Abstract
Introduction
Alcohol hangover experiences in young adulthood have been shown to predict more subsequent alcohol problems. Hangover susceptibility appears to be partially heritable and related to family history of alcohol use disorders. However, very little is known about the developmental course of these associations and whether they are accounted for by an individual’s drinking history. The goal of this study is to investigate the prospective and unique relationships between family history of alcohol use disorders, severity of alcohol hangover experiences in adolescence, and later alcohol use and related problems measured over 13 years.
Methods
Participants were first assessed on family history at age 12–14, prior to initiating drinking, and re-assessed annually on hangover severity, drinks per drinking day (DPDD), and alcohol-related problems throughout the 13-year follow-up period (n=205; 59% male).
Results
In mixed effects negative binomial regression models, greater family history density scores predicted more future DPDD (Incidence Rate Ratio [IRR]=1.19, p=0.04), alcohol problems (IRR=1.64, p=0.05), and future hangover severity (IRR=1.24; p=0.01). In turn, greater hangover severity predicted more future DPDD (IRR=1.03; p=0.002) and alcohol problems (IRR=1.12, p<0.001), and hangover severity mediated the relationship between family history and alcohol use/problems. All models controlled for participant age, sex, and past drinking behavior (where relevant).
Conclusions
These results advance the alcohol hangover experience during late adolescence as a clinically relevant and uniquely informative marker of future alcohol use and problems, above and beyond that of prior personal or familial drinking history.
Keywords: Alcohol, hangover, family history of alcoholism, longitudinal, alcohol use disorder, adolescence
1. INTRODUCTION
Alcohol hangover is a little studied, yet potentially informative sequelae of alcohol consumption (Stephans & Verster, 2010). Hangover is often defined as the cluster of aversive physiological and behavioral symptoms that occur after the end of a drinking episode, peaking when the blood alcohol concentration (BAC) reaches zero (Rohsenow et al., 2007). Some of the most commonly reported symptoms include fatigue, headache, and thirst, although numerous others have been described (Penning, McKinney, & Verster, 2012; Vatsalya, Stangl, Schmidt, & Ramchandani, 2016).
The experience of an alcohol hangover following a heavy drinking episode is common - 76% of healthy adults endorsed symptoms following a peak BAC of .11 g% (Howland et al., 2008). However, the association between average drinking levels and hangover occurrence appears to decrease with increasing age (Huntley et al., 2015; Shorter, Murphy, & Cunningham, 2017; Tolstrup, Stephens, & Gronbaek, 2014). Given this age-related variability, research on the effects of hangover during the adolescent period of development may be particularly informative.
Alcohol continues to be a widely used substance among adolescents, with 23% of students having tried alcohol by 8th grade (age 13–14 on average), which increases to 61% by grade 12 (age 17–18) (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2017). Further, almost 50% of 12th graders report having been “drunk” at least once in their life and 52% do not consider weekly binge drinking (defined as consuming five or more drinks once or twice each weekend) as carrying great risk. As one might expect, binge drinking is strongly associated with hangover frequency during adolescence (Maney, Higham-Gardill, & Mahoney, 2002). Hangovers during this time period are often perceived as neutral or positive experiences (Fjær, 2012; Mallett, Bachrach, & Turrisi, 2008), possibly increasing the adolescents’ willingness to engage in recurrent binge drinking practices that result in more frequent and severe hangover experiences. Binge drinking during adolescence has been associated with significant neurological and neuropsychological consequences (Carbia, Cadaveira, Caamano-Isorna, Rodriguez-Holguin, & Corral, 2017; Correas et al., 2016; Squeglia, Schweinsburg, Pulido, & Tapert, 2011; Squeglia et al., 2012); however, the impact of hangovers, over and above that of quantity of alcohol consumed, has yet to be studied during this vulnerable neurodevelopmental period.
The literature is divided regarding the predictive relationship between alcohol hangover experiences and an individuals’ later alcohol consumption and alcohol-related problems. Given that hangover is an adverse consequence of heavy drinking, one might infer that the experience of hangovers would ultimately discourage future alcohol consumption, and thus reduce alcohol use disorder (AUD) liability. Preliminary genetic (Wall, Shea, Luczak, Cook, & Carr, 2005) and same day/event-level (Huntley et al., 2015) data provide initial support for this model. Further, insensitivity to hangover has been advanced as a potential risk marker of future alcohol problems in a sample of college seniors (Rohsenow et al., 2012), although this effect was only observed at a trend-level of significance (p = .09).
The majority of findings in this area suggest, instead, that alcohol hangover serves as a risk factor, or at least a peripheral marker, of greater future alcohol drinking and related problems (for a review see Piasecki, Robertson, & Epler, 2010). Specifically, hangovers are more common in individuals with an AUD and in those with more drinking-related problems (Epler et al., 2014), and higher average hangover severity is associated with greater amounts of alcohol consumption (Huntley et al., 2015). Engaging in frequent heavy drinking necessarily affords greater opportunity to experience hangovers; yet, the possibility that hangovers in turn confer added risk for future heavy drinking should not be discounted without further inquiry. In fact, a longitudinal study of college students found that the experience of more frequent hangovers is positively predictive of AUD diagnosis at long-term follow-up, even after controlling for baseline drinking practices (Piasecki, Sher, Slutske, & Jackson, 2005). However, more long-term prospective studies are needed to disentangle the potential predictive relationships between early hangover experiences and later alcohol use and problems.
Genetics likely underlie some of the variability of the hangover phenotypes, as 40–45% of past-year hangover frequency and 16–24% of past-year hangover susceptibility variance (defined as the residual variance in hangover frequency after accounting for intoxication frequency) is attributable to genetic factors (Slutske, Piasecki, Nathanson, Statham, & Martin, 2014). An additional study estimated the heritability of hangover frequency at 55% (Wu, Guo, Viken, Reed, & Dai, 2014). Studies investigating the relationship between family history of alcohol problems and hangovers have also observed relationships between the two constructs (for a review see Piasecki, Robertson, et al., 2010). Even after controlling for alcohol drinking practices, those with a positive family history (FHP) of AUD experience greater hangover frequency (Newlin & Pretorius, 1990; Piasecki et al., 2005; Slutske, Piasecki, & Hunt-Carter, 2003) and greater hangover effects following acute alcohol challenge (McCaul, Turkkan, Svikis, & Bigelow, 1991; Span & Earleywine, 1999) as compared to those without family histories of AUD. College students who had parents with alcohol problems were also more likely to endorse “hangover-like experiences” above and beyond their personal alcohol consumption (Piasecki, Slutske, Wood, & Hunt-Carter, 2010). Nevertheless, a recent report challenged these earlier findings on methodological grounds, reporting that FHP was associated with increased estimates of hangover frequency the previous year, but not concurrent hangover severity. The authors suggested that FHP individuals may have become sensitized to past hangover experiences and demonstrate a “retrospective bias” in that they report greater frequency of hangovers versus individuals with no family history of AUD, despite having similar hangover experiences overall (Stephens et al., 2017).
The effects of family history on hangover, however they manifest, appear to vary across the lifespan. Piasecki and colleagues (2005) noted that the effects of family history diminish after the college years, and frequency of hangovers during young adulthood may partially mediate the relationship between family history and the development of an AUD. These findings suggest that hangover frequency may be a developmentally limited risk marker for later alcohol problems and highlight the need for greater hangover-related prospective research on younger samples.
Given the mounting evidence suggesting the importance of age as a key factor in the effects of hangovers, and the relative dearth of long-term prospective studies on the topic, the present study sought to investigate the prospective and unique relationships between family history of alcohol use disorders, severity of alcohol hangover experiences in adolescence, and later alcohol use and related problems as measured across a 13-year span. In line with the literature reviewed above, hangover severity was hypothesized to mediate the predictive relationship between family history assessed at baseline and later alcohol use/problems.
2. METHODS
2.1 Participants & Procedures
Data for the current study was acquired from a larger ongoing longitudinal substance use and neuroimaging project (Nguyen-Louie et al., 2015; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009). At baseline, participants were healthy 12–14 year-olds with very little to no experience with alcohol and drug use, recruited through San Diego area schools. Data for the current project consists of the first 13 years of annual follow-up assessments from this ongoing study. Baseline exclusionary criteria included: any report of prenatal alcohol (>2 drinks during a given week) or illicit drug exposure; premature birth prior to 35th gestational week; history of any neurological or DSM-IV (American Psychiatric Association, 2000) Axis I disorder; head trauma or loss of consciousness (>2 minutes); chronic medical illness; learning disability or mental retardation; psychoactive medication use; history of alcohol use (defined as ≥10 total lifetime drinking days, or > 2 drinks per week); history of drug use (defined as ≥3 lifetime experiences with marijuana or use in the past 3 months, ≥5 lifetime cigarette uses, or any other intoxicant use); inadequate English comprehension; and non-correctable sensory problems. Follow-up exclusion criteria included: emergent Axis I disorder as measured by a structured diagnostic interview (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). All participants were asked to abstain from alcohol and recreational drug use for at least 24 hours prior to baseline or follow-up assessment, confirmed via breath alcohol concentration and urine drug screen in the laboratory. The study protocol and procedures were approved by the University of California San Diego Human Research Protections Program.
Given the study focus on the predictive utility of the alcohol hangover construct, only individuals who have had the opportunity to experience a hangover during the study period were selected for the analyses. Thus, the subsample included only participants who reported consuming at least 1 alcoholic drink during the 13-year follow-up period (n = 205).
2.2 Measures
At baseline, eligible youths were administered comprehensive interviews assessing demographics, family history of AUD, alcohol and drug use, and psychopathology. An informant (a biological parent, or a close relative/friend in a minority of cases) was also interviewed on demographic background and family history to corroborate the report of the youth. Annual follow-up assessments were administered in a similar manner.
2.2.1 Demographics
Participant age and sex at the time of assessment were acquired as part of the standard interview procedure. In addition, the Hollingshead Index of Social Position score (Hollingshead, 1965), an index of socioeconomic status (SES), was calculated for each subject at baseline using parental socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) to characterize the youth’s rearing environment. Higher values indicate lower SES (possible range 11–77).
2.2.2 Family history of AUD
The Family History Assessment Module (Rice et al., 1995) was administered to assess family history of AUD. A family history density (FHD) score was calculated for each participant by assigning a weight of 0.50 to each biological parent and 0.25 to each biological grandparent reported to have experienced AUD symptoms (possible range 0–2).
2.2.3 Hangover Severity
The Hangover Symptoms Scale (HSS) (Slutske et al., 2003) was administered at each time point to assess hangover experiences in the past year. A past year symptom count using a dichotomous scoring method was calculated for each participant for each time point assessed, per the scale developer’s recommendation (possible range 0–13).
2.2.4 Alcohol use and problems
Alcohol consumption and the experience of alcohol-related problems, as well as additional substance use information, were assessed via the Customary Drinking and Drug Use Record structured interview (Brown et al., 1998). The past year alcohol consumption variable was calculated by creating an average of past year alcoholic drinks consumed per drinking day (DPDD) for each time point assessed. Past year alcohol-related problems was captured via a count of DSM-IV and DSM-5 symptoms and alcohol-related consequences for each time point assessed (possible range 0–19).
2.3 Data Analyses
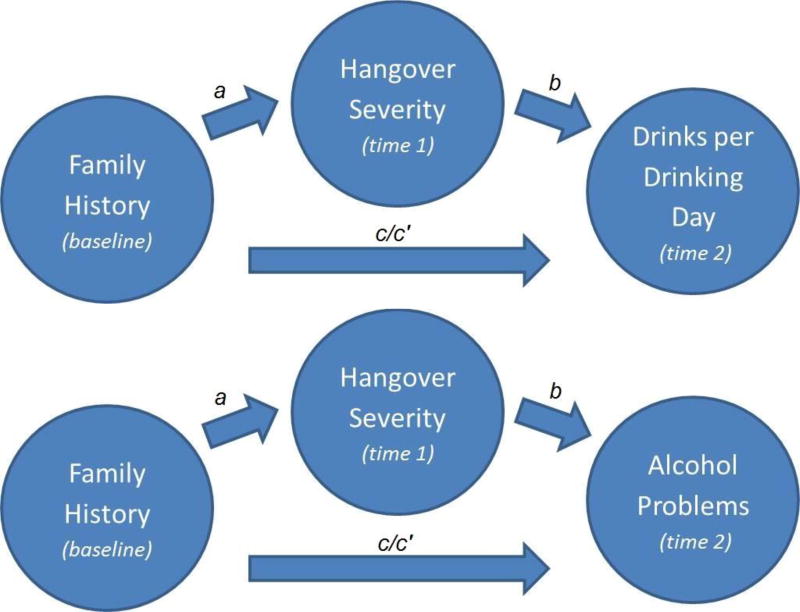
The hypothesized relations between family history of AUD, hangover severity, and alcohol use/problems were tested with multilevel models. All models incorporated random intercepts to account for within-person clustering of repeated observations. Missing data was assumed missing at random. This assumption that was supported by analyses showing that visits with missing outcomes were not related to sex (p = .07) or age (p = .62). All available data were included and analyzed with maximum-likelihood estimation, a preferred approach for handling missing data (Schafer & Graham, 2002). In addition to the primary variables of interest, all statistical models controlled for sex and age given the expected sex (Piasecki, Slutske, et al., 2010; Slutske et al., 2003; Vatsalya et al., 2016) and age-related differences (Huntley et al., 2015; Shorter et al., 2017; Tolstrup et al., 2014) in hangover and alcohol variables. Sex was a person-level (“Level 2”) covariate while age was a time-level (“Level 1”) covariate. Separate statistical models examined each distinct path in the mediation model (see Figure 1).
Figure 1.
Hypothesized theoretical models relating family history of alcohol use disorder (assessed at baseline), hangover severity (assessed annually during follow-up), and alcohol outcomes (assessed annually during follow-up). Note that all available follow-up annual assessments where hangover assessments preceded alcohol problems were included in the model. a denotes the predictor-mediator model and b denotes the mediator-outcome models. c refers to the direct effect of AUD family history on the outcome when hangover severity is not accounted for and c’ refers to the indirect effect of AUD family history on the outcome while accounting for hangover severity.
First, a pair of models used AUD family history density as a person-level predictor of future DPDD or alcohol problems (see c paths in Figure 1). Then, AUD family history density was used as a person-level predictor of future time-varying hangover severity (a paths). A pair of models separately controlled for corresponding measures of past-year DPDD or alcohol problems as time-varying covariates, to estimate the unique effects of family history on hangover severity that were independent from related differences in DPDD or alcohol problems. The final pair of models predicted DPDD or alcohol problems from family history density (c’ paths) and prior hangover severity (b paths), while controlling for the corresponding prior alcohol measure (DPDD, alcohol problems). This model estimated the effects of hangover severity on future DPDD or alcohol problems that were above and beyond related effects of family history density and prior DPDD/ alcohol problems. All time points that offered a hangover assessment paired with a future alcohol measure were included in the models, with the hangover assessment time point as the outcome in the predictor-mediator model (a path), and the alcohol measure time point as the outcome in the mediator-outcome models (b paths). Participants contributed from 1 to 9 data points (mean = 2.1). First hangover assessments (administered after initial alcohol use) occurred at a mean age of 18.4 (SD = 2.0; range: 13–24). All hangover assessments were paired with the earliest available future alcohol measure, which most commonly occurred one year later (46% of data points) with a mean lag time of 2.8 years. Multilevel negative binomial models were used to account for the count-type measures and positively skewed distributions of hangover severity, DPDD, and alcohol problems. Model paths were considered significant at p < .05. All statistical analyses were conducted in Stata 14.0.
3. RESULTS
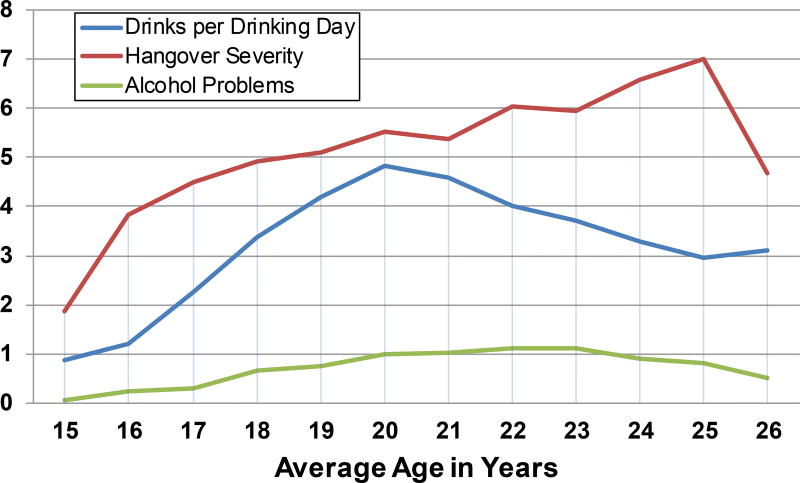
Sample characteristics and demographics are presented in Table 1. Of note, the sample is roughly evenly split between sexes and predominately Caucasian (70.2%). The majority (89.3%) met heavy drinking cutoffs at some point during the follow-up period (Squeglia et al., 2009), and approximately 28% of the sample used marijuana more than 1 day per week at some point during the follow-up. As shown in Figure 2, alcohol consumption in terms of DPDD peaked at 20 years old (Mean = 4.82, SD = 3.33) and began to decline the following years, whereas alcohol problems stayed largely consistent and low across the follow-up period with a minor peak at 22 years old (Mean 1.11, SD = 2.19). Hangover severity appears to increase across the follow-up period, peaking at 25 years old (Mean = 7.00, SD = 3.77) and declining sharply after. Consistent with the adult literature (Penning et al., 2012; Vatsalya et al., 2016), the top three hangover symptoms endorsed across the follow-up period were headache, fatigue, and thirst (see Supplementary Figure S1).
Table 1.
Demographic information on the full sample (N=205).
| Variable | N (%) | M (SD) |
|---|---|---|
|
| ||
| Age at baseline | 13.63 (0.75) | |
| Sex | ||
| - Female | 84 (41%) | |
| - Male | 121 (59%) | |
| Ethnicity | ||
| - Asian | 10 (4.9%) | |
| - Pacific Islander | 1 (0.5%) | |
| - African American | 5 (2.4%) | |
| - Caucasian | 144 (70.2%) | |
| - More than one race | 35 (17.1%) | |
| - Unknown | 10 (4.9%) | |
| SES at baseline (Hollingshead Index - parents) | 23.22 (14.10) | |
| AUD family history density | 0.22 (0.32) | |
| Transitio ns into AUD* | 8 (3.9%) | |
| Alcohol use group* (Squeglia et al., 2009) | ||
| - Control | 4 (2.0%) | |
| - Moderate drinker | 18 (8.8%) | |
| - Heavy drinker | 183 (89.3%) | |
| Regular tobacco smoker (≥10 cigs/day)* | 6 (2.9%) | |
| Marijuana user* | ||
| - > 1 day/month | 80 (39.0%) | |
| - > 1 day/week | 57 (27.8%) | |
Criteria for the specified variable met at any point during the follow-up period
Figure 2.
Alcohol consumption, hangover severity scores, and alcohol problem scores across the follow-up period.
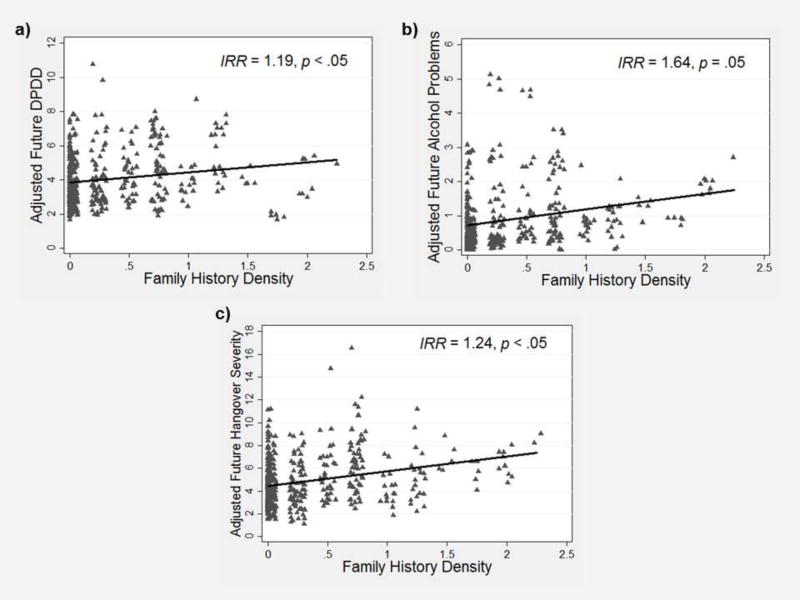
The first set of statistical models estimated the effects of AUD family history density on alcohol outcomes (c paths). As shown in Figure 3a, greater family history density predicted greater future DPDD (IRR = 1.19, p < .05, 95% CI [1.01, 1.40]). As shown in Figure 3b, greater family history density also predicted greater future alcohol problems, but only at a trend level of statistical significance (IRR = 1.64, p = .05, 95% CI [0.99, 2.72]). Overall these results confirmed our expectations that adolescents with greater family history density would have greater alcohol use (and to some extent greater problems) during the follow-up period.
Figure 3.
Scatterplot depicting the prospective relationship between family history of alcohol use disorder density scores assessed at baseline and (a) past-year average drinks per drinking day (DPDD), (b) past-year average alcohol problems, and (c) past-year average hangover severity assessed during follow-up. IRR = incidence rate ratio.
In models estimating the effects of AUD family history density on hangover severity (a path), greater family history density predicted greater future hangover severity (IRR = 1.23, p < .05, 95% CI [1.04, 1.47]). This effect of family history on future hangover severity was independent from the statistically-significant effects of either past-year DPDD (IRR = 1.08, p < .001, 95% CI [1.05, 1.10]) or past-year alcohol problems (IRR = 1.10, p < .001, 95% CI [1.07, 1.13]) on hangover severity. Overall, these results indicate that time points with greater DPDD or alcohol problems were also associated with greater hangover severity, but AUD family history density predicted more severe hangovers above and beyond these effects (see Figure 3c).
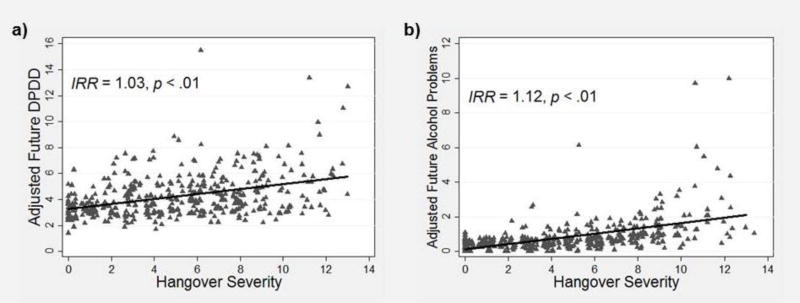
The final set of models estimated future alcohol outcomes as a function of AUD family history density, time-varying prior hangover severity, and time-varying prior alcohol outcomes, controlling for sex and age. As shown in Figure 4a, greater hangover severity was a statistically-significant predictor of greater future DPDD (IRR = 1.03, p < .01, 95% CI [1.01, 1.05]) when controlling for the significant effects of prior DPDD (IRR = 1.06, p < .001). As shown in Figure 4b, greater hangover severity also significantly predicted greater future alcohol problems (IRR = 1.22, p < .001, 95% CI [1.09, 1.36]) when controlling for prior alcohol problems (IRR = 1.21, p < .001). In these models family history did not significantly predict DPDD or alcohol problems (c’ paths). The results indicated that more severe hangovers were positively predictive of higher levels of DPDD and alcohol problems at subsequent time points (b paths), and that this effect was distinct from the related differences in drinking, problems, and family history associated with more severe hangovers.
Figure 4.
Scatterplot depicting the prospective relationship between past-year average hangover severity and (a) later past-year average drinks per drinking day (DPDD) and (b) later past-year average alcohol problems. IRR = incidence rate ratio.
4. DISCUSSION
This study sought to characterize the predictive effects of alcohol hangovers on future alcohol consumption and alcohol-related problems in a longitudinal sample of 205 adolescents. Consistent with the hypotheses, the results revealed significant prospective relationships between hangover severity and both alcohol use and alcohol problems across the 13-year follow-up period. Hangover severity was also found to mediate the positive predictive relationship between family history of AUD and the alcohol outcomes. Importantly, these relationships held after controlling for previous drinking behavior and alcohol-related problems suggesting there is something unique to the hangover phenomenon that predisposes the adolescent to problematic drinking practices later on.
In this sample, the experience of greater hangover severity was related to greater future alcohol consumption and problems across the follow-up period. This finding appears to contradict the existing literature which reported a trend-level relationship between less hangover severity (termed hangover insensitivity) and greater future alcohol problems after controlling for past drinking behavior (Rohsenow et al., 2012). It has been proposed that insensitivity to hangover might be an indicator of a insensitivity to alcohol more broadly (Piasecki et al., 2012; Rohsenow et al., 2012; Vatsalya et al., 2016); however, the history of alcohol drinking and the development of alcohol tolerance likely play an important role in one’s sensitivity to hangovers (Vatsalya et al., 2016). This assertion is further supported by the observed decrease in the association between average drink amount and hangovers with increasing age (Huntley et al., 2015; Shorter et al., 2017; Tolstrup et al., 2014). In contrast to the Rohsenow and collegues’ (2012) study on college seniors aged 21 to 24 at baseline, the current sample consisted of adolescents who were relatively alcohol-naïve at baseline. The 13-year follow-up of these participants enabled assessment of their first experiences with alcohol consumption and hangovers, which occurred as early as 13 years old. Thus, the present results could be capturing a developmentally earlier, more “pure” hangover effect which may change over the course of an individual’s experiences with alcohol.
The mechanisms by which hangovers relate to future drinking outcomes remain unknown. The experience of more hangover symptoms during late adolescence (ages 16–19), controlling for drinking frequency and duration, has been associated with significant neurological changes, including decreased white matter integrity in the corpus callosum, frontal lobe projection fibers, and cerebellar tracts (McQueeny et al., 2009). Given the vast neurodevelopment occurring during the adolescent time period, it is possible that the inflammatory responses that occur as a result of an alcohol hangover (Penning, van Nuland, Fliervoet, Olivier, & Verster, 2010) lead to lasting neural adaptations that confer risk for greater alcohol drinking and AUD development. Furthermore, family history for AUD may somehow function to predispose the adolescent to greater hangover induced neural alterations, possibly accounting for the mediation effect observed in the present study.
Alternatively, the predictive component of hangovers could reflect a more general behavioral risk process such as inability to learn from punishment, heightened impulsivity, or reduced drink refusal self-efficacy; thus, functioning as a non-causal marker of AUD liability (Epler et al., 2014; Piasecki, Robertson, et al., 2010; Piasecki et al., 2005). However, the relationship between these behavioral risk processes and hangover over and above direct measures of alcohol consumption remains to be demonstrated.
The results presented must be taken in context with the study strengths and limitations. Significant strengths include the subjects’ alcohol naivety at baseline and the within-subject modeling approach which provide assurances of confound control that have complicated the literature on adults (e.g., alcohol tolerance, varying histories of use/drinking patterns). The reliance on self-report measures of past year hangover experiences represents a limitation of the study as it is not possible to assess the occurrence of reporting biases or control for differences in alcohol metabolism. The study also lacks a measure of past year hangover frequency which would have provided further clarification on the differential roles of hangover frequency versus severity in predictions of future alcohol use and problems. We were also unable to implement the most modern analytic approaches for the estimation of mediated effects given that they have not been validated for multilevel negative binomial models. Therefore, we could not definitively test the hypothesis that hangover severity mediated the effects of family history, although the pattern of findings and effect sizes do support a potential mediated effect.
In conclusion, the hangover experience in adolescence is a uniquely informative marker of elevated risk for greater alcohol use and problems into young adulthood. It also appears to explain the path between family history for AUD and alcohol outcomes, although the mechanisms by which this occurs remain to be discovered. Given this is the first study to investigate the prospective effects of hangovers in an adolescent sample, replication is needed; however, the evidence is mounting in support of the alcohol hangover as a clinically relevant phenomenon that warrants greater attention in the alcohol research field.
Supplementary Material
HIGHLIGHTS.
Hangover severity in adolescence predicts greater future alcohol use and problems
Hangover severity mediates the path between family history and alcohol outcomes
Hangovers are a clinically relevant marker of future alcohol outcomes
Acknowledgments
Role of Funding Sources
This research was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants T32 AA013525 (PI: Riley) and R01 AA13419 (PI: Tapert). NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author SFT designed the study and wrote the protocol. Author KEC developed the hypothesis and wrote the first draft of the manuscript. Author MW conducted the statistical analysis. Author NC collected the data and assisted in literature searches and statistical analysis. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare they have no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Carbia C, Cadaveira F, Caamano-Isorna F, Rodriguez-Holguin S, Corral M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS One. 2017;12(2):e0171393. doi: 10.1371/journal.pone.0171393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas A, Cuesta P, Lopez-Caneda E, Rodriguez Holguin S, Garcia-Moreno LM, Pineda-Pardo JA, et al. Functional and structural brain connectivity of young binge drinkers: A follow-up study. Sci Rep. 2016;6:31293. doi: 10.1038/srep31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epler AJ, Tomko RL, Piasecki TM, Wood PK, Sher KJ, Shiffman S, et al. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcohol Clin Exp Res. 2014;38(5):1461–1469. doi: 10.1111/acer.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjær EG. The day after drinking: interaction during hangovers among young Norwegian adults. Journal of Youth Studies. 2012;15(8):995–1010. [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Howland J, Rohsenow DJ, Allensworth-Davies D, Greece J, Almeida A, Minsky SJ, et al. The incidence and severity of hangover the morning after moderate alcohol intoxication. Addiction. 2008;103(5):758–765. doi: 10.1111/j.1360-0443.2008.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley G, Treloar H, Blanchard A, Monti PM, Carey KB, Rohsenow DJ, et al. An event-level investigation of hangovers' relationship to age and drinking. Exp Clin Psychopharmacol. 2015;23(5):314–323. doi: 10.1037/pha0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2016: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- Mallett KA, Bachrach RL, Turrisi R. Are all negative consequences truly negative? Assessing variations among college students' perceptions of alcohol related consequences. Addict Behav. 2008;33(10):1375–1381. doi: 10.1016/j.addbeh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DW, Higham-Gardill DA, Mahoney BS. The alcohol-related psychosocial and behavioral risks of a nationally representative sample of adolescents. J Sch Health. 2002;72(4):157–163. doi: 10.1111/j.1746-1561.2002.tb06538.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res. 1991;15(1):94–101. doi: 10.1111/j.1530-0277.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Pretorius MB. Sons of alcoholics report greater hangover symptoms than sons of nonalcoholics: A pilot study. Alcohol Clin Exp Res. 1990;14(5):713–716. doi: 10.1111/j.1530-0277.1990.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of emerging alcohol and marijuana use behaviors on adolescents' neuropsychological functioning over four years. J Stud Alcohol Drugs. 2015;76(5):738–748. doi: 10.15288/jsad.2015.76.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning R, McKinney A, Verster JC. Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol Alcohol. 2012;47(3):248–252. doi: 10.1093/alcalc/ags029. [DOI] [PubMed] [Google Scholar]
- Penning R, van Nuland M, Fliervoet LA, Olivier B, Verster JC. The pathology of alcohol hangover. Curr Drug Abuse Rev. 2010;3(2):68–75. doi: 10.2174/1874473711003020068. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KJ, Shiffman S, et al. Low sensitivity to alcohol: Relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. J Stud Alcohol Drugs. 2012;73(6):925–932. doi: 10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Robertson BM, Epler AJ. Hangover and risk for alcohol use disorders: Existing evidence and potential mechanisms. Curr Drug Abuse Rev. 2010;3(2):92–102. doi: 10.2174/1874473711003020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. J Abnorm Psychol. 2005;114(2):223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Slutske WS, Wood PK, Hunt-Carter EE. Frequency and correlates of diary-measured hangoverlike experiences in a college sample. Psychol Addict Behav. 2010;24(1):163–169. doi: 10.1037/a0017148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA. The Acute Hangover Scale: A new measure of immediate hangover symptoms. Addict Behav. 2007;32(6):1314–1320. doi: 10.1016/j.addbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Winter M, Bliss CA, Littlefield CA, Heeren TC, et al. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. J Abnorm Psychol. 2012;121(1):270–275. doi: 10.1037/a0024706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shorter GW, Murphy M, Cunningham JA. Understanding the hangover experience in Canadian adults: A latent class analysis of hangover symptom patterns and their alcohol-related correlates. Drugs: Educ Prev Polic. 2017;24(2):189–196. [Google Scholar]
- Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of Hangover Symptoms in college students. Alcohol Clin Exp Res. 2003;27(9):1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Nathanson L, Statham DJ, Martin NG. Genetic influences on alcohol-related hangover. Addiction. 2014;109(12):2027–2034. doi: 10.1111/add.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span SA, Earleywine M. Familial risk for alcoholism and hangover symptoms. Addict Behav. 1999;24(1):121–125. doi: 10.1016/s0306-4603(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcohol Clin Exp Res. 2011;35(10):1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220(3):529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans R, Verster JC. The importance of raising the profile of alcohol hangover research. Curr Drug Abuse Rev. 2010;3(2):64–67. doi: 10.2174/1874473711003020064. [DOI] [PubMed] [Google Scholar]
- Stephens R, Holloway K, Grange JA, Owen L, Jones K, Kruisselbrink D. Does familial risk for alcohol use disorder predict alcohol hangover? Psychopharmacology (Berl) 2017 doi: 10.1007/s00213-017-4585-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstrup JS, Stephens R, Gronbaek M. Does the severity of hangovers decline with age? Survey of the incidence of hangover in different age groups. Alcohol Clin Exp Res. 2014;38(2):466–470. doi: 10.1111/acer.12238. [DOI] [PubMed] [Google Scholar]
- Vatsalya V, Stangl BL, Schmidt VY, Ramchandani VA. Characterization of hangover following intravenous alcohol exposure in social drinkers: methodological and clinical implications. Addict Biol. 2016 doi: 10.1111/adb.12469. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Shea SH, Luczak SE, Cook TA, Carr LG. Genetic associations of alcohol dehydrogenase with alcohol use disorders and endophenotypes in white college students. J Abnorm Psychol. 2005;114(3):456–465. doi: 10.1037/0021-843X.114.3.456. [DOI] [PubMed] [Google Scholar]
- Wu SH, Guo Q, Viken RJ, Reed T, Dai J. Heritability of usual alcohol intoxication and hangover in male twins: The NAS-NRC Twin Registry. Alcohol Clin Exp Res. 2014;38(8):2307–2313. doi: 10.1111/acer.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.