Abstract
Objective
While the trajectory of weight change during/after behavioral weight management interventions is believed to include a period of weight loss followed by maintenance and later regain, the sparse data produced by existing study designs (conducting assessments at 3–6 month intervals) has limited investigation into the precise pattern.
Methods
Seventy-five adults were asked to self-weigh daily via “smart” scales during a 12 week, Internet-based weight loss program and 9 months with no further intervention. Longitudinal change-point mixed-effect models were used to characterize overall weight change patterns and identify when individuals moved from weight loss to maintenance/regain.
Results
Analyses suggested a 3-phase model. During the first phase, participants lost weight at a (mean±SE) rate of −0.46±.04 kg/week; after 77.66±3.96 days, they transitioned to regain (0.07±0.02 kg/week). The next transition occurred at 222.55±7.23 days, after which the rate of regain decreased slightly (0.06±.02 kg/week). Exploratory analyses identified baseline/demographic factors predicting the timing of transition points and slope of weight change within phases.
Conclusion
In contrast to the hypothesized trajectory, results demonstrated that participants transitioned immediately from weight loss to regain (with no “maintenance” period) and later to a slower rate of regain. Future studies should investigate whether extended-care programs change or merely delay this pattern.
Keywords: Weight Loss, Weight Regain, Obesity, Overweight, Intervention
Introduction
Behavioral weight management interventions consistently produce weight losses of 7–10% of initial body weight during initial treatment (typically 4–6 months) in adults with overweight and obesity;1 however, successful weight loss maintenance following the end of intervention remains a challenge. While some individuals are able to successfully maintain their weight losses, data collected at longer-term follow-up visits demonstrate that participants, on average, tend to regain one third to one half of weight lost within a year of the end of treatment.1,2
Although the general trend for weight regain after the end of weight management treatment has been well documented,2,3 investigation into the precise timing of weight regain (and the individual variability in this timing) has been challenging given that most trials utilize study designs that space follow-up assessments at 3–6 month intervals. Thus, while the individual trajectory of weight change during/after behavioral weight management interventions is generally believed to include a period of weight loss followed by a period of maintenance and then a later shift to regain, the sparse data produced by existing study designs has prevented further investigation in to this pattern.
Recent technological advances, such as the development of “smart” scales that can be used in participants’ homes and send weight data back to research servers via WiFi or cellular networks, have made the collection of more frequent weight data possible. These scales have been demonstrated to have good concordance compared to “official” assessment weights measured in-person4 and thus, coupled with study protocols that ask participants to weigh themselves weekly or daily, data from these scales can allow researchers to investigate the time course of weight loss and regain with much more precision.
The current study used data collected from these “smart” scales during a 12-week, Internet based weight management program followed by a 9-month “maintenance” period during which no additional intervention was provided. Participants were asked to weigh themselves daily during the entire year of observation, and we hypothesized that these data would demonstrate a three-phase pattern of weight change, including a phase of initial weight loss, a phase of maintenance (or a plateau in weight loss) and then regain. As exploratory aims, we proposed to investigate the association between the rate of weight change during these phases and the timing of transition points at which an individual moved from one phase to the next (e.g., do participants who lose weight at a faster rate during the first phase transition to the next phase sooner than those with a slower rate of initial weight loss?), the association between the rate of weight change during phase one and subsequent phases (e.g., do participants who lose weight at a faster rate initially also regain weight faster during later phases?), and the association between the rate of weight change during phase one and overall weight loss during the 1-year study period (replicating existing research which has demonstrated a strong association between initial weight loss and long-term weight loss outcomes5,6). Finally, we proposed to investigate whether any baseline or demographic characteristics were associated with the timing of transition between phases or the rate of weight change during these phases.
Methods
Participants
The current study was conceptualized as an observational trial of weight loss, maintenance, and regain. Participants were 75 employees or dependents of employees of a large healthcare corporation in Providence, Rhode Island, with ages between 18 and 70 years and body mass indices (BMIs) at baseline equal or greater to 25 kg/m2 (considered “overweight” or “obese”).7 Participants had previously enrolled in a workplace healthcare reward program, had expressed interest in weight loss, and were contacted through e-mails, texts, and advertisements on the worksite intranet. If interested, participants were pre-screened via an online questionnaire and initially eligible individuals were scheduled to attend an in-person orientation visit where they received more information about the study, provided written informed consent, and completed final screening measures. Participants were excluded if their body weight was > 150 kg (a limitation of the in-home “smart” scales that were used) or if they reported medical conditions that would contraindicate changes in eating/physical activity for weight loss (e.g., uncontrolled hypertension or diabetes, treatment for cancer, recent history of coronary heart disease, self-report of an eating disorder, inability to walk at least 2 blocks without stopping), participation in another weight loss study at our Center in the past 2 years, weight loss of ≥ 4.5 kg in the month prior to enrollment, or any other factors that would make it unlikely that they would complete the study (e.g., plans to move out of the area during the study period, substance abuse, terminal illness, severe psychiatric conditions, or dementia).
Intervention
A complete description of the 12-week, Internet-based behavioral weight management program that was provided to participants has been published previously.8 Briefly, as part of this program, participants attended a one-time, in-person group visit to receive basic weight management education and to receive tailored calorie, dietary fat, and physical activity goals. At this visit, participants were further taught how to self-monitor eating and activity habits and were provided with an in-home “smart” scale that sent weights directly back to our research servers. Participants were taught how to use these scales, and instructed to weigh themselves once each day. Participants were then asked to log into the study website each week for 12 weeks to receive interactive, multimedia-based weight management lessons (12–15 minutes each) based on content adapted from the Diabetes Prevention Program9 and Look AHEAD.10 Participants also self-reported their self-monitoring data weekly during the 12-week intervention and received automated, tailored feedback based on these data. After the end of the 12-week program, participants were asked to continue to log into the study website weekly to provide summaries of their self-monitoring habits and to answer brief questionnaires; however, they no longer had access to the intervention content and were no longer provided with the automated, tailored feedback. As the parent study was designed to assess factors associated with weight maintenance and weight regain, participants were given small financial incentives both during the 12-week intervention and during the 9-month no-contact observation period for providing self-monitoring data (note: the self-monitoring itself was not incentivized, as participants could receive the incentive for reporting that they did not self-monitor). Incentives ranged from $1–10 per week, were delivered in a pattern unknown to participants, and averaged $3.50 per week (participants could earn up to a total of $156 over the course of the year). Further, participants were allowed to keep the “smart” scales after the end of the study; however, the data link to our center was extinguished.
Measures
At the initial in-person intervention visit, participants were provided with a BodyTrace smart scale (BodyTrace Inc., New York, NY) to use in-home and were asked to weigh themselves once each day, first thing in the morning, after voiding but prior to having anything to eat/drink. These scales transmitted weight data directly to a research server, and stored values in pounds (we converted lbs to kg at 1 lb = 0.45359237 kg), with stated accuracy to 0.1 kg.11 Demographic information was collected at baseline using a self-report questionnaire.
Statistical Analyses
Analyses were conducted using R version 3.1.312 and SAS version 9.4 for Windows.13 Weight data were prepared for analyses by removal of duplicate entries (i.e., more than one weight measurement recorded in a single day) and outliers (frequently indicative of another person in the household using the scale). To identify outliers, generalized additive models of weight over time were fit by individual, and points where residuals from this model were greater than 2.27 kg from predicted values were visually inspected and removed if not consistent with the weights measured during the prior two and following two observations. If multiple weights were recorded on a single day, the first (earliest) non-outlier weight was retained while the others were removed.
Longitudinal change-point mixed-effect models were used to characterize weight change over time for each participant, using all observed weights. While we hypothesized that a model with two change points (representing three distinct phases of weight change, maintenance, and loss) would provide the best fit, we used Akaike Information Criterion (AIC) to evaluate whether a two-phase (one change point) model provided a better fit. Between change points, changes in body weight were characterized as a linear function of time, and it was assumed that body weight would be a continuous function of time (that is, the “phases” within an individual would be connected together). For each individual (i), we let τi1, τi2 denote the two change points that delimit the three phases, and the line segment in each phase will be formulated as a linear function of time t with intercept and slope aik + bikt. With body weight observed at a set of time points , and with ni being the total number of observed weights for the i-th individual, then the observed body weight at time tij was modeled by:
with continuity constraints:
For each individual, parameters τi1, τi2, bi1, bi2, bi3 were modeled using a random effect model (assuming that that τi1, τi2, bi1, bi2, bi3 were independent observations from the distribution N(μ, σ2):
For any individuals who demonstrated only two phases, we assumed that parameter estimates also came from the above distributions, with τi2 and bi3 considered missing.
Linear regressions were used to assess the association between the timing of the first change point (τ1) and the timing of the second change point (τ2), the association between the slope (rate of weight change) between the change points and the timing of the change points, the association between the rate of weight loss in phase one (b1) and subsequent phases (b2 and b3), and the association between rate of initial weight loss (b1) and overall weight change from baseline to one year. Finally, we used stepwise linear regression models to conduct exploratory analyses investigating whether any baseline and demographic factors (baseline BMI, age, gender, income, education level, and marital status) were associated with the change points and slopes of weight change identified in the main model.
Results
Of the 75 participants initially enrolled in the study, 70 were included in the final model (data from 5 participants were removed due to missing data which prevented model fit). The 5 participants removed from analyses all had less than 100 days of weight data (mean±SD of 40.60 ± 29.13 days, range = 8 to 79 days). Demographic and baseline characteristics of included and excluded participants have been provided in Table 1; there were no differences between participants who were included or excluded from the current analyses in terms of age, baseline weight, BMI, gender, race/ethnicity, marital status, household income, or education.
Table 1.
Baseline and demographic characteristics.
| Included Participants n=70 |
Excluded Participants n=5 |
p | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Age | 50.84 | 10.31 | 49.60 | 12.50 | 0.442 |
| Weight (kg) | 85.64 | 16.63 | 83.32 | 18.87 | 0.566 |
| BMI (kg/m2) | 30.86 | 4.57 | 31.00 | 3.84 | 0.822 |
| n | % | n | % | ||
| Gender | 0.639 | ||||
| Female | 49 | 70.0 | 3 | 60.0 | |
| Male | 21 | 30.0 | 2 | 40.0 | |
| Race/Ethnicity | 0.593 | ||||
| African American | 4 | 5.7 | 0 | 0.0 | |
| Asian | 1 | 1.4 | 0 | 0.0 | |
| Caucasian | 59 | 84.3 | 4 | 80.0 | |
| Hispanic | 2 | 2.9 | 0 | 0.0 | |
| Other/Multiple | 4 | 5.7 | 1 | 20.0 | |
| Marital Status | 0.179 | ||||
| Single | 4 | 5.7 | 1 | 20.0 | |
| Married or living with a partner | 57 | 81.4 | 4 | 80.0 | |
| Separated/Divorced/Widowed | 9 | 12.9 | 0 | 0.0 | |
| Household income, dollars | 0.454 | ||||
| 25,000 – 50,000 | 7 | 10.0 | 0 | 0.0 | |
| 50,001 – 75,000 | 13 | 18.6 | 3 | 60.0 | |
| 75,001 – 100,000 | 17 | 24.3 | 1 | 20.0 | |
| 100,001 – 125,000 | 10 | 14.3 | 0 | 0.0 | |
| 125,000 + | 21 | 30.0 | 1 | 20.0 | |
| Not reported | 2 | 2.9 | 0 | 0.0 | |
| Education | 0.061 | ||||
| High school or less | 5 | 7.1 | 1 | 20.0 | |
| Vocational training | 2 | 2.9 | 0 | 0.0 | |
| Some college | 11 | 15.7 | 3 | 60.0 | |
| College or university degree | 33 | 47.1 | 1 | 20.0 | |
| Graduate degree | 19 | 27.1 | 0 | 0.0 | |
Adherence to the self-weighing protocol was excellent; after the removal duplicate and outlier weight values, included participants weighed themselves on an average (mean±SD) of 272.20 ± 64.09 days, or 74.58 ± 17.56% of potential days (median = 287 days or 78.6% of days; range = 105 to 365 days, maximum possible days = 365).
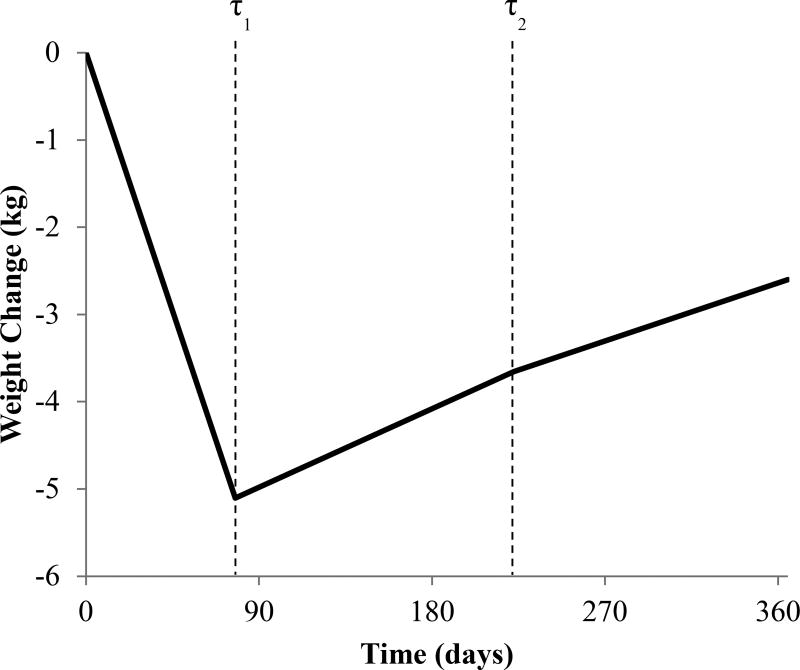
The data fit a three-phase model for 65 of the 70 participants (see Figure 1) and a two-phase model for 5 participants. Participants lost, on average (mean ± SE), −0.46 ± .04 kg/week during the initial phase. Over all participants, the first phase lasted an average of 77.66 ± 3.96 days (at which time the first change point occurred). During the second phase, participants began regaining an average of 0.07 ± .02 kg/week. The second change point occurred at 222.55 ± 7.23 days, after which the slope of weight regain decreased slightly to 0.06 ± .02 kg/week.
Figure 1.
Modeled change in weight over time, across all participants with a three-phase model (n=65).
For the 65 participants with a three-phase model, there was no association between the initial rate of weight change during phase 1 and the timing of the first change point, p = .529. Further, there was no association between the initial rate of weight loss during phase one and the rate of weight regain in phase two, p = .146, or phase three, p = .224 (and the rate of regain in phase 2 was also not significantly associated with the rate of regain in phase 3, p = .107). There was, however, an association between the rate of initial weight loss and overall weight loss, F(1,64) = 97.88, β = 0.28, R2 = 0.61, p < .001, such that participants who had the fastest rate of weight loss during the first phase had the largest weight losses overall between baseline and one year. Likewise, we found no significant association between the timing of the first change point and the slope of weight regain during the second phase (that is, participants who began regaining sooner did not necessarily begin regaining faster), p = .991, or between the timing of the first change point and the length of the second phase (the time between the first change point and the second change point), p = .155. A stepwise regression identified several baseline and demographic factors associated with model parameters (i.e., the model-identified change points and slopes of weight change between the change points; see Table 2). For example, having a higher baseline BMI was associated with a faster rate of initial weight loss and a later transition to the weight regain phase from the weight loss phase, but also a later transition to phase three (the phase with a slower rate of regain) from phase two (the phase of more rapid regain, immediately after weight loss) and a faster rate of regain during phase three. Men lost weight at a faster rate than women during the first phase, but also regained weight at a faster rate during phase two. Compared to younger participants, older participants regained weight at a slower rate during phase two, and further transitioned from phase two (the phase of more rapid regain) to phase three (the phase of less rapid regain) sooner.
Table 2.
Association between demographic and baseline characteristics and the timing of transition points and the slope of weight change between these transition points.
| Variables | β | t | p |
|---|---|---|---|
| Factors associated with greater initial weight loss (b1) | |||
| Baseline BMI (Higher BMI = faster rate of weight loss) | −0.026 | −4.26 | < .001 |
| Gender (Male = faster rate of weight loss) | −0.228 | −3.69 | < .001 |
| Income (Higher income = faster rate of weight loss) | −0.056 | −2.75 | .008 |
| Factors associated with later transition from phase one to phase two (τ1) | |||
| Baseline BMI (Higher BMI = later transition) | 1.647 | 2.05 | .045 |
| Income (Lower income = later transition) | −7.838 | −2.70 | .009 |
| Factors associated with slower weight regain during phase two (b2) | |||
| Baseline Age (Older adults = slower rate of regain) | 0.003 | 2.18 | .034 |
| Gender (Female = slower rate of regain) | 0.088 | 2.24 | .029 |
| Marital Status (Married/living with a partner = slower rate of regain) | 0.092 | 2.14 | .037 |
| Factors associated with transition from phase two to phase three (τ2) | |||
| Baseline BMI (Lower BMI = later transition) | 3.231 | 2.18 | .034 |
| Baseline Age (Younger adults = later transition) | −2.213 | −3.04 | .004 |
| Race/Ethnicity (Non-Hispanic White participants = later transition) | 44.213 | 2.24 | .290 |
| Factors associated with slower weight regain during phase three (b3) | |||
| Baseline BMI (Lower BMI = slower rate of regain) | 0.012 | 2.49 | .016 |
Discussion
In contrast to the expected pattern of weight loss followed by a period of maintenance and then a later shift to weight regain, the results of this study demonstrated that the pattern of weight change for adults with obesity who took part in a 12-week, Internet-based behavioral weight loss program included a period of weight loss followed by a period of weight regain, which later transitioned to a slower rate of regain. While the shape of this trajectory matches the “check mark” pattern of group mean change observed during follow-up visits (spaced at 3–6 months) of many existing behavioral weight management trials,2,3 it was previously unknown whether individual trajectories followed this pattern in the times between the formal assessment visits. Further, it is not consistent with the mathematical models and simulations of weight loss and regain developed by Hall and colleagues,14 which suggest a pattern of weight loss, a plateau, and eventual regain. These models and simulations, however, were developed initially to accommodate the metabolic adaptation that occurs as an individual loses weight, which the authors noted would not occur for most individuals until several years after the initiation of weight loss. Further, Hall et al.14 noted that the weight regain demonstrated in behavioral weight management programs (typically observed far before this time frame, around 6–9 months after the initiation of treatment) is likely due to nonadherence to the changes in dietary intake and physical activity which produced the initial weight loss. While we did not investigate changes in dietary intake and physical activity following treatment in the current study, decreases in adherence to these behavioral changes is a likely driver of the early transition to weight regain observed in the current results.
It is important to note that the transition from weight loss to regain occurred earlier in this study (at 11 weeks) compared to the 6–9 months documented in other behavioral weight management trials.3 This may have been a function of the length of initial treatment; our initial treatment was conducted over 12 weeks, while many existing trials provided intervention (including initial intervention and, frequently, “extended-care” maintenance sessions) for 6–12 months. Alternatively, it may be due to the fact that most trials include assessments at 6 months, possibly missing this first inflection point. Future research should investigate whether this same pattern (a transition from weight loss to weight regain, without an intermediate plateau or maintenance period) exists after longer (6–12 month) weight management interventions and, given the increase in conceptualization of obesity as a chronic condition necessitating ongoing care,15,16 whether this pattern exists when participants are provided with “extended-care” or maintenance programs after the cessation of initial intervention.
Other key findings of the current study include the replication of the association found between initial weight loss success and long-term weight loss outcomes,5,6 as we found that rate of weight loss during the first phase was significantly associated with overall weight loss during the course of the intervention and follow-up period (a total of one year). Further, the rate of weight loss during this initial phase averaged around a pound per week, consistent with the clinical goals of the intervention and other existing intervention protocols.9,10 Finally, our exploratory analyses identified a number of baseline and demographic factors (e.g., baseline BMI, gender, and age) that predicted both the timing of the transition points between phases and the slope of weight change within each phase. These findings are a starting point for the identification of factors that may, with further research, serve as treatment tailoring variables. As increasing access to mobile technology provides the potential to deliver intervention on an individually-tailored level, when and where it is needed,17 an important next step will be to identify what factors (especially those that are modifiable) more proximally predict the transition point from weight loss to weight regain.
Despite these findings, the current study had several important limitations. First, as we intended to study and observe the course of weight maintenance and regain, we did not provide an extended-care intervention after the end of initial treatment, despite evidence that providing additional contact after the end of treatment can improve maintenance and long-term weight loss outcomes.16 Future studies should investigate weight change trajectories during and after the provision of extended-care programs, and investigate whether these interventions are able to change or merely delay the pattern of weight loss and regain observed in our data. Second, the current study utilized an Internet-based behavioral intervention, and while several studies have documented the efficacy of the intervention program used for weight loss,18–20 few have investigated the long-term impact of these programs and if weight regain varies compared to gold-standard, face-to-face group-based behavioral interventions.21 Thus, these results should be replicated in other behavioral interventions, across treatment-delivery modalities. Finally, the sample predominately consisted of non-Hispanic White women, which limits generalizability of the results to the broad population.
Despite these limitations, study strengths included a prospective study design (this study was designed specifically to evaluate the pattern of weight loss, maintenance, and regain), excellent adherence to daily self-weighing protocol (especially given lack of reminders or intervention contact during the 9-month maintenance period after the end of the 12-week Internet-based behavioral weight management program), and the collection of an entire year of objective weight data using in-home “smart” scales that sent weights directly back to our research center (compared to the reliance on self-report data). While most behavioral weight management interventions conduct follow-up assessments at 3–6 month intervals, the current study represents the first attempt to investigate the pattern of weight loss and weight regain on a more proximal level, using daily objective weights to characterize this pattern over time.
Conclusion
The results from the current study challenge our understanding of the shape of the trajectory of weight loss, maintenance, and regain in adults; while it is commonly assumed that a period of maintenance follows initial weight loss prior to the onset of weight regain, our data demonstrated that weight regain began immediately for many participants, with a later decrease in the rate of regain. Given the development of new technologies and methods that allow for individual tailoring of interventions (e.g., just-in-time adaptive interventions delivered via mobile devices), an important next step is to identify the factors that more proximally predict the transition points at which people move from a weight loss trajectory to a weight regain trajectory, and investigate whether this pattern can be modified.
What is already known about this subject?
The trend for individuals to regain weight after the end of behavioral weight management interventions has been well documented; however, little is known about the individual trajectories of weight loss/maintenance/and regain in the time periods between in-person assessments, commonly conducted at 3–6 month intervals.
Newer technologies, including “smart” scales that send data directly back to research servers, offer promise for increasing the precision with which researchers can investigate patterns of weight loss/regain over time.
What does this study add?
While it is commonly assumed that a period of maintenance follows initial weight loss prior to the onset of weight regain, our data demonstrated that weight regain began immediately for many participants, without a plateau or maintenance period, and was followed later by a decrease in the rate of regain.
The average transition point from weight loss to weight regain occurred earlier than expected, at 11 weeks, which may be associated with the length of the initial intervention (which was provided over 12 weeks).
Exploratory analyses identified key baseline and demographic predictors of the rate of weight loss and regain and the timing of transition from weight loss to regain, including baseline BMI, gender, and age.
Acknowledgments
The authors would like to thank all study participants and research staff, and additionally thank Richard N. Jones, Sc.D., for his feedback on initial study design and assistance managing/cleaning the BodyTrace scale dataset.
Funding: This research was supported by the National Institute of Diabetes Digestive and Kidney Diseases (National Institutes of Health) under award number R21DK109205 awarded to KMR, and by the Lifespan Corporation.
Footnotes
Disclosure: The authors report no conflict of interest.
References
- 1.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, Wing RR. Long-term maintenance of weight loss: Current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 3.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity. 2014;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross KM, Wing RR. Concordance of in-home ‘smart’ scale measurement with body weight measured in-person. Obes Sci Pract. 2016;2:224–248. doi: 10.1002/osp4.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: Does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: Factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Physical status: The use and interpretation of anthropometry. World Health Organization; Geneva: 1995. [PubMed] [Google Scholar]
- 8.Ross KM, Wing RR. Implementation of an Internet weight loss program in a worksite setting. J Obes. 2016;2016:1–7. doi: 10.1155/2016/9372515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 11.BodyTrace, Inc. [Accessed April 24, 2017];BodyTrace scale: Frequently asked questions. [Web page]. http://www.bodytrace.com/medical/faq.html.
- 12.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. http://www.R-project.org/ [Google Scholar]
- 13.SAS Institute Inc. SAS Version 9.4. Cary, NC: 2013. [Google Scholar]
- 14.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. The Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford Press; New York, NY: 2002. pp. 357–379. [Google Scholar]
- 16.Ross Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev. 2012;13:509–517. doi: 10.1111/j.1467-789X.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 17.Nahum-Shani S, Smith SN, Tewari A, Witkiewitz K, Collins LM, Spring B, et al. Just-in-time adaptive interventions (JITAIs): An organizing framework for ongoing health behavior support. The Methodology Center, Penn State; University Park, PA: 2014. [Google Scholar]
- 18.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. J Am Med Assoc. 2001;285:1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JG, Leahey TM, Wing RR. An automated Internet behavioral weight-loss program by physician referral: A randomized controlled trial. Diabetes Care. 2015;38:9–15. doi: 10.2337/dc14-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leahey TM, Thomas G, Fava JL, Subak LL, Schembri M, Krupel K, et al. Adding evidence-based behavioral weight loss strategies to a statewide wellness campaign: A randomized clinical trial. Am J Public Health. 2014;104:1300–1306. doi: 10.2105/AJPH.2014.301870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev. 2010;11:306–321. doi: 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]