the presence of a single peak on the dissociation curve. Specific amplification conditions are available upon request. Measurements were performed in triplicate and from at least three independent experiments.
Total cell number, cell proliferation and apoptosis
1 × 105 HEPM cells were encapsulated using sequential concentrations of PEG-MAL hydrogel and cast in the bottom of a 24-well cell culture plate. Cells were cultured in Alpha-MEM supplemented with 10% FBS, penicillin and streptomycin at 37°C at 5% CO2 for 3 days. Cells metabolic activity was measured using the CellTiter Aqueous One 96 Kit (Promega) as recommended by the manufacturer. The conversion of 3–(4,5-Dimethylthiazol-2-yl)-5–(3-carboxymethox-yphenyl)-2–(4-sulfophenyl)-2 H-tetrazolium to formazan by metabolically viable cells was monitored by measuring the absorbance at 490 nm. Apotosis was measured by the activity of the effector caspase 3 and caspase 7 using the Apo-One Homogeneous Caspase3/7 kit (Promega) according to the manufacturer’s instructions. Proliferation and Apoptosis were normalized by the number of total cells quantified by crystal violet staining assay.
Statistics: Each experiment was independently repeated three times on independent biologic samples and differences between groups (control and JAGGED1-stimulation) tested by two-way analysis of variance with 2 degrees of freedom for the treatment main effect.
RESULTS
Influence of PEG-MAL hydrogel density on human embryonic palate mesenchymal (HEPM) cell survival
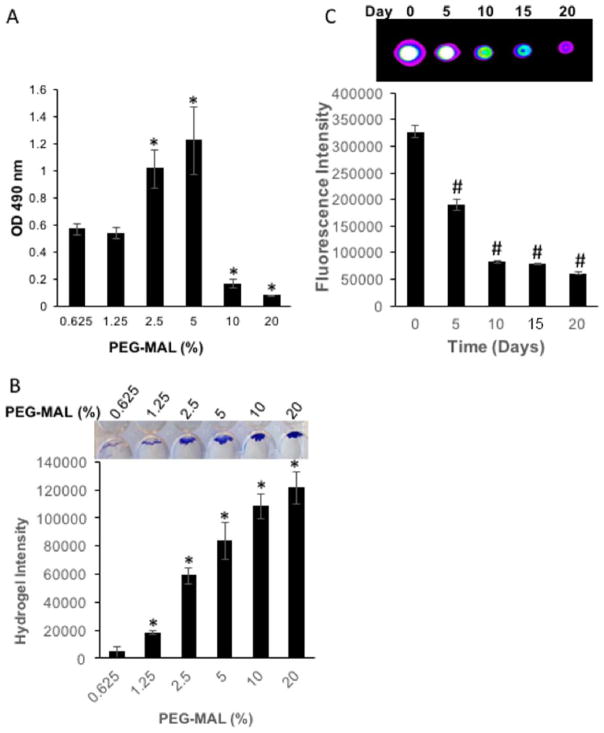
The goal of this article was to evaluate the osteogenic effect of JAGGED1 delivery using PEG-MAL hydrogels. To determine the optimum PEG-MAL density, cell survival potential in various densities of PEG-MAL in vitro were determined (Fig. 1). HEPM cells were encapsulated in PEG-MAL at the density of 1 × 105 cells/mL and cell survival was assessed by Cristal violet staining 3 days post encapsulation. Cell number increased gradually with increased PEG-MAL density reaching the maximum at 5% PEG-MAL. However, PEG-MAL density greater that 5% showed poorer cell number with lower cells density compared to cells in PEG-MAL lower than 5%. [Fig. 1(A)].
FIGURE 1.
Influence of PEG-MAL density on encapsulated cells number. HEPM cells encapsulated within PEG-MAL hydrogels at various densities (0.625% to 20%). (A) Cell viability assessed by MTS assay 3 days post encapsulation. (B) Cells were removed by trypsin treatment and the remained hydrogel stained with Crystal violet. (C) 5%PEG-MAL labeled with VivoTag-S 645 NIR fluorescent dye and polymerized by GPQ cross-linker was incubated in PBS. PEG-MAL degradation products released in PBS were imaged on a BRUKER infrared fluorescent imaging system and quantified by Image J. *p <0.05 versus 0.625% PEG-MAL and #p <0.05 versus day 0.
In vitro degradation of the PEG-MAL hydrogel
PEG-MAL degradation occurs based on the density of the hydrogel and the cross linker used. To measure the stability of this hydrogel construct, we measured the degradation characteristics of the hydrogel over time. HEPM cells were encapsulated in PEG-MAL at various concentrations using GPQ crosslinker and the remaining hydrogel construct at 20 days in culture was stained with crystal violet and quantified to compare in vitro degradation [Fig. 1(B)]. The stability of the PEG-MAL hydrogel in culture increased with the increased gel density, where gels at lower density (0.625% and 1.25%) are quickly degraded.
Incorporation of JAGGED1 in a polyethylene glycol maleimide (PEG-MAL) hydrogel
Incorporation of JAGGED1 into the 5% PEG-MAL hydrogel was accomplished by reacting the sulfhydryl groups on the JAGGED1-Fc peptide immobilized on Protein-G micro-beads with the PEG-MAL. The PEG-MAL was then cross-linked using a degradable cross linker, GPQ, to complete the reaction. To examine the degradation profile of PEG-MAL hydrogel in vitro, 5% PEG-MAL was labeled with Vivo-tag 645-MAL near infrared (NIR) fluorescent dye and incubated in PBS over 20 days [Fig. 1(C)]. Evaluation of hydrogel degradation was compared using Bruker imaging, where there was maximal degradation at day 5 and there was progressive degradation over the initial 10 days. After 10 days, the hydrogel degradation reached equilibrium and remained stable up to 20 days.
JAGGED1 release kinetics from the PEG-MAL hydrogel and HEPM cells proliferation and apoptosis in response to JAGGED1 delivery
To establish the release profile of JAGGED1 from the PEG-MAL JAGGED1-PEG-MAL hydrogel was cultured with PBS and the media was collected every other day for 20 days. JAGGED1 release measurement using ELISA evaluation of the collected media demonstrated a slow release of JAGGED1 over time [Fig. 2(A)], with about 40–60% of the amount of JAGGED1 incorporated remained in PEG-MAL over 10 days in culture and totally released from the gel at day 20. No significant difference was found in HEPM viability or apoptosis with varying concentrations of JAGGED1 incorporation (2.5 μg/mL to 10 μg/mL), as evaluated by caspase3/7 and MTT assays, respectively, [Fig. 2(B,C)]. These results demonstrated that the JAGGED1-PEG-MAL does not induce any cytotoxic effect on HEPM cells in vitro culture.
FIGURE 2.
JAGGED1 release profile and Notch signaling activation. (A) Prefunctionalized PEG-MAL with JAGGED1 (10 ug/m) and RGD peptide were cross-linked and incubated for 20 days in PBS. Released JAGGED1 from PEG-MAL collected every 2–3 days and quantified by ELISA. (B–C). HEPM cells were encapsulated in functionalized JAGGED1–5%PEG-MAL hydrogels and cultured in growth media for 3 days. HEPM cells proliferation (B) and Apoptosis were measured using the CellTiter Aqueous One 96 Kit and Apo-One Homogeneous Caspase3/7 kit, respectively. (n = 3) *p <0.05 versus IgG control (JAGGED1 (0 ug/mL) and #p <0.05 versus day 0.
Incorporation of JAGGED1 in PEG-MAL induces notch signaling and osteoblast differentiation in vitro
To detect functional JAGGED1 protein, the JAGGED1-PEG-MAL hydrogel was seeded with fluorescent CHO reporter cells expressing Notch1, a receptor for JAGGED1. Fluorescence of the CHO cells was compared between the control and the JAGGED1-PEG-MAL hydrogels and there was significantly more fluorescence observed in the JAGGED1-PEG-MAL hydro-gels, suggesting that there was activation of Notch signaling by intact JAGGED1 [Fig. 3(A)]. Analysis of gene expression using qPCR after 2 days of culture with JAGGED1-PEG-MAL hydrogels in vitro demonstrated robust activation of the Notch signaling pathway genes including NOTCH1, HES-1, HES-3, HEY-1, HEY-2, and HEY-L [Fig. 3(B)].
FIGURE 3.
Activation of Notch Signaling Pathway by Jagged-1PEG-MAL. HEPM cells or Notch reporter CHO cells were encapsulated in functionalized JAGGED1–5%PEG-MAL hydrogels and cultured for 3 days. (A) Notch signaling activation in CHO cells analyzed by YFP fluorescence and quantified using Image J. (bar: 100 μm). (B) Notch-associated gene expression in HEPM cells were assessed by RT-PCR. (n = 3) *p <0.05 versus IgG control (JAGGED1 (0 μg/mL).
To determine the osteoinductivity of the JAGGED1-PEG-MAL, HEPM cells were incorporated into JAGGED1-PEG-MAL and cultured in osteogenic media for 7 days. The JAGGED1-PEG-MAL treated HEPM demonstrated increased ALP activity [Fig. 4(A)] and osteoblast gene expression compared to controls, including ALP, COL1, RUNX2, and BSP [Fig. 4(B)]. These results indicate the JAGGED1-PEG-MAL can induce HEPM cell commitment to osteoblast cell fate and is a proof of principle for in vivo approaches to induce local bone regeneration.
FIGURE 4.
JAGGED1-PEG-MAL stimulates the differentiation of HEPM cells in vitro. HEPM cells were encapsulated in functionalized JAGGED1–5%PEG-MAL hydrogels and cultured in osteogenic media for 7 days. (A) HEPM differentiation analyzed by ALP activity using p-nitrophenyl phosphate (pNPP) as a substrate which turns yellow when dephosphorylated by ALP. (B) Expression of osteoblast marker genes was assessed by qPCR (n = 3) *p <0.05 versus IgG control (JAGGED1 (0 μg/mL).
DISCUSSION
The role and requirement of JAGGED1 in multiple biologic processes including cardiac, biliary and vascular development have been well described.32 Recent evidence highlights the importance of JAGGED1 in human bone development and maintenance.27,33 Notch ligand signaling occurs in a cell-to-cell manner, therefore delivery of Notch ligands in a regenerative strategy requires them to be immobilized to signal effectively.28 In this study, we explored the delivery of JAGGED1 immobilized to a PEG-MAL to induce HEPM cell commitment to osteoblasts in vitro. The use of PEG-MAL hydrogels to deliver growth factors is well described.34 The PEG-MAL hydrogel is an attractive regenerative construct as it has tunable degradation, elicits minimal host inflammation, and it binds to the surrounding tissue. We successfully determined the optimal concentration of PEG-MAL to allow HEPM viability and maximize the longevity of hydrogel permanence (Fig. 1). Similarly, Phelps et al. demonstrated that 4% PEG-MAL was optimal for the viability and function of C2C12 myoblast assays in vitro.30 Cardiac progenitor cell in vitro models using self-assembling hydrogel culture demonstrated that 2% hydrogel allowed optimal cell viability, suggesting that the hydrogels should be tailored to the proposed cell line being used.35 The optimal concentration of the 5% PEG-MAL hydrogel in Figure 1 is similar to those found by other investigators as expected.36
The effective incorporation and release of JAGGED1 within the PEG-MAL hydrogel delivered a consistent release profile (Fig. 2). The benefit of the PEG-MAL is the tunable therapeutic delivery that is dependent on the concentration of the hydrogel and the cross-linker used. Similar to our findings, Phelps et al. demonstrated the controlled local delivery of VEGF using a PEG-MAL hydrogel in a controlled fashion.37 Using a similar approach, Boopathy et al. used self-assembling peptide gels to deliver a JAGGED1 mimic to cardiac progenitor cells in a controlled fashion.36 Similar to Boopathy et al. we demonstrated the functional incorporation of JAGGED1 within the PEG-MAL hydrogel using a Notch1 reporter yellow fluorescent protein (YFP) assay (Fig. 3).36 Dishowitz et al. reported the delivery of JAGGED1 using a diethylene glycol diacrylate and isobutylamine (A6) scaffold for delivery to induce bone formation.38 In their article, Dishowtiz et al. demonstrated that cellular viability and cell number were superior with direct conjugation of JAGGED1 to the A6; however, they did not evaluate how varying the concentration of the scaffold affected cellular viability.
PEG-MAL hydrogel delivery of growth factors, molecules, and cells has been used reliably in animals to induce regeneration of tissues, including bone. Specifically the delivery of BMP2 using PEG-MAL has reproducibly led to new bone formation in vivo.39,40 The ability to control the degradation rate of the PEG-MAL hydrogel provides a distinct advantage over other delivery systems, and can be adjusted to maximize the regenerative effect including prolonged growth factor release to induce bone formation. In our model the transition to in vivo implantation of the PEG-MAL hydrogel degradation and release kinetics will likely be different and will have to be determined empirically. Despite the advances in PEG-MAL hydrogel development, there are still limitations in using this construct including maintaining precise spatial control of the scaffold in vivo, avoiding the burst effect associated with growth factor delivery, and controlling the dynamic cellular microenvironment after in vivo delivery.41
Notch pathway signaling induction of Hes1 and Hey1 gene expression in response to JAGGED1 incorporation with the A6 scaffold demonstrated 4–6 fold changes in the Dishowitz study. In Figure 3 we demonstrated markedly higher induction of Notch pathway genes Hes1 and Hes3, that were 50-fold and 6-fold increased compared to controls, suggesting a robust induction of the Notch signaling pathway by the JAGGED1-PEG-MAL hydrogel. Induction of the bone pathway genes was demonstrated by the JAGGED1-PEG-MAL hydrogel with increased Runx2, alkaline phosphatase, Collagen 1, and Bone Sialoprotein at 6 days (Fig. 4). In contrast to the data presented by Dishowitz et al. where higher JAGGED1 concentrations (10 ng/mL) induced higher bone-related genes, our findings suggest that lower doses of JAGGED1 incorporation (2.5 μg/mL) into the PEG-MAL demonstrated a stronger induction of bone-related genes, particularly alkaline phosphatase, Collagen 1 and Runx2 compared to higher doses of JAGGED1. However, higher doses of JAGGED1 (10 μg/mL) in the PEG-MAL construct were associated with increased BSP production, similar to that found by Dishowitz et al.
The role of JAGGED1 during osteoinduction of human mesenchymal cells is supported by this and other reports, suggesting that JAGGED1 can be used as a potential bone therapy.27,38 In this report, we demonstrated that JAGGED1 incorporation in PEG-MAL strongly induces Notch signaling and bone-related gene transcription. Furthermore, we found that the concentration of the hydrogel used, cross-linking agent and concentration of JAGGED1 is critical for cell survival and that its affect on cellular differentiation and gene expression is likely cell dependent, as other authors have found.27,28,33,38,42 We propose that JAGGED1-PEG-MAL osteoinductive construct provides a novel approach to bone regeneration to be tested in vivo.
Supplementary Material
TABLE I.
List of Primers Used in this Study
| Gene | Forward primer | Reverse primer |
|---|---|---|
| HPRT | ATTGGTGGAGATGATCTCTCTCAACTTT | GCCAGTGTCAATTATATCTTCCACAA |
| ALP | ACAAGCA CTCCCACTTCATCTGGA | TCACGTTGTTCCTGTTCAGCTCGT |
| RUNX2 | GGAGTGGACGAGGCAAGAGTTT | AGCTTCTGTCTGTGCCTTCTGG |
| COL1 | GACATGCTCAGCTTTGTGGA | CTTTGTCCACGTGGTCCTCT |
| BSP | AACCTACAACCCCACCACAA | AGGTTCCCCGTTCTCACTTT |
| NOTCH1 | ATCCTGATCCGGAACCGAG | CGTCGTGCCATCATGCAT |
| HES-1 | ATGGAGAAAAATTCCTCGTCCC | TTCAGAGCATCCAAAATCAGTGT |
| HES-3 | CAT CAA TGT GTC ACT GGA GCA | CAA GGA GTT CTG AAG GCT TCT |
| HEY-1 | GAAACTTGAGTTCGGCTCTAGG | GCTTAGCAGATCCTTGCTCCAT |
| HEY-2 | AGGGGGTAAAGGCTACTTTGA | TGGCGCAAGTGCTGAGATG |
| HEY-L | ATG CAA GCC AGG AAG AAA CGC AGA | AGC TTG GAA GAG CCC TGT TTC TCA |
Acknowledgments
Supported by grants from the Oral Maxillofacial Surgery Foundation and from Children’s Healthcare of Atlanta Research Trust.
We would like to thank Manvinder Kumar and Melissa Oh for their help on this project. This work was supported by grants from the Oral Maxillofacial Surgery Foundation and from Children’s Healthcare of Atlanta Research Trust (SG) and NIH HL127236 (SG, AG, MD).
Footnotes
CONFLICT OF INTEREST
None.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44(Suppl 1):109–116. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Kim HJ, Ryoo HM. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 3.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007;24:613–618. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.de la Croix Ndong J, Stevens DM, Vignaux G, Uppuganti S, Perrien DS, Yang X, Nyman JS, Harth E, Elefteriou F. Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1Osx −/− mice. J Bone Miner Res. 2015;30:55–63. doi: 10.1002/jbmr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talley-Ronsholdt DJ, Lajiness E, Nagodawithana K. Transforming growth factor-beta inhibition of mineralization by neonatal rat osteoblasts in monolayer and collagen gel culture. In Vitro Cell Dev Biol Anim. 1995;31:274–282. doi: 10.1007/BF02634001. [DOI] [PubMed] [Google Scholar]
- 7.Im JY, Min WK, You C, Kim HO, Jin HK, Bae JS. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cells in scaffold. Laboratory Anim Res. 2013;29:196–203. doi: 10.5625/lar.2013.29.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu HC, ELL, Wang DS, Su F, Wu X, Shi ZP, Lv Y, Wang JZ. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide) Tissue Eng A. 2011;17:2417–2433. doi: 10.1089/ten.TEA.2010.0620. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto J, Naruse K, Nagai Y, Kan S, Nakamura N, Hata M, Omi M, Hayashi T, Kawai T, Matsubara T. Efficacy of a self-assembling peptide hydrogel, SPG-178-Gel, for bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng A. 2017 Jul 24; doi: 10.1089/ten.TEA.2017.0025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X, Smith RJ, Jr, Guan H, Ionita CN, Khobragade P, Dziak R, Liu Z, Pang M, Wang C, Guan G, Andreadis S, Yang S. Hybrid biomaterial with conjugated growth factors and mesenchymal stem cells for ectopic bone formation. Tissue Eng A. 2016;22:928–939. doi: 10.1089/ten.tea.2016.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitouni S, Krause U, Clough BH, Halderman H, Falster A, Blalock DT, Chaput CD, Sampson HW, Gregory CA. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med. 2012;4:132ra55. doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripamonti U, Ferretti C, Teare J, Blann L. Transforming growth factor-beta isoforms and the induction of bone formation: implications for reconstructive craniofacial surgery. J Craniofac Surg. 2009;20:1544–1555. doi: 10.1097/SCS.0b013e3181b09ca6. [DOI] [PubMed] [Google Scholar]
- 13.Bodalia PN, Balaji V, Kaila R, Wilson L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: A systematic review. Bone Joint Res. 2016;5:145–152. doi: 10.1302/2046-3758.54.2000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235–239. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14:552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 16.Habibovic P, de Groot K. Osteoinductive biomaterials–properties and relevance in bone repair. J Tissue Eng Regener Med. 2007;1:25–32. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 17.Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66–72. doi: 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247–2270. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tollemar V, Collier ZJ, Mohammed MK, Lee MJ, Ameer GA, Reid RR. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Gene Dis. 2016;3:56–71. doi: 10.1016/j.gendis.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosier CR, Uhrig BA, Willett NJ, Krishnan L, Li MT, Stevens HY, Schwartz Z, Boyan BD, Guldberg RE. Effect of cell origin and timing of delivery for stem cell-based bone tissue engineering using biologically functionalized hydrogels. Tissue Eng A. 2015;21:156–165. doi: 10.1089/ten.tea.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35:2443–2451. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- 23.Taichman DB, Loomes KM, Schachtner SK, Guttentag S, Vu C, Williams P, Oakey RJ, Baldwin HS. Notch1 and Jagged1 expression by the developing pulmonary vasculature. Dev Dyn. 2002;225:166–175. doi: 10.1002/dvdy.10146. [DOI] [PubMed] [Google Scholar]
- 24.Bales CB, Kamath BM, Munoz PS, Nguyen A, Piccoli DA, Spinner NB, Horn D, Shults J, Leonard MB, Grimberg A, Loomes KM. Pathologic lower extremity fractures in children with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;51:66–70. doi: 10.1097/MPG.0b013e3181cb9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys R, Zheng W, Prince LS, Qu X, Brown C, Loomes K, Huppert SS, Baldwin S, Goudy S. Cranial neural crest ablation of Jagged1 recapitulates the craniofacial phenotype of Alagille syndrome patients. Hum Mol Genet. 2012;21:1374–1383. doi: 10.1093/hmg/ddr575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Ping Y, Ma M, Zhang D, Liu C, Zaidi S, Gao S, Ji Y, Lou F, Yu F, Lu P, Stachnik A, Bai M, Wei C, Zhang L, Wang K, Chen R, New MI, Rowe DW, Yuen T, Sun L, Zaidi M. Anabolic actions of Notch on mature bone. Proc Natl Acad Sci U S A. 2016;113:E2152–E2161. doi: 10.1073/pnas.1603399113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill CR, Yuasa M, Schoenecker J, Goudy SL. Jagged1 is essential for osteoblast development during maxillary ossification. Bone. 2014;62:10–21. doi: 10.1016/j.bone.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan L, Priddy LB, Esancy C, Li MT, Stevens HY, Jiang X, Tran L, Rowe DW, Guldberg RE. Hydrogel-based delivery of rhBMP-2 improves healing of large bone defects compared with autograft. Clin Orthop Relat Res. 2015;473:2885–2897. doi: 10.1007/s11999-015-4312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, Garcia AJ. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krantz ID, Colliton RP, Genin A, Rand EB, Li L, Piccoli DA, Spinner NB. Spectrum and frequency of jagged1 (JAG1) mutations in Alagille syndrome patients and their families. Am J Hum Genet. 1998;62:1361–1369. doi: 10.1086/301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Long T, Wang C, Mirando AJ, Chen J, O’Keefe RJ, Hilton MJ. NOTCH-Mediated Maintenance and Expansion of Human Bone Marrow Stromal/Stem Cells: A Technology Designed for Orthopedic Regenerative Medicine. Stem Cells Transl Med. 2014;3:1456–1466. doi: 10.5966/sctm.2014-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia AJ. PEG-maleimide hydrogels for protein and cell delivery in regenerative medicine. Ann Biomed Eng. 2014;42:312–322. doi: 10.1007/s10439-013-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boopathy AV, Che PL, Somasuntharam I, Fiore VF, Cabigas EB, Ban K, Brown ME, Narui Y, Barker TH, Yoon YS, Salaita K, Garcia AJ, Davis ME. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials. 2014;35:8103–8112. doi: 10.1016/j.biomaterials.2014.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boopathy AV, Martinez MD, Smith AW, Brown ME, Garcia AJ, Davis ME. Intramyocardial delivery of notch ligand-containing hydrogels improves cardiac function and angiogenesis following infarction. Tissue Eng A. 2015;21:2315–2322. doi: 10.1089/ten.tea.2014.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34:4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dishowitz MI, Zhu F, Sundararaghavan HG, Ifkovits JL, Burdick JA, Hankenson KD. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J Biomed Mater Res A. 2014;102:1558–1567. doi: 10.1002/jbm.a.34825. [DOI] [PubMed] [Google Scholar]
- 39.Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35:5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Kucharski C, Doschak MR, Sebald W, Uludag H. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials. 2010;31:952–963. doi: 10.1016/j.biomaterials.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin NY, Distler A, Beyer C, Philipi-Schobinger A, Breda S, Dees C, Stock M, Tomcik M, Niemeier A, Dell’Accio F, Gelse K, Mattson MP, Schett G, Distler JH. Inhibition of Notch1 promotes hedgehog signalling in a HES1-dependent manner in chondrocytes and exacerbates experimental osteoarthritis. Ann Rheum Dis. 2016;75:2037–2044. doi: 10.1136/annrheumdis-2015-208420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.