Abstract
Objective
To investigate the relationship between adiposity and cognition using mean accuracy, mean reaction time, and intraindividual variability (IIV) among preadolescents.
Methods
Children 7–9 years old (N=233, 133 females) underwent dual-energy x-ray absorptiometry and a VO2peak test to assess whole-body adiposity and aerobic fitness relative to fat-free mass (VO2FF), respectively. Attentional inhibition was assessed using a modified flanker task. IIV was assessed as standard deviation (SDRT) and coefficient of variation (CVRT) of response time. Hierarchical linear regression analyses were performed to examine the relationships between adiposity and cognitive measures following adjustment of significant demographic factors, intelligence quotient, and VO2FF.
Results
Whole-body adiposity was negatively related to congruent trial mean accuracy and reaction time and to CVRT in both the congruent and incongruent trials. Differences in cognitive function across weight status were selectively evident for measures of IIV such that children with overweight/obesity (≥85th BMI-for-age percentile) exhibited higher CVRT for both the congruent and incongruent trials.
Conclusion
This work provides additional evidence linking childhood obesity to poorer cognitive function and includes novel data extending the negative influence of adiposity to measures of intraindividual response variability in cognitive control, even after accounting for intellectual abilities, aerobic fitness and demographic factors.
Keywords: Obesity, DXA, Dual-energy X-ray Absorptiometry, Flanker, IIV, Intraindividual Variability, CVRT, Coefficient of Variation of Reaction Time, SDRT, Standard Deviation of Reaction Time
Introduction
Currently, one in three children in the United States has overweight/obesity (≥85th BMI-for-age percentile)1, which is concerning given that obesity contributes to numerous chronic diseases including cardiovascular disease, metabolic syndrome, and diabetes mellitus type 22. In addition to its cardiovascular and metabolic implications, obesity in midlife is also a known risk factor for adverse cognitive health outcomes including greater risk for Alzheimer’s disease and dementia3. Further, psychosocial consequences of obesity include poorer educational attainment, higher rates of poverty, and lower household income4. Given the detrimental relationship of obesity on cardiovascular disease and cognitive heath in adulthood, the question of whether these relationships are evident in childhood has received increased scrutiny.
A converging body of literature indicates that greater aerobic fitness, as early as preadolescence, promotes superior cognitive performance, and alterations in brain structure and function5. Cognitive control encompasses a complex set of goal-directed processes including attention, memory, learning, and perception6, and has been shown to be positively related to greater aerobic fitness. Improved cognitive control during development is predictive of later academic achievement and has been linked to greater educational attainment, higher income and socioeconomic status, as well as better access to health care7. In contrast to fitness effects on the brain, mechanisms underlying how obesity or excess fat mass influence cognitive control are not clear, although indirect mechanisms involving adipocyte-induced neuroinflammation have received considerable attention8. Excess adiposity can lead to increased levels of circulating free fatty acids, pro-inflammatory cytokines, and immune cells, which in turn may contribute to neuroinflammation8,9. For example, proinflammatory cytokines such as IL-6 and TNFα are known to exert neurodegenerative effects in several brain diseases8,10. Additionally, inflammation and oxidative stress often co-exist and oxidative stress is associated with astrocyte activation, brain pro-inflammatory cytokine production, and cognitive impairment8,11. Although additional research is needed to illustrate the mechanisms by which adiposity affects cognitive function, correlational and longitudinal studies often support a negative relationship between obesity and cognitive control12. Given the inverse relationship between obese weight status and poorer aerobic fitness, these physiological factors likely impart counteractive effects on cognitive control, yet the two are seldom examined together. Therefore, relatively little is known about the influence of adiposity on children’s cognitive function while accounting for fitness.
In addition to limited research examining the cognitive implications of childhood obesity while accounting for fitness, additional work is required to characterize the specific measures of cognitive function susceptible to the influence of childhood obesity. Previous cognitive and neuropsychological research has disproportionately focused on measures of central tendency, such as mean differences in performance13, across individuals while neglecting measures of within individual variability, therefore limiting our understanding of the true extent to which obesity may influence children’s cognitive function. Intraindividual variability (IIV) provides metrics of within-person fluctuations in behavioral performance and offers insight into the degree of consistency in cognitive control during task performance14. Specifically, intraindividual standard deviation of reaction time (SDRT) and intraindividual coefficient of variation of reaction time (CVRT) can serve as useful indices of patterns of behavioral responses that underlie the consistency of cognitive control performance and have been previously shown to have relevance for a number of cognitive abilities in everyday life as well as to the study of neurological disease15. However, the extent to which childhood obesity may impact IIV during cognitive control tasks has not been directly examined.
Accordingly, the present study examined the relationships between adiposity and cognitive performance using measures of central tendency and intraindividual variability, while accounting for demographical factors and aerobic fitness. We hypothesized that greater adiposity would be related to poorer mean performance as well as higher IIV among preadolescent children. We also anticipated that children with overweight and obesity would have significantly higher IIV relative their healthy weight counterparts.
Methods
Participants
Participants were 7–9-year-old preadolescent children recruited as part of the FITKids2 randomized controlled trial, a physical activity after-school intervention program assessing the effect of daily exercise on cognitive function between 2013 and 2017 (NCT01619826). Children who completed all tasks (N=233) were included at their baseline measurement, prior to randomization and intervention. Exclusion criteria included neurological disorders, physical disabilities, and psychoactive medication use, as reported by parents in an eligibility questionnaire. All participants were required to have normal or corrected-to-normal vision. Participants provided written assent and their legal guardians provided written consent in accordance with the ethical standards and regulations of the Institutional Review Board (IRB) at the University of Illinois at Urbana-Champaign (IRB #12321).
Procedure
Testing occurred over two laboratory visits. During the first visit, participants completed informed assent/consent, the Woodcock Johnson Test of Cognitive Abilities to estimate intelligence quotient (IQ), measurement of height and weight, and a maximal oxygen consumption test (VO2peak)16 to assess aerobic fitness. Concurrently, parents completed surveys assessing demographics, health history, and pubertal status according to the modified Tanner Staging Scales17,18. Socioeconomic status (SES) was determined from eligibility for school meal-assistance programs, maternal and paternal education levels, and the number of parents with full-time employment. During the second visit, participants completed a modified flanker task19 designed to assess attentional inhibition, and a Dual-Energy X-ray Absorptiometry (DXA) assessment of whole body and visceral adiposity.
Intelligence Quotient Assessment
The Woodcock Johnson Test of Cognitive Abilities was used to estimate IQ. Tests include audio recordings, subject response booklet, and subject response pages. The test is individually administered by a trained examiner based on the guidelines provided in the Examiner’s Manual20. Basal and ceiling criteria are listed in the Test Book for each subtest and raw scores are calculated for each test. Test and cluster scores are then calculated using the Woodcock Johnson III Normative Update Compuscore and Profiles program (Compuscore; Schrank & Woodcock, 2007).
Pubertal Stage Assessment
The modified Tanner Staging Scales were presented to the parents as a document with five separate line drawings depicting various stages of external genitalia development (males), breast development (females), and pubic hair development (males and females). Parents were asked to identify the line drawing that depicted their child’s developmental status and the average of scores was used to determine the child’s pubertal stage. Previous research has validated the Tanner Scale in different samples of children and has shown good agreement with clinician examination with kappa values ranging from 0.68 to 0.7618,21.
Anthropometric and Adiposity Assessment
Participants height and weight were measured, without shoes, using a stadiometer (model 240; Seca, Hamburg, Germany) and a Tanita WB-300 Plus digital scale (Tanita, Tokyo, Japan), respectively. Each measurement was taken three times and the average was used for analyses. BMI-for-age-percentile cut-offs from the CDC were used to determine weight status22. Fat mass and muscle mass were measured using DXA with a Hologic Discovery A bone densitometer (software version 12.7.3; Hologic, Bedford, MA). Whole-body adiposity (%Fat) was expressed using the standard software measure23.
Cardiorespiratory Fitness Assessment
Maximal aerobic capacity (VO2peak) was assessed using a modified Balke treadmill protocol16. This modification involved maintaining a constant speed of the treadmill while increasing the workload (i.e., grade) of the treadmill. The modified Balke protocol follows the ACSM Guidelines for Exercise Testing and Prescription24,25 in children and is regarded as valid and reliable for estimating cardiorespiratory fitness in children26. Children were then fitted with a heart rate monitor (Polar WearLink + 31, Polar Electro, Finland) or the duration of the assessment. Children started with a warm-up period, and then jogged at a constant speed with increasing grade increments of 2.5% every 2 minutes until perceived exhaustion. Oxygen consumption was measured using a computerized indirect calorimetry system (True Max 2400; ParvoMedics, Sandy, Utah) with averages for oxygen uptake and respiratory exchange ratio assessed every 20 seconds. Concurrently, ratings of perceived exertion (RPE) were measured every 2 minutes using the children’s OMNI rating of perceived exertion scale. VO2peak was defined as the highest oxygen consumption corresponding to a minimum of 2 of the following 4 criteria: (1) a peak heart rate ≥185 beats per minute, (2) a respiratory exchange ratio > 1.0, (3) a RPE score of ≥8, and/or (4) a plateau in oxygen consumption corresponding to an increase of <2 mL/kg/min despite an increase in workload16. Aerobic fitness percentiles were determined by using normative values for VO2peak27. Absolute VO2peak (L/min) was adjusted for fat-free mass (from DXA) to calculate fat-free VO2peak (VO2FF). Prior to VO2peak assessment, all participants completed the Physical Activity Readiness Questionnaire (PAR-Q) to screen for contraindications to physical activity28. Further, each assessment was conducted by a minimum of at least 3 trained staff members with certification in cardiopulmonary resuscitation (CPR) and automated external defibrillator (AED) administration.
Attentional Inhibition Assessment
A modified flanker task19 presented a target stimulus (cartoon fish) amid an array of four flanking stimuli. Participants were asked to respond to the centrally presented target with the flanking stimuli irrelevant to the task. This modified version of the flanker task consisted of both congruent trials, where the flanking fish faced the same direction as the target fish (> > > > >), and incongruent trials, where the flanking fish faced the opposite direction from the target fish (> > < > >)29. Congruent and incongruent trials were equiprobable and random. Participants responded to the direction of the target fish, left or right, with their consonant thumb. Participants completed 54 practice trials followed by two blocks of 84 trials. The viewing distance was 1 meter, the stimulus duration was 250 milliseconds, and the interstimulus interval was jittered at 1600, 1800, or 2000 milliseconds. For behavior data, primary variables of interest included mean response time (time in ms from stimulus presentation until response execution), response accuracy (percentage of correct responses), standard deviation (SDRT), and coefficient of variation (SD/Mean RT) of reaction time (CVRT) for all correct trials types (congruent and incongruent).
Statistical Analysis
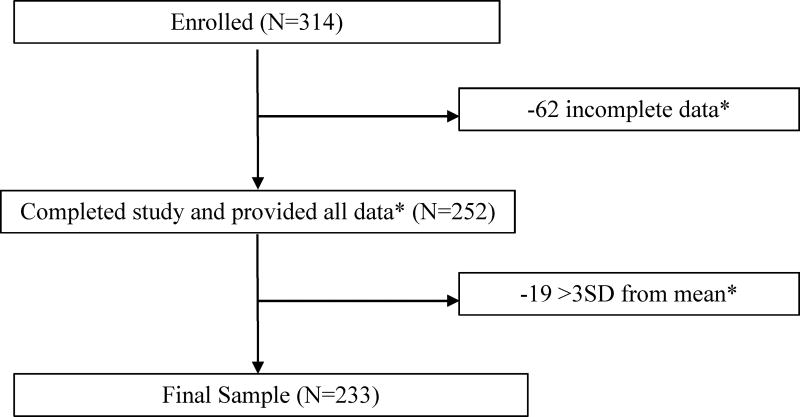
Normality was first assessed for each of the main outcomes using Kolmogorov-Smirnov and Shapiro-Wilk Tests, Skewness and Kurtosis values, as well as visual examination of Normal Q-Q Plots and histograms. Outliers were defined as values ±3 standard deviations from the mean and were removed from subsequent analyses (see Figure 1). To examine the relevance of IIV for behavioral performance, Pearson correlations were used to assess the relationships between SDRT and CVRT with accuracy and reaction time in both congruent and incongruent trials. Pearson correlations were also used initially to assess bivariate relationships between adiposity, cognitive measures, and fitness and demographic variables including BMI, age, pubertal timing, sex, and SES (2-tailed p<0.05 considered significant). Hierarchical linear regression analyses were performed to examine variability in cognitive performance. The demographic and IQ variables that were significant in the bivariate correlations were entered into Step 1. Steps 2 and 3 were used for VO2FF and %fat, respectively, in the models where they correlated in the bivariate analysis. Each predictor was evaluated by studying its significance (α-level, 0.05). Finally, one-way ANOVA were used to determine differences in IIV across weight status grouping utilizing a 2 (type: congruent, incongruent) × 3 (group: health weight, overweight, obese) factorial model. Post hoc analyses included independent samples T-tests with Bonferroni correction. All analyses were completed using SPSS Version 24 (IBM, Armonk, NY).
Figure 1.
Consort Diagram
*All data includes values for age, sex, SES, IQ, Tanner, BMI, VO2peakFF, %Fat, Flanker Compatible Congruent and Incongruent RT, %accuracy, SDRT, and CVRT.
Results
Preadolescent children ages 7 to 9 (N=314) were recruited from the east-central Illinois region. See Figure 1 for consort diagram. The complete breakdown of demographics, body composition, and cognitive performance can be found in Table 1. SES categorization of the participants was 40% low, 36% middle, and 25% high. According to the Tanner pubertal staging questionnaire, 51% of participants were stage 1, 45% were stage 2, and 5% were stage 3. Categorizations for BMI showed 56% of the children were classed as healthy weight, 19% as overweight, and 23% as obese.
TABLE 1.
DEMOGRAPHICS, IQ, ADIPOSITY, AND FLANKER PERFORMANCE
| N=233 | Mean (SD) | |
|---|---|---|
| Age, y | 8.67 (0.54) | |
| Sex | ||
| Male | 42.9% (n=100) | |
| Female | 57.1% (n=133) | |
| SES | ||
| Low | 39.5% (n=92) | |
| Medium | 35.6% (n=83) | |
| High | 24.9% (n=58) | |
| IQ | 108.41 (12.92) | |
| Pubertal Timing | 1.36 (0.47) | |
| BMI, kg/m2 | 18.83 (4.00) | |
| Weight Status | ||
| Underweight | 2.6% (n=6) | |
| Healthy Weight | 56.2% (n=131) | |
| Overweight | 18.5% (n=43) | |
| Obese | 22.7% (n=53) | |
| VO2peak Relative | 42.13 (7.20) | |
| VO2peak %tile | 36.6 (30.40) | |
| VO2FF | 61.00 (7.26) | |
| %Fat | 31.62 (6.88) | |
| Flanker Congruent | ||
| Accuracy, % | 80.26 (12.01) | |
| Reaction Time, ms | 552.35 (103.62) | |
| SDRT, ms | 182.43 (52.23) | |
| CVRT | 0.33 (0.08) | |
| Flanker Incongruent | ||
| Accuracy, % | 72.93 (13.57) | |
| Reaction Time, ms | 600.54 (113.64) | |
| SDRT, ms | 193.84 (56.28) | |
| CVRT | 0.32 (0.08) |
Data presented as mean ± STD unless otherwise indicated
SES, Socioeconomic Status; IQ, Intelligence Quotient; BMI, Body Mass Index; VO2peak %tile, Maximum Aerobic Capacity Age and Sex Percentile; VO2FF, Maximum Lean Aerobic Capacity; %Fat, Whole Body Percent Fat; VAT, Visceral Adipose Tissue; SDRT, Standard Deviation of Reaction Time; CVRT, Coefficient of Variation of Reaction Time
Table 2 shows the results of the bivariate correlations. For the congruent trials, correlations with SDRT showed a negative relationship with accuracy (r=−0.27 p<0.01) and a positive relationship with reaction time (r=0.63, p<0.01); and correlations with CVRT similarly showed a negative relationship with accuracy (r=−0.51, p<0.01). For the incongruent trials, SDRT was negatively correlated with accuracy (r=−0.28, p<0.01) and positively associated with reaction time (r=0.54, p<0.01), and CVRT was negatively associated with accuracy (r=−0.59, p<0.01) but not associated with reaction time. Overall results (i.e., collapsed across congruency) indicated that flanker SDRT was significantly correlated with age (r=−0.230, p<0.01), SES (r=−0.15, p<0.05), IQ (r=−0.23, p<0.01), and VO2FF (r=−0.16, p<0.05). Additionally, CVRT was correlated with age (r=−0.25, p<0.01), IQ (r=−0.23, p<0.01), VO2FF (r=−0.19, p<0.01), and %fat (r=0.20, p<0.01).
TABLE 2.
BIVARIATE CORRELATIONS BETWEEN DEMOGRAPHICS, IQ, WEIGHT STATUS, AND FLANKER PERFORMANCE
| Congruent | Incongruent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Accuracy | RT | SDRT | CVRT | Accuracy | RT | SDRT | CVRT | ||
|
|
|||||||||
| Age | 0.25** | −0.19** | −0.27** | −0.22** | 0.21** | −0.16* | −0.29** | −0.24** | |
| Sex | 0.02 | −0.01 | −0.03 | −0.02 | 0.01 | −0.04 | −0.14* | −0.10 | |
| SES | 0.12 | −0.06 | −0.08 | −0.05 | 0.14* | −0.07 | −0.19** | −0.17* | |
| IQ | 0.24** | −0.06 | −0.23** | −0.23** | 0.26** | −0.04 | −0.20** | −0.20** | |
| Pubertal Timing | −0.04 | −0.13 | −0.08 | -−0.00 | −0.11 | −0.12 | −0.01 | 0.07 | |
| BMI | −0.11 | −0.19** | 0.01 | 0.16* | −0.13* | −0.18** | 0.03 | 0.18** | |
| VO2FF | 0.09 | 0.01 | −0.10 | −0.13* | 0.15* | −0.03 | −0.18** | −0.19** | |
| %Fat | −0.14* | −0.16* | 0.02 | 0.16* | −0.14* | −0.13** | 0.07 | 0.18** | |
| Incongr Congr | SDRT | −0.27** | 0.63** | - | - | −0.18** | 0.54** | - | - |
| CVRT | −0.51** | −0.00 | - | - | −0.51** | −0.06 | - | - | |
| SDRT | −0.34** | 0.47** | - | - | −0.28** | 0.54** | - | - | |
| CVRT | −0.59** | −0.13* | - | - | −0.59** | −0.11 | - | - | |
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
SES, Socioeconomic Status; IQ, Intelligence Quotient; BMI, Body Mass Index; VO2FF, Maximum Lean Aerobic Capacity; %Fat, Whole Body Percent Fat; SDRT, Standard Deviation of Reaction Time; CVRT, Coefficient of Variation of Reaction Time
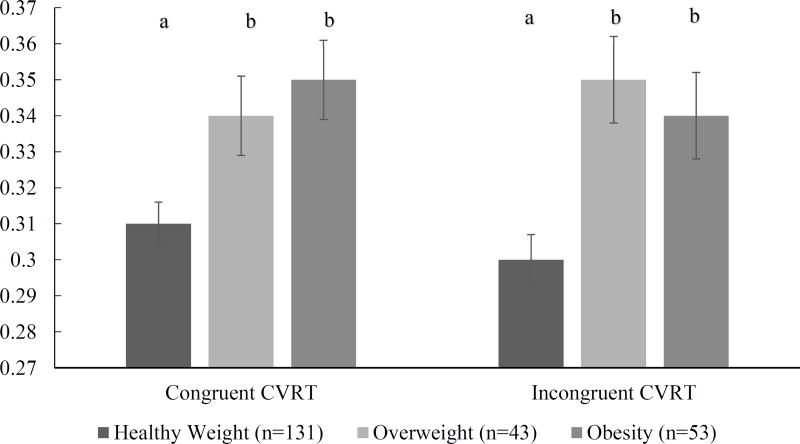
Results of the ANOVA can be seen in Figure 2. Results showed a significant effect of weight status in CVRT for both congruent [F (3, 229) =4.46, p<0.01, η2=0.06] and incongruent [F (3, 229) = 6.77, p<0.01, η2=0.08] trials, with the healthy weight group exhibiting lower variability compared to both overweight and obese groups. Post hoc comparisons using the Bonferroni test indicated that both congruent (p<0.02) and incongruent (p<0.01) trials of CVRT were lower in healthy weight individuals than in individuals with obesity.
Figure 2.
Results of one-way ANOVA for differences in Coefficient of Variation between healthy weight, overweight, and obese individuals
Different letters depict significant difference between groups (p<0.05)
Hierarchical regression results are summarized in Table 3 and Table 4. Whole-body adiposity was a significant predictor of congruent accuracy (β=−0.15, p=0.02), reaction time (β=−0.14, p=0.03), and CVRT (β=0.15, p=0.02), as well as incongruent CVRT (β=0.15, p=0.02). VO2FF was not a significant predictor of variance in any of the final models (all p’s>0.05). Age, IQ, VO2FF, and %fat explained 15% of the variance in congruent CVRT (ΔR2=0.15, F=10.24, p<0.01). Age, SES, VO2FF, and %fat accounted for 18% of the variance in incongruent CVRT (ΔR2=0.18, F=9.70, p<0.01).
TABLE 3.
RESULTS FOR HIERARCHICAL REGRESSION FOR CONGRUENT FLANKER
| Accuracy | Reaction Time | CVRT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| β | R2 | F | β | R2 | F | β | R2 | F | |||
| Step 1 | 0.14** | 19.36** | Step 1 | 0.04** | 8.52** | Step 1 | 0.12** | 16.17** | |||
| Age | 0.30** | Age | −0.19** | Age | −0.27** | ||||||
| IQ | 0.29** | IQ | −0.28** | ||||||||
|
| |||||||||||
| Step 2 | 0.17** | 15.20** | Step 2 | 0.06** | 6.66** | Step 2 | 0.13** | 11.46** | |||
| %Fat | −0.15* | %Fat | −0.14* | VO2FF | −0.09 | ||||||
|
| |||||||||||
| Step 3 | 0.15** | 10.24** | |||||||||
| %Fat | 0.15* | ||||||||||
Significant at the 0.01 level (2-tailed)
Significant at the 0.05 level (2-tailed)
Marginally significant at the 0.10 level (2-tailed)
IQ, Intelligence Quotient; VO2FF, Maximum Lean Aerobic Capacity; %Fat, Whole Body Percent Fat; CVRT, Coefficient of Variation of Reaction Time
TABLE 4.
RESULTS FOR HIERARCHICAL REGRESSION FOR INCONGRUENT FLANKER
|
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Reaction Time | SDRT | CVRT | ||||||||||||
|
| |||||||||||||||
| β | R2 | F | β | R2 | F | β | R2 | F | β | R2 | F | ||||
| Step 1 | 0.14** | 11.96** | Step 1 | 0.03* | 6.36* | Step 1 | 0.19** | 12.94** | Step 1 | 0.13** | 11.79** | ||||
| Age | 0.25** | Age | −0.16* | Age | −0.33** | Age | −0.28** | ||||||||
| SES | 0.07 | Sex | −0.15* | SES | −0.11 | ||||||||||
| IQ | 0.29** | SES | −0.12* | IQ | −0.23** | ||||||||||
| IQ | −0.24** | ||||||||||||||
|
| |||||||||||||||
| Step 2 | 0.15** | 9.66** | Step 2 | 0.04** | 4.81** | Step 2 | 0.20** | 11.07** | Step 2 | 0.15** | 10.39** | ||||
| VO2FF | 0.10 | %Fat | −0.12† | VO2FF | −0.11† | VO2FF | −0.14* | ||||||||
|
| |||||||||||||||
| Step 3 | 0.16** | 8.55** | Step 3 | 0.18** | 9.70** | ||||||||||
| %Fat | −0.12† | %Fat | 0.15* | ||||||||||||
Significant at the 0.01 level (2-tailed)
Significant at the 0.05 level (2-tailed)
Marginally significant at the 0.10 level (2-tailed)
SES, Socioeconomic Status; IQ, Intelligence Quotient; VO2FF, Maximum Lean Aerobic Capacity; %Fat, Whole Body Percent Fat; SDRT, Standard Deviation of Reaction Time; CVRT, Coefficient of Variation of Reaction Time
Discussion
The use of IIV as a marker of cognitive impairment or dysfunction has been demonstrated in clinical studies among patients with brain disorders such as ADHD and Alzheimer’s disease30,31. However, few have attempted to examine IIV in generalizable or non-clinical study populations, particularly in childhood. The results of the study were consistent with our a priori hypothesis given that we observed negative relationships between adiposity and task accuracy. Further, children with greater adiposity exhibited higher IIV indicating that the negative influence of excess fat mass extends to measures of dispersion in attentional inhibition, following adjustment for demographic factors, intelligence, and aerobic fitness. These findings were further supported by comparisons across weight status categories. Children with overweight and/or obesity exhibited greater IIV during both congruent and incongruent trials of the modified flanker task, relative to their healthy weight counterparts. Interestingly, differences across weight status categories were only evident for measures of IIV and not central tendency, providing further evidence supporting the susceptibility of measures of dispersion to the potentially negative influence of childhood obesity.
Although previous studies have observed an inverse relationship between aerobic fitness and IIV, these studies did not consider %fat as a contributing factor14. The results here indicated that fitness significantly contributed to the variation in CVRT during the incongruent trials; however, the inclusion of %fat in the regression models appeared to have a moderating influence on the initial relationships observed for fitness. This moderating influence of %Fat indicates that excess adiposity exerts a considerable negative impact on cognitive control that mitigates some of the positive contribution of fitness to the cognitive measures. Alternatively, the sample studied was relatively homogeneous with regard to fitness, and was predominantly comprised of lower-fit children. Conducting similar analyses in a heterogeneous sample that includes a greater proportion of higher-fit children may reveal a positive influence of fitness, independent of adiposity. Future studies among children with varying levels of fitness are necessary to further confirm the findings observed here.
Emerging evidence indicates that behavioral and emotional problems are more common among children with obesity with the most frequently implicated psychosocial factors including externalizing (e.g., impulsivity) and internalizing (e.g., depression and anxiety) behaviors32. Further, obesity has been linked to poorer ability for cognitive control processes such as attention, memory, and inhibition12. In two systematic reviews, higher BMI was associated with poorer cognitive control performance; however, there was little consistency within and across the different domains of cognitive control12. The conflicting state of knowledge may be, at least in part, due to the metric of performance studied. Virtually all previous studies on obesity and children’s cognitive and neuropsychological function have relied on central tendency measures with little known regarding the influence of behaviors and physiological health on IIV. To our knowledge, the current study is the first to examine the relationship between measures of intraindividual performance and adiposity among preadolescent children.
The findings of the current study provide support linking IIV in cognitive control performance to the interrelated health factors of aerobic fitness and adiposity. However, the mechanisms underlying this observation are not clear. One possibility may be differential trajectories of development or maturation in cognitive control across health factors. For example, considering factors beyond adiposity and fitness, we observed that age was a significant predictor of both IIV and central tendency. As children develop and mature, they exhibit improved performance in cognitive control tasks, displaying both higher response accuracy and shorter response times33. Similarly, IIV performance during cognitive control tasks also decreases throughout childhood and adolescence34. These findings show consistency that younger children exhibit higher variability during cognitive control tasks. Conversely, Myerson et al. observed that older adults (M=73.9 years) exhibited greater IIV in their RT than did younger adults (M=20.9 years)35. In the same study, the older adults also displayed longer RT than the younger adults35. Der and Deary found similar results in a study of 7130 adults participants, they found that reaction time increased throughout the adult age range and reaction time variability decreased in early adulthood but then increased throughout late adulthood36. Collectively, these investigations suggest that the IIV-age relationship follows a U-shaped curve throughout the lifespan with improvements through young adulthood and decrements through older adulthood. These studies provide initial support for the theory of a developmental mechanism contributing to the differences in IIV and the results in the current work show consistency that older children exhibited lower response variability.
Additional insights into the underpinnings of response variability can be gained from magnetic resonance imaging (MRI) studies. Previous studies demonstrate that variability indexes a demand for top-down cognitive control37. Further, patients with damage to the dorsolateral prefrontal cortex or the superior medial frontal cortex exhibited increases in IIV during a cognitive control task that required feature discrimination and integration38. Diffusion tensor imaging studies show that reduced performance variability reflects the maturation of white matter connectivity39. Tamnes et al. reported that irrespective of age, lower IIV was associated with higher fractional anisotropy, lower mean diffusivity, lower axial diffusivity, and lower radial diffusivity; all indicating that children (8–19-year-olds) with more mature white matter exhibit lower degrees of performance variability39. Additionally, increased BMI is associated with a global and distributed decrease in white matter microstructural integrity as well as detectable brain volume deficits in people with obesity, including atrophy in the frontal lobes, anterior cingulate gyrus, hippocampus, and thalamus, when compared to normal-weight subjects40. These findings indicate that obesity is associated with decrease brain volume, supporting the theory that brain structure and development contribute to response time variability.
Limitations to this study include the report of cross-sectional data rather than an intervention approach. The cross-sectional design yields the possibility that observed fitness and adiposity differences may have resulted from a combination of extraneous factors not accounted for in the present investigation such as diet, survey response bias, or preexisting health conditions and undiagnosed mental disorders. Additionally, data were not collected regarding the amount of time that preadolescent children had been exposed to overweight or obesity. Children who have had overweight/obesity longer may have further cognitive impairment and additional research will need to account for this factor. Further, it is possible that the relationship between fitness, adiposity, and IIV is bidirectional. Therefore, additional randomized controlled and longitudinal trials are needed to elucidate the influence of change in health factors (i.e., fitness and fatness) to changes in cognitive control, with the current work highlighting the importance of utilizing dispersion measures, rather than central tendency alone, as perhaps more sensitive markers of obesity-related decrements in cognitive control in children.
In conclusion, the current work is based on a large dataset comprising fitness, adiposity, and cognitive control data in preadolescent children. The findings point to the importance of maintaining healthy weight status in children for better cognitive control. They also indicate that that increased adiposity, regardless of fitness level, exhibits a deleterious relationship with aspects of children’s cognitive control. The association between adiposity and IIV points to the important influence of excess fat mass on markers of cognitive control, which may serve a developmental barrier and contribute to long-term decrements in cognitive function.
Study Importance.
1. What is already known about this subject?
Relationship between adiposity and cognitive function in children is equivocal
Measures of response variability during task performance may provide a useful index of cognitive function beyond measures of central tendency
Knowledge on the influence of adiposity on intraindividual response variability is unknown
2. What does your study add?
Children with overweight/obesity exhibited greater intraindividual response variability during an attentional inhibition task
Higher adiposity was directly associated with more response variability, even after accounting for aerobic fitness levels
Measures of response variability may serve as sensitive markers of adiposity-related cognitive decrements in childhood
Acknowledgments
Funding: National Institute of Health R01 HD06938 (Drs. Kramer and Hillman).
Footnotes
Clinical Trial Registration: Registered at www.clinicaltrials.gov (identifier NCT01619826)
Disclosure: The authors of this document report no conflicts of interest associated with the collection, dissemination, or interpretation of this research. No patents, copyrights, or royalties are involved or included in this work.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Key findings What was the prevalence of obesity among adults in 2011–2014? 2011 [Google Scholar]
- 2.Meigs JB, Wilson PWF, Fox CS, et al. Body Mass Index, Metabolic Syndrome, and Risk of Type 2 Diabetes or Cardiovascular Disease. J Clin Endocrinol Metab. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and Late-Life Obesity and the Risk of Dementia. Arch Neurol. 2009;66(3):1337–1342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan NA, Hillman CH. The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. Pediatr Exerc Sci. 2014;262:138–146. doi: 10.1123/pes.2013-0125. [DOI] [PubMed] [Google Scholar]
- 6.Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. 2013;7:97. doi: 10.3389/fnhum.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer R, Gergen P. Health and Social Characteristics and Children’s Cognitive Functioning: Results from a National Cohort. [Accessed March 13, 2017]; doi: 10.2105/ajph.85.3.312. http://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.85.3.312. [DOI] [PMC free article] [PubMed]
- 8.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2011;29(1):415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 10.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore RD, Wu C-T, Pontifex MB, et al. Aerobic fitness and intra-individual variability of neurocognition in preadolescent children. Brain Cogn. 2013;82(1):43–57. doi: 10.1016/j.bandc.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unsworth N. Consistency of attentional control as an important cognitive trait: A latent variable analysis. Intelligence. 2015;49:110–128. doi: 10.1016/j.intell.2015.01.005. [DOI] [Google Scholar]
- 16.ACSM’s Guidelines for Exercise Testing and Prescription. 7. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. Medicine AC of S. [Google Scholar]
- 17.Tanner JM. Growth at adolescence. 1962 [Google Scholar]
- 18.Taylor Whincup, Hindmarsh Lampe, Odoki Cook. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- 20.Woodcock RW. Woodcock-Johnson Tests of Achievement. Itasca, IL: 2001. [Google Scholar]
- 21.Norris SA, Richter LM. Usefulness and Reliability of Tanner Pubertal Self-Rating to Urban Black Adolescents in South Africa. J Res Adolesc. 2005;15(4):609–624. doi: 10.1111/j.1532-7795.2005.00113.x. [DOI] [Google Scholar]
- 22.Kuczmarski RJ. CDC Growth Charts: United States. Adv data. 2000;314:1–27. [PubMed] [Google Scholar]
- 23.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-Energy X-Ray Performs as Well as Clinical Computed Tomography for the Measurement of Visceral Fat. Obesity. 2012;20(5):1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arena R, editor. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription / American College of Sports Medicine. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 25.Durstine JL American College of Sports Medicine; American College of Sports Medicine. ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities. Human Kinetics; 2009. [Google Scholar]
- 26.Skinner JS. Exercise Testing and Exercise Prescription for Special Cases: Theoretical Basis and Clinical Application. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 27.Shvartz E, Reibold R. Aerobic fitness norms for males and females aged 6 to 75 years: A review. Aviat Sp Env Med. 1990;61(1):3–11. [PubMed] [Google Scholar]
- 28.Chisholm DM, Collis ML, Kulak LL, Davenport W, Gruber N, Stewart GW. PAR-Q validation report: the evaluation of a self-administered pre-exercise screening questionnaire for adults. Victoria Canada BC Minist Heal Welf. 1978 [Google Scholar]
- 29.Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH. Exercise Improves Behavioral, Neurocognitive, and Scholastic Performance in Children with Attention-Deficit/Hyperactivity Disorder. J Pediatr. 2013;162(3):543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathalon D, Bennett A, Askari N, Gray E, Rosenbloom M, Ford J. Response-monitoring dysfunction in aging and Alzheimer’s disease: An event-related potential study. Neurobiol Aging. 2003;24:675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 31.Neale BM, Chen W. Reaction time performance in ADHD : improvement under fast-incentive condition and familial effects. 2017 doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purder JJ, Munsch S. Psychological correlates of childhood obesity. Int J Obes. 2010;34:S37–S43. doi: 10.1038/ijo.2010.238. [DOI] [PubMed] [Google Scholar]
- 33.Mezzacappa E. Alerting, Orienting, and Executive Attention: Developmental Properties and Sociodemographic Correlates in an Epidemiological Sample of Young, Urban Children. Child Dev. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. http://web.b.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=1&sid=d087c492-027c-4a0d-9f61-d632d096f519%40sessionmgr107&hid=129. [DOI] [PubMed] [Google Scholar]
- 34.Williams BR, Strauss EH, Hultsch DF, Hunter MA. Reaction Time Inconsistency in a Spatial Stroop Task: Age-Related Differences Through Childhood and Adulthood. Aging, Neuropsychol Cogn. 2007;14(4):417–439. doi: 10.1080/13825580600584590. [DOI] [PubMed] [Google Scholar]
- 35.Myerson J, Robertson S, Hale S. Aging and Intraindividual Variability in Performance: Analyses of Response Time Distributions. J Exp Anal Behav. 2007;88(3):319–337. doi: 10.1901/jeab.2007.88-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Der G, Deary IJ. Age and Sex Differences in Reaction Time in Adulthood: Results From the United Kingdom Health and Lifestyle Survey. Psychol Aging. 2006;21(1):62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- 37.West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of Intention and Performance Variability Reveal Age-Related Increases in Fluctuations of Executive Control. Brain Cogn. 2002;49(3):402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- 38.Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126(11):2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 39.Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Behavioral/Systems/Cognitive Becoming Consistent: Developmental Reductions in Intraindividual Variability in Reaction Time Are Related to White Matter Integrity. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2009;31(3) doi: 10.1002/hbm.20870. NA-NA. [DOI] [PMC free article] [PubMed] [Google Scholar]