Abstract
Dietary nitrate (NO3−) and nitrite (NO2−) support nitric oxide (·NO) generation and downstream vascular signaling responses. These nitrogen oxides also generate secondary nitrosating and nitrating species that react with low molecular weight thiols, heme centers, proteins and unsaturated fatty acids. To explore the kinetics of NO3− and NO2− metabolism and the impact of dietary lipid on nitrogen oxide metabolism and cardiovascular responses, the stable isotopes Na15NO3 and Na15NO2 were orally administered in the presence or absence of conjugated linoleic acid (cLA). The reduction of 15NO2− to 15NO was indicated by electron paramagnetic resonance spectroscopy detection of hyperfine splitting patterns reflecting 15NO-deoxyhemoglobin complexes. This formation of 15NO also translated to decreased systolic and mean arterial blood pressures and inhibition of platelet function. Upon concurrent administration of cLA, there was a significant increase in plasma cLA nitration products 9- and 12-15NO2-cLA. Co-administration of cLA with 15NO2− also impacted the pharmacokinetics and physiological effects of 15NO2−, with cLA administration suppressing plasma NO3− and NO2− levels, decreasing 15NO-deoxyhemoglobin formation, NO2− inhibition of platelet activation, and the vasodilatory actions of NO2−, while enhancing the formation of 9- and 12-15NO2-cLA. These results indicate that the biochemical reactions and physiologic responses to oral 15NO3− and 15NO2− are significantly impacted by dietary constituents such as unsaturated lipids. This can explain the variable responses to NO3− and NO2− supplementation in clinical trials and reveals dietary strategies for promoting the generation of pleiotropic nitrogen oxide-derived lipid signaling mediators.
Keywords: nitrate, nitrite, nitric oxide, conjugated linoleic acid, pharmacokinetics, blood pressure, platelet activation
Introduction
Inorganic nitrite (NO2−) undergoes reactions that yield vasodilatory products that contribute to blood pressure regulation and hypoxic signaling1, 2. The entero-salivary microbiome-mediated reduction of NO3− to NO2− and ·NO in mammals also promotes vascular relaxation and other pleiotropic pharmacologic responses3–6. These reactions of NO3−/NO2−/·NO both complement L-arginine/·NO synthase/·NO/cGMP signaling and expand the spectrum of nitrogen oxide intermediates that instigate signaling responses beyond the activation of guanylate cyclase. This new understanding contrasts with the previous perspective that tissue NO3− and NO2− represented potentially toxic and relatively stable ·NO oxidation products.
There are an abundance of metabolic and inflammatory reactions that NO3− and NO2− undergo that can differentially impact downstream signaling responses1, 2, 4 (Figure 1A). While NO3− is formed upon the oxidation of ·NO by oxyhemoglobin, much greater endogenous NO3− levels are achieved upon the ingestion of dietary NO3− sources, including leafy green vegetables, roots (e.g., beets) and fruits7. Once consumed, NO3− is readily reduced to NO2− by commensal oral bacterial nitrate reductase activities8. While the majority of NO3− is reduced in the oral cavity by commensal bacteria, xanthine oxidoreductase can reduce NO3− to NO2−9. Up to 25% of plasma NO3− can be stored and concentrated by the salivary glands, thus providing a facile route for entero-salivary recycling10–13. Also acquired from both endogenous ·NO oxidation reactions and dietary sources, the more intrinsically-reactive NO2− can undergo multiple reactions such as protonation to nitrous acid (HNO2) in the low pH environments of the GI tract, inflammatory foci and the mitochondrial intermembrane compartment. Nitrite is also absorbed into the circulation and tissues where it can react with a) deoxyhemoglobin and other heme proteins, as well as molybdopterin-containing enzymes to form ·NO 14, 15,16, 17 or b) heme peroxidases to yield the nitrating species nitrogen dioxide (·NO2)18. During intermediary metabolism and inflammatory responses, partially reduced oxygen species such as superoxide (O2·−), hydrogen peroxide (H2O2) and lipid peroxyl radicals will also undergo a variety of reactions with ·NO and NO2− to accelerate rates of production of nitrosating and nitrating species19. ·Nitric oxide, NO2− and HNO2 will also undergo a variety of reactions that lead to the generation of symmetric and asymmetric dinitrogen trioxide (ONONO and OONNO)20,21. These unstable intermediates rapidly decay to yield secondary nitrosating (addition of ·NO) and nitrating (addition of ·NO2) species, as well as ·NO. These and other redox reactions are responsible for propagating downstream signaling and pathogenic responses that are a consequence of oxidative, nitrosative and nitrative post-translational protein modification (PTM) reactions.
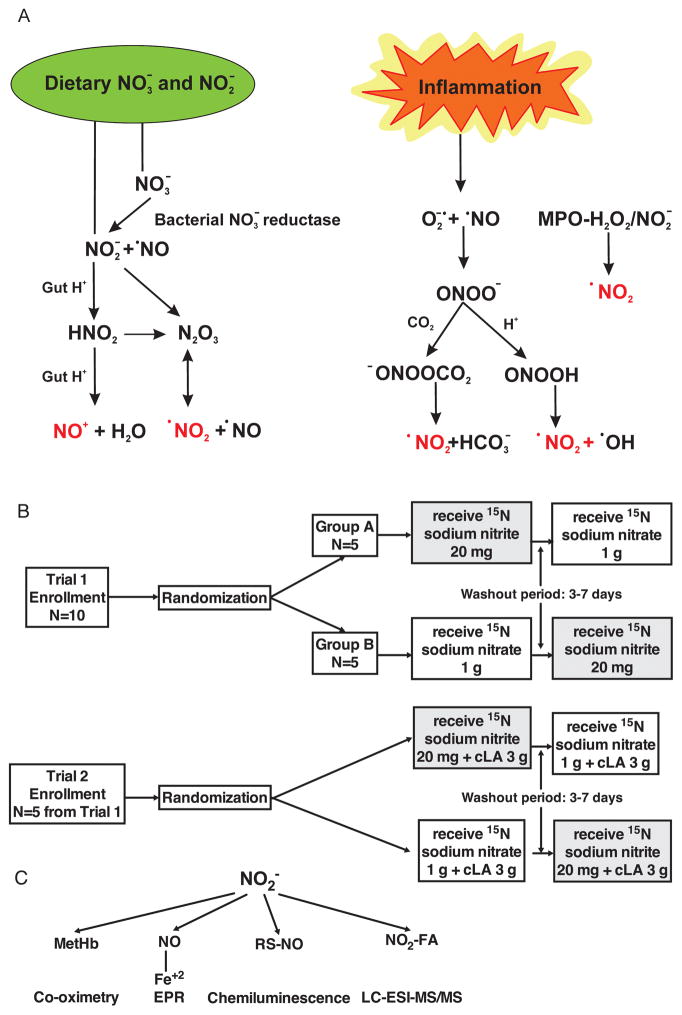
Figure 1. Trial schemes and NO2− signaling pathways.
(A) Multiple metabolic and inflammatory reactions yield nitrogen dioxide. Nitration (·NO2) and nitrosation (NO+) reactions produce an array of bioactive nitrogen oxide products, including NO2-FA and S-nitrosothiol derivatives that have incompletely-characterized biological activities. (B) To understand the metabolism of single doses of heavy nitrogen labeled sodium NO3− and NO2− and to characterize their signaling pathways and physiologic effects, cross-over drug design studies were conducted. Ten healthy volunteers were randomized into one of two subject cohorts to receive a single dose of each study drug, oral 15N-labeled sodium NO3− and NO2−, in random order, separated by a washout period. Five of the 10 healthy volunteers who completed Trial 1 returned and were randomized to receive a single dose of each drug, oral 15N-labeled sodium NO3− and NO2− plus conjugated linoleic acid (cLA), in random order, separated by a washout period in Trial 2. (C) To track NO2− metabolism in vivo, methemoglobin (MetHb), 15NO bound to the heme of hemoglobin (15NO-Hb), RS-NO and 15NO2-fatty acid (FA) formation were examined.
Human studies and animal models reveal that up to 15 day courses of inorganic and dietary NO3− and NO2− administration induce robust responses that include decreased blood pressure (BP)1, 2, 11, 22, hypoxic vasodilation1, 23, 24, modulation of mitochondrial function under hypoxic or exercise stress25, 26, prevention of endothelial dysfunction27 and inhibition of platelet aggregation28, 29. It remains uncertain as to whether these effects are mediated by ·NO, secondary nitrating and nitrosating products or a combination thereof that will induce functionally-significant PTMs such as thiol S-nitrosation or thiol alkylation30, 31.
One class of of nitrogen oxide metabolites are electrophilic fatty acid nitroalkene derivatives (NO2-FA)32, formed by radical addition reactions of ·NO2 with alkenyl carbons of unsaturated fatty acids. Once formed, these lipid electrophiles rapidly react with the nucleophilic amino acids Cys, and to a lesser extent His, residues via reversible Michael addition33. Endogenously present at low nM concentrations in healthy human plasma and urine, the rates and extents of production of NO2-FA can be increased by an array of metabolic and inflammatory-related nitration reactions (Fig. 1C)34. Upon the PTM of functionally-significant hyperreactive Cys residues in critical enzymes and transcriptional regulatory proteins, NO2-FA influence gene expression and inflammatory responses35. For example, specific pro-inflammatory and blood pressure regulation enzymes are directly inhibited by NO2-FA, including xanthine oxidoreductase, cyclooxygenase-2 and soluble epoxide hydrolase (sEH)36–38. Notably, NO2-FA mediate pleiotropic signaling actions: this includes the PTM of p65 inhibit nuclear factor-κB to inhibit pro-inflammatory cytokine expression, the activation of heat shock factor-1 dependent heat shock protein expression, the partial agonism of peroxisome proliferator-activating receptor-γ and the activation of nuclear factor (erythroid-derived) 2-regulated anti-inflammatory gene expression39–43.
The most prevalent endogenous NO2-FA, nitro-conjugated linoleic acid (NO2-cLA), is detected as both the free acid, complex lipid-esterified and as protein-adducts34, 44. Conjugated linoleic acid (predominantly 18:2, cis-9, trans-11) is a dietary polyunsaturated fatty acid prevalent in dairy products, meats and plants. Endogenous generation of cLA in humans occurs by the isomerization of bis-allylic linoleic acid (LA) to a conjugated diene and by the desaturation of oleic acid to cLA, with both reactions catalyzed by the gut microbiome45. Importantly, the external flanking carbons of the conjugated diene of cLA are 3–4 orders of magnitude more reactive than bis-allylic LA44. This promotes facile addition reactions of radical species such as ·NO2 to yield NO2-cLA. It will always be a daunting challenge to dissect which products of nitrogen oxide metabolism, such as ·NO, metal-NO complexes, S-nitrosothiols (RS-NO) or NO2-FA, are proximally responsible for modulating the physiological actions of nitrogen oxides in humans, such as the regulation of inflammatory responses, BP regulation and platelet function.
We hypothesized that ingested NO3− is reduced to the redox-active metabolite NO2− in vivo, which is then metabolized to both ·NO and NO2-cLA. Since ·NO and NO2-cLA are both signaling molecules known to modulate BP and platelet function3, 38, 46–50, we evaluated nitrogen oxide levels and clinical responses upon consumption of oral15NO3− and 15NO2− by healthy adults, with and without cLA supplementation. The use of FDA Investigational New Drug (IND)-approved (IND #115926) oral formulations of 15NO3− and 15NO2− allowed for specific metabolite tracking in vivo, and demonstrated that 15NO-Hb and 15NO2-cLA formation primarily occurred following 15NO2− consumption, and that the physiological responses and nitrogen oxide product profiles were strongly influenced by the concomitant presence of cLA.
Material and Methods
Study design: Two pharmacokinetic (PK) studies of 15NO3− and 15NO2− metabolism were conducted, without (Trial 1) and with cLA (Trial 2) supplementation
In Trial 1, ten subjects were enrolled. To understand the precise mechanism of how the metabolites signal and exert their effects, these subjects were invited to return to participate in a modified PK study, Trial 2, to serve as a direct paired control. Five subjects completed Trial 2. Subjects were randomized in both trials as shown in Figure 1B. All study subjects were ages 18–60 years and had a normal BP defined as systolic BP≤130 and diastolic BP≤85 mmHg. The study was approved by the University of Pittsburgh Institutional Review Board and the U.S. Food and Drug Administration for use of these INDs. Prior to performing any of the research study procedures or interventions, subjects provided written informed consent and procedures were followed in accordance with institutional guidelines.
The selected drug doses were 1 g Na15NO3− (11.8 mmol) and 20 mg Na15NO2− (0.29 mmol) with and without 3 g cLA. With each drug dose, plasma samples were collected for PK analysis of NO3− and NO2− and methemoglobin (MetHb) was assessed using non-invasive co-oximetry (Masimo Corp., Irvine, CA), at times 0 (baseline or trough prior to study drugs), 0.5, 1, 2, 3, 6 and 24 hr post-drug administration. Prior to the start of each PK study visit, subjects fasted for 10–12 hr. BP and mean arterial blood pressure (MAP) were measured for 30–45 min to ensure subjects were at a steady state prior to the time 0 MAP and administration of the study drugs. BP, MAP, respiratory rate and heart rate monitoring were measured every 15 min during the first 2 hr, then at 3, 6, and 24 hr post-drug administration.
To track NO2− metabolism in vivo (Figure 1C), PK evaluations were utilized to examine total (15N and 14N) plasma NO3−, NO2− and RS-NO (S-nitrosothiols) by gas phase reductive ozone-based chemiluminescence detection. 15NO was differentiated from endogenous 14NO in blood using electron paramagnetic resonance spectroscopy (EPR) via the formation of the 15NO· ligand to deoxyhemoglobin (15NO-Hb). This iron-nitrosyl paramagnetic species has a distinctive hyperfine absorbance measured by EPR which produces a doublet for NO-Hb labeled with 15N. To elucidate the overall metabolic fate of 15NO3− and 15NO2− without and with cLA, plasma and urinary 15NO2-cLA was differentiated from endogenous 14NO2-cLA using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described44. A detailed description of the urinary measures is published separately34. Platelet activity was monitored at time 0, 6 and 24 hr after 15N drug with and without cLA. Comprehensive methods are described in Supplemental Material.
Statistical analysis
Detailed statistical analyses for each data set are included in the individual figure legends. Where differences existed between trial drug treatments, post-hoc multiple comparisons were performed using Bonferroni correction. Measurements shown represent mean ± SEM. A p value <0.05 was considered significant.
Results
15NO3−/15NO2− pharmacokinetics with and without cLA
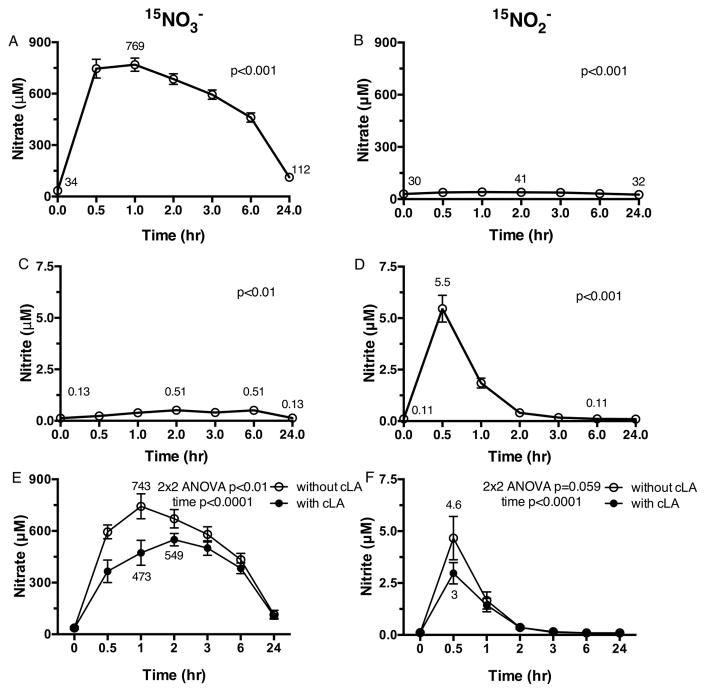
Ten healthy adult volunteers (baseline characteristics in Table S1) who fulfilled the inclusion/exclusion criteria were randomized into one of two subject cohorts of 5 subjects to receive a single dose of each study drug, oral Na15NO3− (1 g) and Na15NO2− (20 mg) in random order (Figure 1B). PK visits were separated by a 3–7 day washout period to ensure washout of nitrogen oxides and metabolites. Total plasma NO3− concentrations were significantly increased after 15NO3− dosing (peak level of 769 ± 38 μM, p<0.001, Figure 2A). Upon dosing with 15NO2−, total plasma NO3− concentrations rose over 3 hr compared to baseline (p<0.001, Figure 2B). When the same subjects received oral 15NO3−, total plasma NO2− concentrations increased over 6 hr compared to baseline (p<0.01, Figure 2C). In contrast, when subjects received oral 15NO2−, total plasma NO2− concentrations rapidly peaked at 0.5 hr (5.5 ± 0.7 μM, p<0.001, Figure 2D) and returned to basal concentrations after 3 hr.
Figure 2. 15NO3− and 15NO2− PK without and with cLA.
(A) Following oral 15NO3−, plasma NO3− concentrations increase at all time points compared to baseline. (B) Following oral 15NO2−, plasma NO3− concentrations rise through 3 hr compared to baseline. (C) Following oral 15NO3−, plasma NO2− concentrations increase through 6 hr compared to baseline. (D) Following oral 15NO2−, plasma NO2− concentrations increase at 0.5 hr and return to baseline after 6 hr. (E) When plasma NO3− concentrations were examined following oral 15NO3− treatment alone compared to 15NO3− with cLA, lower plasma NO3− concentrations were achieved with 15NO3− plus cLA. (F) A trend towards lower plasma NO2− concentrations was seen through 1 hr with 15NO2− plus cLA compared to 15NO2− alone. Repeated measures ANOVA with time as the within-subject effect was used to evaluate response to drug treatment for the endpoint measures in A–D. 2×2 repeated measures ANOVA with time as the within-subject effect and trial drug(s) (without vs. with cLA) as the between subject effect was used to compare the endpoint measures between Trial 1 and Trial 2 in E–F.
Five of the 10 healthy volunteers who completed Trial 1 returned for Trial 2 and were randomized to receive a single dose of each study drug, Na15NO3− (1 g) and Na15NO2− (20 mg), plus 3 g cLA, in random order, separated by a 3–7 day washout period so that metabolic responses could be compared to those from Trial 1 (Figure 1B). Total plasma NO3− concentrations were lower following 15NO3− + cLA at 1 hr (473.2 ± 72.4 μM, Figure 2E closed circles) compared to 15NO3− supplementation (743.1 ± 72.2 μM, p<0.01, Figure 2E open circles). Peak plasma NO3− concentration occurred at 2 hr for 15NO3− + cLA administration and 1 hr for 15NO3− alone. No significant differences in plasma NO2− concentrations were observed for 15NO3− treatment alone vs. 15NO3− + cLA (Figure S1). While total plasma NO2− concentrations increased over time after 15NO2− + cLA (p=0.001), a trend towards lower total plasma NO2− concentrations was seen at 0.5 and 1 hr (Figure 2F closed circles) when compared to 15NO2− alone (p=0.059, Figure 2F open circles). Peak plasma NO2− concentration was 3.0 ± 0.5 μM for 15NO2− + cLA and 4.6 ± 1.0 μM for 15NO2− at 0.5 hr.
Formation of MetHb and 15NO-Hb
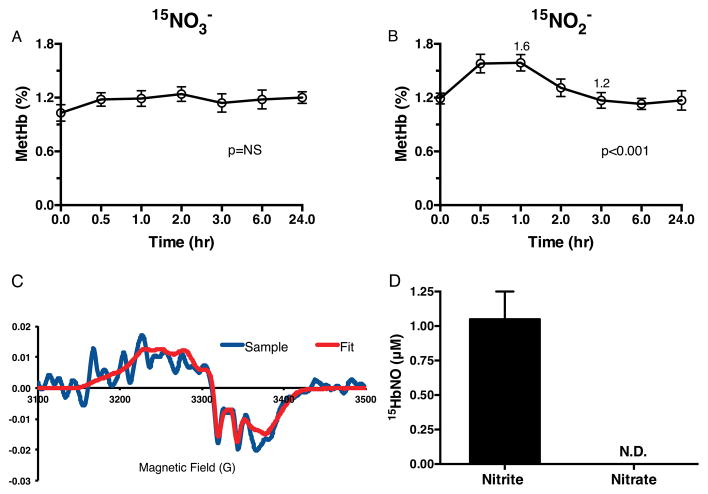
Further metabolism of 15NO2− was also reflected by MetHb, 15NO-Hb, plasma RS-NO and 15NO2-cLA generation (Figure 1C). Following 15NO3− administration, no significant increase in MetHb was observed (Figure 3A). Following 15NO2− dosing, a small but significant increase in MetHb occurred from baseline over 1 hr (baseline 1.2 ± 0.1%, peak at 1 hr 1.6 ± 0.1%, p<0.001, Figure 3B) and returned to baseline by 3 hr. cLA supplementation did not impact MetHb levels (not shown). The metabolism of 15NO2− to ·15NO was measured by the formation of 15NO-Hb in red blood cells sedimented from venipuncture blood samples. A representative EPR spectra (red) shows the hyperfine splitting characteristic of 15NO-Hb from one subject (raw data in blue, Figure 3C). There was no evidence for the formation of 14NO-Hb (Figure S2B). 15NO-Hb formation was only detected between 0.5 and 1 hr after 15NO2− supplementation (Figure 3D). 15NO-Hb derivatives were not detectable after 15NO2− + cLA and 15NO3− + cLA administrations (not shown).
Figure 3. NO2− signaling and methemoglobin and 15NO-Hb formation.
(A) Following oral 15NO3−, no significant increase in methemoglobin (MetHb) occurs. (B) Following oral 15NO2−, a significant rise in MetHb occurs through 1 hr. (C) A representative EPR spectra (red) shows the characteristic hyperfine splitting of 15NO-Hb from one subject following oral 15NO2− (raw tracings shown in blue). The fit is composed of 42% pentacoordinate 15N alphanitrosyl Hb, 21% hexacoordinate alphanitrosyl Hb, and 38% betanitrosyl Hb. Inclusion of pentacoordinate 14N alphanitrosyl Hb does not substantially improve the fit (Fig. S3B). (D) 15NO-Hb formation is detected with oral 15NO2− treatment only at 0.5 to 1 hours, with no 15NO-Hb detected with oral 15NO3− treatment. NS = not significant, N.D. = not detected. Repeated measures ANOVA with time as the within-subject effect was used to evaluate response to drug treatment for the endpoint measures in A–B.
Formation of RS-NO
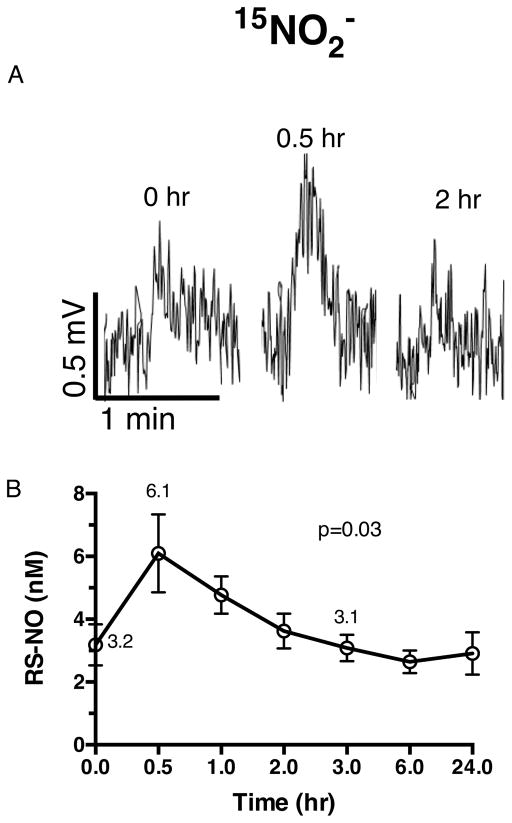
Representative plasma RS-NO traces are shown at baseline and for peak concentrations of RS-NO at 0.5 hr after 15NO2− dosing, followed by an additional time point after 15NO2− treatment (Figure 4A). 15NO2− consumption significantly increased plasma RS-NO concentrations (baseline 3.2 ± 1.0 nM, peak concentration of 6.1 ± 1.0 nM at 0.5 hr, p<0.05, Figure 4B) and returned to basal concentrations after 3 hr. In contrast, after 15NO3− dosing, there was no significant impact on RS-NO concentration (Figure S3A open circles). There was also no impact of cLA supplementation on plasma RS-NO concentrations after dosing in combination with either 15NO3− or 15NO2− compared to 15NO3− or 15NO2− alone (Figure S3A and B).
Figure 4. NO2− signaling and RS-NO.
(A) Representative plasma RS-NO traces at baseline and the time when peak RS-NO concentrations were detected with oral 15NO2− followed by a time point after 15NO2− treatment. (B) Following oral 15NO2−, plasma RS-NO concentrations increase significantly, but after 3 hr, approach baseline. Repeated measures ANOVA with time as the within-subject effect was used to evaluate response to drug treatment for the endpoint measure in B.
Formation of NO2-cLA
HPLC-MS/MS analysis of the structural regioisomers, β-oxidation metabolites, Michael addition products and pharmacokinetics of 15NO3− and 15NO2−-induced fatty acid nitration in Trials 1 and 2 has been reported34. Endogenous plasma 14NO2-cLA concentrations averaged 1.58 ± 0.2 nM after 15NO3− and 15NO2− and rose significantly to 2.84 ± 0.24 nM upon cLA supplementation. Mean plasma 15NO2-cLA concentrations were maximal at 24 hr after 15NO3− + cLA (3.06 ± 1.79 nM), and 15NO2− + cLA supplementation resulted in maximal 15NO2-cLA concentrations that were sustained between 1–6 hr (6.61 ± 4.84 nM to 4.86 ± 2.39 nM). These 15NO2− related levels paralleled the levels of free plasma cLA. These data support that free cLA concentrations are limiting for fatty acid nitration. A representative LC-ESI-MS/MS chromatogram of one volunteer’s plasma lipid extract (Figure 5) shows the 15NO2-cLA regioisomers generated upon dosing with 15NO2− + cLA34.
Figure 5. NO2− signaling and NO2-cLA formation.
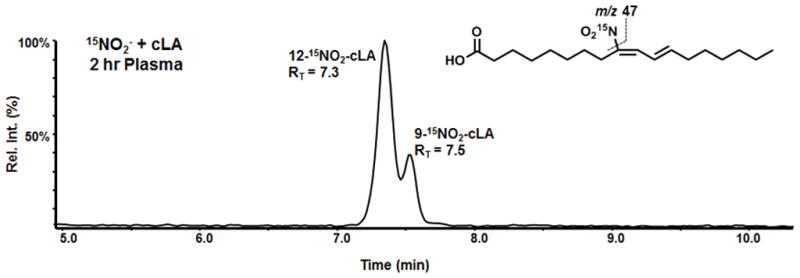
A representative LC-ESI-MS/MS chromatogram of plasma lipid extract shows 15NO2-cLA present in plasma of volunteers treated with oral 15NO2− plus cLA (Adapted with permission from Delmastro-Greenwood et al. Nitrite and Nitrate-Dependent Generation of Fatty Acid Nitroalkenes. Free Radic Biol Med. 2015;89:333–341.).
Physiologic responses to 15NO3− and 15NO2− supplementation with and without cLA supplementation
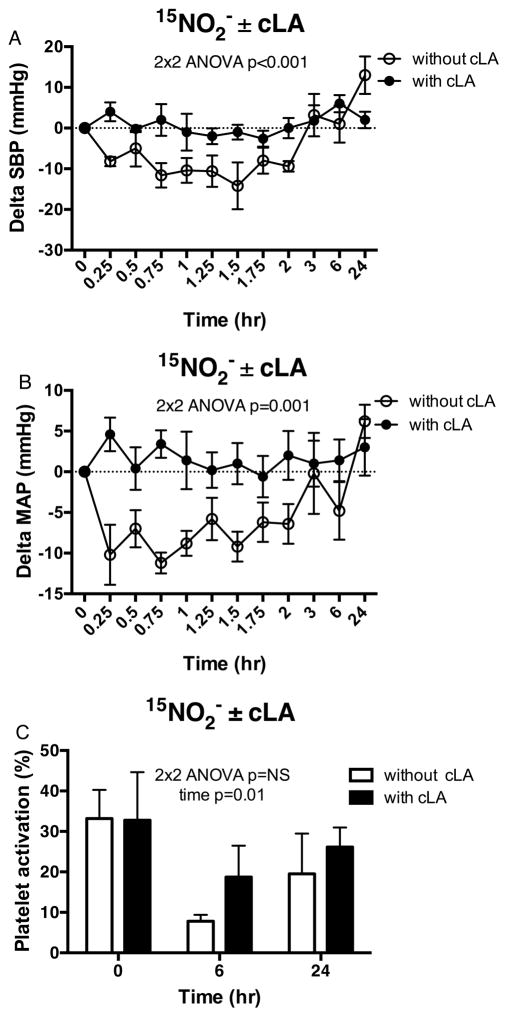
In Trial 1, oral 15NO2− dosing induced vasodilation, decreasing systolic (Δ−12.1±3.3 mmHg, p<0.001), diastolic (Δ−8.1±2.0 mmHg, p=0.002) and mean arterial pressure (Δ−10.4±1.2 mmHg, p=0.001) (Figure 6A and B open circles) with no differences in heart rate (data not shown). In this 15NO2− dosed cohort, the reduction in SBP and MAP correlated with the change in plasma NO3− concentration (Figure S4A and C). Also, the greater the reduction in DBP at 1 hr, the greater the rise in total NO3− concentration at 0.5 hr (Figure S4B). Notably, the significant decreases in SBP and MAP that persisted for up to 2 hr after subjects were treated with 15NO2− were abolished upon co-administration of cLA in Trial 2 (Figure 6A and B closed circles).
Figure 6. Physiologic effects and NO2− signaling without and with cLA.
Significant decreases in SBP (A) and MAP (B) but not DBP that persisted through 1.5 hr after subjects were treated with oral 15NO2− alone (open circles) were abolished by cLA supplementation (closed circles). (C) No differences in the % of platelet activation were detected when comparing oral 15NO2− treatment alone (open bars) versus oral 15NO2− with cLA (dark bars) over 24 hr. 2×2 repeated measures ANOVA with time as the within-subject effect and trial drug(s) (without vs. with cLA) as the between subject effect was used to compare the endpoint measures between Trial 1 and Trial 2 in A–C.
Inhibition of platelet activation in whole blood was observed 6 hr following 15NO2− dosing (baseline 33.2 ± 7.1%, 6 hr post-dosing 7.8 ± 1.6%, p=0.02, Figure 6C open bars), an effect that was eliminated when subjects were dosed with 15NO2− + cLA, (Figure 6C dark bars). Following 15NO3− dosing in all 10 subjects who completed Trial 1, there was significant inhibition of platelet activation 6 hr after 15NO3− administration (17.0 ± 4.1%, 6 hr post-dosing 9.0 ± 1.7%, p=0.029, Figure S5A). With co-administration of 15NO3− + cLA in Trial 2, there was no significant inhibition of platelet activation in whole blood at 6 hr (27.5 ± 12.3%, 6 hr post-dosing 20.0 ± 7.5%, Figure S5B).
In subjects given oral 15NO3−, plasma 15NO2− concentrations were significantly increased from 1–2 hr post dosing (Figure S1, open circles), with a maximum of 1.40 μM reached. In Trial 1, oral 15NO3− dosing induced a small reduction in DBP at 3 hr compared to baseline (ΔDBP = −6.3±1.2 mmHg, p=0.003, Figure S6B open circles) with no significant change in SBP and MAP (Figure S6A and C open circles) or heart rate (data not shown). No significant correlations were noted between changes in SBP, DBP or MAP and absolute NO3− or NO2− concentrations after 15NO3− administration (not shown). Following 15NO3− + cLA dosing, there were no significant changes in SBP, DBP and MAP (Figure S6A, B and C closed circles) or heart rate (data not shown).
Discussion
There are a panoply of physiological reactions that nitrogen oxides can undergo, all uniquely influenced by factors such as changes in pH, oxygen tension, CO2/H2CO3 levels, rates of production of reactive inflammatory mediators and redox-reactive metalloprotein levels. The relative significance of these mitigating factors will change as basal metabolism transitions to metabolic stress and inflammatory responses. This will critically impact the specific reactions that occur between ·NO/NO2−, molecular oxygen, partially reduced oxygen species and metal centers. In the context of ·NO signaling, the physiological outcomes of these reactions are manifested by the diversion of ·NO away from canonical guanylate cyclase activation and cGMP-dependent signaling responses to the generation of a spectrum of highly reactive and transient secondary oxidizing, nitrosating and nitrating products (Figure 1A)51. At high concentrations, these reactive products may be pathogenic, but under physiological conditions these chemically-reactive intermediates a) represent a metastable ·NO reserve that still signals via guanylate cyclase activation and cGMP-dependent mechanisms and b) react with and modify the structure and function of cell targets (e.g. unsaturated fatty acids, proteins). These latter reactions instigate an array of PTMs and non-cGMP-dependent signaling responses31. In particular, the nucleophilic amino acid Cys confers proteins with a sensitivity for reduction-oxidation (redox)-induced PTMs that include Cys oxidation, glutathionylation, S-nitrosation, and alkylation upon Michael addition by electrophilic species52. These PTMs intimately link metabolic and inflammatory status with changes in cell and organ function, since many enzymes, receptors and transcriptional regulatory proteins that regulate metabolism and inflammation are endowed with functionally-significant hyperreactive Cys moieties. Herein, we report that oral co-administration of 3 g of the polyunsaturated fatty acid cLA with 15NO2− significantly redirects the vasodilatory and platelet inhibitory actions of NO2− to alternative pathways.
Nitrite and its reduction product ·NO are typically viewed to induce vasodilation via cGMP-dependent mechanisms1, 2. In the human brachial artery, forearm blood flow increases after NO2− infusion, in concert with a simultaneous rise in ·NO formation1. Abundant clinical studies have demonstrated significant reductions in BP in healthy adults following both single ingestion11, 53, repetitive 15 day22, 54, 55 courses of NO3− rich foods56 or consumption of a traditional Japanese diet57, another rich source of dietary NO3−. Various dosages of pure NO3− and NO2− preparations also significantly reduced BP in healthy adults53,58,59. These human studies have largely been limited to testing single or short-term doses of NO3− or NO2−, with no evaluation of downstream metabolites. Animal models also recapitulate canonical vasodilatory and BP lowering effects of ·NO stemming from NO2− and NO3−60, 61. There are no statistically significant differences between RS-NO concentrations with 15NO2 alone vs. 15NO2 + cLA, with peak concentrations of 15NO-Hb being ~150 times greater than peak concentrations of RS-NO. Thus, these human NO2− PK data collectively demonstrate elevated plasma 15NO2− concentrations (Figure 2D) and 15NO2− reduction to 15NO· (Figure 3D) in vivo as the most likely mediators of the simultaneous systolic and mean arterial blood pressure reductions upon 15NO2 administration (Figure 6A and B, open circles). Oral administration of 15NO2 in concert with cLA blunted hemodynamic responses (Figure 6A and B, closed circles), with no detectable formation of 15NO-Hb adducts. Our lack of blood pressure response with cLA addition does not indicate that our nitrite drug dose is not vasodilating, but that we are reducing the nitrite concentrations enough to reduce the blood pressure lowering extreme. A reduction in blood pressure requires a systemic increase in blood flow (or a decrease in systemic vascular resistance - SVR) that cannot be compensated by an increase in stroke volume and heart rate (MAP - CVP = CO x SVR). We have previously measured vasodilation in the human forearm at nitrite concentrations as low as 150–200 nM (0.1–0.2 uM)1.
Oral administration of single doses of 15NO3− and 15NO2− gave detectable increases in plasma nitrogen oxide concentrations, with each nitrogen oxide displaying unique pharmacokinetic and metabolite profiles. Moreover, co-administration of cLA significantly affected these parameters. Oral 15NO3− reached its highest plasma NO3− concentration 1 hr after dosing and yielded a peak concentration of plasma NO2− 2–6 hr after dosing (Figure 2A and C). This concentration of plasma NO2− with oral 15NO3− was not sufficient to increase MetHb or to yield detectable red blood cell 15NO-Hb levels (Figure 3A and D) or to induce (Figure S6A and C) or sustain (Figure S6B) reductions in BP parameters. Dosing of 15NO3− in concert with cLA suppressed peak plasma NO3− levels by ~30% (Figure 2E closed circles). For oral 15NO2−, the highest plasma concentration of NO2− was measured 0.5 hr after dosing (Figure 2D), giving a modest but significant increase in plasma NO3− metabolite concentrations 2–3 hr later (Figure 2B). Notably, the co-administration of cLA with 15NO2− also suppressed peak plasma NO2− concentrations by ~30% (Figure 2F closed circles).
Nitrite reacts with oxyHb to form NO3− and MetHb, and with deoxyhemoglobin to ultimately form NO-Hb16. Upon NO2− protonation to HNO2 and reaction with ·NO, both symmetric (ONONO) and asymmetric (OONNO) dinitrogen trioxide intermediates are generated, serving as proximal mediators of biomolecule nitrosation and nitration20, 62. After 15NO2− administration, evidence of all of these reactions was noted, with a significant 25% increase in MetHb levels, the formation of ~1 μM paramagnetic 15NO-Hb species and a 3 nM increase in mean plasma protein RS-NO levels 0.5 hr post-dosing (Figures 3B and D, 4A and B). With cLA supplementation, mean plasma NO2-cLA concentration increased by ~5 nM34. Only a few studies have reported changes in plasma ·NO concentrations at baseline and following dietary NO3− or NO2− supplementation. For example, after a single 5.6 mmol dose of NO3− rich beetroot juice53, plasma cGMP, an indicator of ·NO generation, increased within 3 hr. Following a single 24 mmol dose of KNO3, plasma cGMP increased within 3 hr and remained elevated through 24 hr53.
Following entero-salivary reduction of NO3− to NO2−, NO2− is readily protonated (pKa=3.4) to nitrous acid (HNO2) in the acidic gastric compartment. This promotes a “redirection” of nitrite chemistry where HNO2 in turn gives rise to secondary N2O3 species that mediate nitrosation, nitration and oxidation of susceptible targets. These reactions can be blunted by administering proton pump inhibitors such as esomeprazole, thus inhibiting gastric acid secretion and NO2− protonation to HNO2, – a pH response that typifies the pharmacologic action of PPIs63. Esomeprazole also inhibited the blood pressure-lowering effects of nitrite. The more basic conditions in the stomach after esomeprazole administration limited the protonation of nitrite to HNO2, a species that undergoes both dismutation and nitric oxide reactions to yield the nitrosating and nitrating products symmetric and asymmetric dinitrogen trioxide (ONONO and ONNOO)20. The pathways leading to NO2-cLA formation first require entero-salivary reduction of NO3− to NO2−. In the present study, where nitrite and nitrate were orally consumed in the presence of cLA, stomach pH would either remain the same or be lowered by the acidic cLA (pKa = 4.0). The suppression of detectable plasma NO3− and NO2− by ~30% upon cLA co-administration indicated that the greater availability and reactivity of cLA favored its nitration, thus supporting the generation of NO2-cLA derivatives rather than the accumulation of NO3− and NO2− in plasma and presumably other tissue compartments. Our present report supports the concept that the addition of cLA and its facile nitration by nitrite-derived species is a consequence of these acid-catalyzed reactions. Our data indicates that this concomitantly leads to an attenuation of NO-forming reactions that would otherwise result in the vasodilation and inhibition of platelet function that was observed when nitrite alone was administered. There may also have been an impact of cLA on gut nitrogen oxide absorption or microbial NO3− reduction. The small increase in plasma RS-NO derivatives observed in the 15NO2− treated cohort in Trial 1 at 0.5 hr post-administration (Figure 4B) was not significantly different compared to Trial 2 with addition of cLA (Figure S3B). Dosing of volunteers with 15NO3− with cLA had no impact on plasma RS-NO levels (Figure S3A).
In healthy, hypertensive and high BMI subjects, cLA supplementation (3–6.8g/day, 5–26 wk), compared to control or placebo displays no significant impact on BP in humans64,65,66,67. Herein, we observed that the decreased BP induced by NO2− was abrogated by concomitant cLA administration (Figure 6AB). In addition to promoting a shift in nitrosative and nitrative chemistries, cLA might also be modulating NO2− responses by impacting endogenous ·NO production68,69, 70. The present data do not diminish the significance of heme proteins such as deoxyHb in mediating reactions of nitrite that lead to changes in vascular function. Rather, our results further affirm the impact that other biomolecules such as conjugated diene-containing fatty acids that readily undergo radical addition reactions, can have on nitrogen oxide reaction pathways and downstream signaling responses. This is manifested by the fact that Fe-NO Hb levels in nitrite-treated subjects are decreased to undetectable levels upon nitrite + cLA administration (EPR analysis has a limit of quantitation of ~500 nM for Fe-NO complexes). These findings indicate that cLA supplementation and other dietary constituents have the ability to modulate the biochemical reactions of NO2−, the trafficking of downstream nitrogen oxide metabolites and the physiological concentrations of this pluripotent signaling mediator. Moreover, a more chronic exposure to elevated cLA and fat in the diet has the potential to also influence the entero-salivary microbiome and its impact on nitrogen oxide metabolism71.
Along with vasodilatory actions, ·NO formed by the reduction of NO2− inhibits platelet function, including platelet adhesion to the endothelium47 and platelet aggregation72. In contrast, partially reduced oxygen species such as O2·− and H2O2 propagate platelet activation73, 74. The mechanism by which ·NO affects platelet activity is by activating cGMP-dependent signaling. While dietary NO3− sources (beetroot juice or KNO3−) reduce platelet activation or aggregation11, 29, 75, 76, to date only in vitro studies of human platelet function have addressed the effects of NO2− sources on platelet activation or aggregation28, 29, 77, 78. Herein, the inhibition of platelet activation occurred in whole blood for 6 hr following 15NO2− dosing, with the inhibition of platelet activation still evident well beyond the physiologic plasma NO2− peak and the time of 15NO-Hb detection. Previous in vitro studies revealed that two synthetic nitro-fatty acids, nitro-linoleic acid and nitro-arachidonic acid (at high concentrations in buffered saline), inhibited platelet aggregation and activation, respectively79, 80. In the more clinically-relevant study herein, 15NO2− administration in vivo decreased platelet activation in whole blood at 6 hr. The whole blood basal platelet activation levels of subjects were moderately increased, which are attributed to an artifact of the venipuncture sampling. There may be a threshold plasma nitrite concentration where sufficient ·NO formation subsequently reduces platelet activation with 15NO2− dosing, whereas oral 15NO2− and 15NO3− in combination with cLA diverted nitrogen oxide metabolites to support the formation of 15NO2-cLA, thus consistently attenuating NO2− and NO3− inhibition of platelet activation.
Study Limitations and Strengths
The data presented here cannot exclude dietary cLA contribution during Trial 1 and dietary NO3− and NO2− contributions also influencing baseline plasma NO3− and NO2− concentrations in both trials. Additionally, no formal power calculations were performed as these were exploratory Phase I studies. While this study may be underpowered to detect an effect of 15NO3− on BP, given that there were significant reductions in all BP parameters with 15NO2−, the abolishment of the 15NO2− BP effect with the addition of cLA can be assumed to be due to the impact of cLA and not insufficient power. Traditional human NO3− intakes of 40–100 mg/day81, 82, NO2− intakes of 0–20 mg/day83 and cLA intakes of 150–200 mg/day84 are quite low, but daily intakes of up to 2.6 g of cLA are reported in the literature. Therefore, it may be difficult to achieve all three of these intakes simultaneously through diet on a daily basis, so as to be comparable to those provided in capsule formulations herein. However, our goal with cLA supplementation was to define whether the biochemical reactions and physiologic responses to oral 15NO3− and 15NO2− were modulated by cLA. Our investigations are strengthened by utilizing paired PK studies in the same subjects so direct comparisons could be made using 15N sodium NO3− and NO2− with and without cLA supplementation to track ·NO metabolism and the source of ·NO and NO2-cLA species formation in vivo with each drug.
Perspectives
The oral administration of cLA altered the metabolic fate of oral 15NO3− and 15NO2− and modulated the ·NO-signaling and vasodilatory properties of these nitrogen oxides. The cGMP-dependent signaling actions of NO3− and NO2− can be further transformed by digestive reactions, the entero-salivary microbiome and metalloprotein-catalyzed reactions to yield ·NO and additional nitrogen oxides that elicit redox signaling responses via nitration, nitrosation and oxidation reactions. One would expect that the spectrum of nitrogen oxide products formed will depend on diet, metabolic and inflammatory status, acidic microenvironments and NO3−-reducing salivary bacteria. The concurrent administration of cLA in the diet decreased peak plasma NO3− and NO2− concentrations, enhanced the formation of 15NO2-cLA, and decreased detectable ·NO concentrations in the vascular compartment, thus effectively diverting nitrosative reactions to nitration events. This diversion of nitrosative reactions to nitrative chemistries resulted in the increased generation of NO2-FA, with concomitant attenuation of the inhibition of platelet activation and abrogation of the vasodilatory properties of NO2−. This affirms that the metabolic and the physiological responses to oral NO3− and NO2− can be significantly modulated by the composition of the diet, particularly with meat and dairy products that are rich in cLA.
Supplementary Material
Novelty and Significance.
What is new?
We developed oral capsule formulations of sodium nitrate (NO3−) and nitrite (NO2−) with the stable [15N] isotope allowing for specific metabolite tracking in vivo and to investigate the human vascular responses.
What is relevant?
Oral 15NO2− consumption resulted in ·NO formation, vasodilation and acute inhibition of platelet activation
Conjugated linoleic acid (cLA) with oral 15NO3−/NO2− diverted metabolic products to NO2-cLA
Addition of dietary cLA with oral 15NO2− decreased ·NO formation and vasodilation and attenuated the acute inhibition of platelet activation
Summary
Concurrent cLA in the diet altered the metabolic fate of oral 15NO3− and NO2− with decreased plasma NO3−/NO2− levels, enhanced formation of NO2-FA species and decreased ·NO formation. This diversion of the downstream reactions of NO3− and NO2− to NO2-cLA attenuated the inhibition of platelet activation and abrogated the vasodilatory properties of NO2−.
Acknowledgments
We thank Ram Agarwal, Deborah Sperling, and Judy Starling for their work in the development of the oral sodium nitrate and nitrite capsules; Nydia Chien for FDA IND submission oversight, Sean Barrett for study drug dispensing, and Alex Despines, Sergey Ivanovich, and Yinna Wang for their technical assistance in the clinical trial. We also thank Andreas Perlegas for help with EPR analyses.
Funding sources: The research was supported by 5K12-HD052892, UL1-TR000005 and UL1-RR024153 (University of Pittsburgh Clinical and Translational Science Institute), the McKamish Family Foundation and the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (KSH), R01-HL064937 (BAF), R37-HL058115 (BAF), P01-HL103455 (MTG, BAF) and R37-HL058091 (DBK-S).
Footnotes
Clinical Trial Registration - http://www.clinicaltrials.gov, NCT01681836
Disclosures: MTG and DBK-S are co-inventors on a US government patent for the use of sodium nitrite for the treatment of cardiovascular diseases and have received royalties from the US government. BAF and SGW acknowledge interest in Complexa, Inc. The other authors report no conflicts.
References
- 1.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 2.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 3.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr. 2013;33:129–159. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 6.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Amer J Clin Nut. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 8.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 11.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin L, Liu X, Sun Q, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci USA. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg JO. Nitrate transport in salivary glands with implications for NO homeostasis. Proc Natl Acad Sci USA. 2012;109:13144–13145. doi: 10.1073/pnas.1210412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Wajih N, Liu X, et al. Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem. 2015;290:1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 18.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph V, Freeman BA. Cardiovascular consequences when nitric oxide and lipid signaling converge. Circ Res. 2009;105:511–522. doi: 10.1161/CIRCRESAHA.109.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitturi DA, Minarrieta L, Salvatore SR, Postlethwait EM, Fazzari M, Ferrer-Sueta G, Lancaster JR, Jr, Freeman BA, Schopfer FJ. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat Chem Biol. 2015;11:504–510. doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 22.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 23.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO., 3rd Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci USA. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 25.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 26.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–8. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 28.Apostoli GL, Solomon A, Smallwood MJ, Winyard PG, Emerson M. Role of inorganic nitrate and nitrite in driving nitric oxide-cGMP-mediated inhibition of platelet aggregation in vitro and in vivo. J Thromb Haemost. 2014;12:1880–1889. doi: 10.1111/jth.12711. [DOI] [PubMed] [Google Scholar]
- 29.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocha BS, Gago B, Pereira C, Barbosa RM, Bartesaghi S, Lundberg JO, Radi R, Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Curr Drug Targets. 2011;12:1351–1363. doi: 10.2174/138945011796150334. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, Shiva S, Schopfer FJ, Gladwin MT, Freeman BA, Gelhaus Wendell S. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic Biol Med. 2015;89:333–341. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Ann Rev Physio. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, Schopfer FJ, Lancaster JR, Jr, Freeman BA, Tarpey MM. Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. J Biol Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trostchansky A, Bonilla L, Thomas CP, O’Donnell VB, Marnett LJ, Radi R, Rubbo H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J Biol Chem. 2011;286:12891–12900. doi: 10.1074/jbc.M110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schopfer FJ, Cole MP, Groeger AL, et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate Nrf2 by a Keap1 cysteine 151-independent mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J Biol Chem. 2012;287:44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishino S, Takeuchi M, Park SB, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 47.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 48.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haas JA, Khraibi AA, Perrella MA, Knox FG. Role of renal interstitial hydrostatic pressure in natriuresis of systemic nitric oxide inhibition. American Journal of Physiology - Renal Physiology. 1993;264:F411–F414. doi: 10.1152/ajprenal.1993.264.3.F411. [DOI] [PubMed] [Google Scholar]
- 52.Go YM, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med. 2015;84:227–245. doi: 10.1016/j.freeradbiomed.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 54.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 55.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 56.Hobbs DA, Goulding MG, Nguyen A, Malaver T, Walker CF, George TW, Methven L, Lovegrove JA. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: a randomized controlled trial. J Nutr. 2013;143:1399–1405. doi: 10.3945/jn.113.175778. [DOI] [PubMed] [Google Scholar]
- 57.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 59.Hunault CC, van Velzen AG, Sips AJ, Schothorst RC, Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol Lett. 2009;190:48–53. doi: 10.1016/j.toxlet.2009.06.865. [DOI] [PubMed] [Google Scholar]
- 60.Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953;108:129–143. [PubMed] [Google Scholar]
- 61.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AE, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Basu S, Grubina R, Huang J, et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 63.Montenegro MF, Sundqvist ML, Larsen FJ, Zhuge Z, Carlstrom M, Weitzberg E, Lundberg JO. Blood pressure-lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension. 2017;69:23–31. doi: 10.1161/HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]
- 64.Iwata T, Kamegai T, Yamauchi-Sato Y, Ogawa A, Kasai M, Aoyama T, Kondo K. Safety of dietary conjugated linoleic acid (CLA) in a 12-weeks trial in healthy overweight Japanese male volunteers. J Oleo Sci. 2007;56:517–525. doi: 10.5650/jos.56.517. [DOI] [PubMed] [Google Scholar]
- 65.Raff M, Tholstrup T, Sejrsen K, Straarup EM, Wiinberg N. Diets rich in conjugated linoleic acid and vaccenic acid have no effect on blood pressure and isobaric arterial elasticity in healthy young men. J Nutr. 2006;136:992–997. doi: 10.1093/jn/136.4.992. [DOI] [PubMed] [Google Scholar]
- 66.Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9, trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Amer J Clin Nut. 2010;91:175–183. doi: 10.3945/ajcn.2009.28192. [DOI] [PubMed] [Google Scholar]
- 67.Laso N, Brugue E, Vidal J, Ros E, Arnaiz JA, Carne X, Vidal S, Mas S, Deulofeu R, Lafuente A. Effects of milk supplementation with conjugated linoleic acid (isomers cis-9, trans-11 and trans-10, cis-12) on body composition and metabolic syndrome components. Br J Nutr. 2007;98:860–867. doi: 10.1017/S0007114507750882. [DOI] [PubMed] [Google Scholar]
- 68.Jenko KJ, Vanderhoek JY. Conjugated linoleic acids and CLA-containing phospholipids inhibit NO formation in aortic endothelial cells. Lipids. 2008;43:335–342. doi: 10.1007/s11745-008-3160-y. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Archives of Biochemistry and Biophysics. 2007;466:250–259. doi: 10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 70.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. 2007;89:169–177. doi: 10.1016/j.biochi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 71.Chaplin A, Parra P, Serra F, Palou A. Conjugated linoleic acid supplementation under a high-fat diet modulates stomach protein expression and intestinal microbiota in adult mice. PloS One. 2015;10:e0125091. doi: 10.1371/journal.pone.0125091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcus AJ, Silk ST, Safier LB, Ullman HL. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977;59:149–158. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canoso RT, Rodvien R, Scoon K, Levine PH. Hydrogen peroxide and platelet function. Blood. 1974;43:645–656. [PubMed] [Google Scholar]
- 75.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG, Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Amer J Clin Nut. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson G, Hicks SL, O’Byrne S, Frost MT, Moore K, Benjamin N, McKnight GM. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 77.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PloS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akrawinthawong K, Park JW, Piknova B, Sibmooh N, Fucharoen S, Schechter AN. A flow cytometric analysis of the inhibition of platelet reactivity due to nitrite reduction by deoxygenated erythrocytes. PloS One. 2014;9:e92435. doi: 10.1371/journal.pone.0092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 80.Bonilla L, O’Donnell VB, Clark SR, Rubbo H, Trostchansky A. Regulation of protein kinase c by nitroarachidonic acid: impact on human platelet activation. Archives of Biochemistry and Biophysics. 2013;533:55–61. doi: 10.1016/j.abb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Mensinga TT, Speijers GJ, Meulenbelt J. Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev. 2003;22:41–51. doi: 10.2165/00139709-200322010-00005. [DOI] [PubMed] [Google Scholar]
- 82.Gangolli SD, van den Brandt PA, Feron VJ, Janzowsky C, Koeman JH, Speijers GJ, Spiegelhalder B, Walker R, Wisnok JS. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 83.Pennington J. Dietary exposure models for nitrates and nitrites. Food Control. 1998;9:385–395. [Google Scholar]
- 84.Ritzenthaler KL, McGuire MK, Falen R, Shultz TD, Dasgupta N, McGuire MA. Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J Nutr. 2001;131:1548–1554. doi: 10.1093/jn/131.5.1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.