Abstract
Objective
The current study aimed to identify how sex influences neurobiological responses to food cues, particularly those related to hedonic eating, and how this relates to obesity propensity, using functional magnetic resonance imaging (fMRI).
Methods
Adult men and women who were either obesity-resistant (OR) or obesity-prone (OP) underwent fMRI while viewing visual food cues (hedonic foods, neutral foods, and non-food objects) in both fasted and fed states.
Results
When fasted, a significant sex effect on the response to hedonic vs. neutral foods was observed, with greater responses in women than men in the nucleus accumbens (p=0.0002) and insula (p=0.010). Sex-based differences were not observed in the fed state. No significant group effects (OP vs. OR) or group by sex interactions were observed in fasted or fed states.
Conclusions
Greater fasted responses to hedonic food cues in reward-related brain regions were observed in women compared to men, suggesting that women may be more sensitive to reward value of hedonic foods than men when fasted. This may indicate sex-dependent neurophysiology underlying eating behaviors.
Keywords: sex-based differences, obesity-prone, obesity-resistant, functional magnetic resonance imaging (fMRI)
Introduction
Sex-based differences in eating behaviors are consistently observed. For example, studies suggest women are more likely to diet than men, express greater concern about weight control, report more frequent consumption of fruits and vegetables, and attribute greater importance to healthy eating1–3. Women also report more behaviors associated with eating disorders4, and have higher rates of obesity, particularly severe obesity5. Differences in eating behaviors and obesity rates between men and women involve a number of factors, such as gonadal hormones, social pressures and norms, and physical activity engagement6. These factors also interact with neuronal processes involved in eating behaviors.
Multiple brain regions and networks are involved in eating-related processes, including those involved in sensory perception (e.g., primary visual cortex, fusiform gyrus), reward and interoceptive processing (e.g., insula, nucleus accumbens, anterior cingulate cortex [ACC]), and cognitive control (e.g., dorsolateral prefrontal cortex [dlPFC], parietal cortex)7,8. Women consistently demonstrate greater response to visual food cues than men in a number of regions related to eating behaviors, including the insula9, ACC10,11, dlPFC12, and fusiform gyrus10,12,13. Previous studies have found sex-based differences when comparing neuronal responses to visual cues of foods vs. non-food objects in normal-weight individuals in the fasted state (dlPFC, fusiform, parietal cortex: women > men [W>M])12 and across fasted and fed states (fusiform: W>M)13. Sex-based differences have also been observed in neuronal responses in normal-weight participants to high-calorie visual food cues (fusiform: fasted > fed in women only)10 and to high- vs. low-calorie food cues in a hunger-neutral (1 hour post-prandial) state (insula, orbitofrontal cortex, middle/posterior cingulate gyrus: W>M)9. Similar sex-based differences to high- vs. low calorie food cues have also been observed in participants with obesity in fasted (fusiform, caudate nucleus: W>M; parietal cortex: M>W)11 and fed states (ACC: W>M; fusiform: M>W)11.
Hedonic eating, or eating beyond homeostatic needs, may be particularly associated with obesity14. As such, the current study investigated sex-based differences in neuronal responses to hedonic foods. This was assessed in both fasted and fed states, using functional magnetic resonance imaging (fMRI). This study is the first to investigate whether sex-based differences in neuronal responses to food cues vary based on hedonic properties of foods, by comparing responses to foods with high vs. neutral hedonic value. This is also the first study to determine if sex-based differences in neuronal responses to food cues vary based on propensity to obesity, with the inclusion of both obesity-prone and obesity-resistant study groups. We hypothesized that women would show greater neuronal response to food cues than men, with obesity-prone women more responsive than obesity-resistant women.
Methods
Participant characteristics
Fifty-six adults 25–40 years old were included in the study, drawn from a larger study investigating effects of obesity-proneness on metabolism. Half the study participants were recruited as having a propensity to be resistant to weight-gain and obesity (OR; N=28) and half were recruited as being prone to weight gain and obesity (OP; N=28), as previously described15–19. Briefly, OR participants had a body mass index (BMI) between 17–25 kg/m2, responded to advertisements for “naturally thin people,” and reported no first-degree relatives with obesity, never being overweight themselves, having weight stability despite few to no attempts to lose weight, and not having high levels of physical activity. OP participants had a BMI between 20–30 kg/m2, responded to advertisements for “people who struggle with their weight,” and reported at least one first-degree relative with obesity, a history of weight fluctuations despite efforts to lose or maintain weight, but were not actively attempting to lose weight and were weight-stable for at least 3 months prior to participation. All participants were free of significant medical and psychiatric disease, including eating disorders, as assessed by medical history, physical examination, blood testing, and behavioral questionnaires (Eating Attitudes Test20; Center for Epidemiologic Studies Depression Scale [CES-D]21). All participants were right-handed, with no contraindications to MRI scanning, and reported being weight-stable +/− 5 lbs for the 3 months prior to the study. Data from three participants were excluded from analyses due to technical problems (N=2) or head movement >2 mm (N=1) during fMRI scanning. Final analyses included 25 women (11 OR, 14 OP) and 28 men (14 OR, 14 OP). Participants provided written informed consent and all procedures were in accordance with and approved by the Colorado Multiple Institutional Review Board.
Study design
As previously described16,18,19, body composition was assessed by dual-energy X-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp.) and eating behaviors were assessed with the Three Factor Eating Questionnaire (TFEQ22). One participant did not complete the TFEQ, resulting in a reduced sample size for the OR group for this measure (10 women, 14 men). Participants then completed a four-day eucaloric run-in diet (50% carbohydrate, 30% fat, 20% protein; estimation of energy needs made using lean body mass plus an activity factor23) prior to fMRI scanning, to ensure energy and macronutrient balance. Food was prepared by the Clinical Translational Research Center (CTRC) metabolic kitchen at the University of Colorado Anschutz Medical Campus. Participants presented to the CTRC every morning during the diet period, then were weighed, ate breakfast, and were given the remainder of their daily meals. Participants were asked to maintain usual patterns of physical activity and not to consume alcoholic or calorie-containing beverages. They were regularly questioned regarding activity and compliance. In women, study measures were performed in the follicular phase of their menstrual cycle.
On the study day, participants presented to the CTRC after an overnight fast of at least 10 hours. A visual analog scale (VAS) measured hunger (“how hungry are you?” from “not at all hungry” to “extremely hungry”), satiety (“how full do you feel right now?” from “not at all” to “extremely”), and prospective food consumption (“how much do you think you could eat right now?” from “nothing at all” to “a large amount”). Following this, participants were escorted to the Brain Imaging Center at the University of Colorado Anschutz Medical Campus. Following fasting fMRI measures (described below), participants consumed a liquid breakfast meal over 20 min, the caloric content of which was equal to 25% of the energy provided during run-in diet days, with identical macronutrient composition. Repeat fMRI measures were performed 30 min post-meal. VAS measures were repeated 30, 90, 120, 150, and 180 min post-meal (60 min ratings omitted due to being in the scanner).
fMRI data acquisition
As previously described16,19, fMRI was performed using a GE 3.0 T MR scanner. A high-resolution, T1-weighted 3D anatomical scan was acquired for each participant, after which functional images were acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with the following parameters: TR=2000 ms, TE=30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. An inversion-recovery echo-planar image (IR-EPI; TI=505 ms) volume was acquired to improve coregistration between the echo-planar images and gray matter templates used in preprocessing. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark).
Functional imaging was performed while participants viewed visual stimuli using a projector and screen system. Previously validated visual stimuli consisted of three categories: nonfood-related objects (e.g., animals, trees, furniture), foods of high hedonic value (e.g., chocolate cake, eggs and bacon, pizza), and foods of neutral hedonic value (e.g., bagels, fruit, cereal). High vs. neutral hedonic value was determined in a previous study in which participants rated the images for “food appeal”24. Those in the top food appeal tertile were selected as “high hedonic,” with those in the bottom tertile selected as “neutral.” To reduce habituation potential, different but similar images were used in each scanning session (fasted and fed). The primary analysis compared foods of high hedonic value to those of neutral hedonic value. A secondary analysis compared all foods (hedonic and neutral) to non-food objects. Two runs were performed, each consisting of a pseudo-randomized block design with 6 blocks of pictures of each category. Seven blocks of a low-level baseline (fixation cross) were also included in each run. Each block consisted of 4 stimuli shown for 4 s each for a total of 16 s per block. Four additional scans were acquired at the beginning of each run to minimize saturation effects. Subjects were asked to lie quietly while viewing images.
Data analyses
fMRI data were preprocessed and analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK), as previously described16,19. Functional data were realigned to the first echo-planar image, normalized to the Montreal Neurological Institute (MNI) EPI template, using the gray-matter-segmented IR-EPI as an intermediate to improve registration, and smoothed with a 6 mm full width at half maximum (FWHM) Gaussian kernel. Movement parameters derived from the realignment procedure were included in the model to reduce effects of residual motion-related noise. The hemodynamic response was modeled with a double gamma function, without temporal derivatives, using the general linear model in SPM8. A 128 s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal. To account for within-group and within-subject variance, a random effects analysis was implemented. Parameter estimates for each individual’s first-level analysis (SPM contrast images) contrasting hedonic to neutral food cues were entered into second-level repeated measures ANOVA. For a secondary analysis, all food cues (hedonic and neutral) were contrasted to non-food objects. The main group comparison was sex (women vs. men), with obesity-propensity group comparisons (OR vs. OP) as a secondary aim. These were evaluated using directional contrasts (SPM t-contrasts) in a full factorial model. A priori regions of interest (ROIs; insula, nucleus accumbens, dlPFC, ACC, and fusiform gyrus) were selected as those consistently observed to be involved in visual food cue processing7,25–27 and based on previous findings of sex-based differences in neuronal response to visual food cues9–13. ROIs were anatomically defined using the WFU Pickatlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas) and examined using the MarsBaR toolbox (http://marsbar.sourceforge.net/) in SPM. For each ROI, one value was extracted (mean across all values in the ROI). Results were corrected for multiple comparisons by increasing the statistical significance threshold to p=0.01, reflecting a Bonferroni correction for an alpha of 0.05 (five ROIs were examined).
Analyses of behavioral and body composition measures were performed with SPSS 24 (IBM Corp., Armonk, NY). Total area under the curve for appetite VAS ratings using all post-meal time points was used. A general linear model in SPSS assessed effects of sex (women vs. men), group (OR vs. OP), and sex by group interactions, with alpha=0.05. For correlations between fMRI and behavioral/body composition data, regression analyses were performed in SPSS, using the peak fMRI signal extracted from each ROI.
Results
Behavioral and body composition measures
Main effects of sex were observed for BMI, lean body mass, and percent body fat, with BMI and lean body mass greater in men and percent body fat greater in women (see Table 1). As previously reported, group effects were observed for BMI, fat mass, and percent body fat, such that all were significantly greater in OP compared to OR16,28. OP also had significantly greater lean body mass compared to OR.
Table 1.
Participant characteristics.
| Characteristic | Women | Men | Sex Effect | Group Effect | Sex x Group | ||
|---|---|---|---|---|---|---|---|
| OR (N=11) | OP (N=14) | OR (N=14) | OP (N=14) | p1 | p1 | p1 | |
| Age (years)2 | 31.7 ± 2.7 | 30.5 ± 3.9 | 31.2 ± 3.9 | 29.9 ± 3.9 | 0.595 | 0.221 | 0.977 |
| BMI (kg/m2)2 | 19.4 ± 0.8 | 25.9 ± 3.3 | 22.0 ± 2.0 | 26.5 ± 2.5 | 0.022 | <0.001 | 0.140 |
| Lean body mass (kg)2 | 40.7 ± 3.6 | 46.6 ± 6.7 | 56.5 ± 9.1 | 62.7 ± 7.0 | <0.001 | 0.003 | 0.930 |
| Fat mass (kg)2 | 11.6 ± 1.9 | 24.0 ± 7.7 | 17.2 ± 18.2 | 23.6 ± 14.3 | 0.461 | 0.010 | 0.396 |
| Body fat (%)2 | 21.5 ± 3.0 | 32.9 ± 6.7 | 16.4 ± 4.4 | 24.2 ± 7.3 | <0.001 | <0.001 | 0.268 |
Significant p-values in bold;
Mean ± SEM
OP: obesity-prone; OR: obesity-resistant.
No significant effects of sex were observed for appetite or food-related measures (see Table 2). As previously reported, significant effects of group on all TFEQ subscales were observed, with greater restraint, disinhibition, and hunger ratings in OP compared to OR28.
Table 2.
Appetite and food-related behaviors.
| Measure | Women | Men | Sex Effect | Group Effect | Sex x Group | ||
|---|---|---|---|---|---|---|---|
| OR (N=11) | OP (N=14) | OR (N=14) | OP (N=14) | p1 | p1 | p1 | |
| TFEQ: Restraint2,3 | 4.1 ± 2.2 | 10.6 ± 4.7 | 4.2 ± 3.2 | 8.1 ± 4.0 | 0.277 | <0.001 | 0.233 |
| TFEQ: Disinhibition2, 3 | 3.0 ± 2.4 | 7.4 ± 3.6 | 3.2 ± 2.1 | 7.6 ± 3.2 | 0.763 | <0.001 | 0.966 |
| TFEQ: Hunger2, 3 | 4.3 ± 2.8 | 5.3 ± 2.2 | 4.4 ± 2.2 | 7.2 ± 3.2 | 0.170 | 0.014 | 0.229 |
| Hunger VAS2 | 68.3 ± 19.5 | 71.9 ± 34.2 | 60.8 ± 20.7 | 68.5 ± 16.5 | 0.463 | 0.450 | 0.780 |
| Hunger AUC4 | 6586.7 ± 3011.8 | 7696.7 ± 4667.0 | 6769.0 ± 3065.0 | 9491.2 ± 2863.8 | 0.370 | 0.087 | 0.464 |
| Satiety VAS2 | 11.7 ± 20.4 | 29.7 ± 36.1 | 26.6 ± 18.0 | 19.25 ± 15.6 | 0.761 | 0.475 | 0.094 |
| Satiety AUC4 | 9331.7 ± 3946.5 | 10281.7 ± 4680.6 | 8527.5 ± 1431.2 | 7543.7 ± 2570.0 | 0.100 | 0.987 | 0.363 |
| PFC VAS2 | 69.8 ± 19.6 | 65.3 ± 26.1 | 61.4 ± 17.8 | 74.0 ± 16.6 | 0.982 | 0.526 | 0.189 |
| PFC AUC4 | 10256.7 ± 4474.3 | 8331.7 ± 4330.0 | 9963.0 ± 2825.0 | 10388.7 ± 2656.4 | 0.443 | 0.514 | 0.308 |
Significant p-values in bold;
Mean ± SEM;
Sample size for OR group women reduced to N=10 for TFEQ measures;
Mean total area under the curve (mm x 180 min)
AUC: area under the curve; OP: obesity-prone; OR: obesity-resistant; PFC: prospective food consumption; TFEQ: Three Factor Eating Questionnaire; VAS: visual analog scale.
fMRI
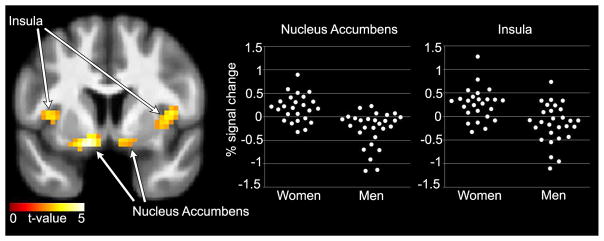
In the fasted state, a significant effect of sex on the response to hedonic compared to neutral foods was observed, with a greater response in women than men in the nucleus accumbens (p=0.0002) and insula (p=0.010), as shown in Figure 1. A trend in this direction was observed in the ACC (p=0.039), but did not survive multiple comparison correction. Sex differences in response to hedonic vs. neutral foods were not observed in the fed state. No significant group effects (OP vs. OR) or sex by group interactions were observed when comparing hedonic to neutral foods in fasted or fed states.
Figure 1.
Greater neuronal response to hedonic vs. neutral foods in the nucleus accumbens and insula in women compared to men, in the fasted state. Statistical maps thresholded at a voxel-wise threshold of p < 0.01 for visualization and overlaid onto the group averaged anatomical image. Data are shown in the neurological convention (right hemisphere on the right).
When comparing all foods (hedonic plus neutral foods) to non-food objects, no significant sex effects were observed in either fasted or fed states. No significant effects of group were observed in the fasted state, but in the fed state, response to foods vs. non-food objects was greater in OP compared to OR in dlPFC (p<0.001), ACC (p=0.005), and insula (p=0.008). A significant interaction between group and sex in the nucleus accumbens (p=0.005) in the fed state food vs. object comparison was also observed, such that although there were no OP vs. OR differences in women, greater nucleus accumbens response was observed in OP vs. OR men (p=0.009). Although there was no significant sex by group interaction in these regions (p>0.05), a greater OP vs. OR response was also observed in men only for ACC (p=0.009) and marginally for insula (p=0.018). Greater dlPFC response in OP vs. OR was observed for men (p=0.008) and marginally for women (p=0.017).
Correlations between fMRI and behavioral/body composition measures
A significant correlation between nucleus accumbens response to hedonic > neutral foods in the fasted state and percent body fat was observed, with greater response associated with greater percent body fat, r=0.40, p=0.003. However, when sex was included as a covariate, the correlation between response and body fat was no longer observed. Similarly, significant correlations were observed between insula response to hedonic > neutral foods in the fasted state and both percent body fat (r=0.30, p=0.030) and lean body mass (r=−0.29, p=0.035). As in the nucleus accumbens, these associations were not observed when sex was included as a covariate, suggesting that the observed correlations between brain response and body fat/lean body mass were driven by sex-based differences.
Discussion
Sex-based differences in neuronal responses to hedonic compared to neutral foods were observed in the fasted state, with greater responses in women compared to men in the nucleus accumbens and insula. Given the prominent roles of these regions in reward processing related to food8,29,30, study results could indicate that women are more sensitive to salient and rewarding aspects of hedonic foods than men when fasted, potentially indicating sex-dependent neurophysiology underlying eating behaviors. Previous studies have found that nucleus accumbens response to visual food cues relates to subsequent snack food consumption31. Greater nucleus accumbens response to food cues has also been associated with subsequent weight gain in women32 and less success in a weight-loss program27. Greater insula response to food cues has also been found to predict reduced weight-loss success27.
In the fed state, sex differences were not observed, suggesting that this hyper-responsivity is specific to the fasted state. This corresponds to previous findings that fasting enhances food image valence for women, but not men33. It has been suggested that this may reflect greater sensitivity to hunger state in women, with men having greater internal food representation stability33. Furthermore, although fasted sex-based differences (women > men) were observed when comparing hedonic to neutral foods, this was not observed for the foods vs. objects comparison, i.e., this was specific to the more distinct comparison of hedonic to neutral foods, rather than foods as a whole. This may suggest that while both men and women are similarly responsive to food vs. objects in general, when fasted, women may find hedonic foods specifically to be more rewarding than men. Hormonal differences between men and women may relate to these effects, as gonadal hormones have been implicated in nucleus accumbens activity modulation34. For example, estrogen signaling at beta estrogen receptors in the brain is thought to be relevant to hedonic eating, with studies finding evidence of dense beta estrogen receptor distribution in the nucleus accumbens30. However, behavioral effects of centrally-acting gonadal hormones, and how they relate to obesity propensity, are not yet fully understood. For example, estrogen plays an important role in the central regulation of food intake, energy expenditure, fat distribution, and is thought to be associated with weight gain, particularly during menopause, but underlying mechanisms are still unclear35,36. No OP vs. OR differences in the response to hedonic vs. neutral foods were observed, nor were interactions between group and sex, suggesting that women as a whole may be more sensitive to rewarding aspects of food in the fasted state, regardless of weight gain propensity.
These findings are consistent with previous observations of greater insula response to visual food cues in a neutral hunger state (1 hour post-prandial) in women compared to men9. Previous studies have also found greater fasted response to visual food cues in women compared to men in other ROIs included in the current study, specifically the dlPFC12 and fusiform gyrus10–12. Additionally, studies have observed greater fed state responses in women compared to men in the ACC11 and in men compared to women in the fusiform gyrus11, while others have observed no sex-based differences in neuronal response to food cues37,38. A number of factors may contribute to differences between previous and current findings. An important consideration is menstrual cycle phase, which was controlled in the current study. To the best of our knowledge, our previous investigation of sex-based differences in fasted normal-weight individuals is the only other study of sex-based differences in visual food cue responses that has held menstrual cycle phase constant12. Differences in neuronal response to visual food cues have been demonstrated in different cycle phases, so this may considerably impact results39–41. Other differences between previous and current findings may be explained by different MR field strengths11,13,37, which can impact signal-to-noise ratio, inclusion of only lean participants or only participants with overweight/obesity11–13,37, lack of controlled meal composition and size prior to fed state measurements13, different meal administration timing9,11,13,37, and lack of a pre-study run-in diet9,11,13,37,38. The inclusion of a four-day eucaloric run-in diet, controlled study meals, and both fasted and fed measurements are strengths of the current study, as is the inclusion of groups varying in obesity propensity.
The secondary aim of the current study was to assess the impact of obesity propensity on sex-based differences in neuronal response to food cues. Although OP vs. OR differences, across sex, were not observed when comparing hedonic to neutral foods, in either fasted or fed states, greater ACC, dlPFC, and insula responses were observed in OP vs. OR when comparing foods to objects while fed. This suggests a continued reward response to foods in OP post-satiation, not observed in OR. A sex by group interaction observed in the nucleus accumbens (fed state, foods > objects) suggests this group difference was driven by the men in the sample, with greater OP vs. OR response in men, but not women. Similar results were observed in the ACC and insula, with greater OP response in men, but not women. This further supports that weight propensity may play a lesser role in food responses in women than in men. While women in the sample had greater percent body fat than men, this does not appear to drive the observed sex-based differences in neuronal responses (i.e., women > men to hedonic foods in nucleus accumbens and insula), as associations between neuronal response and percent body fat were not observed when accounting for sex.
A potential limitation of the current study is that although women were scanned in the same menstrual cycle phase, we did not assess variations in sex-based differences across different menstrual cycle phases. In future studies, assessing women during multiple phases would allow for investigation of how menstrual cycle phase affects sex-based differences in response to food cues. It could also be useful to include gonadal hormone measures to determine how estrogen and testosterone impact food cue responses and how this interacts with sex-based differences. Order of hunger state was not counterbalanced in the current study, which may also be a limitation. Sample sizes in the current study are another potential limitation. As they were not large enough to provide appropriate power for a whole-brain analysis, it is possible that additional regions with sex differences could have been missed with the ROI approach used in the current study. Sample sizes for testing interactions may also have been too small. Also, while the secondary analysis of obesity-proneness aimed to assess effects independent of obesity that make an individual prone to obesity, the multivariate nature of this construct makes it difficult to parse which aspects of obesity proneness (e.g., BMI, genetic propensity, frequency of past dieting behavior, history of weight instability) drive differences in brain response. Additionally, although sex-based differences in dietary restraint were not observed in the current study, this has been observed in previous studies42. Given that higher levels of dietary restraint may relate to hyperactive response to food cues in reward-related brain regions43, it is possible that sex-based differences in neuronal response to food cues may relate to differences in dietary restraint. Future studies with larger sample sizes can investigate this further.
In conclusion, the current study found sex-based differences (women > men) in the response to hedonic foods while participants were in the fasted state, but not in the fed state. These differences were specific to the more nuanced comparison of hedonic vs. neutral foods and were not present when simply comparing all-foods to non-food objects. These results suggest that women may be more sensitive than men to salient and rewarding aspects of food when fasted. The current findings also suggest that weight gain propensity may have less of an impact on visual food cue responses in women, as response differences were observed between OP and OR men that were not seen for women. This suggests potentially different mechanisms underlying weight gain and maintenance in women compared to men, supporting the use of different weight loss and maintenance strategies. These findings also suggest that neurophysiology underlying obesity propensity may be distinct between the sexes. An approach targeting the neurophysiology underlying food cue responses in obesity-prone compared to obesity-resistant men may thus not be appropriate for women, whose reward-related food cue response may have less to do with weight gain propensity. Additional study is warranted to further delineate relationships between sex and neuronal responses to food cues, such as studies with larger sample sizes, which would allow for whole-brain analyses, and studies that can examine neuronal responses in different menstrual cycle phases.
Study Importance Questions.
What is already known about this subject?
Sex-based differences are observed in obesity rates and eating behaviors.
Previous studies have inconsistently observed sex-based differences in neuronal responses to foods.
What does this study add?
The current study is the first to investigate if sex-based differences in neuronal responses to food cues are related to the hedonic properties of foods, and if these effects are related to an individual’s propensity to obesity.
Findings suggest women are more sensitive to rewarding aspects of hedonic foods than men, and that obesity propensity has less influence on food cue responses in women compared to men.
Acknowledgments
Funding: This work was supported by NIH Colorado CTSI grant UL1TR00154, NIH Nutrition Obesity Research Center grant P30DK48520, and NIH grants R01MH102224 (JRT), R01DK103691 (JRT), R01DK089095 (MAC and JRT), R01DK072174 (MAC), R21DK102052 (JRT), and K01DK100445 (KTL).
We thank the research participants, and Debra Singel and Yiping Du for their assistance with fMRI data collection.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Courtenay WH, McCreary DR, Merighi JR. Gender and ethnic differences in health beliefs and behaviors. J Health Psychol. 2002;7:219–231. doi: 10.1177/1359105302007003216. [DOI] [PubMed] [Google Scholar]
- 2.Baker AH, Wardle J. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40:269–275. doi: 10.1016/s0195-6663(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 3.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27:107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- 4.Striegel-Moore RH, Rosselli F, Perrin N, et al. Gender difference in the prevalence of eating disorder symptoms. Int J Eat Disord. 2009;42:471–474. doi: 10.1002/eat.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 6.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 7.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cogn. 2016;110:20–42. doi: 10.1016/j.bandc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport. 2010;21:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity (Silver Spring) 2012;20:2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornier MA, McFadden KL, Thomas EA, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013;110–111:122–128. doi: 10.1016/j.physbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt SL, Kealey EH, Horton TJ, VonKaenel S, Bessesen DH. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int J Obes (Lond) 2013;37:1192–1197. doi: 10.1038/ijo.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas EA, Bechtell JL, Vestal BE, et al. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite. 2013;65:96–102. doi: 10.1016/j.appet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornier MA, Shott ME, Thomas EA, et al. The effects of energy balance, obesity-proneness and sex on the neuronal response to sweet taste. Behav Brain Res. 2015;278:446–452. doi: 10.1016/j.bbr.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The Eating Attitudes Test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 22.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 23.Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO. Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res. 2003;11:752–759. doi: 10.1038/oby.2003.105. [DOI] [PubMed] [Google Scholar]
- 24.Burger KS, Cornier MA, Ingebrigtsen J, Johnson SL. Assessing food appeal and desire to eat: the effects of portion size & energy density. Int J Behav Nutr Phys Act. 2011;8:101. doi: 10.1186/1479-5868-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Bessesen DH, Tregellas JR. Propensity to obesity impacts the neuronal response to energy imbalance. Front Behav Neurosci. 2015;9:52. doi: 10.3389/fnbeh.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 30.Van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update. 2010;16:276–292. doi: 10.1093/humupd/dmp051. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 32.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoeckel LE, Cox JE, Cook EW, 3rd, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48:139–144. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 34.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 35.Clegg DJ. Minireview: the year in review of estrogen regulation of metabolism. Mol Endocrinol. 2012;26:1957–1960. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lizcano F, Guzman G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta S, Melhorn SJ, Smeraglio A, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens MJ, Born JM, Lemmens SG, et al. Increased sensitivity to food cues in the fasted state and decreased inhibitory control in the satiated state in the overweight. Am J Clin Nutr. 2013;97:471–479. doi: 10.3945/ajcn.112.044024. [DOI] [PubMed] [Google Scholar]
- 39.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 2010;1363:81–92. doi: 10.1016/j.brainres.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Alonso M, Ziemke F, Magkos F, et al. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr. 2011;94:377–384. doi: 10.3945/ajcn.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnoni-Bauer Y, Bick A, Raz N, et al. Is it me or my hormones? Neuroendocrine activation profiles to visual food stimui across the menstrual cycle. J Clin Endocrinol Metab. 2017;102:3406–3414. doi: 10.1210/jc.2016-3921. [DOI] [PubMed] [Google Scholar]
- 42.Ernst B, Wilms B, Thurnheer M, Schultes B. Eating behaviour in treatment-seeking obese subjects: influence of sex and BMI classes. Appetite. 2015;95:96–100. doi: 10.1016/j.appet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011;55:233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]