Abstract
Objective
To characterize obesity-related sex-differences in intrinsic activity/connectivity of the brain’s reward networks.
Methods
Eighty-six women (N=43) and men (N=43) completed a 10-minute resting functional MRI scan. Sex differences and commonalities in BMI-related frequency power distribution and reward seed-based connectivity were investigated using partial least squares analysis.
Results
Whole Brain Activity
In both men and women, increased BMI was associated with increased slow-5 activity in left globus pallidus and substantia nigra. In women only, increased BMI was associated with increased slow-4 activity in right globus pallidus and bilateral putamen.
Seed-Based Connectivity
In women, increased BMI was associated with reduced slow-5 connectivity between left globus pallidus/putamen and emotion and cortical regulation regions, but in men was increased with medial frontal cortex. In both men and women, increased BMI was associated with increased slow-4 connectivity between right globus pallidus/bilateral putamen and emotion regulation and sensorimotor-related regions.
Conclusions
The stronger relationship between increased BMI and decreased connectivity of core reward network components with cortical and emotion regulation regions in women may be related to the greater prevalence of emotional eating. The present findings suggest the importance of personalized treatments for obesity that consider the sex of the affected individual.
Keywords: sex differences, body mass index (BMI), resting state functional connectivity, reward regions, sensorimotor regions, emotional regulation regions
Introduction
Over one third of American adults are now considered clinically obese (1). Moreover, obesity rates are still rising, along with associated comorbid conditions including type 2 diabetes, cardiovascular diseases, depression and an increased risk of mortality (1). Despite various efforts directed towards identifying the biological mechanisms underlying obesity, our current understanding of human obesity remains limited. One barrier to progress includes the inconsistent consideration of sex differences in underlying mechanisms. Increasingly, sex is understood to be an important basic variable that influences the quality and generalizability of biomedical research (2).
In the United States, the rates of obesity are similar in men and women unless stratified by race, education, residential status, or inequalities (3). However, global studies show that there are greater prevalence rates of obesity in women (4). Although women have an increased propensity to gain total body fat, especially subcutaneous abdominal fat when compared to men, men tend to have more visceral adipose tissue, which has been associated with increased metabolic and cardiovascular disease (5). Sex differences in eating behaviors have also been identified in both rodent and human experiments (6). Food cravings, whether cue-induced, trait-related, or state-related, can lead to increased hedonic eating behaviors, which has also been associated with increased risk of obesity (7). Differences between women and men have been observed in the types of food craved (women crave sweets such as chocolates, while men crave savory foods), in the intensity and frequency of the cravings (women report more trait- and state-related cravings and show greater responses in the orbital frontal cortex and insula in response to high calorie food cues), and in the ability to cognitively regulate or restrain food cravings (more difficult for women)(7). These sex differences can also be observed in eating behaviors, with men taking larger bites, and chewing faster and more forcefully when compared to women (8). These ingestive patterns could translate to women eating more often and men consuming larger meals.
Recent neuroimaging studies have identified obesity-related alterations in brain activity and connectivity, supporting the importance of brain alterations in the pathophysiology of obesity (9, 10). These studies have demonstrated associations between increased weight and structural and functional alterations in regions of the core reward network (e.g. nucleus accumbens [NAcc], orbital frontal cortex) as well as the extended reward network, which encompasses regions in the executive control, salience, emotional arousal, and sensorimotor networks that interact with the core reward network (11). Furthermore, sex-specific activations in response to food intake have been observed in cognitive, emotional, and reward-related regions (12, 13). For example, women exhibit greater post-prandial activation in the dorsal anterior cingulate cortex (dACC; a region of the salience and emotional-arousal networks), while men exhibit greater post-prandial activation in the supplementary motor area (SMA; a region of the sensorimotor network), which may influence the risk of engaging in maladaptive eating behaviors (14). Using graph theory, body mass was observed to impact topological measures of centrality for regions of the reward and salience networks in women, and in reward and sensorimotor networks in men (15). When viewed together, these findings suggest the importance of investigating sex differences in obesity-related alterations in the core and extended reward networks.
Resting state or ‘resting-scan’ magnetic resonance imaging (rsMRI) capitalizes on the wealth of connectivity versus activation information that can be extracted from the intrinsic fluctuations of the BOLD signal, without requiring the presence of a task. The advantage of this is that the investigation of intrinsic connectivity patterns can identify alterations in brain organization and coordination. Specifically, the investigation of the amplitude of low frequency fluctuations can identify brain areas with altered spontaneous neural activity and abnormal local functioning (16, 17). The purpose of the current study was to identify commonalties and differences between men and women in the relationship between body mass index (BMI) and intrinsic activity/connectivity of the brain, using both amplitude- and connectivity-based approaches, with a focus on regions within the core and extended reward networks. Given our focus on both core and extended reward networks, which include cortical and basal ganglia regions, we examined two frequency bands, slow-5 (0.01-0.027 Hz; Low-Frequency), slow-4 (0.027-0.073 Hz; Medium-Frequency), as these bands are thought to represent different neuronal oscillation classes (18), and are differently distributed across the brain, with slow-5 predominating in cortex and slow-4 predominating in basal ganglia (23). We hypothesized that increased BMI would be associated with alterations in the intrinsic connectivity between core and extended reward regions. Moreover, we hypothesized that women show greater BMI-related alterations in reward, salience and emotion regulation regions, while men show additional BMI-related alterations in sensorimotor regions.
Methods
Participant Selection
Forty-three healthy females (mean age, 31.79±10.62 years) and 43 healthy males (mean age, 29.84±7.45 years) were recruited through the University of California, Los Angeles and local community advertisements. All subjects were classified as healthy by a nurse practitioner after an examination and clinical assessment, including the MINI mental state exam+5.0 and Hospital Anxiety and Depression (HAD) scale (19). Individuals with substance abuse, tobacco dependence, psychiatric illness, or eating disorders, such as anorexia or bulimia, were excluded. In addition, individuals who were pregnant or taking medications that interfere with the central nervous system (full dose antidepressants including SSRIs, NSRIs, sedatives or anxiolytics, and opioids) were excluded. Participants were also excluded if they had undergone any obesity-reduction surgery. All subjects were right-handed. All women were premenopausal and were scanned during the follicular phase of the menstrual cycle (i.e., 4-12 days after the first day of the last menstrual period), as sex hormones are known to affect brain structure and function. The subjects provided written informed consent and all procedures were reviewed and approved by the UCLA Medical Institutional Review Board.
Resting Scan MRI acquisition and preprocessing
Subjects underwent a 10-minute rsMRI scan while resting with their eyes closed. Images were acquired on a Siemens 3 Tesla Trio scanner using an echo planar sequence with the following parameters: TE = 28 ms, TR = 2000 ms, flip angel = 77 degrees, FOV = 220 mm, slices = 40, slice thickness = 4.0mm. A standard T1-weighted magnetization prepared-rapid acquisition gradient echo (MP-RAGE) scan was acquired with the following parameters: TE = 3.26ms, TR = 2200ms, slices= 176, slice thickness = 1.0mm, voxel size = 1×1×1mm.
Image preprocessing and data analysis were performed using Statistical Parametric Mapping-8 (SPM8) software (Wellcome Department of Cognitive Neurology, London) and the Data Processing Assistant for Resting-State fMRI toolbox (DPARSF). The first two volumes were discarded to allow for stabilization of the magnetic field. Data were slice-time and motion corrected. All subjects were confirmed to have motion less than 3 mm in any direction. In addition to motion correction, the framewise displacement (FD) was examined to determine any spurious correlations between time courses, and potentially any group differences (20). There were no significant differences between men and women for any of the FD measures of motion during the scan (mean, number, and percent; all p’s>0.05). In addition, there were no significant differences between BMI status (normal vs. overweight/obese), and no significant interaction between sex and BMI status for any of the FD measures of motion (mean, number, and percent; all p’s>0.05). Nuisance covariate regression was performed to minimize physiological noise using six head motion parameters, white matter signal and corticospinal fluid signal. Data was then spatially normalized to the Montreal Neurological Institute (MNI) template using the structural scans. Spatial smoothing with a 5 mm3 Gaussian kernel occurred after the calculation of frequency and connectivity maps (see below).
Frequency Power Analyses (whole brain)
The BOLD signal was subdivided into 2 frequency bands, referred to as slow-5 (Low-Frequency: 0.01-0.027 Hz) and slow-4 (Medium-Frequency: 0.027-0.073 Hz), using band-pass filtering in the DPARSF toolbox. Relative power within each frequency band was computed for each voxel (in the DPARSF toolbox) using the fractional amplitude of low frequency fluctuations (fALFF) technique, which involves summing the oscillatory amplitudes across a particular frequency range (i.e., 0.027-0.073 Hz), then dividing by the amplitude summed across the total range (i.e., 0-.25 Hz). fALFF thereby assesses the ratio of the power for a particular frequency band to the power of the entire frequency range. A gray matter mask was applied to restrict analyses to gray matter regions.
Individual subject frequency power maps were then entered into partial least squares (PLS) analyses to identify sex differences and/or commonalities in the relationship between BMI and regional frequency power distribution. Separate PLS analyses were performed for the slow-5 and slow-4 frequency bands. PLS (http://www.rotman-baycrest.on.ca) is a multivariate statistical technique similar to principle component analysis (PCA), where solutions are restricted to the part of the covariance structure that is attributable to covariate measures (behavioral PLS)(21). Correlation maps representing the across-subject correlation between BMI and frequency power for each voxel were calculated for men and for women. The PLS analysis determines the most robust patterns (latent variables) in these across-subject correlations maps, negating the need for specifying contrasts of interest (22). PLS is considered a random effects model as it employs permutation tests (500 permutations) that assess generalizability of the pattern and bootstrapping (500 samples) to identify the stable or most reliable elements of the pattern (21, 22). Clusters with a peak voxel bootstrap ratio (BSR) exceeding ±3.3 (p<.001) and an extent of at least 60 voxels are reported.
In order to investigate the intersex similarities or differences in the high BMI group (participants with BMIs in the overweight/obese range) compared to the low BMI group (participants with BMIs in the normal range), supplementary analyses were run. Whole-brain frequency power partial least squares (PLS) analyses separately on the subset of participants with BMI in the overweight/obese range and then on participants in the normal weight group, in order to examine whether similar BMI-related relationships as observed in the full sample analysis would be obtained (See Supplementary Materials, Figure S1 and Figure S2).
Reward seed-based intrinsic connectivity analyses
Core reward network regions displaying altered frequency power in the above analysis were further examined for BMI-related intrinsic connectivity changes. Regions of Interest (ROIs) were created from the cluster maps of the frequency power-based PLS analyses (Figure 1). Intrinsic connectivity maps were created in the DPARSF toolbox using slow-5/slow-4 filtered timecourse data, as appropriate for the ROI. These frequency band-specific intrinsic connectivity maps were then entered into PLS analyses (one for slow-5 ROIs and one for slow-4 ROIs) to identify sex differences and/or commonalities in the relationship between BMI and frequency-specific intrinsic connectivity of core reward regions. As in the above analysis, latent variable significance was determined using 500 permutations and voxel reliability was assessed using 500 bootstrap samples. Clusters with a peak voxel bootstrap ratio (BSR) exceeding ±3.3 (p<.001) and an extent of at least 60 voxels are reported.
Figure 1. Regions of Interest used in the seed connectivity analyses.
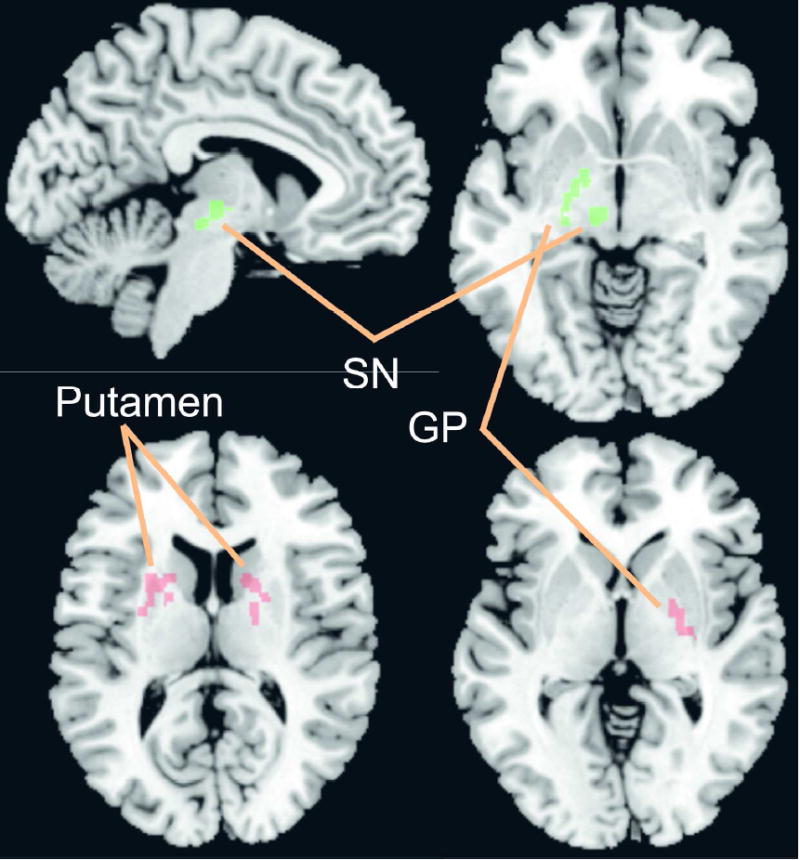
Slow 5: Left globus pallidus (GP) and left substantia nigra (SN)
Slow 4: Right globus pallidus (GP) and bilateral putamen
Behavioral analyses
Behavioral analyses were performed using Statistical Package for the Social Sciences (SPSS) software (version 19, Armonk, NY, IBM Corp). Group differences in age, BMI, and behavioral measure scores (anxiety and depression) were evaluated using independent sample t-tests.
Results
Sample and behavioral characteristics
Mean age, BMI, and anxiety and depression ratings are provided in Table 1. The BMI range for female subjects was 18.2 - 43.6 kg/m2; for male subjects, the BMI range was 19.2-34.7 kg/m2. There were no significant sex differences in age, BMI, HAD anxiety scores, or HAD depression scores (t(84) > .05). There were 45 normal weight subjects (<25 kg/m2), of which 27 were female and 18 were male. There were 41 subjects who were overweight or obese (≥ 25kg/m2), of which 16 were female and 25 were male.
Table 1.
Sample and behavioral characteristics
| Female | Male | T-value | p-value | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |||
| N | 43 | 43 | Total = 86 | |||
| Age (yrs) | 29.84 (7.45) | 21-49 | 31.79 (10.62) | 19-51 | 9.91 | .002 |
| BMI (kg/m2) | 25.60 (5.79) | 18.19-43.59 | 25.62 (3.32) | 19.20- 34.70 | 9.31 | .003 |
| Number of Normal Weight (<25kg/m2) | 27 | 18 | Total = 45 | |||
| Number of Obese and Overweight (≥25kg/m2) | 16 | 25 | Total = 41 | |||
| Anxiety (HAD) | 2.88 (2.89) | 0-12 | 3.14 (2.69) | 0-10 | .32 | .57 |
| Depression (HAD) | .91 (1.56) | 0-6 | 1.58 (2.52) | 0-14 | 2.67 | .11 |
Abbreviations: Body mass index, BMI; Hospital Anxiety and Depression Scale, HAD N= Sample Size; SD, standard deviation; Significance p<.05
Sex Differences in Frequency power analyses (whole brain)
Slow-5 (Low Frequency) activity
The first latent variable (accounting for 65.24% of the cross-block correlation; p<0.001) reflected similar (in the same direction) correlations between BMI and slow-5 frequency power in men (r=0.48) and women (r=0.81), which was expressed in a set of regions including the left globus pallidus (GP) and left substantia nigra (SN) (Table 2A; Figure 2A). Although the correlation between BMI and the latent variable was in the same direction for men and women, it was significantly stronger in women (Figure 2A). The left GP and left SN were chosen as slow-5 ROIs (Figure 1).
Table 2.
Whole Brain frequency power activity correlated with BMI in both men and women
| A: Whole Brain Slow-5 frequency power correlated with BMI in both men (r=0.48) and women (r=0.81) | ||||||||
|---|---|---|---|---|---|---|---|---|
| SLOW – 5 FREQUENCY | ||||||||
| Region | Side | Coordinates | Size | BSR | Correlation | |||
| X | Y | Z | ♂ | ♀ | ||||
| Subcortical | ||||||||
| Globus Pallidus | left | -14 | 2 | 0 | 140 | 8.26 | .10 | .69 |
| Midbrain (SN) | left | -8 | -18 | -4 | 62 | 7.39 | .45 | .52 |
| Frontal Cortex | ||||||||
| Frontal Sup medial (BA 32) | left | -10 | 48 | 28 | 82 | 8.16 | .17 | .68 |
| Frontal Sup medial (BA 10) | left | -16 | 62 | 2 | 120 | 6.60 | .13 | .55 |
| Frontal Sup (BA 32) | right | 16 | 50 | 18 | 76 | 6.92 | .28 | .61 |
| Rectus Gyrus (BA 11) | bilat | -2 | 36 | -16 | 624 | 6.33 | .51 | .44 |
| SMA (BA 6) | left | -4 | -16 | 60 | 162 | -5.02 | -.10 | -.46 |
| Insular Cortex | ||||||||
| aINS | right | 30 | 10 | -12 | 65 | 5.44 | .27 | .49 |
| Limbic/Cingulate Cortex | ||||||||
| PHG | right | 28 | -28 | -22 | 135 | 5.87 | .31 | .54 |
| Retrosplenial (BA 30) | bilat | 2 | -52 | 16 | 298 | -7.53 | -.44 | -.53 |
| Temporal Cortex | ||||||||
| Temporal Mid (BA 21) | left | -58 | -50 | 8 | 66 | -5.51 | -.14 | -.50 |
| Temporal Mid (BA37) | left | -48 | -70 | 14 | 138 | -5.40 | -.35 | -.50 |
| Temporal Mid (BA 37) | right | 56 | -66 | 6 | 109 | -5.23 | -.05 | -.48 |
| Occipital Cortex | ||||||||
| Cuneus (BA 18/19) | left | -8 | -88 | 30 | 547 | -8.09 | -.17 | -.62 |
| Calcarine (BA 17) | left | -12 | -78 | 6 | 76 | -6.58 | -.28 | -.52 |
| Lingual (BA 18/19) | left | -18 | -64 | 4 | 573 | -6.08 | -.22 | -.51 |
| Cerebellum | ||||||||
| Cerebellum Crus2 | left | -36 | -62 | -38 | 115 | 6.70 | .31 | .64 |
| Cerebellum Vermis8 | bilat | 2 | -74 | -36 | 64 | 5.18 | .18 | .48 |
| 2B: Slow-4 frequency power correlated with BMI in women (r=0.76) but not men (r=0.08) | ||||||||
| SLOW-4 FREQUENCY | ||||||||
| Region | Side | Coordinates | Size | BSR | Correlation | |||
| X | Y | Z | ♂ | ♀ | ||||
| Subcortical | ||||||||
| Putamen | right | 18 | -4 | 14 | 166 | 6.71 | .25 | .55 |
| Putamen | left | -26 | 6 | 10 | 160 | 6.35 | -.14 | .65 |
| Globus Pallidus | right | 22 | -4 | -2 | 129 | 5.49 | -.21 | .59 |
| Frontal Cortex | ||||||||
| Frontal Med Orb (BA 11) | left | -12 | 40 | -12 | 63 | 5.35 | -.26 | .51 |
| Frontal Mid (BA 46) | left | -28 | 38 | 24 | 76 | 5.33 | -.37 | .55 |
| Limbic/Cingulate Cortex | ||||||||
| Retrosplenial (BA 30) | right | 10 | -44 | -2 | 595 | -8.66 | -.02 | -.67 |
| Temporal Cortex | ||||||||
| Temporal Mid (BA 22) | left | -52 | -54 | 24 | 121 | 6.53 | 0 | .35 |
| Temporal Sup (BA 22) | left | -62 | -24 | 4 | 82 | -6.37 | -.03 | -.45 |
| Temporal Pole Sup (BA 21) | left | -46 | 2 | -20 | 90 | -6.27 | .17 | -.60 |
| Occipital Cortex | ||||||||
| Lingual (BA 18) | bilat | -12 | -52 | -6 | 1709 | -9.22 | .06 | -.65 |
| Lingual (BA 18) | bilat | -4 | -76 | 26 | 76 | -7.13 | .06 | -.50 |
| Lingual (BA 18) | left | -28 | -92 | -14 | 61 | -5.00 | -.24 | -.48 |
| Cuneus (BA 18/19) | bilat | -6 | -86 | 28 | 264 | -7.98 | .02 | -.58 |
| Calcarine (BA 18) | left | -10 | -96 | -10 | 424 | -7.01 | .07 | -.57 |
| Occipital_Sup (BA 17) | left | -16 | -94 | 12 | 69 | -6.15 | -.16 | -.53 |
| Cerebellum | ||||||||
| Cerebellum 8 | right | 20 | -60 | -46 | 100 | 7.81 | .07 | .60 |
| Cerebellum 6 | right | 28 | -44 | -34 | 62 | 6.90 | .24 | .60 |
| Cerebellum 8 | left | -40 | -48 | -46 | 62 | 5.77 | .06 | .44 |
| Cerebellum 6 | left | -16 | -72 | -24 | 249 | 5.61 | .34 | .48 |
| Cerebellum Crus2 | right | 16 | -80 | -32 | 67 | 5.41 | 0 | .57 |
The correlation columns refer to the correlation between BMI and slow-5 frequency power at the peak voxel. The correlations in the table title refer to the correlation between BMI and the overall spatial pattern of slow-5 frequency power distribution.
BA, Brodmann area; BSR, bootstrap ration; aINS, anterior insula; PHG, parahippocampal gyrus; SMA, supplementary motor area
The correlation columns refer to the correlation between BMI and slow-4 frequency power at the peak voxel. The correlations in the table title refer to the correlation between BMI and the overall spatial pattern of slow-4 frequency power distribution.
BA, Brodmann area; BSR, bootstrap ration
Figure 2. Whole-Brain BMI-related intrinsic activity/connectivity in men and women.
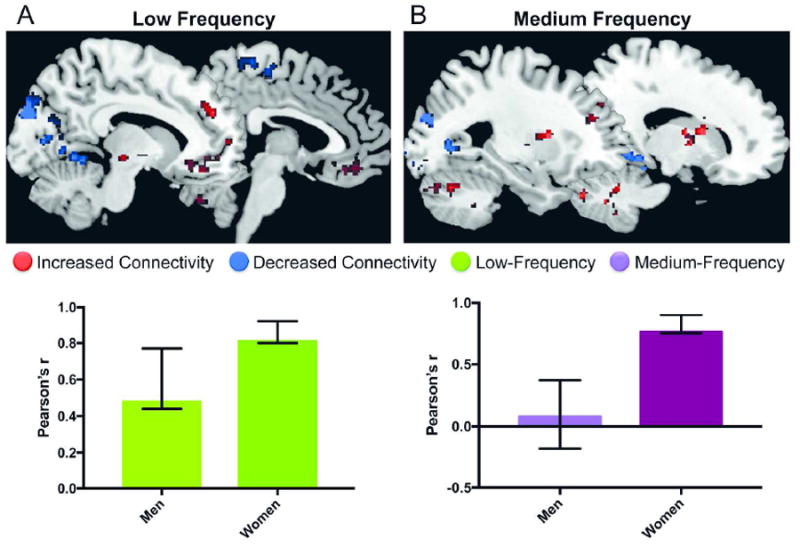
A: BMI-related slow-5 (low frequency) activity. The first latent variable in the partial lest squares analysis reflected sex similarities in the relationship between slow-5 power and BMI. However, the relationship between BMI and slow-5 power was significantly stronger in women compared to men.
Red, positive correlation between BMI and frequency power; Blue, negative correlation between BMI and frequency power
B: BMI-related slow-4 (medium frequency) activity. The first latent variable in the partial lest squares analysis reflected sex differences in the relationship between slow-4 power and BMI. The identified brain regions (top of the figure) were correlated with BMI in women but not in men. Red, positive correlation between BMI and frequency power; Blue, negative correlation between BMI and frequency power
Slow-4 (Medium Frequency) activity
The first latent variable (accounting for 64.24% of the cross-block correlation; p=0.002) reflected a significant correlation between BMI and slow-4 frequency power in women (r=0.76), but not men (r=0.08), which was expressed in a set of regions including the right GP and bilateral putamen (Table 2B; Figure 2B). The right GP and bilateral putamen were chosen as slow-4 ROIs (Figure 1).
Sex Differences in Reward seed-based functional connectivity analyses
Slow-5 (Low Frequency) connectivity
The first latent variable (accounting for 55.64% of the cross-block correlation; p=0.012) reflected significant sex differences in the correlation between BMI and slow-5 intrinsic connectivity of the left GP and left SN (Figure 3A). In women, increased BMI was weakly associated with decreased left GP/SN slow-5 intrinsic connectivity (left GP, r=-0.16; left SN, r=-0.27) with a set of regions including emotion regulation regions (such as bilateral anterior cingulate cortex [ACC]) and with the posterior mid cingulate cortex, and with frontal cortical regions (such as the medial orbital cortex and inferior frontal cortex) (Table 3A, Figure 3A). In contrast, in men, increased BMI was associated with increased left GP/SN slow-5 intrinsic connectivity (left GP, r=0.42; left SN, r=0.31) with a set of regions, largely consisting of the medial frontal cortex (Table 3A, Figure 3A).
Figure 3. Reward seed-based frequency connectivity with BMI in men and women.
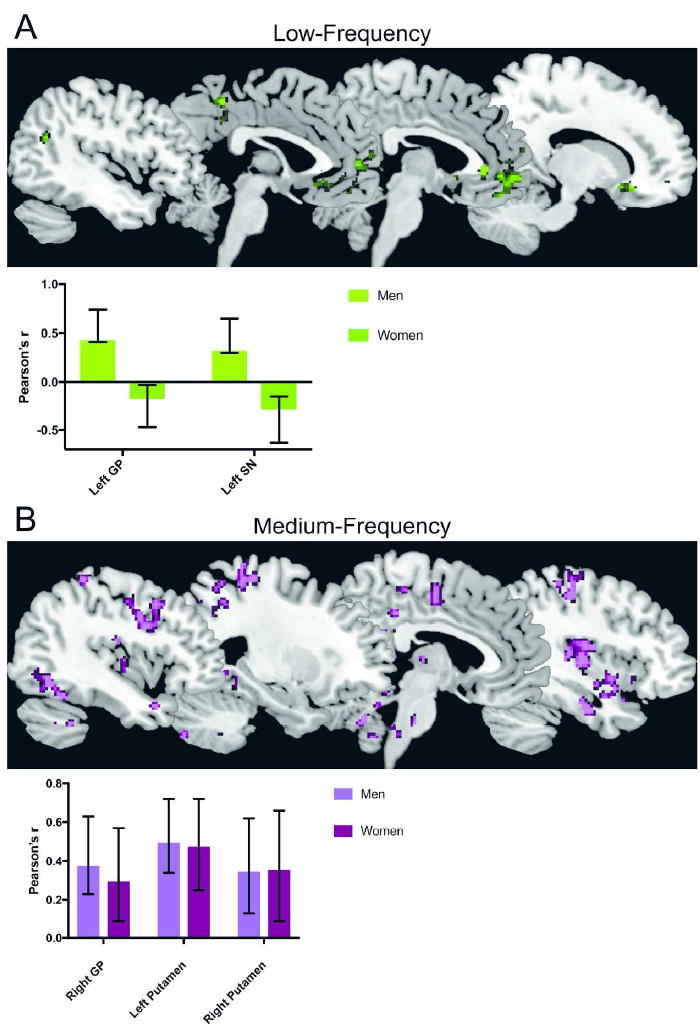
A: BMI-related slow-5 (low frequency) reward seed-based connectivity. The first latent variable in the partial lest squares analysis reflected sex differences in the relationship between BMI and slow-5 connectivity of the left globus pallidus (GP) and substantia nigra (SN). Positive correlation between BMI and connectivity in men, negative/zero correlation between BMI and connectivity in women
B: BMI-related slow-4 (medium frequency) reward seed-based connectivity. The first latent variable in the partial lest squares analysis reflected sex commonalities in the relationship between BMI and slow-4 connectivity of the right globus pallidus (GP) and bilateral putamen. Purple, positive correlation between BMI and connectivity in men and women
Table 3.
Reward seed-based frequency connectivity with BMI in men and women
| A: Sex differences in BMI-related left GP/SN slow-5 connectivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Side | Coordinates | Size | BSR | Correlation ♂ | Correlation ♀ | ||||
| X | Y | Z | L GP | L SN | L GP | L SN | ||||
| Frontal Cortex | ||||||||||
| Frontal Med Orb (BA 11) | bilat | -8 | 28 | -10 | 540 | 5.83 | .65 | .44 | 0 | -.02 |
| Frontal Sup Med (BA 32) | left | -10 | 50 | 20 | 89 | 5.30 | .46 | .41 | -.20 | .09 |
| Frontal Inf Orb (BA 47) | left | -34 | 40 | -16 | 89 | 5.62 | .55 | .28 | -.03 | -.40 |
| Frontal Inf Orb (BA 11) | right | 24 | 20 | -18 | 125 | 5.01 | .50 | .37 | -.19 | -.08 |
| Limbic/Cingulate Cortex | ||||||||||
| ACC (BA 25) | right | 4 | 36 | 2 | 68 | 5.81 | .51 | .21 | -.23 | -.39 |
| ACC (BA 10/32) | left | -8 | 44 | 0 | 63 | 4.48 | .48 | .32 | -.03 | -.19 |
| pMCC (BA 23) | bilat | -2 | -42 | 50 | 67 | 4.58 | .31 | .34 | -.38 | -.22 |
| Temporal Cortex | ||||||||||
| Angular Gyrus (BA 39) | left | -38 | -58 | 28 | 86 | 4.54 | .29 | .19 | -.30 | -.37 |
| Occipital Cortex | ||||||||||
| Calcarine (BA 17) | right | 10 | -96 | 4 | 69 | 4.32 | .46 | .42 | -.18 | -.20 |
| Occipital Mid (BA 17) | left | -18 | -102 | 0 | 63 | 4.96 | .47 | .40 | -.14 | -.24 |
| B: Sex commonalities in BMI-related right GP/putamen slow-4 connectivity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Side | Coordinates | Size | BSR | Correlation ♂ | Correlation ♀ | ||||||
| X | Y | Z | R GP | L Put | R Put | R GP | L Put | R Put | ||||
| Subcortical | ||||||||||||
| Thalamus | right | 18 | -24 | -8 | 261 | 4.59 | .33 | .37 | .18 | .30 | .41 | .45 |
| Brainstem | bilat | 0 | -28 | -34 | 336 | 4.66 | .40 | .41 | .36 | .41 | .39 | .40 |
| Frontal Cortex | ||||||||||||
| Frontal Med Orb (BA 10) | left | -8 | 60 | -4 | 92 | 5.06 | .44 | .54 | .42 | .22 | .31 | .40 |
| Frontal Med Sup (BA 8) | left | -6 | 24 | 46 | 139 | 5.57 | .54 | .45 | .31 | .20 | .26 | .41 |
| Precentral (BA 4) | left | -60 | 0 | 34 | 431 | 6.20 | .32 | .64 | .59 | .31 | .33 | .30 |
| Precentral (BA 4/6) | right | 58 | 2 | 40 | 129 | 5.09 | .32 | .48 | .37 | .13 | .42 | .29 |
| Parietal Cortex | ||||||||||||
| Postcentral (BA 3/4) | right | 36 | -26 | 50 | 508 | 5.12 | .26 | .58 | .46 | .12 | .38 | .27 |
| Postcentral (BA 3/4) | left | -28 | -30 | 60 | 401 | 4.93 | .24 | .32 | .23 | .42 | .49 | .51 |
| Supramarginal (BA 40) | right | 64 | -44 | 40 | 104 | 4.69 | .32 | .42 | .34 | .13 | .45 | .13 |
| Precuneus (BA 5) | right | 10 | -58 | 56 | 95 | 4.67 | .35 | .59 | .44 | .20 | .51 | .10 |
| Precuneus (BA 5) | right | 8 | -44 | 54 | 240 | 4.30 | .35 | .50 | .36 | .14 | .32 | .32 |
| Parietal Sup (BA 5/7) | left | -20 | -56 | 56 | 89 | 4.31 | .25 | .45 | .36 | .42 | .40 | .38 |
| Limbic/Cingulate Cortex | ||||||||||||
| Amygdala | right | 26 | 2 | -24 | 543 | 5.44 | .36 | .51 | .27 | .47 | .41 | .21 |
| PCC (BA 23) | bilat | 6 | -46 | 24 | 358 | 5.21 | .36 | .40 | .26 | .32 | .32 | .40 |
| aMCC (BA 23/24) | right | 4 | -10 | 52 | 182 | 4.06 | .36 | .47 | .40 | .29 | .32 | .47 |
| Insula/Operculum | ||||||||||||
| pINS | right | 34 | -10 | 16 | 1011 | 5.43 | .35 | .53 | .29 | .15 | .35 | .37 |
| pINS | left | -40 | -10 | -2 | 111 | 4.95 | .39 | .55 | .44 | .29 | .47 | .23 |
| Rolandic Oper | left | -46 | -2 | 18 | 61 | 4.88 | .24 | .17 | .36 | .30 | .36 | .49 |
| Temporal Cortex | ||||||||||||
| Temporal Pole Sup (BA 38) | right | 44 | 14 | -22 | 98 | 5.29 | .48 | .62 | .44 | .17 | .35 | -.02 |
| Temporal Pole Sup (BA 21) | left | -46 | 8 | -22 | 74 | 4.93 | .34 | .50 | .07 | .32 | .38 | .56 |
| Temporal Inf (BA 37) | right | 54 | -56 | -4 | 61 | 4.50 | .28 | .40 | .37 | .30 | .48 | .48 |
| Occipital Cortex | ||||||||||||
| Lingual/Fusiform/Cerebellum 4/5 (BA 19/37) | right | 28 | -34 | -20 | 3699 | 6.06 | .44 | .58 | .51 | .43 | .48 | .24 |
| Lingual (BA 18) | left | -24 | -82 | -4 | 376 | 5.74 | .34 | .50 | .32 | .53 | .38 | .17 |
| Occipital Inf (BA 19) | left | 36 | -72 | -8 | 196 | 5.47 | .29 | .48 | .28 | .20 | .36 | .38 |
| Occipital Mid (BA 7/19) | left | -30 | -64 | 38 | 148 | 4.71 | .42 | .52 | .44 | -.13 | .28 | .05 |
| Cerebellum | ||||||||||||
| Cerebullum 9 | bilat | -4 | -56 | -42 | 1697 | 5.80 | .58 | .51 | .43 | .10 | .36 | .37 |
| Cerebellum 9 | right | 12 | -48 | -34 | 90 | 4.70 | .34 | .48 | .31 | .46 | .35 | .33 |
The correlation columns refer to the correlation between BMI and slow-5 left GP/SN connectivity at the peak voxel. The correlations in the text refer to the correlation between BMI and the overall spatial pattern of slow-5 left GP/SN connectivity.
BA, Brodmann area; BSR, bootstrap ration; ACC, anterior cingulate cortex; pMCC, posterior mid cingulate cortex
The correlation columns refer to the correlation between BMI and slow-4 right GP/putamen connectivity at the peak voxel. The correlations in the text refer to the correlation between BMI and the overall spatial pattern of slow-4 right GP/putamen connectivity.
BA, Brodmann area; BSR, bootstrap ration; pINS, posterior insula; PCC, posterior cingulate cortex; aMCC, anterior mid cingulate cortex
Slow-4 (Medium Frequency) connectivity
The first latent variable (accounting for 75.22% of the cross-block correlation; p<0.001) reflected sex commonalities in the correlations between BMI and slow-4 intrinsic connectivity of the right GP and bilateral putamen (Figure 3B). In men and women, increased BMI was associated with increased GP/putamen slow-4 intrinsic connectivity (right GP, r=0.37; left putamen, r=0.49; right putamen, r=0.34) with a set of regions, largely consisting of sensorimotor regions such as bilateral precentral gyrus, bilateral postcentral gyrus, supramarginal gyrus, and posterior insula, but also including emotion regulation regions such as amygdala, medial orbital frontal cortex, and precuneus (Table 3B, Figure 3B).
Discussion
In the current study, we examined the relationship between BMI and low frequency resting state activity/connectivity in men and women. We examined two low frequency bands, slow-5 (0.01-0.027 Hz), slow-4 (0.027-0.073 Hz), which are largely found in gray matter and are thought to reflect different neuronal classes (23). Consistent with previous reports that slow-5 predominates in cortex, while slow-4 predominates in basal ganglia (23), we found more widespread BMI-related alterations involving the basal ganglia in the slow-4 band compared to that in the slow-5 band. For both women and men, increased BMI was associated with increased slow-5 frequency power in the left GP and SN; however, the correlation was stronger in women compared to that in men. In addition, with increasing BMI, increased slow-4 frequency power in the right GP and bilateral putamen was observed in women, but not in men. Sex-related similarities and differences were observed in BMI-related alterations in resting state activity/connectivity of the core reward network. To our knowledge, this is the first study demonstrating the influence of sex on BMI-related alterations associated with resting state activity/connectivity in two different low frequency bands.
Sex differences in the associations between frequency power distribution and BMI
Past research has shown that individuals with obesity (both men and women) have more low frequency power in subcortical regions of the reward circuitry including the putamen, claustrum, and insula (24, 25). Furthermore, several neuroimaging studies have demonstrated functional alterations in the reward network in relationship to ingestive behaviors and obesity, especially in core reward regions such as the basal ganglia (9). The basal ganglia (putamen, caudate nucleus, globus pallidum, and nucleus accumbens), along with the corticostriatal pathways, are a critical component of the reward network (26), and influence memory and value assessment processes attributed to hedonic ingestion and obesity (27). According to the reward-deficiency model, hedonic eating occurs as a result of decreased dopamine receptor availability in the reward regions with increased BMI (9). Together with the present results, a body of evidence supports the concept that similar to drug addiction, key regions of the reward network are activated (9, 10), as a way to compensate for a dopamine deficiency, and therefore, are responsible for an increased drive in pleasure-seeking rewarding behaviors, food intake, and hedonic eating (28).
In addition to these insights, the present results demonstrate for the first time that the correlations between frequency power distribution in the reward regions (GP and putamen) and BMI are especially strong in women. Multiple female-specific studies (7, 13) have reported increased low frequency-activity in obese women in reward regions, such as the putamen, compared to normal-weight counterparts, which is not modulated by food intake. Coveleskie et al. (29) also reported greater low frequency-activity in the medium frequency band in obese women in several reward regions. Furthermore, alterations in resting state fMRI have been associated with eating behaviors and have been used to predict weight gain in women (30). The dorsal striatum (consisting of the putamen and caudate nucleus) is a key relay region, receiving input from the prefrontal cortical regions and projecting to other portions of the basal ganglia and sensorimotor cortex, and influences reward-related behaviors (9, 26). In addition, reduced dopamine signaling in the striatum is associated with reinforcing the rewarding properties (31) and the value of food (9, 26). In fact, it appears that alterations in dopamine availability in the striatum are an important factor driving greater impulsivity and hedonic eating in preschool girls (32). Our results suggest that women experience less restraint and self-regulation, which may start at an early age and may play a role in the persistence of hedonic eating behavior later in life. These findings have important implications for understanding the increased risk of obesity and hedonic ingestion in women, especially in the context of an obesogenic environment with easy access to highly palatable and energy-dense foods. Past neuroimaging studies have highlighted that greater activity in regions involved with interoception and awareness of body states (particularly fullness) in men (10, 33). This points to a different mechanism in males where compulsive eating related to obesity is dictated by the integration of information from the autonomic system (10, 15, 34). It is possible that we were unable to detect these differences due to limited BMI ranges and unbalanced samples in the overweight/obese versus normal BMI groups.
Sex differences in associations between reward seed-based connectivity and BMI
For women, with increasing BMI, slow-5 connectivity between the left GP/SN and cortical regions decreased in several frontal regions (including the medial orbital and inferior frontal cortices) as well as in emotion regulation regions (including the ACC and posterior mid cingulate cortices). On the other hand, for men, increased BMI was associated with increased slow-5 connectivity between the left GP/SN and the medial frontal cortex.
Reduced impulse control has been associated with alterations in cortical regions, including the orbital frontal cortex and ACC, corresponding to lower self-restraint with higher BMI (35, 36). Activation of the inferior frontal cortices is associated with top-down control and inhibition processes involved in the self-regulation of food cravings and impulses associated with hedonic ingestion (37). A negative association between frontal cortical-basal ganglia functional connectivity has been linked to reduced hunger control (9, 10), increased weight gain (14) and appetite (6, 8) in obesity. When viewed together, our results may explain why the modulation of impulse control is more associated with increased hedonic ingestion and obesity in women than in men. Impulsivity is a major predictor for the development of obesity, and explains more of the variance associated with increased hedonic ingestion than genetic factors (such as FTO, alpha-ketoglutarate dependent dioxygenase)(38). This important role of impulsivity may relate to the observed sex differences in functional connectivity between the striatum and the cortical regions. Reduced connectivity in prefrontal and anterior cingulate cortical regions has been implicated in increased cravings and desire to eat high-fat foods, and decreased self-control over eating (39). In addition, alterations in the posterior cingulate cortex have been implicated in self-referential processes involving body appearance (40). One may speculate that reduced functional connectivity in reward, cortical, and emotion regulation regions reflects alterations in body image and self-perception in women with higher BMIs. Furthermore, sex differences in hedonic eating and impulse control may result in greater emotional eating observed in women.
Sex commonalities in associations between reward seed-based connectivity and BMI
Contrary to our hypothesis, increased BMI in both men and women was associated with increased slow-4 connectivity between the right GP/putamen and a wide range of cortical regions, including the precuneus, amygdala, and sensorimotor-related regions. Although the correlations between BMI and right GP/putamen connectivity with sensorimotor-related regions displayed a general pattern of being slightly stronger in men than in women, the differences in the correlations were not significant.
Abnormal connectivity of the precuneus has been related to hunger ratings and to restrictive eating scores (41). Another key region in the central control of eating behaviors is the amygdala which is closely connected to key homeostatic and reward regions such as the hypothalamus, nucleus of the solitary tract, ventral tegmental area, NAcc, and parabrachial nucleus (42). The amygdala is also connected with striatal and sensorimotor cortical regions, including the postcentral gyrus, precentral gyrus, and supplementary motor area (11, 15, 43), which have been associated with disinhibited eating (44). Together, these regions play important roles in the regulation of feeding behaviors and satiation (45).
Limitations
Although we collected data for women in the follicular phase of the menstrual cycle by self-report, we did not measure female sex hormone levels. Future studies should evaluate associations between the observed BMI-related brain alterations and eating behaviors, eating preferences, and diet information. All our findings represent associations rather than causal relationships. In order to address the issue of causality between the observed brain changes and obesity, future longitudinal and mechanistic studies linking the brain to peripheral markers (e.g. the gut microbiome/metabolites and inflammatory factors) and psychosocial variables (e.g. stress, race) are required to determine whether the observed BMI-related brain alterations reflect a premorbid state or are a consequence of remodeling secondary to obesity. When whole-brain frequency power PLS analyses were performed in a subset of participants with BMI in the overweight/obese range and in the normal range, we found that our whole-brain results are more clearly evident among those with BMI in the overweight/obese range than in those with BMI in the lean range, suggesting that some nonlinearities may exist/the more limited range in the lean analysis may have affected power. This would suggest future studies focused on wider BMI ranges and more balanced breakdown of males and females in the overweight/obese and normal BMI groups.
Supplementary Material
Summary and Clinical Implications.
The present results confirm the hypothesis that high BMI is associated with alterations in the reward network in both men and women, and emphasizes the importance of considering sex-related differences in these alterations. The decreased connectivity of reward regions with cortical and emotion regulation regions in women with higher-BMI may explain the greater prevalence of emotional and compulsive behavior related to increased hedonic ingestion in women. Sex and sex hormones are known to modulate responses of the mesolimbic dopamine system (involved in reward) to stress and drug use, and are thought to underlie sex differences in the pathophysiology of drug addiction. The present results suggest that similar sex effects on the mesolimbic reward system may also be at play in obesity. However, longitudinal studies are needed to determine whether a high BMI and associated metabolic changes play a causal role rewiring brain circuits, or if is genetic and epigenetic factors influence brain development in a way to increase the vulnerability to develop maladaptive eating behaviors in the context of easy access to high caloric foods. These findings may have implications for more effective treatments for obesity, which take sex related differences in eating behavior into consideration.
What is already known about this subject?
Neuroimaging studies have shown evoked functional brain alterations in homeostatic and reward-related brain networks in individuals with obesity.
Sex differences have been observed in individuals with obesity, with women having an increased propensity to gain total body fat, and men having more visceral adipose tissue.
Sex differences in eating behaviors, food cravings, and types of food craved have been identified, which could reflect sex-specific activations in cognitive, emotional, and reward-related brain regions.
What does this study add?
This manuscript identifies sex-related commonalities and differences in obesity-related alterations in the intrinsic brain activity and connectivity of key regions of the reward, salience, and sensorimotor networks, which previous neuroimaging studies have identified as contributing to eating behaviors that override homeostatic needs, overeating, and obesity.
The effects of sex hormones on modulating responses of the mesolimbic dopamine system (involved in reward) to stress and drug use, are similar to the sex-differences observed in the pathophysiology of obesity and hedonic ingestion.
These sex-difference findings in the brain’s extended reward system have implications for more effective treatments for obesity, which take sex-related differences in eating behavior into consideration.
Acknowledgments
Funding Support: This research was supported by grants from the National Institutes of Health including K23 DK106528 (AG), R01 DK048351 (EAM), P50 DK064539 (EAM), P30 DK041301, and pilot funds were provided for brain scanning by the Ahmanson-Lovelace Brain Mapping Center.
Footnotes
Author Contributions:
Arpana Gupta: Study concept and design, analysis and interpretation of data, drafting and revision of manuscript, study funding
Emeran Mayer: Drafting and critical review of manuscript, approval of final version of the manuscript, study funding
Jennifer S. Labus: analysis and interpretation of data
Ravi Bhatt: Analysis of data
Amanat Bal: Literature review
Kirsten Tillisch: Review of manuscript, study funding
Bruce Naliboff: Review of manuscript, study funding
Claudia P. Sanmiguel: Drafting and critical review of manuscript
Lisa A. Kilpatrick: Study concept and design, analysis and interpretation of data, drafting and revision of manuscript
Preliminary data was reported in an abstract presented as a poster at Digestive Diseases Week (DDW) 2017 and as an oral presentation at The Obesity Week (TOS) 2016.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Ward ZJ, Long MW, Resch SC, Gortmaker SL, Cradock AL, Giles C, et al. Redrawing the US Obesity Landscape: Bias-Corrected Estimates of State-Specific Adult Obesity Prevalence. PloS one. 2016;11:e0150735. doi: 10.1371/journal.pone.0150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton JA, Tannenbaum C. Reporting Sex, Gender, or Both in Clinical Research? JAMA : the journal of the American Medical Association. 2016;316:1863–1864. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 3.Gartner DR, Taber DR, Hirsch JA, Robinson WR. The spatial distribution of gender differences in obesity prevalence differs from overall obesity prevalence among US adults. Ann Epidemiol. 2016;26:293–298. doi: 10.1016/j.annepidem.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garawi F, Devries K, Thorogood N, Uauy R. Global differences between women and men in the prevalence of obesity: is there an association with gender inequality? Eur J Clin Nutr. 2014;68:1101–1106. doi: 10.1038/ejcn.2014.86. [DOI] [PubMed] [Google Scholar]
- 5.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. International journal of obesity. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 6.Lalitha V, Pal GK, Pal P, Parija SC, Murugaiyan SB. Gender Difference in the Role of Posterodorsal Amygdala on the Regulation of Food Intake, Adiposity and Immunological Responses in Albino Wistar Rats. Ann Neurosci. 2016;23:6–12. doi: 10.1159/000443550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallam J, Boswell RG, DeVito EE, Kober H. Gender-related Differences in Food Craving and Obesity. Yale J Biol Med. 2016;89:161–173. [PMC free article] [PubMed] [Google Scholar]
- 8.Park S, Shin WS. Differences in eating behaviors and masticatory performances by gender and obesity status. Physiol Behav. 2015;138:69–74. doi: 10.1016/j.physbeh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends in cognitive sciences. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, et al. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. Neuroimage Clin. 2015;7:506–517. doi: 10.1016/j.nicl.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57:421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Mayer EA, Hamadani K, Bhatt R, Fling C, Alaverdyan M, et al. Sex differences in the influence of body mass index on anatomical architecture of brain networks. International journal of obesity. 2017 doi: 10.1038/ijo.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egorova N, Veldsman M, Cumming T, Brodtmann A. Fractional amplitude of low-frequency fluctuations (fALFF) in post-stroke depression. Neuroimage Clin. 2017;16:116–124. doi: 10.1016/j.nicl.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YC, Xia W, Luo B, Muthaiah VP, Xiong Z, Zhang J, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in chronic tinnitus. Front Neural Circuits. 2015;9:67. doi: 10.3389/fncir.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh AR. Tracing the route to path analysis in neuroimaging. Neuroimage. 2012;62:887–890. doi: 10.1016/j.neuroimage.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, et al. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. International journal of obesity. 2016;40:1687–1692. doi: 10.1038/ijo.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Tian D, Yu C, Zhang J, Tian X, von Dneen KM, et al. Altered baseline brain activities before food intake in obese men: a resting state fMRI study. Neuroscience Letter. 2015;584:156–161. doi: 10.1016/j.neulet.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 29.Coveleskie K, Gupta A, Kilpatrick LA, Mayer ED, Ashe-McNalley C, Stains J, et al. Altered functional connectivity within the central reward network in overweight and obese women. Nutr Diabetes. 2015;5:e148. doi: 10.1038/nutd.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong D, Jackson T, Wang Y, Chen H. Spontaneous regional brain activity links restrained eating to later weight gain among young women. Biol Psychol. 2015;109:176–183. doi: 10.1016/j.biopsycho.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silveira PP, Portella AK, Kennedy JL, Gaudreau H, Davis C, Steiner M, et al. Association between the seven-repeat allele of the dopamine-4 receptor gene (DRD4) and spontaneous food intake in pre-school children. Appetite. 2014;73:15–22. doi: 10.1016/j.appet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Rapuano KM, Huckins JF, Sargent JD, Heatherton TF, Kelley WM. Individual Differences in Reward and Somatosensory-Motor Brain Regions Correlate with Adiposity in Adolescents. Cerebral cortex. 2016;26:2602–2611. doi: 10.1093/cercor/bhv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A, et al. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2013;14:315–323. doi: 10.1111/obr.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. 2011;101:579–592. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith R, Alkozei A, Killgore WDS. Conflict-related dorsomedial frontal cortex activation during healthy food decisions is associated with increased cravings for high-fat foods. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9726-7. [DOI] [PubMed] [Google Scholar]
- 40.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y, Ikuta T. Caudate-Precuneus functional connectivity is associated with obesity preventive eating tendency. Brain Connect. 2017 doi: 10.1089/brain.2016.0424. [DOI] [PubMed] [Google Scholar]
- 42.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 43.Baek K, Morris LS, Kundu P, Voon V. Disrupted resting-state brain network properties in obesity: decreased global and putaminal cortico-striatal network efficiency. Psychol Med. 2017;47:585–596. doi: 10.1017/S0033291716002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J, Li M, Zhang Y, Song H, von Deneen KM, Shi Y, et al. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: an fMRI study. Brain Imaging Behav. 2016 doi: 10.1007/s11682-015-9491-4. [DOI] [PubMed] [Google Scholar]
- 45.Anderberg RH, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol Behav. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


