Abstract
Intermittent fasting (IF) is a term used to describe a variety of eating patterns in which no or few calories are consumed for time periods that can range from 12 hours to several days, on a recurring basis. Here we focus on the physiological responses of major organ systems, including the musculoskeletal system, to the onset of the metabolic switch – the point of negative energy balance at which liver glycogen stores are depleted and fatty acids are mobilized (typically beyond 12 hours after cessation of food intake). Emerging findings suggest the metabolic switch from glucose to fatty acid-derived ketones represents an evolutionarily conserved trigger point that shifts metabolism from lipid/cholesterol synthesis and fat storage to mobilization of fat through fatty acid oxidation and fatty-acid derived ketones, which serve to preserve muscle mass and function. Thus, IF regimens that induce the metabolic switch have the potential to improve body composition in overweight individuals. Moreover, IF regimens also induce the coordinated activation of signaling pathways that optimize physiological function, enhance performance, and slow aging and disease processes. Future randomized controlled IF trials should use biomarkers of the metabolic switch (e.g., plasma ketone levels) as a measure of compliance and the magnitude of negative energy balance during the fasting period.
Keywords: Weight Loss, Lipid Metabolism, Ketone Bodies, Caloric Restriction, Body Composition
Introduction
Calorie restriction (CR), a reduction in caloric intake without malnutrition, has consistently been found to produce reductions in body weight and extend healthy life span across a variety of species,(1) including non-human primates.(2) Studies conducted in overweight humans indicate short-term CR (6-months) can significantly improve several cardiovascular risk factors, insulin-sensitivity, and mitochondrial function.(3) Thus, there is emerging evidence from human clinical trials to indicate in overweight adults, CR may have a number of beneficial effects, in addition to weight loss. Unfortunately, findings from obesity intervention trials over the past several decades indicate the vast majority of humans have significant difficulty sustaining daily CR for long periods of time.(4)
In recent years, intermittent fasting (IF) has gained popularity as an alternative to continuous CR and has shown promise in delivering similar benefits in terms of weight loss and cardiometabolic health.(5–9) In contrast to traditional caloric restriction paradigms, IF is a dietary approach that requires fasting for varying periods of time, typically for 12 hours or longer.(6, 10, 11) For example, alternate-day fasting (ADF) is a form of IF where individuals alternate between not consuming any calories for one day and eating without restriction the next.(8) Similarly, alternate-day modified fasting (ADMF) is a form of IF in which individuals alternate between consuming few calories one day (< 25% energy needs) and eating without restriction the next.(12) Table 1 defines the various terms used to describe different types of IF regimens discussed in this review.
Table 1.
Definition of terms used to describe different types of eating patterns in this review.
| Intermittent Fasting (IF) | This eating pattern involves fasting for varying periods of time, typically for 12 hours or longer. |
| Calorie Restriction (CR) | This eating pattern involves a continuous reduction in caloric intake without malnutrition. |
| Time Restricted Feeding (TRF) | This eating pattern involves restricting food intake to specific time periods of the day, typically between an 8 – 12 hours each day |
| Alternate Day Fasting (ADF) | This eating pattern involves consuming no calories on fasting days and alternating fasting days with a day of unrestricted food intake or “feast” day. |
| Alternate Day Modified Fasting (ADMF) | This eating pattern involves consuming less than 25% of baseline energy needs on “fasting” days, alternated with a day of unrestricted food intake or “feast” day. |
| Periodic Fasting (PF) | This eating pattern consists of fasting only 1 or 2 days/week and consuming food ad libitum on 5 to 6 days per week. |
Placing time restrictions on feeding has been shown to have broad systemic effects and trigger similar biological pathways as caloric restriction.(5) For example, IF regimens have been shown to improve cardio-metabolic risk factors (such as insulin resistance, dyslipidemia and inflammation cytokines),(13) decrease visceral fat mass,(6) and produce similar levels of weight loss as CR regimens.(8) In addition to the weight loss effects and metabolic improvements, several other beneficial effects of therapeutic fasting have been described including improvements in lipid profiles,(14) osteoarthritis,(15) healing of thrombophlebitis,(15) healing of refractory dermal ulcers, (16) and tolerance of elective surgery.(17)
One key mechanism responsible for many of these beneficial effects appears to be “flipping” of the metabolic switch. But what is this metabolic switch and how is it flipped? Here, we define the metabolic switch as the body’s preferential shift from utilization of glucose from glycogenolysis to fatty acids and fatty acid-derived ketones. The reason we use the word ‘preferential’ is because there is now a growing body of research to indicate ketones are the preferred fuel for both the brain and body during periods of fasting and extended exercise.(18, 19) Of relevance to weight management, this switch represents a shift from lipid synthesis and fat storage to mobilization of fat in the form of free fatty acids (FFAs) and fatty-acid derived ketones. For this reason, many experts have suggested IF regimens may have potential in the treatment of obesity and related metabolic conditions, including metabolic syndrome and type 2 diabetes.(20)
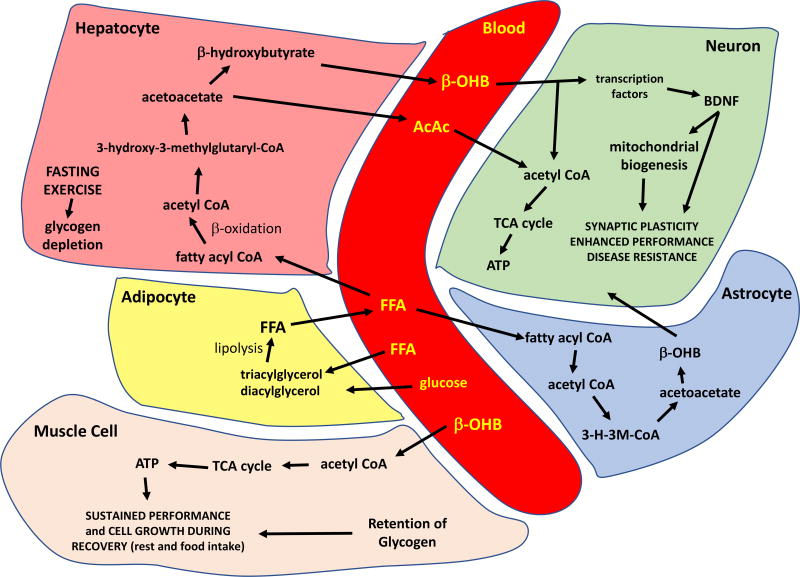
The metabolic switch typically occurs in the third phase of fasting when glycogen stores in hepatocytes are depleted and accelerated adipose tissue lipolysis produces increased fatty acids and glycerol.(21) The metabolic switch typically occurs between 12 to 36 hours after cessation of food consumption depending on the liver glycogen content at the beginning of the fast, and on the amount of the individual’s energy expenditure/exercise during the fast. The lipids in adipocytes (triacylglycerol and diacylglycerol) are then metabolized to FFAs, which are released into the blood (Figure 1). Simultaneously, other cell types may also begin generating ketones, with astrocytes in the brain being one notable example. FFAs are transported into hepatocytes where they are metabolized by β-oxidation to produce the ketones β-OHB and acetoacetate, which may in turn induce mitochondrial biogenesis.(22)
Figure 1.
Summary of the major metabolic pathways involved in the metabolic switch and responses of excitable cells to the ketone β-hydroxybutyrate (β-OHB). See text for description. AcAc, acetoacetate; ATP, adenosine triphosphate; FFA, free fatty acids; TCA, tricarboxylic acid.
The ketones are transported in high amounts into cells with high metabolic activity (muscle cells and neurons) where they are metabolized to acetyl coenzyme A, which then enters the tricarboxylic acid (TCA) cycle to generate ATP. Through these physiological processes, ketones serve as an energy source to sustain the function of muscle and brain cells during fasting and extended periods of physical exertion/exercise.(21) Thus, it appears when the metabolic switch is flipped, the primary energy source for the body shifts from glucose to FFA derived from adipose tissue lipolysis and ketones, which serve to preserve muscle. In support of this, retention of lean mass is increased following IF regimens for weight loss as compared to continuous CR regimens in humans.(8) Additionally, in mice, the decline in muscle mass that occurs during normal aging is prevented by time restricted feeding (TRF) involving 40% caloric restriction.(23)
There are a few potential mechanisms through which a shift to fatty acid and ketone oxidation, relative to glucose oxidation, may serve to preserve muscle mass. Muscle cells store triglycerides in lipid droplets providing a local source of fatty acids that are utilized for β-oxidation and ketone generation during periods of prolonged fasting and extended exercise (see Nakamura et al., 2014 for review).(24) The transcriptional regulator PPAR-α induces the expression of genes that mediate fatty acid oxidation in muscle cells, and also regulates muscle cell mitochondrial biogenesis and glucose metabolism. PPAR-α gene targets that mediate a shift in muscle cell fuel preference from glucose to fatty acids during fasting and endurance exercise include the fatty acid translocase CD36, fatty acid binding protein 3, mitochondrial uncoupling protein 3, PGC-1α, pyruvate kinase dehydrogenase 4 and forkhead box O1A. Mice with a muscle cell-specific knockout of the PPAR-α gene exhibit reduced numbers of oxidative fibers in their tibialis muscle,(25) whereas overexpression of PPAR-α results in increased numbers of oxidative fibers.(26) Mice lacking PGC-1α only in skeletal muscle are exercise intolerant and their muscle cells do not exhibit functional adaptations to exercise.(27) The emerging evidence therefore suggests critical roles for metabolic switch-associated signaling pathways in both acute functional adaptations to bioenergetic challenges and the long-term increases in muscle growth and endurance capacity that accrue from intermittent metabolic switching.
Based on the findings described above, many experts have proposed IF regimens can improve body composition in overweight individuals; however, the effects of this dietary approach on body weight and body composition in humans are not currently well understood. In this paper, we briefly review the evolutionary underpinnings of optimal brain and body function in the fasted state and historical experience with fasting in humans. We then review the effects of different types of IF regimens on cellular, systemic, and performance-based outcomes from pre-clinical studies. In the final section, we review findings from human clinical trials that have tested the effects of IF regimens on changes in body composition, cardiometabolic health, and performance outcomes in humans.
Evolutionary Perspective
Many animals in the wild regularly experience extended time-periods with little or no food. For example, packs of wolves living in the Northern Rocky Mountains of the United States typically kill prey, such as deer, elk or bison only once every one or two weeks. Their success depends upon their brains and bodies functioning at a high level so they can work with their pack mates to ‘formulate’ and execute a strategy to capture and kill the prey animal.(28) In order to survive in such environments, animals have to possess the ability to quickly shift their metabolism from lipidogenesis (fat storage) to fat mobilization for energy through fatty acid β-oxidation. This metabolic flexibility enables individuals to store energy in the form of lipids in fat depots when food is available, and then perform at a high level during extended periods when food is not available.(29) Accordingly, those individuals whose brains and bodies performed optimally in a food deprived/fasted state would have a survival advantage.
Knowledge of early human evolution and data from recent studies of hunter-gatherer societies suggest humans evolved in environments where they intermittently experienced extended time-periods with little or no food.(30, 31) Indeed, one can reasonably hypothesize the superior cognitive capabilities of humans evolved as adaptations that enabled invention of tools and methods for hunting, animal domestication, agriculture and food storage, and processing.(32) To our knowledge, however, there have been no studies in which plasma ketone levels have been measured, or the frequency and duration of metabolic shifts determined, in current-day hunter-gatherers.
Fasting in Humans: Historical Perspective
Historically, fasting has been used as both a religious and a medical practice for thousands of years. Fasting for medical purposes has been suggested since the time of ancient Chinese, Greek and Roman physicians.(33) Throughout the millennia, many have recommended fasting for medical reasons. For example, Benjamin Franklin has been quoted as saying “The best of all medicines is resting and fasting.”(34) Similarly, Mark Twain wrote “A little starvation can really do more for the average sick man than can the best medicines and the best doctors. I do not mean a restricted diet; I mean total abstention from food for one or two days.”(35)
Initial scientific studies conducted in 1914 utilized fasting for the treatment of both type 1 and type 2 diabetes.(36),(37) Subsequently, numerous studies suggested fasting as a treatment for type 2 diabetes. Genuth(38) described a case report of a severely obese woman who had resolution of her diabetes following four weeks of fasting; significantly, her glucose tolerance remained normal for over a year after she regained the lost weight. Jackson et al.(39) found an improvement in glucose tolerance following 17–99 days of fasting, and the improvement in glucose and insulin metabolism was not related to the weight lost during the fast or the weight regained following the fast. Several additional studies found an improved insulin sensitivity and glucose tolerance in people with diabetes immediately following a fast.(40) (41–46)
These potential benefits, however, must be weighed against potential risks as there have also been numerous adverse effects reported in the medical literature from “therapeutic fasting.” These include: nausea and vomiting,(47) edema,(48) alopecia and motor neuropathy,(14) hyperuricemia and urate nephropathy,(49) irregular menses,(49) abnormal liver function tests and decreased bone density,(17) thiamine deficiency and Wernicke’s encephalopathy,(50, 51) and mild metabolic acidosis.(52) Additionally, several deaths have been reported during or immediately following therapeutic fasting with the etiologies including lactic acidosis, small bowel obstruction, renal failure, and cardiac arrhythmias.(53)
The adverse events described above have only occurred during or following extended fasts of several weeks or more, and have not been reported in trials of shorter more frequent fasts such as the ‘5:2’ diet, which consists of eating no more than 500 calories on two days per week (7) ADF diets, or IF diets.(8, 54) Thus, although therapeutic fasting has been popular as recently as the 1950s and 1960s,(21) the potential severity of adverse events following therapeutic fasting limited its use in traditional medicine and led to the development of protein sparing modified fasts that are still in use today. It is only over the last decade that scientific interest in the benefits of short-term fasting approaches, such as IF or ADF, in humans has returned, in part due to the positive findings found with IF in animal models (described below).
Adaptive Cellular and Molecular Responses to Fasting in Animal Models
Three IF protocols that have been most thoroughly studied in laboratory mice and rats are ADF, TRF (8 – 12 hour feeding window each day) and a very low calorie diet three consecutive days/week (4:3 IF). In addition, we include examples of IF studies in which rats or mice were maintained on 30–40% daily CR because they typically consume their entire daily food allotment within a 3 – 6 hour time window of receiving it, and thus fast for at least 18 hours/day.(55, 56) The observed reduction in body fat and elevation of ketone levels in these mice strongly indicates the metabolic switch occurs in animals on 30–40% CR.(57, 58)
An early study showed when rats are maintained on ADF beginning at 10 months of age, their average lifespan is increased by 30% compared to littermate rats fed ad libitum.(59) The ADF rats maintained a lower body weight and, when provided with running wheels, maintained a high level of daily running compared to control rats fed ad libitum. To our knowledge, similar lifespan studies have not been performed on rodents on either TRF or 4:3 IF diets. All three IF eating patterns, however, produce elevations in circulating ketone levels, by amounts and for time periods that are determined by the IF regimen, indicating the metabolic switch is turned on intermittently.(60, 61) In this section, we compare and contrast results of analyses of blood and tissue samples for different organ systems of mice or rats maintained on one of these IF regimens in comparison with control animals fed ad libitum.
Circulating Biomarkers
Compared to control animals fed ad libitum, rats or mice maintained on ADF and/or IF exhibit reduced plasma glucose, insulin and leptin levels, and elevated ketone and adiponectin levels, which are most pronounced on the fasting days.(60, 62–64) Mice fed a high fat diet ad libitum develop obesity, elevated plasma glucose, insulin and leptin levels, and impaired glucose tolerance; TRF mostly normalizes these adverse effects of the high fat diet.(61, 65) Compared to mice fed ad libitum, mice on the 4:3 IF diet also exhibit reductions in plasma glucose levels and elevations of ketone levels, which are most pronounced at the end of the fasting period.(66) Interestingly, levels of insulin-like growth factor 1 (IGF-1) are reduced at the end of the three day fasting period in mice on the 4:3 diet,(66) whereas IGF-1 was not reduced, and even increased, in mice on the ADF diet.(60) In the 4:3 IF diet study the animals lost weight during the IF period, whereas in the ADF study the mice did not lose weight. The reduced IGF-1 levels may result from a long-term negative energy balance in animals on the 4:3 diet, whereas an elevation of IGF-1 levels in mice on ADF may mediate the maintenance of lean mass.
Liver
During the first several hours of food deprivation, and until liver glycogen stores are depleted, glycogenolysis in hepatocytes generates glucose for extrahepatic tissues.(21) The most thorough studies of molecular and biochemical responses of hepatic cells to IF are those of mice on TRF fed either a normal or a high fat diet.(61, 65) Early studies of TRF were aimed at understanding how the timing of food intake affects circadian rhythms.(67, 68) TRF (4 hour feeding period every day) normalized circadian rhythms, improved glucose regulation and reduced weight gain in mice.(68) Similarly, 8 hours/day TRF prevented high fat diet-induced obesity in mice,(65) and 9 hours/day TRF lowered glucose levels, increased insulin levels and insulin sensitivity in a rat model of type I diabetes.(69)
In mice fed a high fat diet, TRF normalizes the expression of genes involved in fatty acid metabolism (Fasn), β-oxidation (Pparγ) and antioxidant defenses (Sod1) in the liver.(61) TRF accentuates diurnal rhythms in the expression of many different genes in liver cells including those encoding Per2, Bmal1 and Cry1. TRF also completely prevents the adverse effects of a high fat diet on the circadian rhythm of phosphorylation CREB (cyclic AMP response element-binding protein), a transcription factor that plays a critical role in gluconeogenesis during fasting.(61) TRF also completely prevents the accumulation of lipids in the liver that occurs in mice maintained on a high fat diet.(61) Moreover, multiple markers of inflammation (TNF-α, IL-6 and IL-1β) are reduced in livers of mice on TRF, and metabolomic analyses indicate multiple alterations in liver metabolites (palmitate, oleate and palmitoleate), bioenergetic pathway molecules (glucose-6-phosphate, citrate and opthalmate), and the antioxidant reduced glutathione, caused by a high fat diet are reversed by TRF.(61) However, IF diets have not always resulted in improvements in health indicators. For example, compared to young male rats fed a high fat diet ad libitum, those fed the same diet for only three hours each day for five weeks exhibited increased insulin resistance despite having reduced amounts of body fat.(70)
The transition in transcriptional programs that occurs in liver cells in response to the metabolic switch is regulated, in part, by sirtuins.(71) SIRT1 suppresses glucose production through inhibiting CRTC2 -mediated gluconeogenesis. Time-course analyses during the fasting period showed SIRT1 is activated during the metabolic switch from glycogenolysis to ketone production, which leads to deacetylation and degradation of CRTC2.(29) In addition, SIRT1 represses lipolysis and cholesterol synthesis by regulating the activity of cholesterol catabolic pathways.(29) SIRT1 acts as a positive regulator of liver oxidation of fatty acids. It increases the rate of fatty acid oxidation by deacetylating PGC-1α(72) and by activating peroxisome proliferators-activated receptor α (PPARα).(73) PPARα promotes fatty acid β oxidation in both the mitochondria and peroxisomes. In addition, SIRT1 interacts with the hepatocyte-derived hormone fibroblast growth factor 21 (FGF21) to coordinate an adaptive response to fasting by inducing ketogenesis, preventing hepatic steatosis and controlling energy expenditure.(74)
Overexpression of SIRT1 in mice, however, did not enhance the ability of ADF to decrease adiposity and increase insulin sensitivity,(75) suggesting there are additional pathways through which IF triggers the metabolic switch. Mitochondrial SIRT3 is critical for fatty acid oxidation and ketogenesis during fasting. It directly regulates the acetylation state and activity of mitochondrial enzymes involved in the metabolic switch including acetyl CoA synthetase 2, long chain acyl-CoA dehydrogenase, ornithine transcarbamoylase and a subunit of Complex 1 in the mitochondrial electron transport chain.(76–78) Mice lacking SIRT3 exhibit hallmarks of fatty-acid oxidation disorders during fasting such as reduced ATP levels and intolerance to cold exposure.(78) Shimazu et al. (2010)(79) found SIRT3 is also necessary for ketogenesis in the hepatocytes; they showed SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 (HMGCS2) which is the rate-limiting enzyme of β-hydroxybutyrate production during fasting. As a consequence, SIRT3 knockout mice have lower plasma ketone levels during fasting.
Muscle
Given that skeletal muscle is a major consumer of energy and utilizes ketones during prolonged fasting, there have been surprisingly few studies in which the effects of IF on muscle tissue have been evaluated at any level, from molecular and biochemical to functional. This is perhaps due to the emphasis on the metabolic responses of muscles to exercise, from which a vast literature has emerged. Several studies have reported changes in gene expression in muscles within 6–12 hours of the onset of food deprivation; the genes include FOXO1, PGC-1α, MyoD and uncoupling protein 3.(80, 81) Interestingly, the decline in muscle mass that occurs during normal aging in mice is prevented by 40% CR/TRF, and this beneficial effect on muscle is associated with increased activity in the 2–3 hours prior to feeding and improved performance in agility tests.(23) When mice are maintained on a high-fat diet they become obese when fed ad libitum, but when maintained on ADF they do not become obese and retain muscle mass.(82) Moreover, mice on a low fat diet gain more muscle when on ADF compared to mice fed the same diet ad libitum.(82)
Accumulating evidence suggests some organ systems exhibit similar cellular and molecular responses to aerobic exercise and IF (e.g., suppression of mTOR, stimulation of autophagy, and mitochondrial biogenesis).(83, 84) It is therefore informative to briefly summarize major responses of muscle cells to exercise before reviewing effects of fasting on muscles. Acetylcholine released from presynaptic terminals of motor neurons activates nicotinic receptors on the muscle cell membrane resulting in membrane depolarization and Ca2+ influx through voltage-dependent channels and Ca2+ release from the endoplasmic (sarcoplasmic) reticulum. The Ca2+ then engages multiple downstream signals including kinases (e.g., CaMKII) and phosphatases (e.g., calcineurin), SIRT1 and SIRT3, reactive oxygen species, AMPK, and the transcription factors CREB, FOXO1, FOXO3 and PGC-1α.(85) Similar to exercise, fasting for time-periods sufficient to flip the metabolic switch results in the activation of AMPK in muscle cells which, in turn, can activate SIRT1.(86) Consequently, gene expression programs and posttranslational modifications of extant proteins that promote mitochondrial biogenesis, autophagy and cellular stress resistance are activated, while mTOR and overall protein synthesis are suppressed.
IF and exercise stimulate mitochondrial biogenesis and mitochondrial stress resistance in muscle cells by mechanisms involving the second messenger Ca2+ and an increase of the AMP/ATP ratio. The elevation of cytoplasmic Ca2+ levels that mediates muscle contraction also activates Ca2+ /calmodulin-dependent protein kinase IV which, in turn, activates the transcription factor CREB. The increase in the AMP/ATP ratio activates AMPK. Both CREB and AMPK up-regulate expression of PGC-1α, a master regulator of the transcription of multiple genes which encode proteins that mediate the division and growth of mitochondria.(87) As in liver cells, fasting induces the expression of SIRT3 in skeletal muscle cells, and SIRT3 knockout mice exhibit reduced activities of AMPK and CREB, and reduced expression of PGC-1α.(88) SIRT3 also protects muscle cells against oxidative stress by deacetylating and activating SOD2. Data also suggest fasting stimulates autophagy in muscle cells by mechanisms involving AMPK-mediated inhibition of mTOR signaling and up-regulation of the autophagy-promoting proteins FOXO3a and ULK1.(89) AMPK also contributes to the increased sensitivity of muscle cells to insulin that occurs in response to IF and exercise. Thus, mice deficient in AMPKα2 are resistant to the insulin-sensitizing effect of a TRF diet in which they are provided an amount of food equivalent to 60% of their ad libitum intake.(90) Moreover, mice with muscle-specific AMPK knockout exhibit hyperglycemia and impaired gluconeogenesis, which may result from impaired autophagy in muscle cells.(91)
Cardiovascular System
Studies of rats and mice have documented robust effects of IF and/or ADF on heart rate and blood pressure. In one study, transmitters were implanted in rats that enabled continuous monitoring of heart rate and blood pressure in their home cages.(92) After 48 hour baseline recordings were acquired, the rats were assigned to either ad libitum control diet or an ADF diet and recordings were made at designated time periods during a six month period. In rats on the ADF diet, but not the ad libitum diet, resting heart rate and blood pressure decreased progressively during the first month of ADF and were thereafter maintained at the lower levels (350 beats/min in ad libitum (AL) rats versus 250 beats/min in ADF rats; mean blood pressure of 120 mm Hg in AL rats and 90 mm Hg in IF rats). Moreover, rats on ADF exhibited superior cardiovascular stress adaptation as indicated by reduced blood pressure and heart rate elevation during and after one hour of immobilization stress.(92) However, it was reported that male rats maintained on ADF for six months exhibit reduced cardiac reserve,(93) although it is unclear whether this is related to a pathological process or is associated with a long-term reduction in cardiac load due to the reduced blood pressure and heart rate in response to ADF.(63)
Heart rate variability is also increased in response to ADF, as a result of enhanced parasympathetic tone.(93) The effects of ADF on heart rate, blood pressure and heart rate variability are very similar to the effects of endurance training, suggesting similar underlying mechanisms.(94) Interestingly, the effects of IF on cardiovascular regulation result from BDNF-mediated enhancement of activity of cholinergic cardiovagal neurons in the brainstem dorsal motor nucleus of the vagus.(95) Because exercise is known to be a potent inducer of BDNF expression in the brain,(96) studies are needed to determine whether brainstem BDNF signaling also mediates the beneficial effects of endurance training on heart rate and blood pressure.
Central Nervous System
The decline in cognitive function with age is forestalled in mice maintained on 40% CR/TRF.(97, 98) For example, an early study showed 40% CR/TRF prevented age-related decrements in motor performance (rotarod test) and maze learning (14 unit T-maze) in mice.(99) Although the cellular and molecular mechanisms by which IF enhances cognitive and motor performance have not been established, the shift to ketone utilization appears to be one of the key biological mechanisms that prevents age-related reductions in brain white matter integrity, and preserves spatial memory.(97)
Emerging findings also suggest other possible mechanisms through which IF can maintain or even enhance cognitive function during aging. For example, both normal weight and obese mice maintained for three months on 40% CR/TRF exhibit an increased density of dendritic spines on hippocampal dentate granule neurons, and this increase in synapse numbers is correlated with an increased level of brain-derived neurotrophic factor (BDNF).(100) It is well known that BDNF plays fundamental roles in learning and memory and also mediates the anxiolytic and antidepressant effects of exercise and antidepressant drugs.(96, 101) BDNF signaling may play important roles in the enhancement of synaptic plasticity by IF, as well as in the production of new neurons from stem cells (neurogenesis) in the hippocampus.(102) Recent findings suggest β-OHB also has important signaling functions involving activation of transcription factors. In neurons, β-OHB induces the expression of BDNF, which, in turn stimulates mitochondrial biogenesis and the formation of new synapses. In these ways, events triggered by the metabolic switch may play major roles in the optimization of performance of the brain and body by IF.
In addition to BDNF, studies have shown ADF increases the expression of fibroblast growth factor 2, heme oxygenase 1 and glucose regulated protein 78 in the cerebral cortex and striatum; these proteins are known to protect neurons against excitotoxic, metabolic and oxidative stress.(103) Studies of mice with a conditional knockout of CREB in the hippocampus and forebrain have demonstrated a critical role for this transcription factor as a mediator of the cognition-enhancing effects of IF (40% CR/TRF).(104) Additional findings suggest 50% CR/TRF can prevent age-related increases in DNA methylation, which may thereby sustain the expression/responsivity of genes involved in adaptive neuroplasticity and cognition.(105) Collectively, the available data suggest IF affects multiple transcription-coupled signaling pathways that result in bolstering of synaptic plasticity and neuronal stress resistance.
Recent studies of laboratory animals provide further support that cognitive function and physical performance are enhanced by IF. For example, when mice were maintained on an ADF diet for 6–8 months, their performance in two different learning and memory tests (an operant conditioning task and a novel object recognition task) was significantly improved compared to control mice fed daily.(106) Similarly, mice maintained on ADF for 11 months exhibited superior cognitive ability in the Barnes maze test of spatial memory.(107) Another study found when old rats (20 months old) were maintained on an ADF diet for three months their locomotor performance on a rotarod test, as well as their learning and memory performance on a water maze test, were significantly improved.(108) Thus, findings from pre-clinical trials suggest IF can improve cognitive and locomotor performance even when initiated late in life.
Numerous studies have shown ADF can protect neurons in the brain against dysfunction and degeneration in animal models of a range of different neurological disorders including epilepsy,(109) Alzheimer’s disease,(110) and Parkinson’s disease (111) and stroke (112). For example, mice maintained on ADF for three months prior to transient occlusion of the middle cerebral artery (an animal model of focal ischemic stroke) exhibit highly significant reductions in brain damage and neurological deficits, and a reduction in stroke-induced reactive neurogenesis.(112) The underlying mechanisms likely involve both activation of adaptive stress response signaling pathways in neurons by neurotrophic factors and neurotransmitters,(112) and circulating factors. It is well established that the ketone β-OHB can suppress epileptic seizures, and emerging findings suggest β-OHB may also play a role in the neuroprotective effects of IF in animal models of Alzheimer’s and Parkinson’s diseases and stroke. As evidence, it was reported that β-OHB stimulates transcription of the gene encoding BDNF in hippocampal neurons.(113) With regards to future translational studies it will be of considerable interest to determine whether IF is beneficial when initiated after the onset of symptoms in animal models first, and then in human patients.
Impact of Intermittent Fasting on Changes in Body Composition in Humans
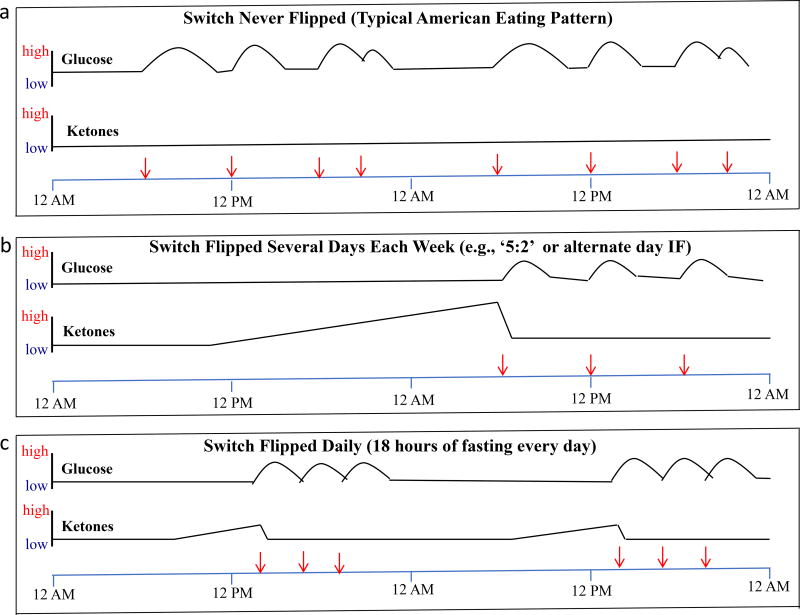
Individuals with a typical Western eating pattern of three or more meals per day never flip the metabolic switch and thus their ketone levels remain continuously low. Additionally, as their insulin resistance increases with excess weight and diabetes, the time it takes to flip the switch is prolonged (Figure 2). The different IF eating patterns described in this article all flip the metabolic switch with varying frequencies and durations (Figure 2). Compared to an eating pattern in which food is consumed over long time intervals (typically 12 or more hours daily), IF eating patterns may result in a wide range of beneficial effects on health including improved glucose metabolism,(7, 10, 114–116) reduced inflammation,(117, 118) reduced blood pressure,(12, 75, 115, 119) improved cardiovascular health,(120–123) and increased resistance of cells to stress and disease in humans (Figure 3).(118, 124) These effects have been clearly established in animal studies as described above, but only some of these adaptations to IF have been investigated in humans, and then typically in overweight or obese subjects.
Figure 2.
Profiles of circulating glucose and ketone levels over 48 hours in individuals with a typical American eating pattern or two different IF eating patterns. (a) In individuals who consume three meals plus snacks every day the metabolic switch is never ‘flipped’ and their ketone levels remain very low, and the area under the curve for glucose levels is high compared to individuals on an IF eating pattern. (b) In this example, the person fasted completely on the first day and then at three separate meals on the subsequent day. On the fasting day ketones are progressively elevated and glucose levels remain low, whereas on the eating day ketones remain low and glucose levels are elevated during and for several hours following meal consumption. (c) In this example the person consumes all of their food within a 6-hour time window every day. Thus, the metabolic switch is flipped on following 12 hours of fasting and remains on for approximately six hours each day, until food is consumed after approximately 18 hours of fasting. Modified from Mattson et al 2016.(9)
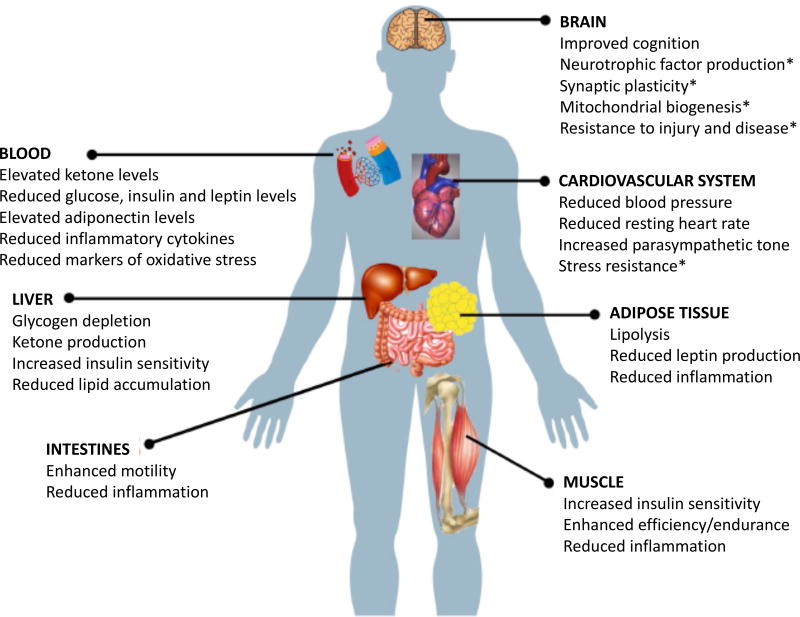
Figure 3.
Examples of functional effects and major cellular and molecular responses of various organ systems to IF. In humans and rodents, IF results in decreased levels of circulating insulin and leptin, elevated ketone levels, and reduced levels of pro-inflammatory cytokines and markers of oxidative stress. Liver cells respond to fasting by generating ketones and by increasing insulin sensitivity and decreasing lipid accumulation. Markers of inflammation in the intestines are reduced by IF. The insulin sensitivity of muscle cells is enhanced and inflammation reduced in muscle cells in response to the metabolic switch triggered by fasting and exercise. Emerging findings further suggest that exercise training in the fasted state may enhance muscle growth and endurance. Robust beneficial effects of IF on the cardiovascular system have been documented including reduced blood pressure, reduced resting heart rate, increased heart rate variability (improved cardiovascular stress adaptation) and resistance of cardiac muscle to damage in animal models of myocardial infarction. Studies of laboratory animals and human subjects have shown that IF can improve cognition (learning and memory); the underlying mechanisms may involve neurotrophic factors, stimulation of mitochondrial biogenesis and autophagy, and the formation of new synapses. IF also increases the resistance of neurons to stress and suppresses neuroinflammation. *Demonstrated in animal models, but not yet evaluated in humans.
Many of the beneficial metabolic and health effects of IF described above may be driven by reductions in body weight and/or body fat. With caloric restriction, approximately one-fourth to one-third of the weight loss is known to be of lean tissue. In a study of 34 healthy men randomly assigned to either a normal control diet or daily TRF (16 hours of daily fasting) and followed for two months during which they maintained a standard resistance training program, the men in the TRF group showed a reduction in fat mass with retention of lean mass and maximal strength.(125) Although findings from the latter and several other previous studies suggest a smaller proportion of lean mass is lost following IF regimens compared to continuous CR for weight loss,(8, 54) there is a need for more research in this area as this approach may not be optimal for all adults and patient populations. This is also a particularly important question for older adults, due to the loss of muscle mass associated with both aging (sarcopenia) and in response to caloric restriction.(126, 127)
In order to gain a better understanding of the effects of IF regimens on changes in body composition, we reviewed the effects of both TRF and ADF regimens on body composition in humans based on findings from clinical trials completed over the past couple decades. Our preset inclusion criteria were the following: 1) interventional clinical trials, 2) sample sizes of at least 10 participants, 3) intervention periods of four weeks or longer, 4) inclusion of adult participants (>18 years), 5) objective measures of body weight and body composition pre- and post-intervention, and 6) article written in the English language. We identified these trials by searching PubMed using the following terms: “fasting” and/or “intermittent fasting” and/or “alternate day fasting” and/or “time restricted feeding” and/or “alternate day modified fasting.” Filters were set to allow only “clinical trials,” “human” studies, and “English” studies to be displayed.
For IF studies involving TRF, defined as fasting for periods of at least 12 hours but less than 24 hours, three of the four eligible studies reported significant reductions in body weight and fat mass. No significant changes in lean mass were reported for any study, which suggests the observed weight losses were primarily comprised of body fat. The magnitude of weight loss, however, was relatively small (< 5.0 kg) in all studies. Table 2 presents findings from clinical trials that examined TRF.(122, 125, 128–130)
Table 2. Time Restricted Feeding (12<×<24 hrs).
Summary and results from clinical trials on time restricted feeding.
| Study | Participants | Duration (Weeks) |
BMI (kg/m2) |
Intervention | Methods | Δ Fat mass (kg) |
Δ Fat Mass (%) |
Δ Lean Mass | Δ Body Weight (kg) |
Δ Body Weight (%) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Carlson et al. (2007)(128) Stote et al (2007)(129) | 10 women, 5 men (Aged 45.0±0.7) | 16 | 23.4±0.5 | Time restricted feeding (TRF). | 1 meal per day during 4-hour time period. | ↓ 2.1kg*** | ↓ 13.0%*** | ↑ 1.5kg | ↓ 1.4kg** | ↓ 2.1%** |
|
| ||||||||||
| Klempel et al. (2012)(122) | 54 obese women (Aged 35–65) | 8 | 30–39.9 | IF and caloric restriction (CR) at 30% total daily caloric needs with either liquids (L) or food (F). | IF CR-L or IF CR-F for 6 days then 1 day fast with only 120kcal of juice powder. | CR-L: ↓ 2.8±1.2kg*** | N/A | CR-L: ↓ 3.9±1.4kg*** | CR-L: ↓ 4.1±1.5%*** | |
| CR-F: ↓ 1.9±0.7kg*** | CR-F: ↓ 2.5±.6kg*** | CR-F: ↓ 2.6± 4%*** | ||||||||
|
| ||||||||||
| Moro et al. (2016)(125) | 34 resistance trained (RT) males (Aged 29.21±3.8) | 8 | n/a | TRF with a 16 hour fasting period. | 100% daily caloric needs in 3 meals within 8 hours (1pm,4pm,8pm). Fast remaining 16 hours. | ↓ 1.62±1.53kg*** | ↓ 16.4%*** | ↑ 0.64kg | ↓ 0.97±1.58kg* | ↓ 1.2%* |
|
| ||||||||||
| Tinsley et al. (2017)(130) | 18 RT men (Aged 22.9±4.1) | 8 | TRF 4 days a week with a 16 hour fasting period. | Unrestricted energy consumption on RT days (3), unrestricted energy consumption in a 4-hour window on other days (4). | ↓ 0.6kg | ↓ 3.4% | ↓ 0.2kg | ↓ 1.0kg | ↓ 0.6% | |
Note.
TRF: time restricted feeding; IF: intermittent fasting; CR: calorie restriction; L: liquids; F: food; RT: resistance trained
p≤.05
p≤.01
p≤.001
For IF studies involving ADMF or ADF, the vast majority (9 of 10) trials tested ADMF interventions with only one trial testing an ADF intervention. Significant reductions in body weight and fat mass were observed in 10 of the 10 eligible trials, and the magnitude of weight losses were quite large with half (5 of 10) of these trials reporting clinically meaningful reductions in body weight (> 5.0 kg). Noteworthy, the five trials that did not report a clinically meaningful weight reduction were of a short-term duration (8–12 weeks), and all five of these trials reported significant weight losses of 3.0 kg or more. The reported effects on lean mass were mixed with three of the 10 eligible trials reporting significant reductions in lean mass. Based on these findings, ADMF regimens appear to produce consistent reductions in body weight and body fat mass but have less consistent effects on changes in lean mass. Table 3 presents findings from clinical trials that examined ADMF and ADF regimens.(12, 54, 119, 131–135)
Table 3. Alternate Day Modified Fasting.
Summary and results from clinical trials on alternate day modified fasting and alternate day fasting.
| Study | Participants | Duration (Weeks) |
BMI (kg/m2) |
Intervention | Methods | Δ Fat mass (kg) |
Δ Fat Mass (%) |
Δ Lean Mass | Δ Body Weight (kg) |
Δ Body Weight (%) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Bhutani et al. (2013)(131) | 64 obese males and females Aged 42±2 (ADMF), 45±5 (combo) | 12 | 35±1 | Alternate day modified fast (ADMF) | ADMF: alternated between fasting days (25% baseline calories) and ad libitum on feast days. | ADMF: ↓ 2.0±1.0kg** | ADMF: ↓ 4.7%** | ADMF: ↓ 1.0±1.0kg | ADMF: ↓ 3.0±1.0kg*** | ADMF: ↓ 3.2%*** |
|
| ||||||||||
| Catenacci et al. (2016)§(54) | 26 healthy obese subjects (Aged 39.6±9.5) | 8 | ADF: 35.8±4 | Alternate day fast (ADF) | ADF: alternated ad libitum feeding and fasting days without food intake | ↓ 3.7±0.5kg*** | ↓ 1.1±0.3%** | ↓ 3.2±0.6kg*** | ↓ 8.2±0.9kg*** | ↓ 8.8±0.9%*** |
|
| ||||||||||
| Eshghinia&Mohammadzadeh (2013)(132) | 15 overweight and obese females (Aged 33±6) | 6 | 33.16±5.02 | Modified fast consuming 25–30% energy needs. | Consumed 25–30% energy needs diet on 3 weekly fasting days (Sat, Mon and Thursday) and consumed a diet of 1700–1800 kcal/d on feast days. | N/A | ↓ 2.8%*** | ↓ 6.0kg*** | ↓ 7.1%*** | |
|
| ||||||||||
| Hoddy et al. (2016)(133) | 54 (47 females, 7 male) | 8 | 34±1 | ADMF consuming 25% energy needs. | Alternating 25% baseline energy needs on fast day, ad libitum on feast days. | Tertile 1: ↓ 2kg*** | Tertile1: ↓ 6±1%*** | Tertile 1: ↓ 1kg*** | Tertile 1: ↓ 3.7kg*** | Tertile 1: ↓ 4±1%*** |
| Tertile 2: ↓ 2kg*** | Tertile 2: ↓ 6±1%*** | Tertile 2: ↓ 2kg*** | Tertile 2: ↓ 3.8kg*** | Tertile 2: ↓ 4±1%*** | ||||||
| (Aged 47±3, 46±2, 47±2) | Tertiles based on degree of insulin resistance. | Tertile 3: ↓ 2kg*** | Tertile 3: ↓ 5±1%*** | Tertile 3: ↓ 1kg*** | Tertile 3: ↓ 3.8kg*** | Tertile 3: ↓ 4±1%*** | ||||
|
| ||||||||||
| Hoddy et al. (2014)(119) | 74 obese | 8 | L: 35± 1 | ADMF consuming 500 calories. | ADMF consuming 500 calories at either lunch or dinner and ad libitum on feast days. | L: ↓ 1.8kg*** | L: ↓ 4.3%*** | L: ↓ 1.3kg*** | L: ↓ 3.5±0.4kg*** | L: ↓ 3.8±0.5%*** |
| (Aged 45±3 lunch (L), 46±2 dinner (D) | D: 34 ± 1 | D: ↓ 2.6kg*** | D: ↓ 6.2%*** | D: ↓ 1.4kg*** | D: ↓ 4.1±0.5kg*** | D: ↓ 4.2±0.5%*** | ||||
|
| ||||||||||
| Klempel et al. (2013)(134) | 35 obese (Aged 25–65) | 8 | 30–39.9 | ADMF consuming 25% energy needs. | ADMF: High Fat (HF) or Low Fat (LF). Alternating between 25% and 125% energy needs. | HF: ↓ 5.4±1.5kg*** | HF: ↓ 11.1%*** | HF: ↑ 1.1±1.3kg | HF: ↓ 4.3±1.0kg*** | HF: ↓ 4.8±1.1%*** |
| LF: ↓ 4.2±0.6kg*** | LF: ↓ 8.8%*** | LF: ↑ 0.5±0.7kg | LF: ↓ 3.7±0.7kg*** | LF: ↓ 4.2±0.8%*** | ||||||
|
| ||||||||||
| Trepanowski et al. (2017) (135) | 100 overweight and obese subjects | 26 | 35 ± 4 | ADMF consuming 25% daily energy needs. | ADMF: 6 months weight loss alternating 25% and 125% of caloric needs. | ↓ 4.2kg* | ↓ 11.1%* | ↓ 1.5kg | ↓ 6.5kg* | ↓ 6.8%* |
| 86 females, 14 males (Aged 44±11) | 52 | Additional 6 months maintenance alternating 50% and 150% of caloric needs. | ↓ 2.0kg | ↓ 5.3% | ↓ 0.9kg | ↓ 5.7kg* | ↓ 6.0%* | |||
|
| ||||||||||
| Varady et al. (2013)(12) | 32 (Aged 35–65) | 12 | 20–29.9 | ADMF consuming 25% daily energy needs. | ADMF: 25% daily caloric intake and ad libitum on feast days. | ↓ 3.6±0.7kg*** | ↓ 13.8%*** | ↓ 1.6 kg | ↓ 5.2±0.9kg*** | ↓ 6.5±1.0%*** |
|
| ||||||||||
| Varady et al. (2009)(123) Klempel et al. (2010)(145) | 16 obese males and females (Aged 46±2) | 8 | 34 ± 1 | ADMF consuming 25% daily energy needs. | ADMF: 25% daily energy needs and ad libitum on feast days. | ↓ 5.4±0.8kg* | ↓ 3.0%* | ↓ 0.1±0.1 kg | ↓ 5.6±1.0kg*** | ↓ 5.8±1.1%*** |
|
| ||||||||||
| Varady et al. (2015) (146) | 29 overweight women (Aged: LF: 43.2±2.3 HF: 42.4±3.0) | 8 | LF: 34.4±8 | ADMF consuming 25% energy needs. | Alternating 25% daily energy needs (1 meal) in either a LF (25% fat) or HF (45% fat) diet and ad libitum on feast days. | LF: ↓ 1.5 kg* | LF: ↓ 4.1%* | N/A | LF: ↓ 4.3 kg* | LF: ↓ 4.9%* |
| HF: 34.6±7 | HF: ↓ 2.9kg* | HF: ↓ 8.0%* | HF: ↓ 4.7 kg* | HF: ↓ 5.3%* | ||||||
Note.
Alternate day fasting which included no food intake;
ADMF: alternate day modified fasting; ADF: alternate day fasting; CR: calorie restriction; L: lunch; D: dinner; HF: high fat; LF: low fat
p≤.05
p≤.01
p≤.001
Impact of Intermittent Fasting on Changes in Cardio-Metabolic Health in Humans
In this section, we briefly review the emerging findings on the effects of IF on cardiometabolic outcomes, in humans. Metabolic syndrome, a combination of insulin resistance, hypertension and abdominal obesity, is a major risk factor for atherosclerotic heart disease and consequent myocardial infarction and heart failure.(136) Each of these defining features of the metabolic syndrome is mitigated by IF in rats and mice,(92, 93) and in models of type 2 (insulin resistance-mediated) diabetes.(63, 69, 137) An emerging literature indicates IF can also ameliorate many of the key features of the metabolic syndrome in humans by decreasing fasting glucose, fasting insulin, and insulin resistance.(8, 138)
In most studies, IF regimens have been shown to reduce overall fat mass and visceral fat both of which have been linked to increased diabetes risk.(139) IF regimens ranging in duration from 8 to 24 weeks have consistently been found to decrease insulin resistance.(12, 115, 118, 119, 122, 123, 131, 132, 134, 140) In line with this, many, but not all,(7) large-scale observational studies have also shown a reduced risk of diabetes in participants following an IF eating pattern. For example, in a study conducted by Horne and colleagues,(120) a series of patients in Utah undergoing coronary artery cauterization were surveyed for the practice of periodic fasting (a common religious practice in the Mormon Church) and for the presence of diabetes based on diagnosis and medication use. Participants who reported periodically fasting had a significantly lower odds ratio for having diabetes compared to those who did not report fasting regularly. In line with this, IF regimens (both TRF and ADMF) have been found to decrease fasting glucose levels by 3 to 6% in individuals with prediabetes.(12, 123, 134) IF regimens, however, do not appear to affect fasting glucose levels in healthy individuals.(141)
In most studies, IF regimens have been shown to reduce traditional cardiovascular risk factors. Reductions in total cholesterol typically range from 6 to 21%, with LDL cholesterol decreasing by 7 to 32% following IF interventions.(12, 115, 118, 122, 123, 134) Similarly, IF regimens have also been shown to decrease triglyceride levels by between 16 to 42% in the majority of studies tested.(12, 115, 118, 119, 122, 123, 131, 132, 134, 140) Significant reductions in systolic (3 to 8%) and diastolic blood pressure (6 to 10%) have also been reported following 6 to 24 weeks of IF, though these changes appear to be driven by weight loss as significant reductions in blood pressure were only reported in studies in which participants lost 6% or more of their body weight.(12, 115, 123, 132)
Not all human trials, however, have demonstrated beneficial effects of TRF on cardiometabolic health indicators. For example, in a small study of middle-age adults, participants were provided three full meals of food corresponding to their estimated daily caloric intake within a 4-hour period (4 – 8 PM) each day for two months. Compared to when participants ate the same three meals spaced at typical breakfast lunch and dinner times, there were very few significant differences in health indicators during a two-month diet period.(129) Where significant, some changes were positive (reduced fat mass and HDL cholesterol) and some were negative (increased blood pressure and LDL cholesterol). When glucose tolerance tests were performed in the morning on the same participants, those on TRF exhibited poorer glucose tolerance compared to participants eating three regularly spaced meals.(128) The latter difference, however, was likely due to the fact the glucose tolerance test was performed within 12 hours of consumption of a full day’s amount of calories as indicated by elevated glucose levels, but normal levels of insulin, leptin, ghrelin and adiponectin in the TRF group.(128)
Impact of Intermittent Fasting on Cognitive and Performance Outcomes in Humans
Human trials of IF that include cognitive and physical performance outcomes are unfortunately limited. Studies of cognition and mood during extended fasts, however, suggest few or no adverse effects, and improvements in performance in some cognitive domains including executive function have been reported.(142–144) In regards to physical performance, a recent randomized controlled trial of IF (20 hours of fasting 4 days/week) during one month of resistance training in men demonstrated superior improvements in upper and lower body endurance in the IF group compared to the control group.(130) Clearly, many additional controlled trials of long-term IF protocols in which brain function and physical performance are evaluated in human subjects are needed to confirm the evidence from evolutionary considerations and anthropological studies.
Future Directions
While our review suggests IF results in both weight and fat loss (even when caloric intake is not limited), as well as increased insulin sensitivity in overweight subjects, there remains an important need for randomized controlled trials (RCTs) of IF in normal weight subjects. Emerging findings indicate that IF when combined with resistance training can produce beneficial changes in body composition and strength in young, healthy males. Additional studies are needed to better understand the effects of combining IF with resistance training on body composition and strength outcomes in other populations.
There remains an important need for interventions that can improve unhealthy changes in body composition that occur during aging. Given the known loss of lean mass that occurs during both aging and continuous CR, IF regimens may be an effective approach to help older adults lose unhealthy weight while retaining larger amounts of lean mass.
When taken together with animal studies, the medical experience with fasting, glucose regulation and diabetes strongly suggests IF can be effective in preventing type 2 diabetes. Thus, RCTs of IF with end points that focus on disease outcomes and/or performance outcomes (e.g., endurance and cognition) should be pursued. The feasibility of such approaches should also be carefully evaluated in such trials, as individuals in some previous trials have reported high levels of hunger and potential discomfort on fasting days.(124, 140)
Of high relevance to the feasibility question, we suggest that future randomized controlled IF trials should use biomarkers of the metabolic switch (e.g., plasma ketone levels) as a measure of compliance and the magnitude of negative energy balance during the fasting period. It is critical for this switch to occur in order to shift metabolism from lipidogenesis (fat storage) to fat mobilization for energy through fatty acid β-oxidation. Moreover, based on the growing body of research that indicates ketones are the preferred fuel for both the brain and body,(18) we hypothesize that once the metabolic switch occurs, both the tolerability and sustainability of IF regimens are greatly increased. Experimental studies, however, are needed to distinguish the effects of caloric restriction versus the metabolic switch induced by IF regimens on fat metabolism, hunger, and overall tolerability.
As the health benefits and therapeutic efficacies of IF in different disease conditions emerge from RCTs, it is important to understand the current barriers to widespread use of IF by the medical and nutrition community and to develop strategies for broad implementation. One argument against IF is that, despite the plethora of animal data, some human studies have failed to show such significant benefits of IF over CR.(54) Adherence to fasting interventions has been variable, some short-term studies have reported over 90% adherence,(54) whereas in a one year ADMF study the dropout rate was 38% vs 29% in the standard caloric restriction group.(135)
There are many reasons some individuals experience difficulties adhering to IF regimens, but most notably is the fact that very few studies ensured that “the switch” was truly flipped and ketosis was achieved. Although some individuals have difficulties in adherence, the majority of studies show similar dropout or non-adherence rates in the treatment and control groups. Finally, despite the many longer-term studies with very few adverse events, safety remains a potential concern. If findings of the research outlined above provide additional support for the metabolic benefits of IF, however, it is foreseeable in the near future that healthcare professionals may recommend IF regimens to patients who are overweight, insulin resistant, and/or hypertensive.
Acknowledgments
Drs. Anton and Leeuwenburgh are supported by the University of Florida’s Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740). Drs. Moehl, Marosi, and Mattson are supported by the National Institute on Aging Intramural Research Program.
We would like to acknowledge both Ju Han Lee (graduate student at the University of Florida) and Madison Goetze (undergraduate student at the University of Florida) for their assistance in putting together the tables and editing this manuscript.
Footnotes
Disclosure: The authors declare no conflict of interest
Reference List
- 1.Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24(6):742–5. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- 2.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14(2):275–87. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheen AJ. The future of obesity: new drugs versus lifestyle interventions. Expert Opin Investig Drugs. 2008;17(3):263–7. doi: 10.1517/13543784.17.3.263. [DOI] [PubMed] [Google Scholar]
- 5.Anton S, Leeuwenburgh C. Fasting or caloric restriction for healthy aging. Exp Gerontol. 2013;48(10):1003–5. doi: 10.1016/j.exger.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164(4):302–11. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–47. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12(7):e593–e601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arguin H, Dionne IJ, Senechal M, et al. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause. 2012;19(8):870–6. doi: 10.1097/gme.0b013e318250a287. [DOI] [PubMed] [Google Scholar]
- 11.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23(6):1048–59. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12(1):146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72(5):308–18. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- 14.Rooth G, Carlstrom S. Therapeutic fasting. Acta Medica Scandinavica. 1970;187(6):455–63. doi: 10.1111/j.0954-6820.1970.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor T, Wells DG. Metabolic hazards of fasting. American Journal of Clinical Nutrition. 1969;22(8):1142–9. doi: 10.1093/ajcn/22.8.1142. [DOI] [PubMed] [Google Scholar]
- 16.Spencer IO. Death during therapeutic starvation for obesity. Lancet. 1968;1(7555):1288–90. doi: 10.1016/s0140-6736(68)92299-x. [DOI] [PubMed] [Google Scholar]
- 17.Fasting and obesity. British Medical Journal. 1978;1(6114):673. [PMC free article] [PubMed] [Google Scholar]
- 18.Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25(2):262–84. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13–20. doi: 10.1080/17461391.2014.959564. [DOI] [PubMed] [Google Scholar]
- 20.Varady KA, Hellerstein MK. Do calorie restriction or alternate-day fasting regimens modulate adipose tissue physiology in a way that reduces chronic disease risk? Nutr Rev. 2008;66(6):333–42. doi: 10.1111/j.1753-4887.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 21.Cahill GF. Fuel Metabolism in Starvation. Annu Rev Nutr. 2005;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 22.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55(11):2211–28. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van NK, Rusli F, van DM, et al. Behavioural changes are a major contributing factor in the reduction of sarcopenia in caloric-restricted ageing mice. J Cachexia Sarcopenia Muscle. 2015;6(3):253–68. doi: 10.1002/jcsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–44. doi: 10.1016/j.plipres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Schuler M, Ali F, Chambon C, et al. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4(5):407–14. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17(15):2299–301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 27.Handschin C, Chin S, Li P, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282(41):30014–21. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 28.MacNulty DR, Tallian A, Stahler DR, Smith DW. Influence of group size on the success of wolves hunting bison. PLoS One. 2014;9(11):e112884. doi: 10.1371/journal.pone.0112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker AK, Yang F, Jiang K, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–17. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crittenden AN, Schnorr SL. Current views on hunter-gatherer nutrition and the evolution of the human diet. Am J Phys Anthropol. 2017;16263(Suppl):84–109. doi: 10.1002/ajpa.23148. [DOI] [PubMed] [Google Scholar]
- 31.De Vynck JC, Anderson R, Atwater C, et al. Return rates from intertidal foraging from Blombos Cave to Pinnacle Point: Understanding early human economies. J Hum Evol. 2016;92:101–15. doi: 10.1016/j.jhevol.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Mattson MP. Challenging oneself intermittently to improve health. Dose Response. 2014;12(4):600–18. doi: 10.2203/dose-response.14-028.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastner M. Fasting. In: Kastner M, Burroughs H, editors. Alternative healing: The complete AZ guide to over 160 different alternative therapies. Las Mesa: Halcyon Publishing; 1993. pp. 92–93. [Google Scholar]
- 34.A Dictionary of Thoughts. Tyron Edwards; 1908. p. 339. [Google Scholar]
- 35.Mark Twain. My Debut as a Literary Person. 1903 [Google Scholar]
- 36.Allen FM. Studies Concerning Diabetes. 1914;63:939–43. [Google Scholar]
- 37.Fitz R. The Treatment of Diabetes Mellitus. Medical Clinics of North America. 1923;7:649–67. [Google Scholar]
- 38.Genuth SM. Insulin secretion in obesity and diabetes: an illustrative case. Annals of Internal Medicine. 1977;87(6):714–6. doi: 10.7326/0003-4819-87-6-714. [DOI] [PubMed] [Google Scholar]
- 39.Jackson IM, McKiddie MT, Buchanan KD. Effect of fasting on glucose and insulin metabolism of obese patients. Lancet. 1969;1(7589):285–7. doi: 10.1016/s0140-6736(69)91039-3. [DOI] [PubMed] [Google Scholar]
- 40.Greenfield M, Kolterman O, Olefsky JM, Reaven GM. The effect of ten days of fasting on various aspects of carbohydrate metabolism in obese diabetic subjects with significant fasting hyperglycemia. Metabolism: Clinical & Experimental. 1978;27(12:Suppl 2) doi: 10.1016/s0026-0495(78)80003-1. Suppl-52. [DOI] [PubMed] [Google Scholar]
- 41.Beck P, Koumans JHT, Winterling CA, Stein MF, Daughaday WH, Kipnis DM. Studies of Insulin and Growth Hormone Secretion in Human Obesity. Journal of Laboratory & Clinical Medicine. 1964;64:654. [PubMed] [Google Scholar]
- 42.Jackson IM, McKiddie MT, Buchanan KD. The effect of prolonged fasting on carbohydrate metabolism: evidence for heterogeneity in obesity. Journal of Endocrinology. 1968;40(2):259–60. doi: 10.1677/joe.0.0400259. [DOI] [PubMed] [Google Scholar]
- 43.Jackson IM, McKiddie MT, Buchanan KD. Influence of blood-lipid levels and effect of prolonged fasting on carbohydrate metabolism in obesity. Lancet. 1971;2(7722):450–2. doi: 10.1016/s0140-6736(71)92625-0. [DOI] [PubMed] [Google Scholar]
- 44.Jackson RA, Moloney M, Lowy C, et al. Differences between metabolic responses to fasting in obese diabetic and obese nondiabetic subjects. Diabetes. 1971;20(4):214–27. doi: 10.2337/diab.20.4.214. [DOI] [PubMed] [Google Scholar]
- 45.Yalow RS, Glick SM, Roth J, Berson SA. Plasma Insulin and Growth Hormone Levels in Obesity and Diabetes. Annals of the New York Academy of Sciences. 1965;131:357–73. doi: 10.1111/j.1749-6632.1965.tb34803.x. [DOI] [PubMed] [Google Scholar]
- 46.Schalch DS. Changes in Carbohydrate Tolerance in Obese Diabetics During Starvation. Diabetes. 1966;15:527. [Google Scholar]
- 47.Harrison MT, Harden RM. The long-term value of fasting in the treatment of obesity. Lancet. 1966;2(7477):1340–2. doi: 10.1016/s0140-6736(66)92085-x. [DOI] [PubMed] [Google Scholar]
- 48.Hermann LS, Iversen M. Death during therapeutic starvation. Lancet. 1968;2(7561):217. doi: 10.1016/s0140-6736(68)92649-4. [DOI] [PubMed] [Google Scholar]
- 49.Munro JF, Duncan LJ. Fasting in the treatment of obesity. Practitioner. 1972;208(246):493–8. [PubMed] [Google Scholar]
- 50.Devathasan G, Koh C. Wernicke's encephalopathy in prolonged fasting. Lancet. 1982;2(8307):1108–9. doi: 10.1016/s0140-6736(82)90039-3. [DOI] [PubMed] [Google Scholar]
- 51.Waterston JA, Gilligan BS. Wernicke's encephalopathy after prolonged fasting. Medical Journal of Australia. 1986;145(3–4):154–5. doi: 10.5694/j.1326-5377.1986.tb113778.x. [DOI] [PubMed] [Google Scholar]
- 52.Ross SK, Macleod A, Ireland JT, Thomson WS. Acidosis in obese fasting patients. British Medical Journal. 1969;1(640):380–1. doi: 10.1136/bmj.1.5640.380-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. Western Journal of Medicine. 1982;137(5):379–99. [PMC free article] [PubMed] [Google Scholar]
- 54.Catenacci VA, Pan Z, Ostendorf D, et al. A randomized pilot study comparing zerocalorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring) 2016;24(9):1874–83. doi: 10.1002/oby.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7(2):113–24. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- 56.Duffy PH, Feuers RJ, Hart RW. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int. 1990;7(4):291–303. doi: 10.1080/07420529009064635. [DOI] [PubMed] [Google Scholar]
- 57.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101(7):1353–61. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42(11):1371–8. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- 59.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38(1):36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- 60.Anson RM, Guo Z, de CR, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100(10):6216–20. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144(6):2446–53. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 63.Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17(9):1133–4. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- 64.Wan R, Ahmet I, Brown M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21(5):413–7. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandhorst S, Choi IY, Wei M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1276–R1283. doi: 10.1152/ajpregu.00775.2005. [DOI] [PubMed] [Google Scholar]
- 68.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26(8):3493–502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 69.Belkacemi L, Selselet-Attou G, Bulur N, Louchami K, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in sand rats. III. Post-mortem investigations. Int J Mol Med. 2011;27(1):95–102. doi: 10.3892/ijmm.2010.556. [DOI] [PubMed] [Google Scholar]
- 70.Park S, Yoo KM, Hyun JS, Kang S. Intermittent fasting reduces body fat but exacerbates hepatic insulin resistance in young rats regardless of high protein and fat diets. J Nutr Biochem. 2017;40:14–22. doi: 10.1016/j.jnutbio.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y, Dentin R, Chen D, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–73. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 73.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Wong K, Giles A, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146(2):539–49. doi: 10.1053/j.gastro.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boutant M, Kulkarni SS, Joffraud M, et al. SIRT1 Gain of Function Does Not Mimic or Enhance the Adaptations to Intermittent Fasting. Cell Rep. 2016;14(9):2068–75. doi: 10.1016/j.celrep.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–52. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hallows WC, Yu W, Smith BC, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41(2):139–49. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimazu T, Hirschey MD, Hua L, et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3- methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12(6):654–61. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de LP, Farina P, Moreno M, et al. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. FASEB J. 2006;20(14):2579–81. doi: 10.1096/fj.06-6025fje. [DOI] [PubMed] [Google Scholar]
- 81.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375(Pt 2):365–71. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gotthardt JD, Verpeut JL, Yeomans BL, et al. Intermittent Fasting Promotes Fat Loss With Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology. 2016;157(2):679–91. doi: 10.1210/en.2015-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr Physiol. 2011;1(3):1383–412. doi: 10.1002/cphy.c100042. [DOI] [PubMed] [Google Scholar]
- 84.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–22. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vainshtein A, Hood DA. The regulation of autophagy during exercise in skeletal muscle. J Appl Physiol (1985) 2016;120(6):664–73. doi: 10.1152/japplphysiol.00550.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11(3):213–9. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Z, Huang X, Feng Y, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103(39):14379–84. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palacios OM, Carmona JJ, Michan S, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1(9):771–83. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritzen AM, Frosig C, Jeppesen J, et al. Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle. Cell Signal. 2016;28(6):663–74. doi: 10.1016/j.cellsig.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Wang P, Zhang RY, Song J, et al. Loss of AMP-activated protein kinase-alpha2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes. 2012;61(5):1051–61. doi: 10.2337/db11-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bujak AL, Crane JD, Lally JS, et al. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metab. 2015;21(6):883–90. doi: 10.1016/j.cmet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133(6):1921–9. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 93.Mager DE, Wan R, Brown M, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20(6):631–7. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 94.Plews DJ, Laursen PB, Stanley J, Kilding AE, Buchheit M. Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med. 2013;43(9):773–81. doi: 10.1007/s40279-013-0071-8. [DOI] [PubMed] [Google Scholar]
- 95.Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014;129(4):573–80. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo J, Bakshi V, Lin AL. Early Shifts of Brain Metabolism by Caloric Restriction Preserve White Matter Integrity and Long-Term Memory in Aging Mice. Front Aging Neurosci. 2015;7:213. doi: 10.3389/fnagi.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhla A, Lange S, Holzmann C, et al. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8(7):e68778. doi: 10.1371/journal.pone.0068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42(1):78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 100.Stranahan AM, Lee K, Martin B, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–61. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitre M, Mariga A, Chao MV. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2017;131(1):13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 103.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fusco S, Ripoli C, Podda MV, et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci U S A. 2012;109(2):621–6. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chouliaras L, van den Hove DL, Kenis G, et al. Age-related increase in levels of 5-hydroxymethylcytosine in mouse hippocampus is prevented by caloric restriction. Curr Alzheimer Res. 2012;9(5):536–44. doi: 10.2174/156720512800618035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27(38):10185–95. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh R, Lakhanpal D, Kumar S, et al. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) 2012;34(4):917–33. doi: 10.1007/s11357-011-9289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45(1):8–15. [PubMed] [Google Scholar]
- 110.Halagappa VK, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26(1):212–20. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 111.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57(2):195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 112.Manzanero S, Erion JR, Santro T, et al. Intermittent fasting attenuates increases in neurogenesis after ischemia and reperfusion and improves recovery. J Cereb Blood Flow Metab. 2014;34(5):897–905. doi: 10.1038/jcbfm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marosi K, Kim SW, Moehl K, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769–81. doi: 10.1111/jnc.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halberg N, Henriksen M, Soderhamn N, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol (1985) 2005;99(6):2128–36. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 115.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35(5):714–27. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ziaee V, Razaei M, Ahmadinejad Z, et al. The changes of metabolic profile and weight during Ramadan fasting. Singapore Med J. 2006;47(5):409–14. [PubMed] [Google Scholar]
- 117.Faris MA, Kacimi S, Al-Kurd RA, et al. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32(12):947–55. doi: 10.1016/j.nutres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 118.Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42(5):665–74. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 2014;22(12):2524–31. doi: 10.1002/oby.20909. [DOI] [PubMed] [Google Scholar]
- 120.Horne BD, Muhlestein JB, May HT, et al. Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 2012;109(11):1558–62. doi: 10.1016/j.amjcard.2012.01.379. [DOI] [PubMed] [Google Scholar]
- 121.Horne BD, Muhlestein JB, Lappe DL, et al. Randomized cross-over trial of short-term water-only fasting: metabolic and cardiovascular consequences. Nutr Metab Cardiovasc Dis. 2013;23(11):1050–7. doi: 10.1016/j.numecd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98. doi: 10.1186/1475-2891-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90(5):1138–43. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 124.Wegman MP, Guo MH, Bennion DM, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015;18(2):162–72. doi: 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol. 2013;48(9):888–97. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–83. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–34. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tinsley GM, Forsse JS, Butler NK, et al. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 131.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 2013;21(7):1370–9. doi: 10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- 132.Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12(1):4. doi: 10.1186/2251-6581-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging. 2016;4(1):63–71. doi: 10.3233/NHA-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. Eur J Clin Nutr. 2013;67(7):783–5. doi: 10.1038/ejcn.2013.83. [DOI] [PubMed] [Google Scholar]
- 135.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):930–8. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364–73. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Baumeier C, Kaiser D, Heeren J, et al. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta. 2015;1851(5):566–76. doi: 10.1016/j.bbalip.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 138.Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98. doi: 10.1186/1475-2891-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- 141.St-Onge MP, Ard J, Baskin ML, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135(9):e96–e121. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liebermeister H, Schroter K. Absence of detrimental changes of cognitive parameters during fasting. Int J Obes. 1983;7(1):45–51. [PubMed] [Google Scholar]
- 143.Solianik R, Sujeta A, Terentjeviene A, Skurvydas A. Effect of 48 h Fasting on Autonomic Function, Brain Activity, Cognition, and Mood in Amateur Weight Lifters. Biomed Res Int. 2016;2016:1503956. doi: 10.1155/2016/1503956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Veasey RC, Gonzalez JT, Kennedy DO, Haskell CF, Stevenson EJ. Breakfast consumption and exercise interact to affect cognitive performance and mood later in the day. A randomized controlled trial. Appetite. 2013;68:38–44. doi: 10.1016/j.appet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 145.Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J. 2010;9:35. doi: 10.1186/1475-2891-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Varady KA, Dam VT, Klempel MC, et al. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci Rep. 2015;5:7561. doi: 10.1038/srep07561. [DOI] [PMC free article] [PubMed] [Google Scholar]