Abstract
Background
Transplant-free survival for single right ventricle (RV) lesions remains less than 70% at 3 years. Arrhythmia burden, influence of shunt type at Norwood procedure (RV-to-pulmonary artery shunt [RVPAS] versus Blalock-Taussig shunt [BTS]), and implications for mortality risk are not well defined.
Methods
The authors performed a single-center retrospective analysis of patients with single RV lesions enrolled in a prospective study of arrhythmias after congenital heart surgery.
Results
Fifty-eight patients received a RVPAS and 62 received a BTS, with a median follow-up of 773 days. Overall arrhythmia incidence was 78%, two-thirds of which prompted intervention. Among all types of arrhythmias, only ventricular arrhythmias (VAs) differed by shunt type, which were more common in patients receiving an RVPAS (29% RVPAS versus 14% BTS; p = 0.049). The majority of VAs were transient (69% less than 1 minute), and typically occurred early post-Norwood procedure (median 12 days). No additional variables were associated with development of VAs. Shunt type did not influence transplant-free survival. Within the entire cohort, there was a trend toward increased mortality with prior history of VA (odds ratio, 2.90; 95% confidence interval, 0.99 to 8.90; p = 0.052). For interstage survivors to Glenn palliation, any VA associated with a 14-fold increased risk of death or transplant (hazard ratio, 14.00; 95% confidence interval, 3.66 to 53.40; p < .001). No other tachyarrhythmia or bradyarrhythmia was associated with mortality.
Conclusions
In this cohort with single RV lesions and prospective rhythm surveillance, patients receiving an RVPAS at Norwood surgery had an increased incidence of VAs compared with patients with a BTS. VAs correlated with late mortality in patients who survived the interstage period.
Contemporary palliation of hypoplastic left heart syndrome consists of 3 staged surgeries, beginning with the Norwood procedure [1]. To allow for pulmonary blood flow from the systemic right ventricle (RV), surgical options at time of Norwood include a modified Blalock-Taussig shunt (BTS) from the innominate artery to the branch pulmonary artery or a RV-to-pulmonary artery shunt (RVPAS) [2, 3]. The multicenter, prospective, randomized SVR (Single Ventricle Reconstruction) trial has provided many insights into the differences between these approaches as well as the overall current state of the field for this serious congenital heart defect [4–6]. Nonetheless, the 3-year transplant-free survival rate remains less than 70% [7], and much opportunity remains for identifying modifiable factors contributing to mortality. In this patient population, arrhythmia burden has not been well characterized, nor has any potential association between arrhythmias and shunt type or mortality been fully elucidated. Given the functional anatomic and physiologic differences between the BTS and RVPAS, a number of mechanisms may affect outcomes. With the comparable use at our institution of either shunt type during Norwood, we retrospectively reviewed an ongoing prospectively collected database of postoperative arrhythmias. We aimed to test the primary hypothesis that shunt type affects arrhythmia incidence. As a secondary measure, we gathered data to explore the impact of arrhythmias on outcomes such as transplant-free survival.
Patients and Methods
Study Population
This study included all patients with hypoplastic left heart syndrome and morphologic single RV variants receiving Norwood surgical palliation enrolled in the ongoing prospective, observational PACS (Postoperative Arrhythmias in Congenital Heart Surgery) study at our institution between September 2007 and September 2015 [8]. The study was approved by the local Institutional Review Board, and each patient’s parents or legal guardians provided written informed consent.
Data Collection
All patients underwent continuous telemetry monitoring throughout inpatient admissions following cardiac surgery. PACS study personnel reviewed these telemetry recordings daily, and arrhythmias were identified prospectively and then confirmed or coded by pediatric electrophysiologists. Arrhythmias were classified into 1 of 8 categories (full definitions in Supplemental Table 1), and coded with respect to duration, mechanism, and any associated therapy. Postoperative recurrences of arrhythmias that had occurred preoperatively were excluded. Additionally, arrhythmias that occurred during subsequent medical admissions and during the outpatient period were identified by retrospective review of the medical record. This included a review of daily cardiology inpatient progress notes, electrocardiograms, Holter monitors, and outpatient clinic notes. All arrhythmia events were adjudicated by a pediatric electrophysiologist.
Perioperative data were collected for Norwood surgery. This included both patient demographics and intraoperative data, as well as early postoperative data. All management decisions were made at the discretion of the surgical, anesthesiology, and intensive care unit teams, including a nonrandomized choice of shunt type driven by both patient-specific variables and the shifting wide-scale preferences influenced by emerging literature over time.
Pre-Norwood procedure, ascending aorta diameter and presence of anomalous pulmonary venous return, in addition to qualitative assessment of systemic RV function and atrioventricular (AV) valve regurgitation, were based on retrospective review of echocardiogram reports. Hemodynamic data (systemic RV end-diastolic pressure) were gathered from the pre-Glenn and pre-Fontan cardiac catheterization reports.
Duration of patient follow-up was based on the most recent clinic note or inpatient admission; data collection was censored at the time of heart transplant when applicable. Follow-up of all patients closed September 2015. Cause of death was determined through review of the medical record.
Statistical Analysis
Continuous data are reported as median with interquartile range (IQR). Categorical variables are presented as frequencies with percentages. Demographic and clinical data were analyzed using the Mann-Whitney U test for continuous variables and the chi-square or Fisher’s exact test, as appropriate, for categorical variables. The odds of the development of an arrhythmia as well as the combined endpoint of death or transplant were assessed through both univariate and multivariate logistic regression analyses. The effect of shunt type on transplant-free survival was assessed using multivariate Cox regression. Univariate analysis was performed initially, and significant factors on univariate analysis were then incorporated into multivariate analysis after assessing for multicollinearity. All patients for whom full data on covariates were available were included in the analysis. All multivariate models underwent assessment of fit with the Hosmer-Lemeshow goodness-of-fit test. Data from logistic regression analyses are reported as estimated odds ratio (OR) and 95% confidence interval (CI), whereas data from Cox regression analyses are reported as estimated hazard ratio and 95% CI. Statistical analysis was performed using SPSS statistical package, version 23.0 (IBM Corporation, Armonk, NY).
Results
Arrhythmia Outcomes
From September 2007 to September 2015, 120 patients with single RV lesions were enrolled. Ninety-nine patients were classified anatomically as hypoplastic left heart, 15 patients as Shone’s complex, and 6 patients with unbalanced (right-dominant) AV septal defect. Full baseline characteristics of each cohort are delineated in Table 1. Median post-Norwood follow-up for the entire study population was 773 days (IQR, 131 to 1435). There was longer median follow-up in the BTS cohort (1002 days) compared with the RVPAS cohort (580 days), reflective of evolving local practice with relatively more recent use of the RVPAS. Nine of 120 patients were lost to follow-up.
Table 1.
Cohort Demographics at Time of Norwood Procedure
| All (N = 120) | RVPAS (n = 58) | BTS (n = 62) | p Value | |
|---|---|---|---|---|
| Age at Norwood procedure, days | 5 (3–7) | 5 (4–7) | 5 (3–8) | 0.725 |
| Gestational age at birth, weeks | 39 (37.9–39.3) | 39 (37.8–39.3) | 38.9 (37.9–39.3) | 0.86 |
| Gestational age at Norwood procedure, weeks | 39.6 (38.8–40.1) | 39.8 (39.0–40.1) | 39.5 (38.7–40.1) | 0.455 |
| Weight at Norwood procedure, kg | 3.3 (3.0–3.6) | 3.4 (2.9–3.6) | 3.3 (3.0–3.5) | 0.709 |
| Height at Norwood procedure, cm | 50 (48–52) | 50 (47–51) | 51 (49–52) | 0.125 |
| Male | 66 | 57 | 74 | 0.046 |
| Chromosomal anomaly | 16 | 19 | 13 | 0.363 |
| Noncardiac congenital anomaly | 17 | 24 | 10 | 0.034 |
| Anatomy | 0.564 | |||
| HLHS | 99 (82.5) | 50 (86.2) | 49 (79.0) | |
| Shone’s syndrome | 15 (12.5) | 6 (10.3) | 9 (9.6) | |
| Unbalanced AVSD | 6 (5) | 2 (3.4) | 4 (6.4) | |
| HLHS subtype | 0.061 | |||
| MS/AS | 29 (29.2) | 18 (36.0) | 11 (22.4) | |
| MS/AA | 39 (39.3) | 14 (28.0) | 25 (51.0) | |
| MA/AA | 31 (31.3) | 18 (36.0) | 13 (26.5) | |
| Anomalous pulmonary venous return | 9 (7.5) | 8 (13.8) | 1 (1.6) | 0.014 |
| Ascending aorta diameter, mm | 3.25 (2.10–4.95) | 3.55 (2.00–5.60) | 3.2 (2.30–4.70) | 0.739 |
| Norwood bypass time, min | 165 (144–202) | 180 (157–218) | 152 (134–182) | 0.002 |
| Norwood cross–clamp time, min | 46 (35–69) | 58 (43–78) | 37 (33–49) | <0.001 |
| Required ECMO postoperatively | 27 (22) | 13 (22) | 14 (23) | 0.983 |
| At cardiac ICU admission post-Norwood procedure (n = 99) | ||||
| Initial lactate, mmol/L | 6.1 (4.0–7.8) | 5.8 (3.9–7.4) | 6.4 (4.3–9.3) | 0.444 |
| Epinephrine infusion | 84/99 (85) | 48/53 (91) | 36/46 (78) | 0.089 |
| Milrinone infusion | 89/99 (90) | 49/53 (92) | 40/46 (87) | 0.365 |
| Dopamine infusion | 24/99 (24) | 8/53 (15) | 16/46 (35) | 0.023 |
| Calcium infusion | 57/99 (58) | 33/53 (62) | 24/46 (52) | 0.311 |
| Dexmedetomidine infusion | 14/99 (14) | 11/53 (21) | 3/46 (6) | 0.043 |
Values are median (interquartile range), %, n (%), or n/n (%). Full covariates at time of post-Norwood intensive care unit (ICU) admission available for 99 patients.
AA = ■■■; AS = ■■■; AVSD = atrioventricular septal defect; BTS = Blalock-Taussig shunt; ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; MS = ■■■; RVPAS = right ventricle-to-pulmonary artery shunt.
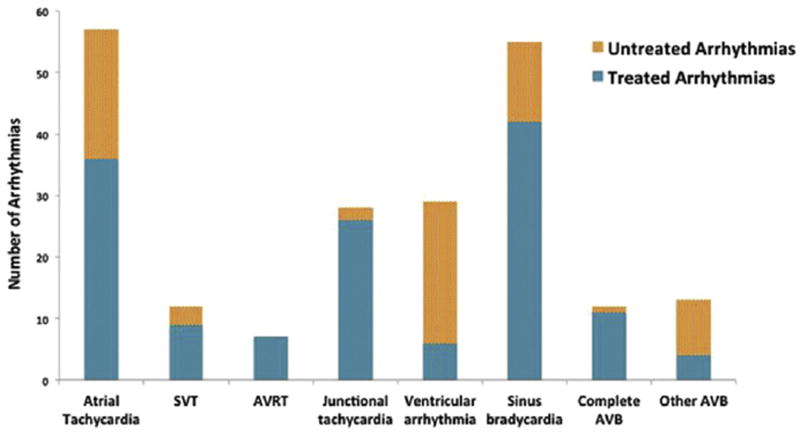
Of the 120 patients, 93 patients developed 213 different arrhythmias (median 1 arrhythmia/patient, range 0 to 7), of which 141 arrhythmias received treatment. Atrial tachycardia and sinus bradycardia were the most common types of arrhythmia, among all events as well as when including only arrhythmias receiving therapy (Fig 1). Common interventions included temporary atrial pacing and administration of antiarrhythmic drugs. Seven patients had AV reciprocating tachycardia supported by an accessory pathway (4 patients) or AV nodal reentry tachycardia (3). Fifty-two of 80 total bradyarrhythmias were transient events lasting less than 24 hours continuously; 22 of these transient events were bradycardia associated with cardiac arrest. Of these 22 arrests, 5 likely represented a primary bradycardic event. Complete (third-degree) AV block developed in 11 patients (4 following surgical AV valvuloplasty), with 12 total episodes; 6 of these patients died. One episode of complete AV block lasted less than 1 minute and did not receive treatment; the rest were supported with temporary or permanent pacing.
Fig 1.
Arrhythmia incidence. Atrial tachycardia, sinus bradycardia, and ventricular arrhythmias were the most commonly observed arrhythmias. Only 6 of 29 ventricular arrhythmias received therapy. (AVB = atrioventricular block; AVRT = atrioventricular reciprocating tachycardia; SVT = supraventricular tachycardia (unspecified).)
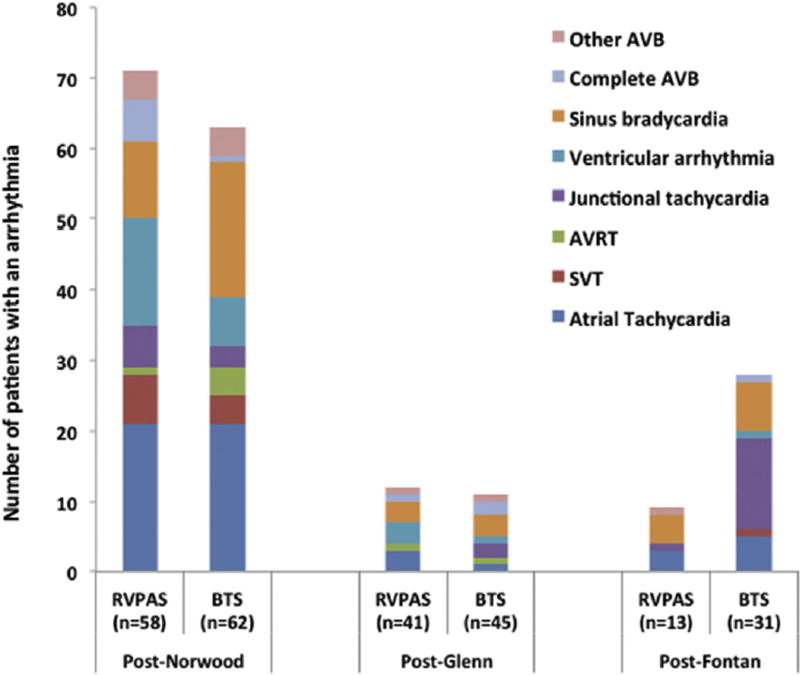
The majority of observed arrhythmias took place during the initial interstage period between the Norwood and Glenn procedures (67% of all arrhythmias). Atrial tachycardia, sinus bradycardia, and ventricular arrhythmias (VAs) were the most common arrhythmias during this time (Fig 2). Junctional tachyarrhythmias were the most commonly observed arrhythmia in the post-Fontan period, which included predominantly BTS patients in light of the previously described differential follow-up duration.
Fig 2.
Arrhythmia incidence by shunt type and stage of palliation. The majority of arrhythmias took place during the post-Norwood period. Patients were recorded multiple times for a given stage if they had multiple types of arrhythmias during that stage. (AVB = atrioventricular block; AVRT = atrioventricular reciprocating tachycardia; BTS = Blalock-Taussig shunt; RVPAS = right ventricle-to-pulmonary artery shunt; SVT = supraventricular tachycardia (unspecified).)
Arrhythmias and Shunt Type
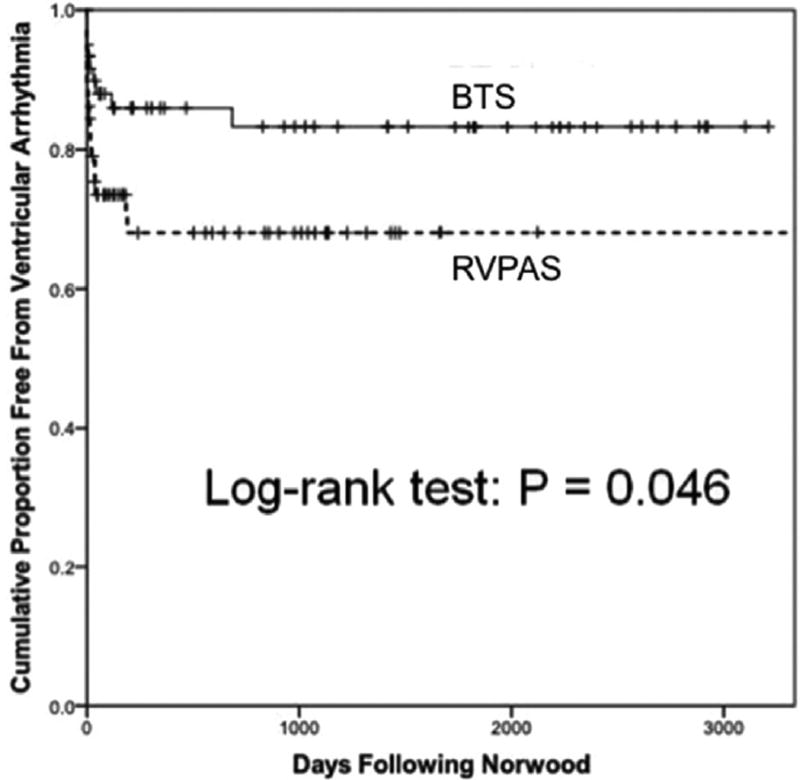
We compared arrhythmia incidence between RVPAS and BTS patients (Table 2). VAs were observed more often in the RVPAS cohort than in the BTS cohort (29% versus 14%; p = 0.049), and the RVPAS was associated with lower freedom from VA on time-to-event analysis (p = 0.046) (Fig 3). Shunt type did not associate with the incidence of cumulative tachyarrhythmias or bradyarrhythmias. Across the entire study population, 26 patients developed 29 different VAs. These included 15 with monomorphic ventricular tachycardia (VT), 1 polymorphic VT, 4 accelerated ventricular rhythm (AVR) prompting intervention, and 9 untreated AVR. The majority of VAs were transient episodes lasting less than or equal to 1 min: 11 monomorphic VT, 1 polymorphic VT, and 7 AVR. Twenty-three of 29 VAs occurred during the post-Norwood period, with most occurring fairly early following Norwood surgery; median time to VA was 10 days post-Norwood procedure among RVPAS patients (IQR, 1 to 36 days) and 13 days post-Norwood procedure among BTS patients (IQR, 1 to 79 days). Six of 29 VAs received therapy (4 AVR, 2 monomorphic VT).
Table 2.
Unadjusted Arrhythmia Frequency by Shunt Type at Norwood Procedure
| All (N = 120) | RVPAS (n = 58) | BTS (n = 62) | p Value | |
|---|---|---|---|---|
| All tachyarrhythmias | 81 (68) | 42 (72) | 39 (63) | 0.266 |
| Tachycardia received treatment | 57 (48) | 28 (48) | 29 (47) | 0.869 |
| VA | 26 (22) | 17 (29) | 9 (14) | 0.049 |
| VA received treatment | 6 (5) | 5 (9) | 1 (2) | 0.078 |
| All bradyarrhythmias | 50 (42) | 21 (36) | 29 (47) | 0.241 |
| Bradyarrhythmia received treatment | 41 (34) | 16 (28) | 25 (40) | 0.142 |
Values are n (%).
BTS = Blalock-Taussig shunt; RVPAS = right ventricle-to-pulmonary artery shunt; VA = ventricular arrhythmia.
Fig 3.
Freedom from ventricular arrhythmia by shunt type. Right ventricle-to-pulmonary artery shunt (RVPAS) patients experienced a higher rate of ventricular arrhythmias than did Blalock-Taussig shunt (BTS) patients. Most ventricular arrhythmias took place fairly early following Norwood surgery.
To determine risk factors for the development of VAs, we examined other variables in addition to shunt type. Age, gestational age, sex, weight, height, anatomy, chromosomal anomaly, noncardiac congenital anomaly, epinephrine, milrinone, dopamine, calcium infusion, dexmedetomidine, post-Norwood lactate, bypass time, cross-clamp time, and necessity for extracorporeal membrane oxygenation (ECMO) during Norwood hospitalization were not associated with VA on univariate analysis (Supplemental Table 2). Likewise, systemic RV systolic dysfunction, AV valve regurgitation, and ventricular end-diastolic pressure were not associated with development of VA (or any arrhythmia), and there was no difference between shunt types in these hemodynamic measurements. We performed multivariate logistic regression evaluating the 3 variables with p less than 0.1 in univariate analysis (shunt type, ECMO requirement, and initial lactate); however, none of the variables was retained in a stepwise logistic regression model with forward selection.
Transplant-Free Survival
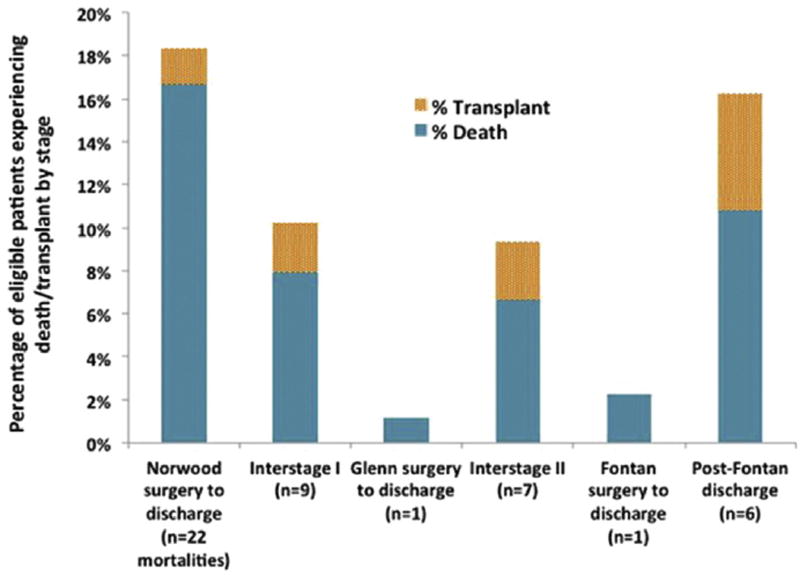
The greatest mortality occurred during the interval between Norwood surgery and post-Norwood discharge (18.3%) (Fig 4). The most common cause of death was cardiopulmonary failure (50% of all deaths), followed by death due to unknown causes (16%, includes unmonitored out-of-hospital deaths) and multisystem organ failure (13%). There was only a single patient with documented arrhythmia deemed to be the primary cause of death (junctional tachycardia leading to hemodynamic instability and cardiac arrest during the post-Fontan period).
Fig 4.
Mortality as a percentage of eligible patients with follow-up at each stage. The post-Norwood period was the time of greatest mortality in our population.
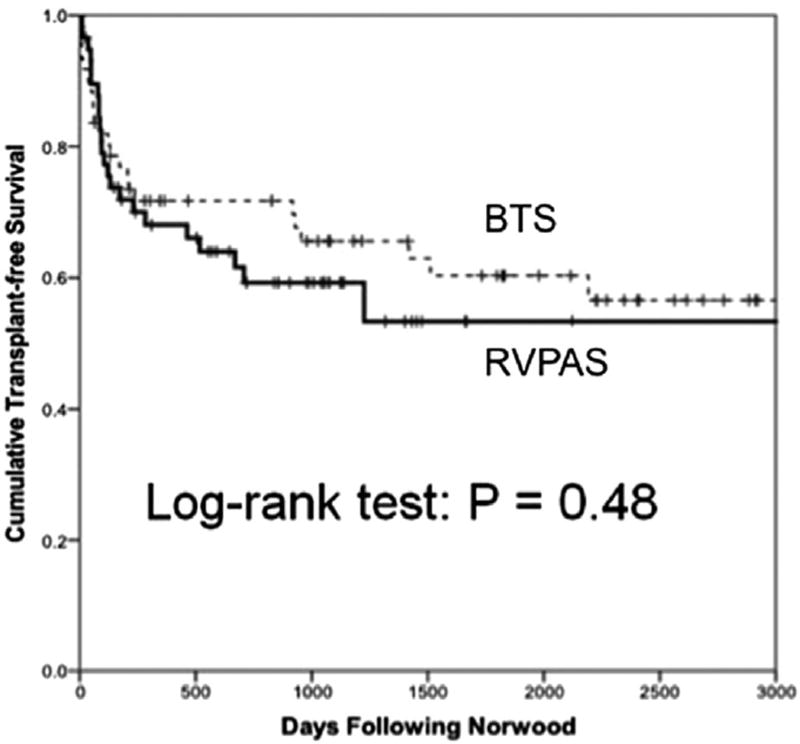
Univariate analysis of factors associated with death or transplant are summarized in Table 3. We observed 6 baseline or perioperative factors that associated with decreased transplant-free survival on univariate analysis: gestational age at birth and at Norwood, presence of a noncardiac congenital anomaly, ascending aorta diameter, Norwood bypass time, post-Norwood lactate, and necessity for ECMO during Norwood hospitalization. Sex, size at Norwood procedure, use of vasoactive drugs, use of digoxin as outpatient, serial hemodynamic parameters, and presence of a chromosomal abnormality were all nonsignificant factors for death or transplant. Notably, shunt type did not associate with transplant-free survival (40% death or transplant among RVPAS patients, 37% death or transplant among BTS patients; p = 0.773) (Fig 5). Likewise, shunt type did not associate with other outcomes during Norwood hospitalization such as ventilator days or necessity for ECMO (Supplemental Table 3).
Table 3.
Univariate and Multivariate Analysis of Death or Transplant
| Death/Transplant (n = 46) |
Alive, No Transplant (n = 74) |
Univariate p Value |
Multivariate p Value |
|
|---|---|---|---|---|
| Gestational age at birth, weeks | 38.6 (37.3–39.0) | 39 (38.0–39.4) | 0.002 | 0.79 |
| Gestational age at Norwood procedure, weeks | 39.5 (38.4–39.9) | 39.7 (39.1–40.3) | 0.02 | 0.79 |
| Noncardiac congenital anomaly | 26.1 | 10.8 | 0.029 | 0.04 |
| Ascending aorta diameter, mm | 2.45 (1.950–4.375) | 3.7 (2.50–5.225) | 0.008 | 0.02 |
| Norwood bypass time, min | 182 (163–220) | 155 (135–183) | <0.001 | 0.08 |
| ECMO during Norwood hospitalization | 35 | 15 | 0.011 | 0.76 |
| Lactate (n = 99 patients), mmol/L | 7.3 (6.0–9.6) (n = 41) | 5.2 (3.4–6.5) (n = 58) | <0.001 | <0.001 |
| Ventricular arrhythmia, any | 35 | 14 | 0.006 | 0.052 |
| Ventricular arrhythmia, treated | 11 | 1 | 0.02 | 0.62 |
Values are median (interquartile range) or %.
ECMO = extracorporeal membrane oxygenation.
Fig 5.
Cumulative transplant-free survival by shunt type. There was no difference in transplant-free survival between shunt types. (BTS = Blalock-Taussig shunt; RVPAS = right ventricle-to-pulmonary artery shunt.)
Among arrhythmias, only VAs were associated with decreased transplant-free survival on univariate analysis (p = 0.006). This observation remained consistent when limiting analysis to only treated VAs. In addition, the duration of VA correlated with rate of death or transplant: there was a progressive increase in death or transplant rate from 32% in patients with no VA to 53% in those patients with history of transient VA (<1 min) and 78% with history of sustained VA (linear-by-linear p = 0.003). Any tachyarrhythmia, tachyarrhythmia prompting treatment, any bradyarrhythmia, and bradyarrhythmia prompting treatment were all nonsignificant factors for death or transplant.
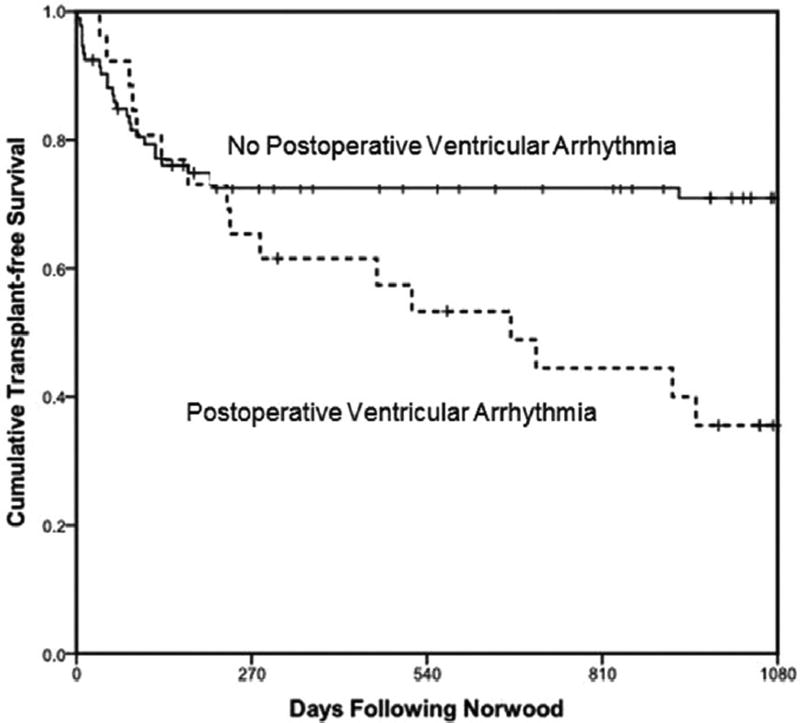
In multivariate analysis, early post-Norwood lactate (OR, 1.40; 95% CI, 1.20 to 1.70; p < .001), presence of a noncardiac congenital anomaly (OR, 3.70; 95% CI, 1.10 to 12.50; p = 0.04), and ascending aorta diameter (OR, 0.70 [as diameter increased]; 95% CI, 0.50 to 0.90; p = 0.02) were independently associated with death or transplant (Table 3). There was a trend toward increased mortality with a history of prior VA (OR, 2.90; 95% CI, 0.99 to 8.90; p = 0.052). In multivariate Cox regression analysis, VAs did not impact time to mortality when considering the entire study population (Fig 6). However, among survivors to Glenn palliation (n = 68 with complete covariate data), a history of a prior VA conferred a markedly increased risk of death or transplant (hazard ratio, 14.00; 95% CI, 3.66 to 53.40; p < .001).
Fig 6.
Cumulative transplant-free survival by presence of postoperative ventricular arrhythmia. Among survivors to Glenn palliation, a history of a prior ventricular arrhythmia conferred a 14-fold increased risk of death or transplant (hazard ratio, 14.00; p < 0.001). Median time of Glenn palliation was 129 days post-Norwood procedure.
Comment
In this single-center cohort of patients with single RV lesions and prospective postoperative arrhythmia surveillance, arrhythmia incidence was 78%. This incidence is higher than that observed in 2 prior retrospective studies analyzing either single-ventricle (right or left) patients following Norwood surgery (50% developed tachyarrhythmias) [9] or hypoplastic left heart syndrome patients (57% developed any arrhythmia) [10]. Several factors might have contributed to this difference. First, the current study included longer follow-up (median 773 days post-Norwood) than prior studies did. Also, the higher incidence of arrhythmias may be attributed in part to the prospective telemetry surveillance. Only 66% of the arrhythmias in this current cohort received therapy, indicating a substantial proportion of the arrhythmias might have been deemed clinically insignificant and thus escaped capture by retrospective review of the medical chart. This is in contrast to the retrospective cohort of Trivedi and colleagues [10], where 83% of arrhythmias received therapy. Finally, in the current study we utilized fairly broad criteria to define an arrhythmia (see Supplemental Table 1), enhancing sensitivity but decreasing what initially appears to be clinically significant specificity.
We did not observe an association between shunt type and overall arrhythmia events, nor did we see an association between selected hemodynamic parameters and arrhythmia incidence. Although the majority of arrhythmias occurred during the post-Norwood period, one-third of arrhythmias took place during the post-Glenn and post-Fontan periods, indicating these patients remain at risk of arrhythmias beyond the initial interstage period. Junctional tachyarrhythmias were the most commonly observed arrhythmia during the post-Fontan period; there was likely insufficient long-term follow-up to capture the well-described progressive development of sinus node dysfunction and atrial tachycardia late after Fontan procedure [11, 12].
However, we did observe the RVPAS was associated with the development of VAs, the majority of which occurred during the early post-Norwood period and did not prompt therapy. No other factors were found to have a similar association with VAs, including perioperative use of vasoactive drugs, other markers of perioperative instability (including lactate and ECMO), or longitudinal hemodynamic measurements. One prior study has described an association between the RVPAS and cumulative tachyarrhythmias in patients with single-ventricle lesions, although this association was not significant when limited to only single RV patients [9]. Those authors hypothesized that there may be an association between the right ventriculotomy of the RVPAS contributing to diastolic dysfunction, increased atrial pressures, and rhythm disorders; we did not observe an association among the RVPAS, RV end-diastolic pressure, and cumulative tachyarrhythmias in the current cohort. Given that many of these VAs occurred early following Norwood surgery, it is possible that the right ventriculotomy may be a contributing factor. Currently there are no longer-term studies to assess the risk of the development of macroreentrant circuits involving the ventriculotomy scar, as has been observed following tetralogy of Fallot repair [13].
Despite the fact that many of the observed VAs were transient events that did not prompt treatment, VAs were associated with death or transplant in univariate analysis. Furthermore, the strength of this association increased in stepwise fashion when considering patients with only transient VAs to those with VAs of greater than 1 minute duration. Although this relationship did not remain independently significant, the apparent late divergence of survival curves on time to event multivariate Cox regression analysis (Fig 6) prompted a post hoc analysis limited to those patients surviving the interstage period to undergo Glenn surgery. For this subset, VAs (the majority of which occurred early post-Norwood procedure) were associated with death or transplant during the late stages of follow-up (post-Glenn procedure and beyond).
The nature of this correlation between VAs and late mortality remains unclear, and there is no support for direct causality. It is likely that VAs represent increased generalized morbidity, with a number of additional independent variables more directly leading to mortality. It is noteworthy that we did not observe an association between selected hemodynamic parameters and either VAs or mortality. Not surprisingly, elevated post-Norwood lactate, presence of a noncardiac congenital anomaly, and smaller ascending aorta diameter were associated with mortality in this cohort.
Although we observed an association between (1) the RVPAS and VAs and (2) VAs and late mortality, we did not demonstrate any direct influence of the RVPAS on mortality. It is likely there were additional factors disproportionately affecting mortality in BTS patients that were not captured within our study but which served to balance the overall death or transplant rates. A previous study had observed an association between bradyarrhythmias and mortality [10], but this was not seen in our cohort. The most common cause of death in our study population was cardiopulmonary failure, consistent with data from the SVR trial [14]. Although only 1 death could be directly attributed to an arrhythmia in our cohort, arrhythmias may have contributed to unmonitored out-of-hospital deaths.
The high rate of post-Norwood mortality remains one of the most pressing problems in the treatment of single RV patients. The rate of death or transplant in our cohort was 38%, with the majority of those mortalities occurring during the post-Norwood period; these findings are consistent with the SVR trial cohort [7]. In contrast to recently published findings, we did not observe an association between digoxin use and improved transplantfree survival [15, 16]. Recent data from the SVR trial have shown that high-grade heart block was a poor prognostic factor; in our study, 6 of 11 patients with complete heart block died [17].
Limitations
There are limitations in this study that must be acknowledged. This was a single-center study encompassing 120 patients, with data derived from both prospective and retrospective components. There was a nonrandomized choice of shunt type as well as nonuniform rhythm surveillance for the retrospective component. A post hoc analysis was performed on a subset of the patients to evaluate the association between VAs and late mortality. Because of evolving local practice with only relatively more recent use of the RVPAS, shunt groups differed in duration of follow-up (BTS cohort median 1002 days versus RVPAS cohort median 580 days), limiting the ability to compare long-term rhythm outcomes by shunt type.
Conclusions
In this cohort of patients with single RV lesions and prospective rhythm surveillance, there was a high incidence of arrhythmia. Patients receiving RVPAS at the time of Norwood surgery had an increased incidence of VAs as compared with patients with BTS. VAs correlated with late mortality in patients who survived the interstage period. However, we did not observe any direct influence of shunt type on mortality.
Supplementary Material
Acknowledgments
This work was funded by the American Heart Association 12CRP10560001, the National Center for Research Resources/NIH 1 UL1 RR024975, the National Center for Advancing Translational Sciences/NIH 1 UL1 TR000445, and the National Institute of Child Health and Human Development/NIH 1R01HD084461.
Footnotes
Presented at the Heart Rhythm Scientific Sessions, San Francisco, CA, May 2016.
The Supplemental Tables can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2017.05.082] on http://www.annalsthoracicsurgery.org.
References
- 1.Norwood WI, Lang P, Casteneda AR, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1981;82:511–9. [PubMed] [Google Scholar]
- 2.Kishimoto H, Kawahira Y, Kawata H, Miura T, Iwai S, Mori T. The modified Norwood palliation on a beating heart. J Thorac Cardiovasc Surg. 1999;118:1130–2. doi: 10.1016/S0022-5223(99)70118-2. [DOI] [PubMed] [Google Scholar]
- 3.Sano S, Ishino K, Kado H, et al. Outcome of right ventricleto-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg. 2004;78:1951–7. doi: 10.1016/j.athoracsur.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, et al. Pediatric Heart Network Investigators. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Allen KR, Tabbutt S, et al. Pediatric Heart Network Investigators. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frommelt PC, Gerstengerger E, Cnota JF, et al. Pediatric Heart Network Investigators. Impact of initial shunt type on cardiac size and function in children with single right ventricle anomalies before the Fontan procedure: the single ventricle reconstruction extension trial. J Am Coll Cardiol. 2014;64:2026–35. doi: 10.1016/j.jacc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newburger JW, Sleeper LA, Frommelt PC, et al. Pediatric Heart Network Investigators. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–20. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuplock JM, Smith AH, Owen J, et al. Association between preoperative dexmedetomidine and arrhythmias after congenital heart surgery. Circ Arrhythm Electrophysiol. 2015;8:643–50. doi: 10.1161/CIRCEP.114.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFerson MC, McCanta AC, Pan Z, et al. Tachyarrhythmias after the Norwood procedure: relationship and effect of vasoactive agents. Pediatr Cardiol. 2014;35:668–75. doi: 10.1007/s00246-013-0836-8. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi B, Smith PB, Barker PC, Jaggers J, Lodge AJ, Kanter RJ. Arrhythmias in patients with hypoplastic left heart syndrome. Am Heart J. 2011;161:138–44. doi: 10.1016/j.ahj.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa R, Sherwin ED, Kovach J, et al. Mechanism and ablation of arrhythmia following total cavopulmonary connection. Circ Arrhythm Electrophysiol. 2015;8:318–25. doi: 10.1161/CIRCEP.114.001758. [DOI] [PubMed] [Google Scholar]
- 12.Balaji S, Daga A, Bradley DJ, et al. An international multicenter study comparing arrhythmia prevalence between the intracardiac lateral tunnel and the extracardiac conduit type of Fontan operations. J Thorac Cardiovasc Surg. 2014;148:576–81. doi: 10.1016/j.jtcvs.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 13.Zeppenfeld K, Schalij MJ, Bartelings MM, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241–52. doi: 10.1161/CIRCULATIONAHA.107.723551. [DOI] [PubMed] [Google Scholar]
- 14.Ohye RG, Schonbeck JV, Eghtesady P, et al. Pediatric Heart Network Investigators. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–14. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oster ME, Kelleman M, McCracken C, Ohye RG, Mahle WT. Association of digoxin with interstage mortality: results from the Pediatric Heart Network Single Ventricle Reconstruction Trial public use dataset. J Am Heart Assoc. 2016;5:002566. doi: 10.1161/JAHA.115.002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DW, Mangeot C, Anderson JB, et al. National Pediatric Cardiology Quality Improvement Collaborative. Digoxin use is associated with reduced interstage mortality in patients with no history of arrhythmia after stage I palliation for single ventricle heart disease. J Am Heart Assoc. 2016;5:002376. doi: 10.1161/JAHA.115.002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mah DY, Cheng H, Alexander ME, et al. Heart block following stage 1 palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2016;152:189–94. doi: 10.1016/j.jtcvs.2016.03.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.