Abstract
Background
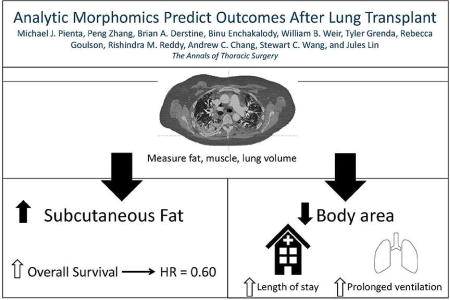
The purpose of this study was to identify morphomic factors on standard, pre-transplant CT scans associated with outcomes after lung transplantation.
Methods
A retrospective review of 200 patients undergoing lung transplantation at a single institution from 2003–2014 was performed. CT scans obtained within 1 year prior to transplant underwent morphomic analysis. Morphomic characteristics included lung, dorsal muscle group, bone, and subcutaneous and visceral fat area and density. Patient data were gathered from institutional and United Network for Organ Sharing databases. Outcomes including initial ventilator support greater than 48 hours, length of stay, and survival were evaluated using univariate and multivariable analyses.
Results
On multivariable Cox regression, subcutaneous fat/total body area (HR 0.60, p=0.001), lung density 3 volume (HR 0.67, p=0.013), and creatinine (HR 4.37, p=0.010) were independent predictors of survival. Initial ventilator support greater than 48 hours was associated with decreased vertebral body to linea alba distance (OR 0.49, p=0.002) and Zubrod Score 4 (OR 14.0, p<0.001). Increased bone mineral density (p<0.001) and increased cross sectional body area (p<0.001) were associated with decreased length of stay while supplemental oxygen (p<0.001), bilateral transplant (p=0.002), cardiopulmonary bypass (p<0.001), and Zubrod Score 3 (p<0.001) or 4 (p=0.040) were associated with increased length of stay.
Conclusions
Morphomic factors associated with lower metabolic reserve and frailty including decreased subcutaneous fat, bone density, and body dimensions were independent predictors of survival, prolonged ventilation, and increased length of stay. Analytic morphomics using pre-transplant CT scans may improve recipient selection and risk stratification.
Keywords: Morphomics, lung transplantation, outcomes, body composition, frailty
Graphical abstract
Lung transplantation aims to improve quality of life and survival for patients with end-stage lung disease. The lung allocation score (LAS) was designed to improve organ allocation and has led to improved outcomes, yet nearly 20% of patients die while awaiting transplant, and a similar percentage die within one year of transplantation (1, 2). Therefore, optimization of patient selection remains of critical importance.
Analytic morphomics is a novel approach using semi-automated image processing to quantitate various aspects of body composition from standard preoperative computed tomography (CT) imaging and correlates those measures to clinical outcomes. Our group has demonstrated the utility of analytic morphomics in predicting outcomes after general surgery, liver transplant, and surgical oncology operations (3–5). Core muscle size has also been shown to be a predictor of postoperative outcomes after lung transplant (6). Despite improvements in patient selection for lung transplant, morbidity and mortality remain high. We hypothesized that using analytic morphomic techniques pioneered by our group to analyze CTs obtained routinely during lung transplant evaluation will identify differences in body composition associated with survival and outcomes after lung transplantation.
PATIENTS AND METHODS
Approval from the Institutional Review Board was obtained. Consecutive patients undergoing lung transplantation from 2003–2014 with a pretransplant chest CT within 1 year were included. Recipient characteristics including age, LAS score, gender, transplant laterality, creatinine, bilirubin, underlying lung disease, pulmonary arterial pressures, diabetes, coronary disease, peripheral vascular disease, cerebrovascular disease, hospitalization status, ischemia times and mechanical ventilation were obtained from our institutional United Network for Organ Sharing (UNOS) data, the University of Michigan Organ Transplant Information System database, and review of the chart.
Lung transplants were performed off bypass when possible, generally through a clamshell incision. Donor lungs were flushed antegrade and retrograde with perfadex, nitroglycerin, and prostaglandin E1 at procurement and retrograde prior to implantation. After extubation, patients were started on clears with a regular diet 1–2 days later. Patients requiring ventilatory support received tube feedings. Steroids were weaned from prednisone 1 mg/kg twice daily to 20 mg/day at discharge and gradually to 5 mg/day at 12 months by protocol. Surveillance bronchoscopy was performed at regular intervals from 3 weeks to 12 months post-transplant. Grade A1B0 rejection was only treated if symptomatic or spirometry decreased. Grade A2 or higher was treated with steroids, and rabbit anti-thymocyte globulin was given for steroid resistant rejection.
Analytic Morphomics
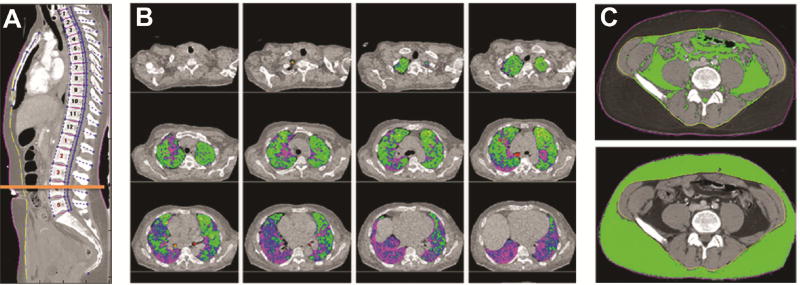
The preoperative chest CT closest to the date of transplant was analyzed. Scans that did not include the entire thorax or with inadequate resolution were excluded. Morphomic variables were acquired using automated algorithms in MATLAB version 13.0 (MathWorks, Natick, MA) (4, 7, 8). Morphomic variables included dorsal muscle group area and density, subcutaneous and visceral fat area, bone density, and body dimensions like cross sectional area. The lung volume was divided into five levels of increasing density: lung density 1 (LD1) < −874 HU (emphysema), LD2 −874 to −725 HU (normal lung), LD3 −724 to −424 HU, LD4 −423 to −174 HU (fibrotic lung), and LD5 > −174 HU (Figure 1). Single slice measurements were obtained at T9. Definitions of all morphomic measures can be found at http://www.med.umich.edu/surgery/morphomics/data_dictionary. While automated algorithms provided standardization between scans, decreasing observer bias, the results were assessed by the morphomics team to confirm accuracy and adjusted as necessary.
Figure 1.
Morphomic analysis using standard chest CTs showing (A) anatomic indexing of the vertebral levels to create an anatomical coordinate system, (B) lung density (LD) measurements where LD3 (green) to LD5 (purple) represent areas of increasing density, and (C) visceral and subcutaneous fat area after delineation of skin (purple) and fascia (yellow).
The primary outcome was overall survival. Secondary outcomes included length of stay and postoperative rehabilitation as well as complications after lung transplant such as initial ventilator support greater than 48 hours, air leak greater than 5 days, grade 3 primary graft dysfunction (PGD) at 72 hours, and acute rejection.
Statistical Analysis
Univariate analysis was used to determine the correlation between clinical and morphomic variables and postoperative outcomes. Two-sample t-test was used to compare continuous variables. Categorical variables were compared using Fisher’s exact test. Univariate Cox regression was used to determine the relationship with post-transplant survival.
A multivariable Cox regression model was developed to investigate survival including all candidate variables. Candidate variables were selected based on clinical factors reported to be associated with post-transplant outcomes. To identify the most relevant morphomic factors, variables were chosen when they were significantly associated with a particular outcome at multiple vertebral levels or several similar morphomic measures, such as body circumference, depth, and width significantly correlated with an outcome. Logistic regression was used for binary outcomes. A Poisson regression model with dependent variable length of stay was fitted. Forward backward selection was used to find a model which optimized the Akiake information criterion (AIC). All morphomic variables were normalized to a mean of 0 and standard deviation of 1 before analysis.
Models were assessed using area under the receiver operating characteristic. Models were developed for each outcome to determine whether a full model containing morphomic and clinical variables could outperform a model using clinical variables alone. Data were analyzed using R statistical software (R Foundation for Statistical Computing, www.r-project.org/foundation/).
RESULTS
Of 209 patients, 200 had appropriate pre-transplant thoracic CTs (Table 1). The mean time from CT to transplant date was 156 days.
Table 1.
Patient Characteristics
| Variable | Value, n (%) (n=200) |
|---|---|
| Age at transplant, mean ± SD | 51.2 ± 12.58 |
| Sex, Male | 144 (72) |
| Disease category | |
| Group A (COPD) | 57 (28.5) |
| Group B (Pulmonary hypertension) | 7 (3.5) |
| Group C (Cystic fibrosis) | 26 (13.0) |
| Group D (Pulmonary fibrosis) | 110 (55.0) |
| Medical condition at transplant | |
| ICU | 21 (10.5) |
| Hospitalized | 5 (2.5) |
| Outpatient | 174 (87.0) |
| Zubrod score | |
| 1 | 82 (42.7) |
| 2 | 73 (38.0) |
| 3 | 20 (10.4) |
| 4 | 17 (8.9) |
| Pre-transplant ECMO | 9 (4.5) |
| Pre-transplant Ventilator | 15 (7.5) |
| BMI, mean ± SD | 26.1 ± 5.03 |
| Serum creatinine, mean ± SD | 0.82 ±0.22 |
| Bilirubin, mean ± SD | 0.71 ± 1.16 |
| Donor CMV+/Recipient CMV− | 60 (30) |
| %Predicted FVC, mean ± SD | 47.7 ± 19.0 |
| LAS at transplant, median (IQR) | 40.98 (IQR 36.1 –52.2) |
| Donor age, mean | 35.1 |
| Transplant organ | |
| Single | 62 (31.0) |
| Bilateral | 138 (69.0) |
| Cardiopulmonary bypass | 85 (42.5) |
| Total ischemic time (minutes), mean | 355.5 |
| Initial ventilator support >48 hours | 46 (23) |
| Postoperative ECMO | 10 (5) |
| Pneumonia | 31 (15.5) |
| Tracheostomy | 32 (16.0) |
| Post-transplant dialysis | 14 (7.0) |
| Feeding tube | 33 (16.5) |
| Induction with basiliximab | 56 (28) |
| Grade 3 primary graft dysfunction | 22 (11) |
| Acute rejection | 117 (58.5) |
| Length of stay (days), median | 17.0 |
| Acute inpatient rehabilitation | 15 (7.5) |
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary Disease; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LAS, lung allocation score; SD, standard deviation
Overall Survival
The 1 and 3-year survival rates were 89.2% and 62.5%. On univariate analysis, age (HR 1.00, p=0.67), FEV1 (HR 0.99, p=0.07), LAS post-transplant survival component at listing (HR 0.98, p=0.14), disease category (HR 0.94, p=0.43), inpatient status (HR1.11, p=0.76), ventilation pre-transplant (HR 1.15, p=0.15), ECMO pre-transplant (HR 1.80, p=0.26), albumin (HR 0.80, p=0.27), bilirubin (HR 0.90, p=0.41), donor CMV positive recipient negative (HR 1.13, p=0.60), BMI (HR 0.97, p=0.15), bilateral lung transplant (0.026), total ischemic time (HR 1.00, p=0.51), donor age (HR 1.00, p=0.71), and donor BMI (HR 1.01, p=0.83) were not associated with survival. Predictors of survival included serum creatinine (HR 2.99, p=0.04), FVC (HR 0.98, p=0.0003), post-transplant dialysis (HR 4.10, p<0.001), LD3 volume (HR 0.73 per SD, p=0.007), LD4 volume (HR 0.74 per SD, p=0.03), subcutaneous fat area (HR 0.76 per SD, p=0.03), subcutaneous fat/total body cross sectional area (HR 0.75 per SD, p=0.02), and visceral fat area/subcutaneous fat area (HR 1.25 per SD, p=0.015). The results of the multivariable model are shown in Table 2. Subgroup analysis of patients undergoing bilateral transplants resulted in a multivariate model with the same significant variables. The C index for the overall model (0.670) was better than the bilateral only model (0.666).
Table 2.
Multivariable Cox Regression (Survival)
| Variable | Hazard Ratio (95% CI) | p value |
|---|---|---|
| Subcutaneous fat area/Total body area* | 0.60 (0.453, 0.81) | 0.001 |
| LAS post-transplant survival component at listing | 0.98 (0.96, 0.99) | 0.006 |
| LD3 volume* | 0.67 (0.48, 0.92) | 0.013 |
| Serum creatinine | 4.37 (1.38, 13.78) | 0.010 |
| Percent predicted FVC | 0.98 (0.96, 0.997) | 0.021 |
| Number of days between CT and transplant | 1.002 (0.999, 1.004) | 0.14 |
Per Standard Deviation (SD)
Initial Ventilator Support Greater than 48 hours
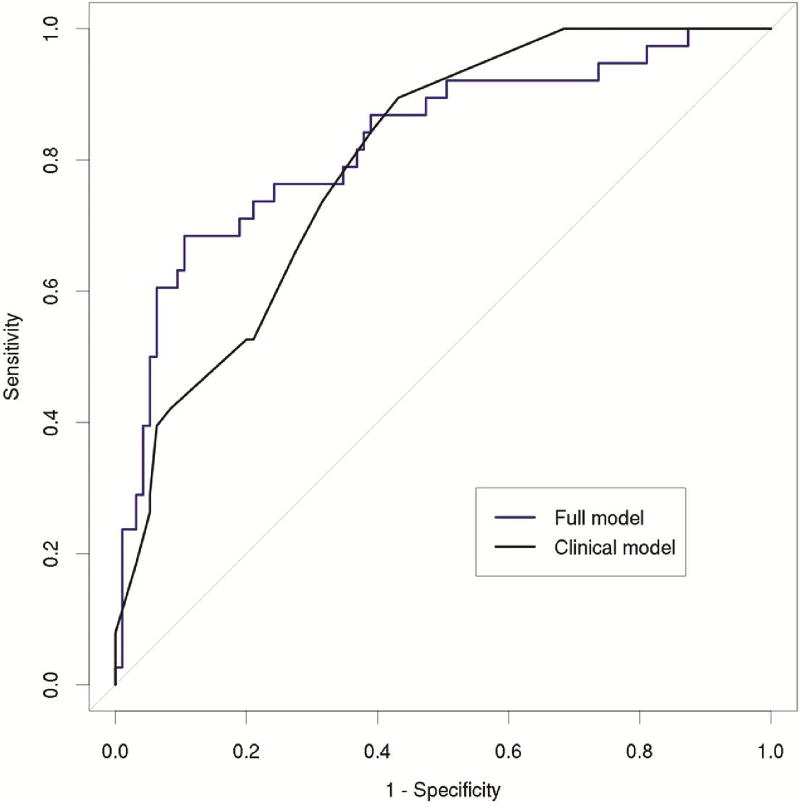
Initial ventilator support greater than 48 hours was required in 23% (46/200) of patients and was associated with inpatient status (OR 5.18, p<0.001), female gender (OR 2.23, p=0.025), Zubrod Score (OR 2.19, p=0.003), LAS score at transplant (OR 1.04, p<0.001), and increased supplemental oxygen (OR 1.09, p=0.004) but not albumin (OR 1.34, p=0.37). The associated morphomic factors were decreased distance from the vertebra to the linea alba (OR 0.54 per SD, p=0.001), decreased distance from the vertebra to the anterior skin (OR 0.58 per SD, p=0.002), decreased cross sectional area within the fascial compartment (OR 0.60 per SD, p=0.003), decreased fascial circumference (OR 0.63 per SD, p=0.009), and decreased mediastinal fat area at the carina (OR 0.64 per SD, p=0.02). The results of the multivariable model are shown in Table 3. The AUC for the full model was 0.786 compared to 0.746 using the clinical variables alone (Figure 2).
Table 3.
Multivariable Logistic Regression Initial Ventilator Support >48 Hours (AUROC 0.786)
| Variable | Odds Ratio (95% CI) | p value |
|---|---|---|
| Distance from vertebral body to linea alba* | 0.49 (0.43, 0.78) | 0.002 |
| Zubrod Score (2 vs. 1) | 1.29 (0.50, 3.36) | 0.60 |
| Zubrod Score (3 vs.1) | 2.41 (0.71, 8.18) | 0.16 |
| Zubrod Score (4 vs. 1) | 14.0 (3.81, 51.48) | 0.0001 |
| Female gender | 2.16 (0.90, 5.21) | 0.086 |
Per Standard Deviation (SD)
Figure 2.
Receiver operating characteristic curves for initial ventilator support greater than 48 hours after transplant. The full model (blue) including morphomic factors was better than the clinical model (black) alone at predicting initial ventilator support greater than 48 hours (AUROC 0.786 vs. 0.746).
Air Leak Greater than 5 Days
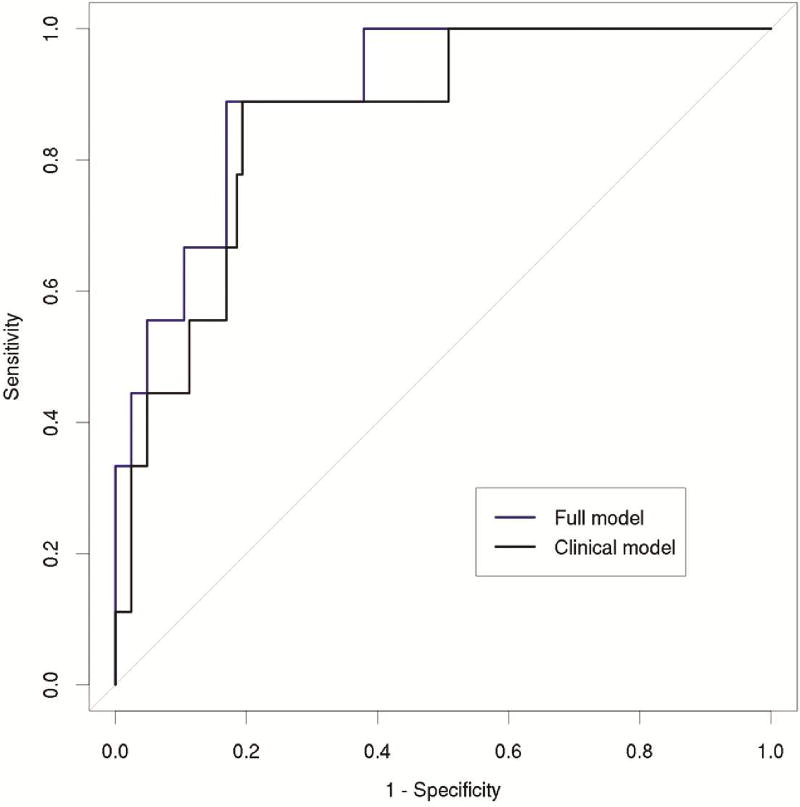
Air leak greater than 5 days was present in 6.5% (13/200) of patients. On univariate analysis, age (OR 0.99, p=0.48), gender (OR 0.45, p=0.31), LAS waitlist urgency component at transplant (OR 1.02, p=0.22), and FEV1 (OR 0.97, p=0.12) were not associated with prolonged air leak. Increased LAS post-transplant survival component at transplant (OR 1.08, p=0.05) and decreased BMI (OR 0.87, p=0.016), visceral fat area (OR 0.29, p=0.01), subcutaneous fat area (OR 0.33p=0.003), visceral fat/total body area (OR 0.34, p=0.008), and total body cross sectional area (OR 0.56, p=0.05) were associated with prolonged air leak. The results of the multivariable model are shown in Table 4. The AUC for the full model was 0.893 compared to 0.857 using the clinical variables alone (Figure 3).
Table 4.
Multivariable Logistic Regression Analysis Air Leak > 5 Days (AUROC 0.893)
| Variable | Odds Ratio (95% CI) | p value |
|---|---|---|
| Visceral fat area* | 0.17 (0.04, 0.79) | 0.023 |
| LAS waitlist urgency component at transplant | 0.986 (0.977, 0.995) | 0.002 |
| LAS post-transplant survival component at transplant | 1.17 (1.02, 1.33) | 0.025 |
| Recipient age at transplant | 1.07 (1.03, 1.14) | 0.039 |
| Female gender | 0.24 (0.04, 1.44) | 0.12 |
Per Standard Deviation (SD);
Abbreviations: LAS, lung allocation score
Figure 3.
Receiver operating characteristic curves for air leak greater than 5 days. The full model (blue) including morphomic factors was better than the clinical model (black) alone at predicting air leak greater than 5 days (AUROC 0.893 vs. 0.857).
Primary Graft Dysfunction and Acute Rejection
Grade 3 primary graft dysfunction (PGD) at 72 hours was present in 22/200 (11%) patients. On univariate analysis, the distance from the vertebra to the linea alba (p=0.018), total ischemic time (p=0.015), and mechanical ventilation prior to transplant (p=0.014) were significantly associated with primary graft dysfunction. Lung density 1 percentage (p=0.19), visceral fat area (p=0.40), subcutaneous fat area/total body fat area (p=0.10), lung density 4 percentage (0.38), and albumin (0.83) were not associated with PGD. Acute rejection occurred in 117/200 (58.5%) of patients. On univariate analysis, acute rejection was related to albumin (p=0.027) and distance from the vertebra to the skin (p=0.035). Factors that were not significantly related to acute rejection included bilirubin (p=0.15), bilateral transplant (p=0.19), donor age (p=0.24), total ischemic time (p=0.25), and induction therapy (0.38).
Length of Stay
The median length of stay was 17.0 days. On univariate analysis, variables associated with increased length of stay were decreased age (p=0.0009), bilateral transplant (p=0.0001), increased Zubrod Score (p<0.0001), LAS at transplant (p=0.0006), supplemental oxygen (p=0.0024), and cardiopulmonary bypass (p=0.0029) while albumin (p=0.088) was not. The morphomic factors associated with length of stay were bone mineral density (p=0.0005) and decreased cross sectional body area (p=0.004), body circumference (p=0.0024), fascial circumference (p=0.0122), and mediastinal fat volume (p=0.002). The results of the multivariable model are shown in Table 5. The Kendall correlation for the selected model was 0.400 (p<0.0001).
Table 5.
Length of Stay (Kendall Correlation 0.403)
| Variable | Coefficient Estimate |
p value |
|---|---|---|
| Zubrod score (2 vs. 1) | −0.26 | <0.001 |
| Zubrod score (3 vs.1) | 0.33 | <0.001 |
| Zubrod score (4 vs. 1) | 0.27 | 0.040 |
| COPD (vs. other disease categories) | 0.50 | <0.001 |
| Supplemental oxygen (L) | 0.030 | <0.001 |
| Transplant organ (Right vs. Left) | −0.09 | 0.41 |
| Transplant organ (Bilateral vs. Left) | 0.28 | 0.002 |
| Cardiopulmonary bypass | 0.14 | <0.001 |
| Bone mineral density* | −0.07 | <0.001 |
| Cross sectional body area* | −0.20 | <0.001 |
Per Standard Deviation (SD)
COMMENT
The goal of this study was to find morphomic predictors of outcomes after lung transplant utilizing standard preoperative CTs. We hypothesized that morphomic variables such as fat area, muscle mass, and bone density may serve as imaging surrogates for clinical risk factors such as frailty. Morphomic variables could also represent other clinical risk factors such as cardiovascular risk (mediastinal calcifications) and physiologic age (bone density, aortic calcifications, and muscle mass), and a combination of morphomic factors could potentially provide a better global assessment of health and functional status.
We have identified several morphomic factors associated with survival following lung transplantation. Weig and colleagues found that psoas area was significantly associated with decreased length of ICU stay after lung transplantation (6). Our group has also reported an association between lean psoas area and postoperative morbidity and mortality following esophagectomy and liver transplant (9, 10). Marquis et al. found that mid-thigh muscle cross sectional area was a better predictor of mortality than BMI in patients with COPD (11). However, most thoracic CTs used for transplant evaluation do not include the psoas or thigh muscles, and there was no significant association between the dorsal (paraspinous) muscle group area and these outcomes in the current study. This is similar to a prior study which found no association between sarcopenia of thoracic muscles and survival after lung transplant (12).
However, our analysis shows that increased subcutaneous fat area was associated with improved survival after lung transplantation. Furthermore, increases in body dimensional factors including the distance from the vertebra to the linea alba and cross sectional body area were associated with decreased incidence of prolonged ventilator support and length of stay, respectively. Increased bone density was associated with decreased length of stay. Improved survival and outcomes in patients with increased subcutaneous fat, body dimensional factors, and bone density may indicate the importance of metabolic reserve in recovery after lung transplant. Prior studies have reported decreased body mass, serum adipokines, and bone density in patients with severe COPD (13, 14).
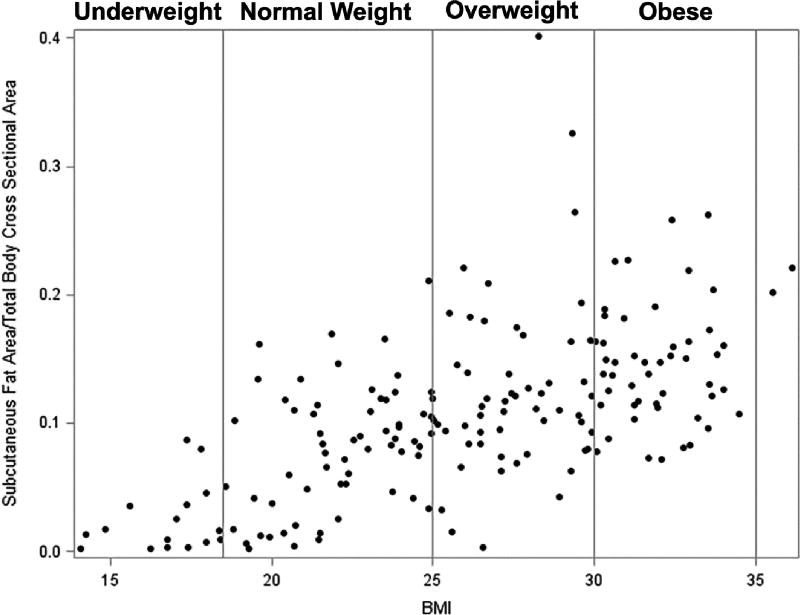
Both underweight and obese patients have increased mortality after lung transplant (15–18), and severe obesity is a relative contraindication (19). While obesity does not correlate with worse outcomes after lung resection, a low BMI<18.5 was associated with increased risk of pulmonary complications and mortality (20). BMI is incorporated into the LAS. However, the use of BMI as an indicator of body composition remains controversial (21). BMI does not accurately discriminate between muscle and fat and can misclassify patients into incorrect weight categories (22). A previous study in healthy subjects identified variation of body fat percentage and metabolic derangements associated with adiposity in patients with a normal BMI (23). We also identified variability between subcutaneous fat area/total body cross sectional area and BMI (Figure 4).
Figure 4.
Scatter plot of the relationship between subcutaneous fat area/total body cross sectional area and BMI demonstrating several patients with low subcutaneous fat/total body cross sectional area despite having a BMI in the normal range. Vertical lines mark boundaries of BMI categories: <18.5 underweight, 18.5–24.9 normal weight, 25–29.9 overweight, and >30 obese.
Subcutaneous fat is an indicator of nutritional status (24, 25), and malnutrition is a risk factor for postoperative complications (26). Lung transplant patients with severe malnutrition have decreased survival (27). Although malnutrition is a relative contraindication to lung transplantation, a patient may not undergo formal nutritional assessment unless they have a BMI outside of the normal range (28). In our study, many patients had low subcutaneous fat area despite a normal BMI (Figure 4). While patients with low subcutaneous fat area had an increased risk of mortality, there was no association between BMI and post-transplant survival. Assessment of the subcutaneous fat area as part of the pre-transplant assessment may identify patients in need of nutritional support.
Increased bone mineral density was associated with decreased length of stay, and low bone density likely reflects frailty. While bone density could be associated with age, recipient age did not correlate with survival in the current study. Increased age has been associated with worse outcomes after lung transplant and was lowest for recipients older than 65 (29). We generally limit transplants to patients less than 65, and older patients are not well-represented in this cohort with only 4 patients older than 65. Individualized assessment of body composition may demonstrate that patients older than the traditional age cutoff have a “younger” physiologic profile and could potentially be considered transplant candidates. Increasing lung density 3 (LD3) volume was also associated with improved survival. The overall cohort includes patients from all diagnosis groups, and this likely reflects that patients with less severe disease (LD3) have improved survival compared to those with more LD1 (emphysema) or LD4 and 5 (severe fibrosis). This study has several limitations.
This is a single-institution, retrospective study over several years, and patient management may have changed over time. Another limitation is that the pre-transplant CT may not reflect changes between when the CT was obtained and the transplant. Ideally the CT would be as close to the transplant date as possible, but this is difficult with the unpredictable nature of transplantation and variability in waitlist times. The study was limited to CTs within 1 year of transplant (mean 156 days). The majority were within 6 months (118 (59%)) while 25.5% were within 50 days. The scan closest to the transplant was analyzed. Higher LAS scores correlated with fewer days between CT and transplant (p<0.001, R2=0.11) suggesting that sicker patients, more likely to have a change in status, were also more likely to have a more recent CT scan. Only 10 (5%) patients were outpatient for their CT but hospitalized at the time of transplant.
The 2015 Scientific Registry of Transplant Recipients (SRTR) Annual Report showed no improvement in outcomes over the past five years highlighting the need to identify additional predictors of survival (29). This will be particularly important as increasingly sicker recipients are being transplanted. Using analytic morphomics to analyze standard chest CTs may allow for assessment of body composition related to metabolic reserve and frailty such as subcutaneous fat area and bone mineral density which may be combined with clinical factors to better select and risk stratify patients, potentially identifying those who may benefit from aggressive nutrition and rehabilitation programs, although identified factors will need to be confirmed in larger, prospective multicenter studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant. 2016;35(4):433–439. doi: 10.1016/j.healun.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: Thirty-second official adult lung and heart-lung transplantation report--2015; focus theme: Early graft failure. J Heart Lung Transplant. 2015;34(10):1264–1277. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Sabel MS, Terjimanian M, Conlon AS, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 2013;108(3):169–175. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 4.Singal AG, Zhang P, Waljee AK, et al. Body composition features predict overall survival in patients with hepatocellular carcinoma. Clin Transl Gastroenterol. 2016;7:e172. doi: 10.1038/ctg.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terjimanian MN, Harbaugh CM, Hussain A, et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant. 2016;30(3):289–294. doi: 10.1111/ctr.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weig T, Milger K, Langhans B, et al. Core muscle size predicts postoperative outcome in lung transplant candidates. Ann Thorac Surg. 2016;101(4):1318–1325. doi: 10.1016/j.athoracsur.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256(2):255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 8.Stidham RW, Waljee AK, Day NM, et al. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in crohn's disease. Inflamm Bowel Dis. 2015;21(6):1306–1313. doi: 10.1097/MIB.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheetz KH, Zhao L, Holcombe SA, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013;26(7):716–722. doi: 10.1111/dote.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Paik HC, Haam SJ, et al. Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients. J Thorac Dis. 2016;8(8):2011–2017. doi: 10.21037/jtd.2016.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fountoulis GA, Minas M, Georgoulias P, Fezoulidis IV, Gourgoulianis KI, Vlychou M. Association of bone mineral density, parameters of bone turnover, and body composition in patients with chronic obstructive pulmonary disease. J Clin Densitom. 2012;15(2):217–223. doi: 10.1016/j.jocd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Vondracek SF, Voelkel NF, McDermott MT, Valdez C. The relationship between adipokines, body composition, and bone density in men with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:267–277. doi: 10.2147/copd.s2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant. 2010;29(9):1026–1033. doi: 10.1016/j.healun.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Lederer DJ, Wilt JS, D'Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180(9):887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the united states. Am J Respir Crit Care Med. 2014;190(9):1012–1021. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upala S, Panichsillapakit T, Wijarnpreecha K, Jaruvongvanich V, Sanguankeo A. Underweight and obesity increase the risk of mortality after lung transplantation: A systematic review and meta-analysis. Transpl Int. 2016;29(3):285–296. doi: 10.1111/tri.12721. [DOI] [PubMed] [Google Scholar]
- 19.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson MK, Im HK, Watson S, Johnson E, Wigfield CH, Vigneswaran WT. Association of body mass index and outcomes after major lung resection. Eur J Cardiothorac Surg. 2014;45(4):e94–99. doi: 10.1093/ejcts/ezu008. discussion e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook JL, Lederer DJ. Selecting lung transplant candidates: Where do current guidelines fall short? Expert Rev Respir Med. 2012;6(1):51–61. doi: 10.1586/ers.11.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah NR, Braverman ER. Measuring adiposity in patients: The utility of body mass index (bmi), percent body fat, leptin. PLoS One. 2012;7(4):e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in bmi-defined normal weight subjects. Nutr Metab Cardiovasc Dis. 2012;22(9):741–747. doi: 10.1016/j.numecd.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 25.White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of nutrition and dietetics and American society for parenteral and enteral nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J Parenter Enteral Nutr. 2012;36(3):275–283. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 26.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Chamogeorgakis T, Mason DP, Murthy SC, et al. Impact of nutritional state on lung transplant outcomes. J Heart Lung Transplant. 2013;32(7):693–700. doi: 10.1016/j.healun.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Madill J, Gutierrez C, Grossman J, et al. Nutritional assessment of the lung transplant patient: Body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant. 2001;20(3):288–296. doi: 10.1016/s1053-2498(00)00315-6. [DOI] [PubMed] [Google Scholar]
- 29.Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 annual data report: Lung. Am J Transplant. 2017;17(Suppl 1):357–424. doi: 10.1111/ajt.14129. [DOI] [PubMed] [Google Scholar]