Abstract
Superinfection is frequently detected among individuals infected by human immunodeficiency virus type I (HIV-1). Superinfection occurs at similar frequencies at acute and chronic infection stages but less frequently than primary infection. This observation indicates that the immune responses elicited by natural HIV-1 infection may play a role in curb of superinfection; however, these responses are not sufficiently strong to completely prevent superinfection. Thus, a successful HIV-1 vaccine likely needs to induce more potent and broader immune responses than those elicited by primary infection. On the other hand, potent and broad neutralization responses are more often detected after superinfection than during monoinfection. This suggests that broadly neutralizing antibodies are more likely induced by sequential immunization of multiple different immunogens than with only one form of envelope glycoprotein immunogens. Understanding why the protection from superinfection by immunity induced by primary infection is insufficient and if superinfection can lead to cross-reactive immune responses will be highly informative for HIV-1 vaccine design.
Keywords: human immunodeficiency virus type I, superinfection, incidence, immune response
Introduction
After infection by an HIV-1 strain, a person can be superinfected by a different HIV-1 strain [1–3]. Superinfection occurs in high-risk and general populations [4–10]. Frequent superinfection raises two important questions: (1) if primary infection induces protective immunity to reduce subsequent heterologous infection and (2) if superinfection leads to broad neutralization responses to heterologous viruses. Frequent superinfection suggests that immune responses induced by primary infection may not be strong or broad enough to prevent subsequent infections by heterologous viruses [7,11–13]. This notion was supported by a study which revealed that neutralizing antibody (nAb) responses were developed prior to superinfection during chronic infection [14]. However, a recent study showed that the polyfunctionality of CD4+ effector memory T cell responses contributed to the suppression of superinfection through mucosal transmission in a rhesus monkey model [15]. Thus far, no evidence was found to support the association of neutralization potency and breadth with the time lag between superinfection and primary infection, but the length of the time that an individual harbored two viruses was found to be correlated with neutralization breadth [16]. A number of other studies also showed that sequential infections led to the augmentation of the broad neutralization responses [16–18]. These observations show that studying superinfection may provide valuable insights into the potential mechanisms that contribute to development of neutralization breadth. Although immune responses can be fundamentally different between HIV-1 infection (damaged CD4+ T cell responses) and vaccination (intact immune system), understanding how superinfection occurs can have important implications in future HIV-1 vaccine design.
Detection of superinfection
Some HIV-1-infected individuals can be infected by two or more HIV-1 strains. Based on the timing of infection, infection with more than one HIV-1 strain can be defined as coinfection and superinfection. Coinfection is for a person who is infected by two different strains at the same time or who is infected by a second strain before development of immune responses against primary infection viruses. Superinfection is for a person who is infected by a heterologous HIV-1 strain after development of immune responses to the primary infection strain [19,20]. Superinfection can occur between two different strains from the same subtypes (intrasubtype) or between strains from different subtypes (intersubtype). The first intersubtype superinfection cases (subtype B and CRF01_AE) were found from two injection drug users (IDUs) in Thailand in 2002 [4]. The first intrasubtype superinfection case (subtype B) was reported in the USA also in 2002 [11]. Since then, superinfection has been reported from a variety of populations and modes of transmission, such as men who have sex with men (MSM) [21–25], IDUs [6,26], heterosexual transmission (HST) [10,27,28], female sex workers (FSWs) [28,29], mother-to-child transmission (MTCT) [30], and general populations [8,10]. More intersubtype superinfection had been reported than intrasubtype superinfection, possibly due to the easier detection of the intersubtype superinfection than intrasubtype superinfection. The variation of env sequences between different subtypes is as high as 30%, much higher than the variation among the viruses from the same subtype (less than10%) [31]. Because some methods are not sensitive enough to distinguish different HIV-1 strains from the same subtype, intrasubtype superinfection is more likely to be underestimated.
Superinfection is determined by detecting the presence of additional distinct viral genomes in an individual who has been infected by the first virus for some time during primary infection. The methods for the detection of superinfection have been improved significantly over the years (Table 1). In the early days, many methods with low sensitivity and low throughput were used, including restriction fragment length polymorphism (RFLP) [6], subtype-specific PCR amplification (ssPCR) [4], restriction fragment analysis, and direct sequencing [32]. Later on, population-based screening strategies were developed for superinfection detection, including the heteroduplex mobility assay (HMA) [31,33–35], the multiregion hybridization assay (MHA) [36], bulk viral sequence analysis, and selective cloning [22,36–38]. Although bulk PCR sequence analysis cannot unequivocally confirm the presence of two distinct HIV-1 strains, its results can indicate a high likelihood of superinfection [22]. Because population-based sequencing is less sensitive to the minority viral population (≤ 20%) than HMA, it underestimates the frequency of superinfection [36]. On the other hand, MHA can only detect intersubtype superinfection and misses the intrasubtype superinfection. HMA, which is sensitive for < 5% minority viral populations, can be used to identify intrasubtype superinfection but often causes false positive results [39]. All these methods require confirmation by cloning and sequencing.
Table 1.
Methods for the identification of superinfection
| Method | Advantage | Limitation |
|---|---|---|
| RFLP | Easy | Low sensitivity |
| Subtype-specific PCR | Low cost | Low throughput |
| MHA | Large-scale screening | Low sensitivity, detect intersubtype SI only |
| Multiple regions | Cloning confirmation | |
| HMA | ≥ 1.5% differences in pairwise distance | False positives |
| Identify intrasubtype SI | Cloning confirmation | |
| Population-based sequencing | Prediction by degenerate base codes | Less sensitive to minority viral population |
| Initial screening method | ||
| Bulk PCR + selective cloning | Improved sensitivity | Expensive |
| Sequences for analysis | Labor intensive | |
| Amount of sequences | ||
| Genomic regions | ||
| Cannot unequivocally confirm | ||
| SGS | More sensitive and accurate than other methods | Expensive |
| No PCR error, bias, and recombination | Labor intensive | |
| NGS | Highly sensitive, 0.25% | Short read length |
| High throughput | Low accuracy in fragment assembly | |
| Multiple regions | Sophisticated data analysis procedure |
Recently, more sensitive and accurate methods have been used to detect superinfection (Table 1). Limiting dilution PCR and single genome sequencing (SGS) methods can determine distinct viral populations in samples [40–43]. However, both methods are costly and labor intensive due to the amount of sequences required [39–41]. The next-generation sequencing (NGS) method is very sensitive for the detection of minority variants, as low as 0.25% of the population in samples. The use of sequence identification tags has significantly reduced the cost and increased the throughput of the NGS method [44–46]. Thus, it has been widely used to detect superinfection in a large number of samples by analyzing multiple genome regions to determine the incidence of superinfection [47]. Moreover, NGS can detect different subtypes or different strains from the same subtype present in 1%–5% of the viral populations [8]. In an MSM acute HIV-1 infection cohort, 15.6% of the cases were found to be superinfected by NGS. Among them, 60% of the superinfected cases were between CRF01_AE and CRF07_BC or subtype B, whereas the other 40% were intrasubtype superinfections [48]. In a cohort of high-risk women in Kenya, 7% of the cases were found to be superinfected by analyzing three genomic regions using the NGS method [47]. Among them, 33.3% were intersubtype superinfections, whereas 66.7% were intrasubtype superinfections. These results demonstrated that NGS can sensitively detect both intersubtype and intrasubtype superinfections and has been increasingly used for the detection of superinfected cases.
Other than assay sensitivity, the size and number of genome regions used for analysis also have a significant impact on the detection of superinfection. The analysis of only small genome regions may lead to an underestimation of the number of superinfected cases [31,34,40]. For example, the examination of additional gag gene sequences identified two additional superinfected cases among 14 high-risk Kenyan women from whom only env gene sequences were initially analyzed [41].
Accurate determination of superinfection incidence
The frequency of superinfection varied significantly among different cohorts (from 1.5% to 19.4%), and the incidence of superinfection also varied from 1.44 to 19.6 per 100 person-years (Table 2). These observations suggest that the incidence of superinfection can be significantly affected by many factors in study cohorts. It also raises a question whether current data reflect the real superinfection frequencies. To precisely determine the frequency of superinfection, some key factors should be well controlled in the cohort design. First, longitudinal cohorts are required for the detection of superinfection. In crosssectional studies, dual infection can be detected, but the timing of infection for each strain cannot be determined. Therefore, superinfection can not be distinguised from coinfection [49,50]. Thus, superinfection can only be clearly defined through the long-term follow-up of HIV-1- infected individuals. The length of follow-up is also an important factor. Superinfection may occur soon after primary infection or during chronic infection [51]. Superinfection can be more frequently detected in a cohort with a longer follow-up period than a shorter one. Second, sampling frequency is critical in longitudinal studies [40]. If intervals are short (e.g., weeks) during follow-up, superinfection can be accurately and timely detected [21,40]. If intervals are long (e.g., months or years), superinfection may be missed due to the rapid recombination between primary and superinfected viruses [35,52–55], which can lead to the misclassification of the superinfection as monoinfection with divergent viruses. Therefore, the incidence or prevalence of superinfection can be underestimated in low-frequency sampling cohorts and might be the reason for the nondetection of superinfection in some studies [34,35,37,56,57]. Moreover, individuals in cohorts should not be on antiretroviral therapy. Ongoing antiretroviral therapy is likely to protect an individual from being infected by new viruses and results in low superinfection frequencies [58–60].
Table 2.
Identification of intrasubtype and intersubtype superinfection among different cohorts
| Cohort | Year | Population | Method | No. of intersubtype/ intrasubtype |
Frequency | Incidence (100 person-years) |
Reference |
|---|---|---|---|---|---|---|---|
| Primary infection cohort, Shenyang, China | 2008–2013 | MSM | NGS | 6/4 | 15.6% | 19.6 | Luan et al. [48] |
| AIEDRP, San Diego and Los Angeles, USA | 1997–2003 | MSM | Population-based sequencing, cloning | 0/3 | 3.8% | 5 | Smith et al. [22] |
| Primary infection cohort, San Diego, USA | 1998–2007 | MSM | NGS | 10/0 | 8.4% | 4.96 | Wagner et al. [21] |
| Mombasa, Kenya | 1993–2000 | HST | HMA cloning | 3/0 | 14.3% | 4.3 | Chohan et al. [31] |
| Mombasa, Kenya | 1993–2004 | HST | ssPCR, SGS | 4/3 | 19.4% | 3.7 | Piantadosi et al. [40] |
| Mombasa, Kenya | 1993–2000 | HST | SGS | 1/1 | 14.3% | 7.7 | Piantadosi et al. [41] |
| Mombasa, Kenya | 1993–2008 | HST | Sanger sequencing | 11/10 | 14.4% | 2.61 | Ronen et al. [47] |
| NGS | |||||||
| Rakai community cohort study | 2002–2005 | HST | NGS | 2/0 | 18.2% | NA | Redd et al. [8] |
| Rakai, Uganda | 1998–2009 | HST | NGS | 3/4 | 4.7% | 1.44 | Redd et al. [10] |
| Durban, South Africa | 2004–2005 | HST | SGS | 0/3 | 9.3% | NA | Sheward et al. [43] |
| Seattle primary infection cohort and FSWs in the South African | 2008–2009 | FSW | HMA, cloning | 0/1 | 1.6% | NA | Gottlieb et al. [33] |
| Bangkok, Thailand | 1995–1996 | IDU | RFLP, ssPCR | 2/0 | 1.5% | 2.2 | Ramos et al. [4] |
| KwaZulu-Natal, South Africa | 2007–2010 | HST | NGS | 0/2 | 2.6% | 1.5 | Redd et al. [28] |
NA, not available.
Superinfection and preexisting immune responses
One critical question is whether weak immune responses against HIV-1 during monoinfection lead to superinfection. HIV-1 superinfection provides a unique opportunity to investigate the relationship between superinfection and preexisting immune responses. nAbs have long been thought to play an important role in the protection from HIV-1 infection [61–63]. The absence of potent nAb responses, especially cross-reactive nAb responses against heterologous viruses, probably predisposes an HIV-1- infected individual to superinfection. Weaker crossprotective and autologous nAb responses were detected in three intrasubtype B superinfected cases before the occurrence of superinfection than in monoinfection controls in the Acute Infection Early Disease Research Program (AIEDRP) in San Diego and Los Angeles, USA [7]. In a subsequent study, the same group further confirmed their findings with a larger number of samples. Ten intrasubtype B superinfected cases had relatively weaker nAb responses against the autologous virus and two neutralization-sensitive heterologous viruses compared to 19 monoinfection controls [64]. An intrasubtype C superinfection study in a discordant couple cohort in Zambia showed that three subtype C intrasubtype superinfected cases had weaker nAb responses against their baseline autologous viruses prior to superinfection compared with monoinfection controls within one year of primary infection [12]. Moreover, antibody-dependent cellular cytotoxicity responses in three intrasubtype superinfected cases were low prior to superinfection [13]. These studies suggest that a relatively weak nAb response is present in superinfected cases within the first year of HIV-1 infection.
Broadly neutralizing antibodies (bnAbs), which are believed to be key in preventing heterologous superinfection, usually take 2–4 years to develop. Because bnAbs are unlikely to develop within one year of infection [65,66,67], understanding whether superinfection is affected during chronic infection in which bnAbs are more likely to be elicited is important. In a high-risk women cohort, nAb responses were examined in six individuals who were superinfected by second strains between 1 and 5 years after the initial infection as well as 18 monoinfection women with similar risk factors [14]. The superinfected women had less neutralization breadth than matched controls at approximately 1 year post infection, but the breadth or potency of nAb responses was not significantly different from the control group just prior to superinfection. In fact, four of the six subjects had relatively broad and potent nAb responses prior to infection by additional strains. Importantly, the detection of nAbs against superinfection viruses in most superinfected individuals prior to their infection suggested that the level of nAbs elicited during primary infection was insufficient to block superinfection. These data indicate that preventing HIV-1 infection by a vaccine will likely require broader and more potent nAb responses than those found during primary HIV-1 infection.
Evidence supporting protection from superinfection by T cell responses is still scarce. In a recent rhesus monkey mucosal superinfection study, the polyfunctionality of CD4+ effector memory T cells, but not nAb responses, was shown to correlate with protection against superinfection [15]. Notably, virus-associated effector memory T cell responses might contribute to the suppression of superinfection. However, in another macaque model study, a series of SIVmac239 CD8+ T cell escape variants failed to infect long-term nonprogressors (LTNP) that had robust SIV-specific CD8+ and CD4+ T responses as well as neutralizing antibodies [68]. In another study, preexisting HIV-1-specific CD8+ T cell responses in one individual failed to protect the host from superinfection [11]. Similar HIV-1-specific cellular immune responses induced during chronic infection also did not appear to significantly contribute to protection from HIV-1 superinfection in a case-control study of high-risk women in Kenyan [69]. Taken together, more studies are warranted to better understand the role of cellular immune responses in preventing subsequent superinfection.
Other immunological factors have also been studied for their potential effects on superinfection. Antibody-dependent cell-mediated virus inhibition activity and frequencies of T cell activation markers were comparable between superinfection and monoinfection cases [70,71]. When immune functions in both plasma and cervical swab supernatants were examined to assess systemic and mucosal responses, no significant associations were detected between any of the immune functions and superinfection acquisition in a sex worker cohort in Kenya [72]. Another study showed that human leukocyte antigen alleles were found to be correlated with the differential superinfection risk in a recent MSM cohort in the USA [73].
Recombination between primary infection viruses and superinfection viruses can be rapidly detected, and recombinants could quickly replace the parental viruses soon after superinfection under the selection of T cell or nAb pressure [35,52–55]. Thus, the rapid recombination between superinfection and primary infection viruses may allow recombinants to quickly escape the preexisting immune responses and be an important cause for the second virus to successfully establish superinfection. However, such recombinant events will not be detected if the genome regions not under selection pressure are used for analysis.
In summary, immune responses elicited by primary HIV-1 infection might have limited impact on superinfection. More potent and broader immune responses than those elicited during monoinfection may be needed for an effective HIV-1 vaccine. However, given the small numbers of cases studied in each of these cohorts and the use of different panels of viruses to determine neutralization activities, well-controlled cohorts with large numbers of cases should be studied to better understand the roles of immune responses in the prevention of superinfection.
Superinfection at different infection stages
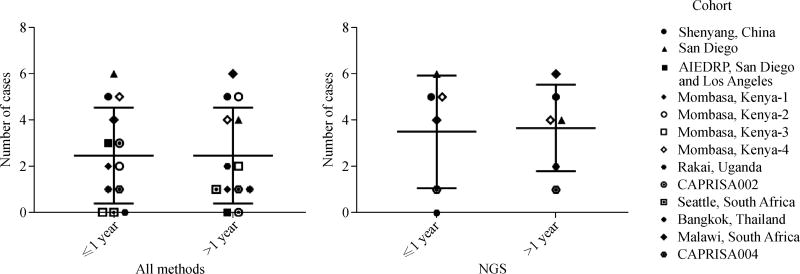
The determination of the timing of superinfection is important for our understanding of the roles of immune responses induced by primary infection in preventing superinfection. A hypothesis is that superinfection occurs more easily at the early infection stage because of weak immunity. This hypothesis was initially supported by some case reports and cohort studies showing superinfection within one year after initial infection [4,7,12,22,28,39,42,64]. However, later studies showed that the number of superinfection within one year after the initial infection was similar to that after one year of infection [11,19,41,51,74]. Moreover, in studies for which superinfection was determined by NGS, no significant differences in superinfection frequencies between acute and chronic infection stages were observed [21,23,40,47,64]. Results from these studies indicate that the immune responses elicited by HIV-1 infection during early and late infection stages similarly failed to protect superinfection (Fig. 1).
Fig. 1.
Comparison of superinfection frequencies at different infection stages. The numbers of superinfected cases detected within one year and after one year were compared when all methods (left) or only NGS method (right) was used (P = 1.0 for both comparisons). Each cohort is indicated by a distinct symbol.
Incidences of superinfection and primary infections
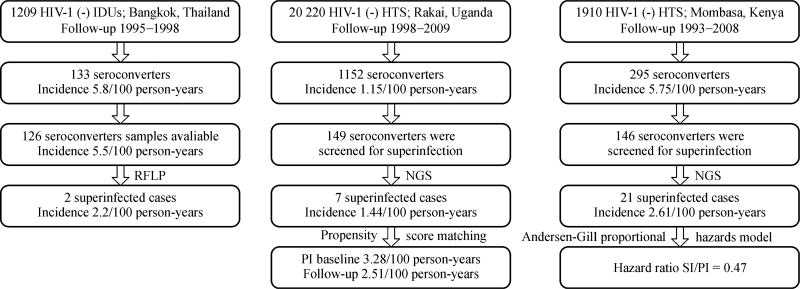
A comparison of incidences of superinfection and primary infections is important to understand whether immune responses elicited by primary infection can be protective from subsequent superinfection. Several studies showed that the incidence of superinfection is lower than that of primary infection. An earlier study using RFLP and subtype-specific primer assays found that the incidence of superinfection (intersubtype) was less than half of that of primary infection (2.2/100 person-years versus 5.8/100 person-years) in an IDU cohort in Thailand [6]. The result from a high-risk women cohort in Kenya also showed that the incidence of superinfection (intersubtype and intrasubtype) was lower than that of primary infection after adjusting variables (such as self-reported sexual risk behavior, place of work, hormonal contraceptive use, genital tract infections, years in sex work, age at first sex, follow-up time in the cohort, and calendar year) previously shown to influence HIV-1 exposure risk. After adjusting these variables, the hazard ratio for superinfection compared with primary infection was 0.47 [47]. Another study on a recent seroconverter cohort from a general population in Uganda also showed that the incidence of superinfection (intersubtype and intrasubtype) was slightly lower than the primary infection incidence at baseline (P = 0.07), but comparable with the primary HIV incidence during follow-up after controlling for differences in sociodemographic and behavioral characteristics [10]. Results from an MSM cohort in the USA showed that the intrasubtype superinfection incidence was similar to that of primary infection [22]. Although the rates of superinfection and primary infection are not consistent among different studies, after the use of a more sensitive NGS method and multiple proper adjustments, the incidence of superinfection is approximately half of the incidence of primary infection (Fig. 2), suggesting that primary infection is only partially protective of superinfection.
Fig. 2.
Comparison of superinfection and primary infection incidence rates.
In summary, results on the incidences of superinfection and primary infection suggest that immune responses elicited by primary infection are only partially protective and not effective enough in fully preventing later superinfection. The comparison of incidences between superinfection and primary infections in a population is very difficult. Except for the methods used, sociodemographic and behavioral factors will also have significant impact on the frequency of superinfection. Superinfected individuals are inherently at a higher risk than people in the control group [10]. Moreover, behavior changes can also significantly affect the incidence. For example, the absence of superinfection one year after infection between 1985 and 1997 coincides with a reduction in sexual risk behavior in the seroincidence in an MSM cohort in Amsterdam [35,37]. Thus, the comparison between incidences of superinfection and primary infections should take into account all major risk factors to ensure that real differences are properly determined in future studies.
Broader immune responses after superinfection
Another important question is whether superinfection can result in broad neutralization activity because the presence of different envelope glycoproteins (Env) from multiple variants may have a better chance to trigger more B cell lineages for broad neutralization responses than a single form of Env [75 – 77]. Viral diversity has been found to be associated with the development of broad neutralization activity [16,53,55,67,78,79]. Dual infection resulted in a significant increase in the neutralization capacity when compared with monoinfection controls within a comparable study period [17]. In a seroincident cohort of high-risk women in Kenya, 12 superinfected individuals were tested for neutralization activities against a panel of eight viruses at matched time points post-superinfection (~5 years postinitial infection) [18]. Superinfected individuals demonstrated significantly broader neutralization responses postsuperinfection when compared with monoinfection individuals. This observation remained the same even after controlling for neutralization breadth developed prior to superinfection, contemporaneous CD4+ T cell counts, and viral loads. This finding suggests that sequential infections may lead to the augmentation of broad neutralization activities. The same group further confirmed this observation by studying nine additional superinfected individuals in the setting of controlling other factors that could potentially influence nAb responses [16]. The results also indicated that the length of time after superinfection could play an important role in development of neutralization breadth. The longer an individual is infected with multiple viruses, the broader nAb responses will develop. In addition, a case study showed that an individual who was superinfected three months post-primary infection mounted broad and potent neutralization responses during three years of post-superinfection [66]. The successful isolation of bnAbs from this individual further demonstrated that bnAbs were indeed elicited [55]. In another study of a superinfected HIV-1 LTNP elite controller, cytotoxic T lymphocyte responses after superinfection were maintained in two samples separated by 9 years, and they were broad and potent [80]. This study demonstrated that strong and sustained cellular and humoral immune responses as well as low replicating viruses are associated with viral control in the superinfected LTNP elite controller. However, broad neutralization activities were not detected in some superinfected cases [52]. It seems that the timing of superinfection and genetic characteristics (diversity, length variation, potential glycosylation site, and others) of superinfection viruses might contribute to development of broad and potent nAb responses [52]. These observations indicate that multiple immunogen variants may be needed to induce cross-reactive immune responses to diverse heterologous viruses.
Summary
Weak immune responses before superinfection and similar incidence of superinfection during acute and chronic infections suggest that immune responses elicited by primary HIV-1 infection are not strong enough to confer protection from superinfection by subsequent heterologous viruses. This finding suggests that hosts with severely damaged CD4+ T cell populations during HIV-1 infection may not be able to mount protective immune responses when they encounter additional viruses, although both T and B cell immune responses are detectable when superinfection occurs. The ineffectiveness to prevent superinfection by primary infection indicates that an HIV-1 vaccine needs to induce more potent and broader immune responses than those elicited by primary HIV-1 infection. However, because healthy people when vaccinated have intact immune system, an HIV-1 vaccine that can induce similar immune responses as those detected in primary infection individuals may be effective to prevent HIV-1 infection. More potent and broader nAb responses detected in superinfection individuals than monoinfection subjects suggest that bnAbs are more likely to be induced by immunization of multiple different immunogens, sequentially or combinationally. Whether superinfection occurs after HIV-1-infected individuals have developed broad neutralization activities and how broadly neutralizing antibodies are induced after superinfection are important for future studies. Well-defined longitudinal cohorts with frequent sampling and standardized assays should be carried out to more precisely identify correlates between superinfection and status of immune responses as well as roles of innate immune responses in protecting superinfection.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31670162 and 81371787), Program for JLU Science and Technology Innovative Research Team (No. 2017TD-05), and a grant from NIAID of NIH (No. AI118571).
Footnotes
Compliance with ethics guidelines Yang Gao, Wen Tian, Xiaoxu Han, and Feng Gao declare no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- 1.Allen TM, Altfeld M. HIV-1 superinfection. J Allergy Clin Immunol. 2003;112(5):829–835. doi: 10.1016/j.jaci.2003.08.037. quiz 836. [DOI] [PubMed] [Google Scholar]
- 2.Smith DM, Richman DD, Little SJ. HIV superinfection. J Infect Dis. 2005;192(3):438–444. doi: 10.1086/431682. [DOI] [PubMed] [Google Scholar]
- 3.Waters L, Smit E. HIV-1 superinfection. Curr Opin Infect Dis. 2012;25(1):42–50. doi: 10.1097/QCO.0b013e32834ef5af. [DOI] [PubMed] [Google Scholar]
- 4.Ramos A, Hu DJ, Nguyen L, Phan KO, Vanichseni S, Promadej N, Choopanya K, Callahan M, Young NL, McNicholl J, Mastro TD, Folks TM, Subbarao S. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J Virol. 2002;76(15):7444–7452. doi: 10.1128/JVI.76.15.7444-7452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manigart O, Courgnaud V, Sanou O, Valéa D, Nagot N, Meda N, Delaporte E, Peeters M, Van de Perre P. HIV-1 superinfections in a cohort of commercial sex workers in Burkina Faso as assessed by an autologous heteroduplex mobility procedure. AIDS. 2004;18(12):1645–1651. doi: 10.1097/01.aids.0000131333.30548.db. [DOI] [PubMed] [Google Scholar]
- 6.Hu DJ, Subbarao S, Vanichseni S, Mock PA, Ramos A, Nguyen L, Chaowanachan T, Griensven Fv, Choopanya K, Mastro TD, Tappero JW. Frequency of HIV-1 dual subtype infections, including intersubtype superinfections, among injection drug users in Bangkok, Thailand. AIDS. 2005;19(3):303–308. [PubMed] [Google Scholar]
- 7.Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, Liu Y, Petropolous CJ, Daar ES, Little SJ, Richman DD. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355(1):1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Redd AD, Collinson-Streng A, Martens C, Ricklefs S, Mullis CE, Manucci J, Tobian AA, Selig EJ, Laeyendecker O, Sewankambo N, Gray RH, Serwadda D, Wawer MJ, Porcella SF, Quinn TC Rakai Health Sciences Program. Identification of HIV superinfection in seroconcordant couples in Rakai, Uganda, by use of next-generation deep sequencing. J Clin Microbiol. 2011;49(8):2859–2867. doi: 10.1128/JCM.00804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redd AD, Ssemwanga D, Vandepitte J, Wendel SK, Ndembi N, Bukenya J, Nakubulwa S, Grosskurth H, Parry CM, Martens C, Bruno D, Porcella SF, Quinn TC, Kaleebu P. Rates of HIV-1 superinfection and primary HIV-1 infection are similar in female sex workers in Uganda. AIDS. 2014;28(14):2147–2152. doi: 10.1097/QAD.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, Tobian AA, Xiao C, Grabowski MK, Nalugoda F, Kigozi G, Laeyendecker O, Kagaayi J, Sewankambo N, Gray RH, Porcella SF, Wawer MJ, Quinn TC. The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda. J Infect Dis. 2012;206(2):267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O’Connor DH, Davis BT, Lee PK, Maier EL, Harlow J, Goulder PJ, Brander C, Rosenberg ES, Walker BD. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420(6914):434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 12.Basu D, Kraft CS, Murphy MK, Campbell PJ, Yu T, Hraber PT, Irene C, Pinter A, Chomba E, Mulenga J, Kilembe W, Allen SA, Derdeyn CA, Hunter E. HIV-1 subtype C superinfected individuals mount low autologous neutralizing antibody responses prior to intrasubtype superinfection. Retrovirology. 2012;9(1):76. doi: 10.1186/1742-4690-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu D, Xiao P, Ende Z, Bere A, Britt WJ, Mulenga J, Kilembe W, Allen SA, Derdeyn CA, Hunter E. Low antibody-dependent cellular cytotoxicity responses in Zambians prior to HIV-1 intrasubtype C superinfection. Virology. 2014;462–463:295–298. doi: 10.1016/j.virol.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blish CA, Dogan OC, Derby NR, Nguyen MA, Chohan B, Richardson BA, Overbaugh J. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82(24):12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun M, Zheng H, Xie Y, Li B, Long H, Guo G, Guo L, Wang J, Ning R, Li Y, Liu L. Functional effector memory T cells contribute to protection from superinfection with heterologous simian immunodeficiency virus or simian-human immunodeficiency virus isolates in Chinese rhesus macaques. Arch Virol. 2017;162(5):1211–1221. doi: 10.1007/s00705-017-3222-7. [DOI] [PubMed] [Google Scholar]
- 16.Cortez V, Wang B, Dingens A, Chen MM, Ronen K, Georgiev IS, McClelland RS, Overbaugh J. The broad neutralizing antibody responses after HIV-1 superinfection are not dominated by antibodies directed to epitopes common in single infection. PLoS Pathog. 2015;11(7):e1004973. doi: 10.1371/journal.ppat.1004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell RL, Kinge T, Nyambi PN. Infection by discordant strains of HIV-1 markedly enhances the neutralizing antibody response against heterologous virus. J Virol. 2010;84(18):9415–9426. doi: 10.1128/JVI.02732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortez V, Odem-Davis K, McClelland RS, Jaoko W, Overbaugh J. HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response. PLoS Pathog. 2012;8(3):e1002611. doi: 10.1371/journal.ppat.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jost S, Bernard MC, Kaiser L, Yerly S, Hirschel B, Samri A, Autran B, Goh LE, Perrin L. A patient with HIV-1 superinfection. N Engl J Med. 2002;347(10):731–736. doi: 10.1056/NEJMoa020263. [DOI] [PubMed] [Google Scholar]
- 20.van der Kuyl AC, Cornelissen M. Identifying HIV-1 dual infections. Retrovirology. 2007;4(1):67. doi: 10.1186/1742-4690-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner GA, Pacold ME, Kosakovsky Pond SL, Caballero G, Chaillon A, Rudolph AE, Morris SR, Little SJ, Richman DD, Smith DM. Incidence and prevalence of intrasubtype HIV-1 dual infection in at-risk men in the United States. J Infect Dis. 2014;209(7):1032–1038. doi: 10.1093/infdis/jit633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Daar ES, Richman DD, Little SJ. Incidence of HIV superinfection following primary infection. JAMA. 2004;292(10):1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- 23.Bezemer D, van Sighem A, deWolf F, Cornelissen M, van der Kuyl AC, Jurriaans S, van der Hoek L, Prins M, Coutinho RA, Lukashov VV. Combination antiretroviral therapy failure and HIV superinfection. AIDS. 2008;22(2):309–311. doi: 10.1097/QAD.0b013e3282f37489. [DOI] [PubMed] [Google Scholar]
- 24.Sidat MM, Mijch AM, Lewin SR, Hoy JF, Hocking J, Fairley CK. Incidence of putative HIV superinfection and sexual practices among HIV-infected men who have sex with men. Sex Health. 2008;5(1):61–67. doi: 10.1071/sh07041. [DOI] [PubMed] [Google Scholar]
- 25.Wagner GA, Pacold ME, Vigil E, Caballero G, Morris SR, Kosakovsky Pond SL, Little SJ, Richman DD, Gianella S, Smith DM. Using ultradeep pyrosequencing to study HIV-1 coreceptor usage in primary and dual infection. J Infect Dis. 2013;208(2):271–274. doi: 10.1093/infdis/jit168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Promadej-Lanier N, Thielen C, Hu DJ, Chaowanachan T, Gvetadze R, Choopanya K, Vanichseni S, McNicholl JM. Cross-reactive T cell responses in HIV CRF01_AE and B′-infected intravenous drug users: implications for superinfection and vaccines. AIDS Res Hum Retroviruses. 2009;25(1):73–81. doi: 10.1089/aid.2008.0169. [DOI] [PubMed] [Google Scholar]
- 27.McCutchan FE, Hoelscher M, Tovanabutra S, Piyasirisilp S, Sanders-Buell E, Ramos G, Jagodzinski L, Polonis V, Maboko L, Mmbando D, Hoffmann O, Riedner G, von Sonnenburg F, Robb M, Birx DL. In-depth analysis of a heterosexually acquired human immunodeficiency virus type 1 superinfection: evolution, temporal fluctuation, and intercompartment dynamics from the seronegative window period through 30 months postinfection. J Virol. 2005;79(18):11693–11704. doi: 10.1128/JVI.79.18.11693-11704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redd AD, Mullis CE, Wendel SK, Sheward D, Martens C, Bruno D, Werner L, Garrett NJ, Abdool Karim Q, Williamson C, Porcella SF, Quinn TC, Abdool Karim SS. Limited HIV-1 superinfection in seroconverters from the CAPRISA 004 Microbicide Trial. J Clin Microbiol. 2014;52(3):844–848. doi: 10.1128/JCM.03143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grobler J, Gray CM, Rademeyer C, Seoighe C, Ramjee G, Karim SA, Morris L, Williamson C. Incidence of HIV-1 dual infection and its association with increased viral load set point in a cohort of HIV-1 subtype C-infected female sex workers. J Infect Dis. 2004;190(7):1355–1359. doi: 10.1086/423940. [DOI] [PubMed] [Google Scholar]
- 30.Redd AD, Wendel SK, Longosz AF, Fogel JM, Dadabhai S, Kumwenda N, Sun J, Walker MP, Bruno D, Martens C, Eshleman SH, Porcella SF, Quinn TC, Taha TE. Evaluation of postpartum HIV superinfection and mother-to-child transmission. AIDS. 2015;29(12):1567–1573. doi: 10.1097/QAD.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chohan B, Lavreys L, Rainwater SM, Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J Virol. 2005;79(16):10701–10708. doi: 10.1128/JVI.79.16.10701-10708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redd AD, Quinn TC, Tobian AA. Frequency and implications of HIV superinfection. Lancet Infect Dis. 2013;13(7):622–628. doi: 10.1016/S1473-3099(13)70066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb GS, Nickle DC, Jensen MA, Wong KG, Grobler J, Li F, Liu SL, Rademeyer C, Learn GH, Karim SS, Williamson C, Corey L, Margolick JB, Mullins JI. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363(9409):619–622. doi: 10.1016/S0140-6736(04)15596-7. [DOI] [PubMed] [Google Scholar]
- 34.Tsui R, Herring BL, Barbour JD, Grant RM, Bacchetti P, Kral A, Edlin BR, Delwart EL. Human immunodeficiency virus type 1 superinfection was not detected following 215 years of injection drug user exposure. J Virol. 2004;78(1):94–103. doi: 10.1128/JVI.78.1.94-103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rachinger A, Stolte IG, van de Ven TD, Burger JA, Prins M, Schuitemaker H, van’t Wout AB. Absence of HIV-1 superinfection 1 year after infection between 1985 and 1997 coincides with a reduction in sexual risk behavior in the seroincident Amsterdam cohort of homosexual men. Clin Infect Dis. 2010;50(9):1309–1315. doi: 10.1086/651687. [DOI] [PubMed] [Google Scholar]
- 36.Rachinger A, van de Ven TD, Burger JA, Schuitemaker H, van’t Wout AB. Evaluation of pre-screening methods for the identification of HIV-1 superinfection. J Virol Methods. 2010;165(2):311–317. doi: 10.1016/j.jviromet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Rachinger A, Manyenga P, Burger JA, Derks van de Ven TL, Stolte IG, Prins M, van’t Wout AB, Schuitemaker H. Low incidence of HIV-1 superinfection even after episodes of unsafe sexual behavior of homosexual men in the Amsterdam Cohort Studies on HIV Infection and AIDS. J Infect Dis. 2011;203(11):1621–1628. doi: 10.1093/infdis/jir164. [DOI] [PubMed] [Google Scholar]
- 38.Templeton AR, Kramer MG, Jarvis J, Kowalski J, Gange S, Schneider MF, Shao Q, Zhang GW, Yeh MF, Tsai HL, Zhang H, Markham RB. Multiple-infection and recombination in HIV-1 within a longitudinal cohort of women. Retrovirology. 2009;6(1):54. doi: 10.1186/1742-4690-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koelsch KK, Smith DM, Little SJ, Ignacio CC, Macaranas TR, Brown AJ, Petropoulos CJ, Richman DD, Wong JK. Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. AIDS. 2003;17(7):F11–F16. doi: 10.1097/00002030-200305020-00001. [DOI] [PubMed] [Google Scholar]
- 40.Piantadosi A, Chohan B, Chohan V, McClelland RS, Overbaugh J. Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog. 2007;3(11):e177. doi: 10.1371/journal.ppat.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piantadosi A, Ngayo MO, Chohan B, Overbaugh J. Examination of a second region of the HIV type 1 genome reveals additional cases of superinfection. AIDS Res Hum Retroviruses. 2008;24(9):1221–1224. doi: 10.1089/aid.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft CS, Basu D, Hawkins PA, Hraber PT, Chomba E, Mulenga J, Kilembe W, Khu NH, Derdeyn CA, Allen SA, Manigart O, Hunter E. Timing and source of subtype-C HIV-1 superinfection in the newly infected partner of Zambian couples with disparate viruses. Retrovirology. 2012;9(1):22. doi: 10.1186/1742-4690-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheward DJ, Ntale R, Garrett NJ, Woodman ZL, Abdool Karim SS, Williamson C. HIV-1 superinfection resembles primary infection. J Infect Dis. 2015;212(6):904–908. doi: 10.1093/infdis/jiv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapland EB, Holmes V, Reeves CD, Sorokin E, Durot M, Platt D, Allen C, Dean J, Serber Z, Newman J, Chandran S. Low-cost, high-throughput sequencing of DNA assemblies using a highly multiplexed nextera process. ACS Synth Biol. 2015;4(7):860–866. doi: 10.1021/sb500362n. [DOI] [PubMed] [Google Scholar]
- 45.Mild M, Hedskog C, Jernberg J, Albert J. Performance of ultra-deep pyrosequencing in analysis of HIV-1 pol gene variation. PLoS One. 2011;6(7):e22741. doi: 10.1371/journal.pone.0022741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kijak GH, Sanders-Buell E, Harbolick EA, Pham P, Chenine AL, Eller LA, Rono K, Robb ML, Michael NL, Kim JH, Tovanabutra S. Targeted deep sequencing of HIV-1 using the IonTorrentPGM platform. J Virol Methods. 2014;205:7–16. doi: 10.1016/j.jviromet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronen K, McCoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, Jaoko W, Mandaliya K, McClelland RS, Richardson BA, Overbaugh J. HIV-1 superinfection occurs less frequently than initial infection in a cohort of high-risk Kenyan women. PLoS Pathog. 2013;9(8):e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luan H, Han X, Yu X, An M, Zhang H, Zhao B, Xu J, Chu Z, Shang H. Dual infection contributes to rapid disease progression in men who have sex with men in China. J Acquir Immune Defic Syndr. 2017;75(4):480–487. doi: 10.1097/QAI.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soares de Oliveira AC, Pessôa de Farias R, da Costa AC, Sauer MM, Bassichetto KC, Oliveira SM, Costa PR, Tomiyama C, Tomiyama HT, Sabino EC, Kallas EG, Sanabani SS. Frequency of subtype B and F1 dual infection in HIV-1 positive, Brazilian men who have sex with men. Virol J. 2012;9(1):223. doi: 10.1186/1743-422X-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chohan BH, Piantadosi A, Overbaugh J. HIV-1 superinfection and its implications for vaccine design. Curr HIV Res. 2010;8(8):596–601. doi: 10.2174/157016210794088218. [DOI] [PubMed] [Google Scholar]
- 51.Yerly S, Jost S, Monnat M, Telenti A, Cavassini M, Chave JP, Kaiser L, Burgisser P, Perrin L Swiss HIV Cohort Study. HIV-1 co/super-infection in intravenous drug users. AIDS. 2004;18(10):1413–1421. doi: 10.1097/01.aids.0000131330.28762.0c. [DOI] [PubMed] [Google Scholar]
- 52.Courtney CR, Mayr L, Nanfack AJ, Banin AN, Tuen M, Pan R, Jiang X, Kong XP, Kirkpatrick AR, Bruno D, Martens CA, Sykora L, Porcella SF, Redd AD, Quinn TC, Nyambi PN, Dürr R. Contrasting antibody responses to intrasubtype superinfection with CRF02_AG. PLoS One. 2017;12(3):e0173705. doi: 10.1371/journal.pone.0173705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ritchie AJ, Cai F, Smith NM, Chen S, Song H, Brackenridge S, Abdool Karim SS, Korber BT, McMichael AJ, Gao F, Goonetelleke N. Recombination-mediated escape from primary CD8+ T cells in acute HIV-1 infection. Retrovirology. 2014;11:69. doi: 10.1186/s12977-014-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Streeck H, Li B, Poon AF, Schneidewind A, Gladden AD, Power KA, Daskalakis D, Bazner S, Zuniga R, Brander C, Rosenberg ES, Frost SD, Altfeld M, Allen TM. Immune-driven recombination and loss of control after HIV superinfection. J Exp Med. 2008;205(8):1789–1796. doi: 10.1084/jem.20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O’Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program. Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz RS, Pardini R, Catroxo M, Operskalski EA, Mosley JW, Busch MP. HIV-1 superinfection is not a common event. J Clin Virol. 2005;33(4):328–330. doi: 10.1016/j.jcv.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty B, Valer L, De Mendoza C, Soriano V, Quiñones-Mateu ME. Failure to detect human immunodeficiency virus type 1 superinfection in 28 HIV-seroconcordant individuals with high risk of reexposure to the virus. AIDS Res Hum Retroviruses. 2004;20(9):1026–1031. doi: 10.1089/aid.2004.20.1026. [DOI] [PubMed] [Google Scholar]
- 58.Gonzales MJ, Delwart E, Rhee SY, Tsui R, Zolopa AR, Taylor J, Shafer RW. Lack of detectable human immunodeficiency virus type 1 superinfection during 1072 person-years of observation. J Infect Dis. 2003;188(3):397–405. doi: 10.1086/376534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin F, Lee J, Thomson E, Tarrant N, Hale A, Lacey CJ. Two cases of possible transmitted drug-resistant HIV: likely HIV superinfection and unmasking of pre-existing resistance. Int J STD AIDS. 2016;27(1):66–69. doi: 10.1177/0956462415571671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowan SA, Gerstoft J, Haff J, Christiansen AH, Nielsen J, Obel N. Stable incidence of HIV diagnoses among Danish MSM despite increased engagement in unsafe sex. J Acquir Immune Defic Syndr. 2012;61(1):106–111. doi: 10.1097/QAI.0b013e31825af890. [DOI] [PubMed] [Google Scholar]
- 61.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Günthard HF. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 62.Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner GA, Landais E, Caballero G, Phung P, Kosakovsky Pond SL, Poignard P, Richman DD, Little SJ, Smith DM. Intrasubtype B HIV-1 superinfection correlates with delayed neutralizing antibody response. J Virol. 2017;91(17):e00475–17. doi: 10.1128/JVI.00475-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7(1):e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore PL, Gray ES, Sheward D, Madiga M, Ranchobe N, Lai Z, Honnen WJ, Nonyane M, Tumba N, Hermanus T, Sibeko S, Mlisana K, Abdool Karim SS, Williamson C, Pinter A, Morris L CAPRISA 002 Study. Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J Virol. 2011;85(7):3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program. Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496(7446):469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinfurter JT, May GE, Soma T, Hessell AJ, León EJ, Macnair CE, Piaskowski SM, Weisgrau K, Furlott J, Maness NJ, Reed J, Wilson NA, Rakasz EG, Burton DR, Friedrich TC. Macaque long-term nonprogressors resist superinfection with multiple CD8 + T cell escape variants of simian immunodeficiency virus. J Virol. 2011;85(1):530–541. doi: 10.1128/JVI.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blish CA, Dogan OC, Jaoko W, McClelland RS, Mandaliya K, Odem-Davis KS, Richardsonb BA, Overbaugh J. Cellular immune responses and susceptibility to HIV-1 superinfection: a case-control study. AIDS. 2012;26(5):643–646. doi: 10.1097/QAD.0b013e3283509a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Forthal DN, Landucci G, Chohan B, Richardson BA, McClelland RS, Jaoko W, Blish C, Overbaugh J. Antibody-dependent cell-mediated virus inhibition antibody activity does not correlate with risk of HIV-1 superinfection. J Acquir Immune Defic Syndr. 2013;63(1):31–33. doi: 10.1097/QAI.0b013e3182874d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blish CA, Dogan OC, Jaoko W, McClelland RS, Mandaliya K, Odem-Davis K, Richardson BA, Overbaugh J. Association between cellular immune activation, target cell frequency, and risk of human immunodeficiency virus type 1 superinfection. J Virol. 2014;88(10):5894–5899. doi: 10.1128/JVI.00187-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ronen K, Dingens AS, Graham SM, Jaoko W, Mandaliya K, McClelland RS, Overbaugh J. Comprehensive characterization of humoral correlates of human immunodeficiency virus 1 superinfection acquisition in high-risk Kenyan women. EBioMedicine. 2017;18:216–224. doi: 10.1016/j.ebiom.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vesa J, Chaillon A, Wagner GA, Anderson CM, Richman DD, Smith DM, Little SJ. Increased HIV-1 superinfection risk in carriers of specific human leukocyte antigen alleles. AIDS. 2017;31(8):1149–1158. doi: 10.1097/QAD.0000000000001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell MS, Gottlieb GS, Hawes SE, Nickle DC, Wong KG, Deng W, Lampinen TM, Kiviat NB, Mullins JI. HIV-1 superinfection in the antiretroviral therapy era: are seroconcordant sexual partners at risk? PLoS One. 2009;4(5):e5690. doi: 10.1371/journal.pone.0005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30(5):423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. HIV-host interactions: implications for vaccine design. Cell Host Microbe. 2016;19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao F. Broad neutralization as a byproduct of antibody maturation during HIV-1 infection: a personal perspective. Infect Dis Transl Med. 2016;2:60–68. [Google Scholar]
- 78.Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009;83(19):10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Euler Z, van den Kerkhof TL, van Gils MJ, Burger JA, Edo-Matas D, Phung P, Wrin T, Schuitemaker H. Longitudinal analysis of early HIV-1-specific neutralizing activity in an elite neutralizer and in five patients who developed cross-reactive neutralizing activity. J Virol. 2012;86(4):2045–2055. doi: 10.1128/JVI.06091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pernas M, Casado C, Arcones C, Llano A, Sánchez-Merino V, Mothe B, Vicario JL, Grau E, Ruiz L, Sánchez J, Telenti A, Yuste E, Brander C, Galíndez CL. Low-replicating viruses and strong antiviral immune response associated with prolonged disease control in a superinfected HIV-1 LTNP elite controller. PLoS One. 2012;7(2):e31928. doi: 10.1371/journal.pone.0031928. [DOI] [PMC free article] [PubMed] [Google Scholar]