Abstract
On-line separations-based sensors employing microdialysis (MD) coupled to microchip electrophoresis (ME) enable the continuous monitoring of multiple analytes simultaneously. Electrochemical detection (EC) is especially amenable to on-animal systems employing MD-ME due to its ease of miniaturization. However, one of the difficulties in fabricating MD-ME-EC systems is incorporating carbon working electrodes into the device. In this paper, a novel fabrication procedure is described for the production of a PDMS/glass hybrid device that is capable of integrating hydrodynamic microdialysis flow with ME-EC using a flow-gated interface and a pyrolyzed photoresist carbon electrode. This fabrication method enables the reuse of carbon electrodes on a glass substrate, while still maintaining a good seal between the PDMS and glass to allow for pressure-driven microdialysis flow. The on-line MD-ME-EC device was characterized in vitro and in vivo for monitoring analytes in the dopamine metabolic pathway. The ultimate goal is to use this device and associated instrumentation to perform on-animal, near-real time in vivo monitoring of catecholamines.
Keywords: On-line, Dopamine, Microfluidics, Lab-on-a-chip, Microdialysis
1 Introduction
Microdialysis is a sampling technique that enables continuous monitoring of small molecules in a variety of biological tissues, including the brain. While there are many advantages of microdialysis sampling for monitoring neurochemicals in the brain, the temporal resolution of the microdialysis measurements is highly dependent on the analytical system used to measure the analytes of interest [1]. For the best temporal resolution, the ideal analytical method exhibits low detection limits and has low sample volume (pL-to-nL) requirements. If the analysis is performed on-line, then fast analysis times are also required. Capillary electrophoresis (CE) and microchip electrophoresis (ME) fulfill these requirements, and have previously been coupled on-line to microdialysis sampling [2, 3]. The resulting “separation-based sensors” allow continuous monitoring of multiple analytes in near-real time.
On-line microdialysis-capillary electrophoresis (MD-CE) was first reported in 1994, where it was used to monitor the pharmacokinetics of an anti-cancer drug in vivo [4]. This study employed laser-induced fluorescence (LIF) detection, due to the ease of integrating optically based techniques with capillary electrophoresis. Later, an on-line MD-CE-EC system was developed using a cellulose acetate decoupler and was employed to monitor the transdermal delivery of nicotine [5]. These CE systems required tubing and valves for introduction of the sample into the CE separation system.
The introduction of lab-on-a-chip devices in the 1990s led to the development of devices that were capable of integrating multiple processing steps (e.g., sampling, separation, and detection) all on a single platform [6]. Later, microchip-based electrophoresis was introduced, making it possible to perform electrophoretic separations in a planar format [7–12]. This technique had many of the advantages of capillary electrophoresis for microdialysis samples, including the ability to perform fast (sub-minute) separations and very small sample volume requirements (pL-to-nL). In addition, ME allows fluid manipulation on-chip, enabling the integration of microdialysis sampling on-line. The first report of on-line MD-ME used a flow-gated, double-t, interface for introducing the microdialysis flow into the separation channel and employed LIF detection [13]. This interface design has since been used for both in vitro [14, 15] and in vivo [16, 17] monitoring of amino acids and other bioactive compounds.
These initial applications of MD-ME all employed LIF detection, due to the ease of focusing the laser directly into the channel, the low detection limits of fluorescence detection, and the inherent isolation of the detector electronics from the separation field. However, in most cases, the analytes of interest needed to be derivatized in order to be detected. Electrochemical detection (EC) for microchip electrophoresis was first described in 1998, and has many unique advantages [18, 19]. These include the direct detection of the analyte of interest and the ability to easily miniaturize the detection electronics, making the entire device portable. Martin’s group was the first to report on-line MD-ME-EC using a PDMS microchip with epoxy-imbedded carbon electrodes and a pneumatic valve interface [20]. More recently, we reported an all-glass device using a flow-gated interface and integrated platinum electrodes for on-animal, on-line MD-ME-EC [21, 22]. Small, portable devices enable the possibility of on-site monitoring of biomarkers of disease states or progression in the clinic [23, 24] or on-animal monitoring of neurochemicals for behavioral research. Currently, the correlation of neurochemicals with behavior is achieved in freely moving animals, which are typically confined to an apparatus such as the Raturn® due to sampling and experimental constraints [3, 25]. This limits the behaviors that can be studied. The ability to place the analytical device on-animal will allow the study of behaviors in the animals’ natural environment, greatly increasing the types of behaviors that can be investigated and correlated with neurochemical information.
The goal of this work is to develop an on-line MD-ME-EC device capable of monitoring catecholamines in vivo and in near-real time using a flow-gated interface. As this device is ultimately intended for on-animal monitoring, the pneumatic valve injection scheme, which involves bulky gas tanks, was not desirable. Additionally, the flow-gated interface using the double-t design adapts more easily to remote activation and control. The electrochemical response for catecholamines is much better on carbon electrodes than on metal-based electrodes [26]; however, carbon electrodes are not currently compatible with all-glass devices, necessitating a novel fabrication procedure to integrate a carbon electrode into a flow-gated device. There are many different types of carbon available for detection in microchips, including carbon fibers, carbon paste, carbon ink, and pyrolyzed photoresist film carbon electrodes (PPF). Of these, PPF electrodes have been reported to possess lower limits of detection and higher sensitivities than electrodes of other carbon materials [27–29]. From a separations standpoint, PPF electrodes are fabricated on a glass substrate (compared to other carbon electrodes fabricated in PDMS substrates), and we have previously demonstrated that a single wall of glass can lend stability to an electrophoretic separation, resulting in more reproducible migration times than with all-PDMS devices [30].
In this paper, the development of a PDMS/glass hybrid microchip with integrated microdialysis sampling, electrophoretic separation, and electrochemical detection using a carbon pyrolyzed photoresist film electrode is described. This device was evaluated in vitro for the continuous monitoring of analytes in the dopamine metabolic pathway (L-DOPA, 3-O-MD, dopamine, HVA, DOPAC, and 3-MT). Preliminary results obtained for the metabolism of L-DOPA in vivo are also described. The ultimate goal is to use this device for on-animal monitoring of catecholamines and correlate this with behavior in large animals, such as sheep.
2 Materials and Methods
2.1 Reagents
The following chemicals were used as received: L-tyrosine (L-Tyr), 3-O-methyldopa (3-O-MD), 3,4-dihydroxy-L-phenylalanine (L-DOPA), homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC), dopamine hydrochloride, 3-methoxytyramine hydrochloride (3-MT), sodium phosphate monobasic, and sodium phosphate dibasic (Sigma-Adrich, St. Louis, MO, USA); NaOH and isopropyl alcohol (IPA) (Fisher Scientific, Fairlawn, NJ, USA); sodium dodecyl sulfate (SDS) (Thermo Scientific, Waltham, MA, USA); AZ 1518 positive photoresist and AZ 300 MIF developer (AZ Electronic Materials, Sommerville, NJ, USA); SU-8 10 and SU-8 developer (Micro-Chem, Newton, MA, USA), and PDMS and curing agent (Sylgard 184 silicon elastomer base and curing agent, Dow Corning Corp., Midland, MI, USA). The following were also employed: high temperature fused silica glass plates (4 in × 2.5 in × 0.085 in, Glass Fab, Rochester, NY, USA); copper wire (22 gauge, Westlake Hardware, Lawrence, KS, USA); hot glue and hot glue gun (ACE Hardware); colloidal silver liquid (Ted Pella, Inc., Redding, CA, USA); PEEK tubing (0.127 mm ID, Index Health & Science); Instech microdialysis connectors (Instech Laboratories, Inc., Plymouth Meeting, PA, USA); 1.0 cm loop microdialysis probes (30 KDa MWCO PAN membrane, 15 cm of FEP tubing before and after the membrane, BASi, West Lafayette, IN, USA); 1.0 mm cannula microdialysis probe (20 KDa MWCO PAES membrane, CMA, Kista, Sweden) with 15 cm of both inlet and outlet tubing (BASi FEP Teflon tubing, 0.12 mm i.d.); and 18.2 MΩ water (Millipore, Kansas City, MO, USA).
Artificial cerebrospinal fluid (aCSF) was comprised of 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, 2.33 mM Na2HPO4 and 0.45 mM NaH2PO4. Stock solutions of 10 mM of each analyte were prepared in 18.2 MΩ water. Analysis solutions were made from these standard solutions and diluted in 15 mM sodium phosphate (pH 7.4) or aCSF at the time of analysis.
2.2 Fabrication of Substrates
PDMS microchip fabrication has been described elsewhere [30, 31]; briefly, a silicon master was created with negative photoresist onto a 4 in silicon wafer using classic photolithography techniques. The master contained 15 µm raised channels, which corresponds to the channel depth in the final PDMS chip. For this flow-gated, double-t design, the separation channel length was 5 cm, each side arm was 0.75 cm, and the top t was 2 cm long. The width of all channels was 40 µm, except for that of the top sampling channel, which was 1.0 mm. The chip design can be seen in Figure 1A. To create the PDMS microchip from the silicon master, PDMS/curing agent was mixed at a 10:1 ratio and poured onto the master to a form a polymer thickness of at least 2 mm. The PDMS was cured overnight at 70°C, after which the PDMS channels were peeled from the wafer. Reservoirs for buffer and pump waste were punched into the PDMS using a 4 mm biopsy punch (Harris Uni-core, Ted Pella).
Figure 1.
Flow-gated interface using double-t microchip design for on-line microdialysis-microchip electrophoresis with electrochemical detection and connection schemes for on-line analysis. (A) Device design, applied voltages, and flow rate. (B) Direct connect mode where the syringe pump containing analytes is connected directly to the microchip using PEEK tubing. (C) Microdialysis connection mode where solution is being sampled through a microdialysis probe.
Pyrolyzed photoresist electrode fabrication has been described previously [27, 28, 30]. Briefly, the electrode design was transferred to the glass substrate with AZ 1518 positive photoresist using classic photolithography techniques. Substrates with photoresist were then placed in a Linden-BlueM Tube furnace (Cole-Parmer, Vernon Hills, IL, USA), with a constant flow of nitrogen gas throughout the pyrolysis procedure. The temperature program was ramped from room temperature to 925°C at 5.5°C/min and held for 1 hour. The furnace was then allowed to cool back down to room temperature. Final electrode dimensions after pyrolysis, as measured using a surface profiler, were 35 µm wide and 0.5 µm in height.
2.3 Microchip Construction
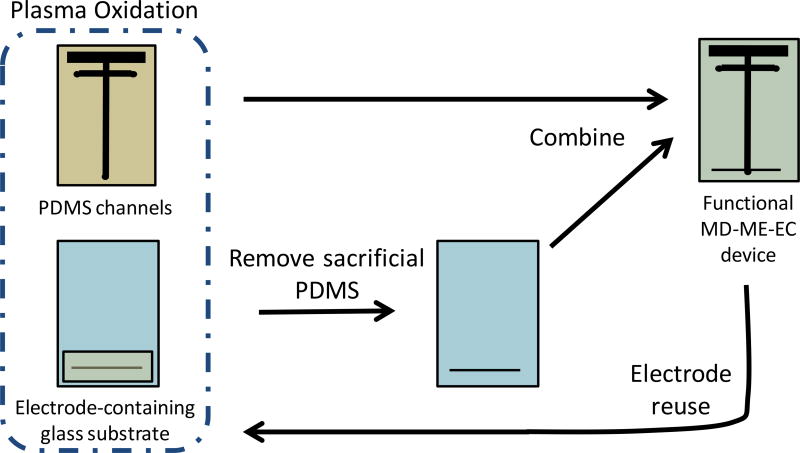
For on-line experiments using the double-t design, a partial irreversible bond between the PDMS channels and glass electrode substrate was created. The microchip was constructed immediately after the PDMS was cured and removed from the oven. Parafilm M®, which is normally used to store PDMS microchips prior to use, leaves a thin residue on the PDMS. This residue interferes with the PDMS/glass bonding described here, so PDMS microchips were used immediately and not stored. PDMS channels and the electrode substrate were simultaneously plasma oxidized (Harrick Plasma Cleaner/Sterilizer PDC-32G, Ithaca, NY, USA) by placing substrates under vacuum for 2 min, followed by oxidation at medium radio frequency (RF) for 60 seconds (also accomplished under vacuum). During this procedure, a piece of sacrificial PDMS (about 6 cm wide and 3 cm in length) was placed over the carbon working electrode and surrounding area. Immediately following oxidation, the sacrificial PDMS was removed and the PDMS channels were placed in conformal contact with the electrode substrate, and slight pressure was applied for 1–2 min to the top portion of the microchip. This procedure created an irreversible bond in the top portion of the microchip to withstand the pressure driven microdialysis flow, and a reversible bond in the bottom portion where the electrode resides to allow the electrode to be reused; the bonding procedure can be seen in Figure 2. Occasionally, when using harsher plasma oxidation conditions, the PDMS portion of the microchip would adhere to the bottom (electrode-containing portion) of the glass substrate. This effect is easily mitigated either through decreasing the intensity of the plasma oxidation or by keeping the lower half of the PDMS substrate and electrode-containing portion of the glass substrate separated until the non-bonded portion of the PDMS reverts back to its native state (typically a few hours). To create the electrical connection, a copper wire was attached to the electrode with silver colloid. This wire was affixed to the glass substrate using hot glue, which permitted stability during experiments as well as easy removal after completion of the experiment.
Figure 2.
PDMS/glass bonding procedure. In this procedure, the PDMS substrate and electrode-containing glass substrate (with sacrificial PDMS) is plasma oxidized. The sacrificial PDMS is removed and substrates placed in conformal contact to create the final device. This procedure creates a fully functional MD-ME-ME device that is irreversibly bonded in the top, MD portion of the microchip and reversibly bonded in the bottom, electrode-containing portion of the microchip. After each experiment, the glass substrate can be cleaned and the electrode reused.
To couple the microchip to the microdialysis pump, a stainless steel 20 gauge blunt needle was used to make a hole in the PDMS for the sample inlet. A 20 gauge 2.0 cm stainless steel connector was used to connect the microchip to either directly to 15 cm of PEEK tubing through Instech microdialysis connectors (Figure 1B) or the 1.0 cm loop microdialysis probe (Figure 1C).
2.4 Experimental Procedure
Prior to electrophoresis experiments, the channels were flushed sequentially with isopropyl alcohol, 0.1 M NaOH, and the run buffer using negative pressure. The top-t was also conditioned and filled with 15 mM phosphate (pH 7.4) using positive pressure produced by using a syringe connected directly to the chip with PEEK tubing (Figure 1B). During experiments, pressure was applied to the 1.0 mL glass syringe using a CMA 102 syringe pump (CMA, Kista, Sweden). Electrophoresis procedures were accomplished using a single Spellman CZE 1000R (Hauppauge, NY, USA) high voltage power supply controlled by LabView software (National Instruments, Austin, TX, USA) written in-house. A flow-gated injection scheme was employed through the application of 2000 V at the buffer reservoir, sample waste and buffer waste reservoirs held at ground, and a microdialysis flow rate of 1.0 µL/min (Figure 1A). Injections were accomplished by floating the buffer voltage for 1.5 s, then reestablishing the voltage for the separation. The separation buffer for all experiments, unless stated otherwise, was comprised of 15 mM phosphate (pH 7.4), 15 mM SDS, and 2.5 mM boric acid. The separation optimization leading to this optimal run buffer has been detailed previously [30].
Electrochemical detection was accomplished using a two-electrode (pyrolyzed photoresist film working, Ag/AgCl reference (BASi, West Lafayette, IN, USA)) system. An electrically isolated potentiostat (10 Hz sampling rate, Pinnacle Technology Inc., Lawrence KS) was used with data visualized using Pinnacle Acquisition Laboratory (PAL 8400) software. This potentiostat has been used previously in our group for in-channel detection [30, 32]. For all experiments described in this paper, the working electrode was held at 1.0 V (versus Ag/AgCl) and placed at the very end of the channel outlet.
2.5 Animal Surgery
All animal experiments were performed in accordance with regulations of the Institutional Animal Care and Use Committee (IACUC) at the University of Kansas, which operates with accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). A Sprague-Dawley rat weighing between 250 and 400 g was anesthetized via the inhalation of isoflurane followed by an i.p. injection of 6–80 mg/kg ketamine, 3–5 mg/kg xylazine, and 1 mg/kg acepromazine diluted in saline. Supplemental doses of ketamine, diluted in saline and delivered i.p., were also administered throughout the experiment to maintain anesthesia.
The rat was placed into a stereotaxic instrument for the placement of the microdialysis guide cannula into the striatum of the brain, following the coordinates (from Bregma) A/P +0.7, M/L −2.7 and V/D −3.4 [33]. The guide cannula and microdialysis probe were held in place using dental acrylic and metal screws. Prior to on-line experiments, the rat was allowed to recover from surgery for at least 1 hr, during which time aCSF was continually perfused through the microdialysis probe at a flow rate of 1.0 µL/min.
For on-line in vivo experiments, the microdialysis probe (1 mm cannula-style probe) was connected to the syringe and microchip using 15 cm of FEP tubing through microdialysis connectors on each side of the probe. A perfusion flow rate of 1.0 µL/min was employed, and the perfusate consisted of either aCSF or 50 µM L-DOPA dissolved in aCSF.
3 Results and Discussion
3.1 Bonding Procedure
When coupling microdialysis sampling on-line to microchip electrophoresis, it is important that the microdialysis-chip interface be designed to accommodate the hydrodynamic flow from microdialysis sampling without leaking or chip delamination due to back pressure. This is accomplished by creating an irreversible bond between the two substrates (channels and base) so that when hydrodynamic pressure is introduced, the two substrates remain in contact. If the irreversible bond is not strong enough to withstand this pressure, it will delaminate and lead to leaks and/or the inability to inject or separate sample.
In the case of glass devices, many bonding procedures exist to irreversibly bond two pieces of glass both with [21, 34–36] and without [37–42] the incorporation of metal electrodes. However, the integration of carbon-based electrodes, which generate better responses to many biologically important analytes, into an all glass device has not yet been reported, due to required tolerances and high-temperature bonding methods. Previous reports of MD-ME chips from our laboratory that used PDMS as a substrate utilized a full, irreversible seal between the PDMS channels and the substrate (glass or PDMS) and detection with LIF [15, 17]. Plasma oxidation or semicuring methods were employed to irreversibly bond these devices so that they would not delaminate during sampling.
The main drawback in irreversibly bonding PDMS channels to a substrate containing a carbon electrode is that the resultant chip will have PDMS that is bonded to the electrode, destroying the electrode for any future use. As the lifetime of PDMS microchips used in MD-ME is relatively short (typically a day or less), after which a new microchip is constructed, a bonding method that enables electrode substrates to be used multiple times is highly desirable. For the optimal detection of catecholamines after an electrophoretic separation, a PPF electrode fabricated on a glass substrate was employed. These electrodes possess high sensitivity and low limits of detection for catecholamines, due to both the high signals generated and low background currents [27–29]. Additionally, we have previously demonstrated that microchips constructed with even a single wall of glass produce much more reproducible separations than those fabricated solely from PDMS [30].
The bonding procedure developed here and outlined in Figure 2 enables the reuse of an electrode substrate for many experiments. In this fabrication approach, the PDMS channels are bonded to all but the electrode-containing portion of the glass substrate. Plasma oxidation was used to alter the surface of the PDMS from –OSi(CH3)2O– to –OnSi(OH)4-n– and remove any organic residues from the glass substrate prior to irreversible bonding [43, 44]. When the two pieces are placed in conformal contact with one another, it is believed that covalent O-Si-O bonds form between the PDMS and glass, creating an irreversible bond [31].
To protect the PPF carbon electrode in this device, a sacrificial piece of PDMS is placed on the glass electrode substrate over the electrode-containing portion of the microchip prior to plasma oxidation. This sacrificial PDMS prevents a small area of the glass substrate from being cleaned through plasma oxidation, making the subsequent bond between PDMS and glass reversible at the detection reservoir. This results in the upper portion of the glass (and therefore microdialysis inlet port and sample introduction channel) being irreversibly bonded to the PDMS, producing a microchip that was stable for at least one week (further time points were not investigated). This part of the chip is then able to withstand the hydrodynamic pressure of the microdialysis flow necessary for on-line sampling. A major advantage of this approach is that the PDMS portion of the microchip can be removed (using a razor blade where irreversibly bonded) and reused. This makes it possible to use the same batch of electrodes for months or years. Generally, a single PPF electrode can easily last for several months, with daily experimentation.
3.2 Method Optimization
The goal of this work was to demonstrate the feasibility of the developed MD-ME-EC device for in vivo, on-animal monitoring, specifically for monitoring conversion of L-DOPA to dopamine and its subsequent metabolism in the brain. To achieve this goal, first the analytes in the dopamine metabolic pathway had to be separated using microchip electrophoresis. Optimization of the ME-EC separation of these compounds (L-DOPA, 3-O-MD, dopamine, HVA, DOPAC, and 3-MT) using a 5 cm simple-t device has been described previously [30]. In transitioning from the simple-t device to the double-t design used for MD-ME-EC, it was important to both optimize the injection procedure and verify the separation integrity prior to the integration of microdialysis sampling for in vitro or in vivo experiments.
Specifically, in order to continuously run MD-ME experiments using this flow-gated interface design, the microdialysis flow rate, separation voltage, and ground placement needed to be optimized. These parameters are all interdependent and critical for establishing a good, stable gate and injection. In these studies, a microdialysis flow rate of 1.0 µL/min was employed, as it is widely used in the literature and in our lab. In addition, the use of lower flow rates (below 0.8 µL/min have previously been shown to result in lower signals at the detector when using the flow-gated injection design [13].
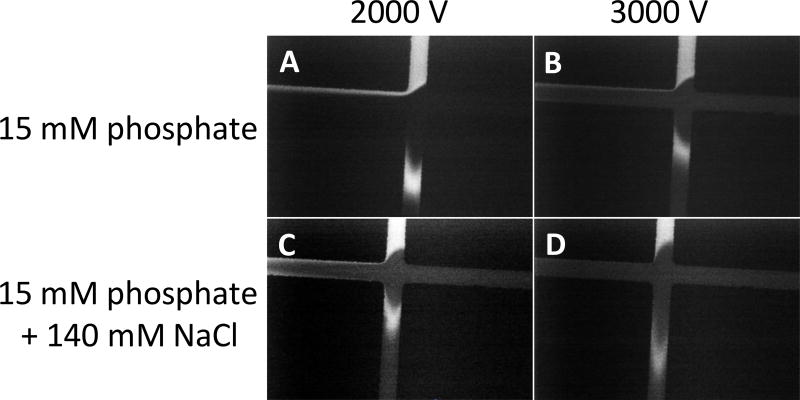
Flow-gated interfaces rely on both the microdialysis pressure and the electroosmotic flow to establish a good gate. In these studies, a separation voltage of 2000 V, which corresponds to field strength of ~130 V/cm as calculated using Kirchhoff’s laws, was used in conjunction with a microdialysis flow rate of 1.0 µL/min [45]. Lower voltages were not considered, as at lower voltages (and, therefore, lower field strengths), the separation is less efficient. Higher voltages, such as 3000 V were investigated, but it was found that less sample was injected. This trend has been previously reported by Huynh et. al [13], and can be seen in Figure 3, where an applied voltage of 2000 V (A and C) results in the injection of a much larger sample plug than with 3000 V (B and D), which has a very strong gate.
Figure 3.
Effect of separation voltage and sample matrix on flow-gated injection. These images focus on the injection-t in the double-t microchip, and the image contrast was increased for clarity. The first column (A and C) represents flow-gated injections with an applied voltage of 2000 V, and the second column (B and D) represents an applied voltage of 3000 V. The first row (A and B) represents a sample matrix of 15 mM phosphate (pH 7.4), and the second row (C and D) represents a sample matrix of 15 mM phosphate (pH 7.4) and 140 mM NaCl. All injections were created by floating the separation voltage for 1.0 s.
Additionally, it quickly became apparent that the ionic strength of the sample matrix heavily influenced the integrity of the gate and amount of sample injected. When sample matrices of higher ionic strength (such as Ringer’s or aCSF) than the separation buffer were employed, the gate was much more intense, with little to no sample reaching the sample waste side arm. Weber’s group has reported a similar effect for a gated (compared to the flow-gated employed here) injection scheme [46]. This effect is demonstrated in Figure 3, where the sample was dissolved in either 15 mM phosphate (A and B) or 15 mM phosphate and 140 mM NaCl (C and D). A solution of 15 mM phosphate and 140 mM NaCl represented the approximate ionic strength of aCSF. Because the ultimate goal of this device is the on-line analysis of brain microdialysis samples, where the aCSF perfusate is high in ionic strength, 2000 V was chosen as the optimal applied voltage for gating and separation with an injection time of 1.5 s.
3.3 Separation and in vitro Analysis
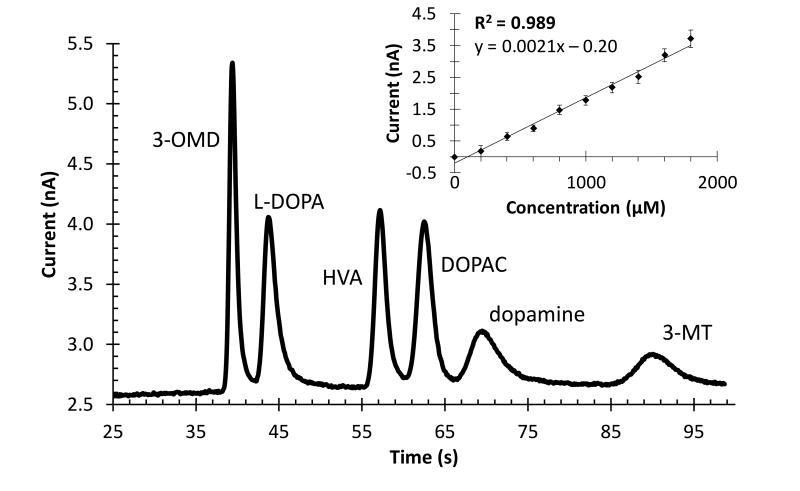
Using the optimized voltage and injection scheme, the separation parameters previously optimized for ME-EC [30] were investigated with the double-t device. These experiments used a direct connection mode, where the syringe containing the sample was directly connected to the microchip using 15 cm of PEEK tubing (Figure 3). As separation conditions in these experiments are similar to those previously published, with the same separation length and only a slight decrease in field strength, the same separation buffer of 15 mM phosphate, 15 mM SDS, and 2.5 mM boric acid was employed. This resulted in near-baseline resolution, and the separation of analytes in the dopamine metabolic pathway can be seen in Figure 4.
Figure 4.
Separation of analytes in the dopamine metabolic pathway and monitoring concentration changes with the online MD-ME-EC system. Separation of analytes (100 µM each) dissolved in 15 mM phosphate. A direct connect scheme was employed and peak identities are indicated in the figure. A separation voltage of 2000 V, injection time of 1.5 s, perfusion flow rate of 1.0 µL/min, and separation buffer of 15 mM phosphate (pH 7.4), 15 mM SDS, and 2.5 mM boric acid were used. Inset demonstrates monitoring concentration change of L-DOPA over time, where the concentration of L-DOPA in a vial was increased through spiking, and sampled and analyzed on-line with the developed device (n = five injections for each concentration and a separation buffer of 15 mM phosphate (pH 7.4), 0.5 mM SDS, and 2.5 mM boric acid).
The successful on-line device must show the ability to monitor concentration changes over time. To demonstrate this ability, the microchip was connected to a linear microdialysis probe that was placed into a vial. The concentration of L-DOPA in the vial was increased over time by spiking the vial with authentic compound. Additions were made every 5 injections so that the concentration of L-DOPA in the vial increased by 200 µM each time. The results of this experiment can be seen in the inset of Figure 4. Using this device, it is clear that increasing the concentration in the vial does increase the signal in a linear fashion (R2 = 0.989).
The lag time, or the time from the change in concentration in the vial until the change was measured by the device, was also calculated for this experiment. Lag time is dependent, in part, on the length of tubing and sampling flow rate employed [47]. With 15 cm of tubing from the probe to the device (a volume of 1.23 mm3) and a flow rate of 1.0 µl/min, the lag time was about 500 seconds (or 5 injections). Theoretically, this time could be dramatically reduced by shortening the length of tubing, as the actual separation of analytes in the dopamine metabolic pathway using this device is completed in under 100 s.
3.4 In vivo Analysis in Anesthetized Rat Following L-DOPA Perfusion
Preliminary proof-of-principle in vivo experiments were performed by placing a cannula microdialysis probe into the rat striatum, allowing the rat to recover from surgery for at least an hour while perfusing aCSF through the probe, and then connecting the microdialysis outlet tubing to the microchip. Initially, aCSF was perfused through the microdialysis probe and into the microchip to establish a baseline. While analytes in the dopamine metabolic pathway do exist endogenously in the brain, their concentrations are below the current detection limits of this method; therefore, when perfusing with only aCSF, no peaks appeared in the corresponding electropherograms.
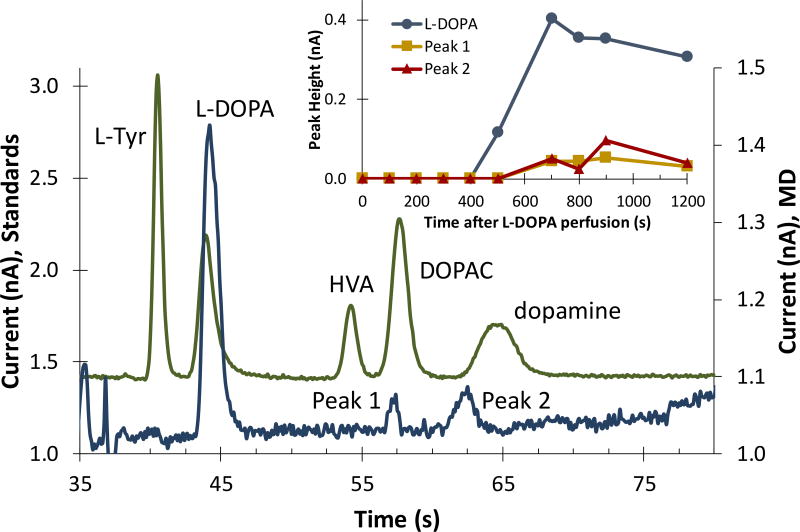
After basal electropherograms were collected, the perfusate syringe was switched to one containing 50 µM L-DOPA dissolved in aCSF. When perfusing L-DOPA, a fraction of the perfused L-DOPA passed through the microdialysis probe and into the brain via retrodialysis. Concurrently, the analytes taken up through the probe were analyzed on-line with the MD-ME-EC method for any L-DOPA metabolites. The results of this experiment can be seen in Figure 5, which shows a representative electropherogram obtained after the start of the L-DOPA perfusion. This in vivo data is overlaid with an electropherogram of standards acquired prior to the in vivo analysis by directly connecting microchip to the syringe pump filled with standards (as in Figure 1B).
Figure 5.
Monitoring the metabolism of L-DOPA using on-line MD-ME-EC device. Electropherogram comparison between analytes dissolved in aCSF (top trace) and microdialysis sampled on-line after the start of an L-DOPA infusion via retrodialysis (bottom trace). Two metabolite peaks are indicated. The inset shows the change in peak height for L-DOPA and the two metabolites as a function of time.
As can be seen in this figure, two metabolite peaks (Peak 1 and Peak 2) appeared after the administration of L-DOPA, and can be monitored over time (Figure 5 inset). When comparing the metabolite migration times to those of the standards, Peak 1 can be tentatively identified as DOPAC and Peak 2 can be tentatively identified as dopamine. It is important to note in this study that both metabolite peaks are near the limits of detection for the current method and, therefore improvements are necessary in this area. Additionally, the future inclusion of a biologically compatible internal standard in the perfusate that is electrophoretically separated from the analytes of interest will enable quantitation with this device. However, these in vivo proof-of-principle data demonstrate that the MD-ME-EC device described in this paper is capable of successfully integrating high ionic strength, pressure-driven microdialysis flow with microchip electrophoresis and electrochemical detection at a carbon electrode for monitoring L-DOPA metabolism in vivo.
4 Conclusions
On-line microdialysis-microchip electrophoresis with electrochemical detection at a carbon electrode was accomplished using a PDMS/glass hybrid device. The effect of the separation voltage and sample matrix on sample injection was investigated. This MD-ME-EC device was found to give a linear response to concentration changes in vitro sampled though microdialysis, and the separation and detection of analytes in the dopamine metabolic pathway was achieved. Finally, in a proof-of-principle study, the metabolism of L-DOPA after its perfusion via retrodialysis into the brain of an anesthetized rat was monitored with the on-line MD-ME-EC device. In the future, this device can be used for on-animal monitoring of neurotransmitters in freely roaming animals, to better correlate neurotransmitter concentrations and behavior.
Acknowledgments
A research grant from the National Institutes of Health (grant R01 NS042929) supported this research. Device fabrication was performed at the Ralph N. Adams Institute COBRE Core Microfabrication Facility (grant P20 GM103638). We thank Nancy Harmony for her editorial support.
Abbreviations
- MD
microdialysis
- ME
microchip electrophoresis
- EC
electrochemical detection
- PDMS
polydimethylsiloxane
- CE
capillary electrophoresis
- LIF
laser-induced florescence
- L-Tyr
L-tyrosine
- 3-O-MD
3-O-methyldopa
- HVA
homovanillic acid
- DOPAC
3,4-dihydroxyphenylacetic acid
- 3-MT
3-methoxytyramine
- L-DOPA
L-3,4-dihydroxyphenylalanine
- SDS
sodium dodecyl sulfate
- aCSF
artificial cerebrospinal fluid
Footnotes
The authors declare no conflict of interest.
References
- 1.Saylor RA, Thomas SR, Lunte SM. In: Compendium of In Vivo Monitoring in Real-Time Molecular Neuroscience Volume 2. Wilson GS, Michael AC, editors. World Scientific Publishing; Singapore: 2017. [Google Scholar]
- 2.Nandi P, Lunte SM. Anal. Chim. Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saylor RA, Lunte SM. Journal of Chromatography A. 2015;1382:48–64. doi: 10.1016/j.chroma.2014.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. Anal. Chem. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Heckert DM, Zuo H, Lunte CE, Lunte SM. Anal. Chim. Acta. 1999;379:307–317. [Google Scholar]
- 6.Manz A, Graber N, Widmer HM. Sens. Actuators, B. 1990;1:244–248. [Google Scholar]
- 7.Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A. Science. 1993;261:895–897. doi: 10.1126/science.261.5123.895. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DJ, Manz A, Fan Z, Luedi H, Widmer HM. Anal. Chem. 1992;64:1926–1932. [Google Scholar]
- 9.Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Luedi H, Widmer HM. J. Chromatogr. 1992;593:253–258. [Google Scholar]
- 10.Seiler K, Harrison DJ, Manz A. Anal. Chem. 1993;65:1481–1488. [Google Scholar]
- 11.Jacobson SC, Hergenroder R, Koutny LB, Ramsey JM. Anal. Chem. 1994;66:1114–1118. [Google Scholar]
- 12.Jacobson SC, Hergenroder R, Koutny LB, Warmack RJ, Ramsey JM. Anal. Chem. 1994;66:1107–1113. [Google Scholar]
- 13.Huynh BH, Fogarty BA, Martin RS, Lunte SM. Anal. Chem. 2004;76:6440–6447. doi: 10.1021/ac049365i. [DOI] [PubMed] [Google Scholar]
- 14.Huynh BH, Fogarty BA, Nandi P, Lunte SM. J. Pharm. Biomed. Anal. 2006;42:529–534. doi: 10.1016/j.jpba.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Nandi P, Scott DE, Desai D, Lunte SM. Electrophoresis. 2013;34:895–902. doi: 10.1002/elps.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandlin ZD, Shou M, Shackman JG, Kennedy RT. Anal. Chem. 2005;77:7702–7708. doi: 10.1021/ac051044z. [DOI] [PubMed] [Google Scholar]
- 17.Nandi P, Desai DP, Lunte SM. Electrophoresis. 2010;31:1414–1422. doi: 10.1002/elps.200900612. [DOI] [PubMed] [Google Scholar]
- 18.Woolley AT, Lao K, Glazer AN, Mathies RA. Anal. Chem. 1998;70:684–688. doi: 10.1021/ac971135z. [DOI] [PubMed] [Google Scholar]
- 19.Gunasekara DB, Wijesinghe MB, Saylor RA, Lunte SM. In: Electrochemical Strategies in Detection Science. Arrigan DWM, editor. Royal Society of Chemistry; 2016. pp. 85–124. [Google Scholar]
- 20.Mecker LC, Martin RS. Anal. Chem. 2008;80:9257–9264. doi: 10.1021/ac801614r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott DE, Grigsby RJ, Lunte SM. ChemPhysChem. 2013;14:2288–2294. doi: 10.1002/cphc.201300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott DE, Willis SD, Gabbert S, Johnson D, Naylor E, Janle EM, Krichevsky JE, Lunte CE, Lunte SM. The Analyst. 2015;140:3820–3829. doi: 10.1039/c4an01928h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oborny NJ, Costa EEM, Suntornsuk L, Abreu FC, Lunte SM. Anal. Sci. 2016;32:35–40. doi: 10.2116/analsci.32.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers ML, Leong CL, Gowers SA, Samper IC, Boutelle MG, Jewell SL, Khan A, McCarthy L, Pahl C, Tolias CM, Walsh DC, Strong AJ, Pahl C, Tolias CM, Walsh DC. J Cereb Blood Flow Metab. 2017;37:1883–1895. doi: 10.1177/0271678X16674486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper JD, Heppert KE, Davies MI, Lunte SM. J. Neurosci. Methods. 2007;160:269–275. doi: 10.1016/j.jneumeth.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams RN. Anal. Chem. 1958;30:1576. [Google Scholar]
- 27.Fischer DJ, Hulvey MK, Regel AR, Lunte SM. Electrophoresis. 2009;30:3324–3333. doi: 10.1002/elps.200900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer DJ, Vandaveer WRIV, Grigsby RJ, Lunte SM. Electroanalysis. 2005;17:1153–1159. [Google Scholar]
- 29.Fischer DJ. "Development of Analytical Methodology for Neurochemical Investigations," Department of Pharmaceutical Chemistry. The University of Kansas; 2010. [Google Scholar]
- 30.Saylor RA, Reid EA, Lunte SM. Electrophoresis. 2015;36:1913–1919. doi: 10.1002/elps.201500150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 32.Gunasekara DB, Hulvey MK, Lunte SM. Electrophoresis. 2011;32:832–837. doi: 10.1002/elps.201000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Steriotaxic Coordinates. Academic Press, Inc; 1986. [Google Scholar]
- 34.Baldwin RP, Roussel TJ, Jr, Crain MM, Bathlagunda V, Jackson DJ, Gullapalli J, Conklin JA, Pai R, Naber JF, Walsh KM, Keynton RS. Anal. Chem. 2002;74:3690–3697. doi: 10.1021/ac011188n. [DOI] [PubMed] [Google Scholar]
- 35.Keynton RS, Roussel TJ, Crain MM, Jackson DJ, Franco DB, Naber JF, Walsh KM, Baldwin RP. Anal. Chim. Acta. 2004;507:95–105. [Google Scholar]
- 36.Pai RS, Walsh KM, Crain MM, Roussel TJ, Jr, Jackson DJ, Baldwin RP, Keynton RS, Naber JF. Anal. Chem. 2009;81:4762–4769. doi: 10.1021/ac9002529. [DOI] [PubMed] [Google Scholar]
- 37.Fan ZH, Harrison DJ. Anal. Chem. 1994;66:177–184. [Google Scholar]
- 38.Carroll S, Crain MM, Naber JF, Keynton RS, Walsh KM, Baldwin RP. Lab Chip. 2008;8:1564–1569. doi: 10.1039/b805554h. [DOI] [PubMed] [Google Scholar]
- 39.Iles A, Oki A, Pamme N. Microfluid. Nanofluid. 2007;3:119–122. [Google Scholar]
- 40.Akiyama Y, Morishima K, Kogi A, Kikutani Y, Tokeshi M, Kitamori T. Electrophoresis. 2007;28:994–1001. doi: 10.1002/elps.200600437. [DOI] [PubMed] [Google Scholar]
- 41.Lima RS, Carneiro Leao PAG, Monteiro AM, Oliveira Piazzetta MH, Gobbi AL, Mazo LH, Carrilho E. Electrophoresis. 2013;34:2996–3002. [Google Scholar]
- 42.Allen PB, Chiu DT. Anal. Chem. 2008;80:7153–7157. doi: 10.1021/ac801059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morra M, Occhiello E, Marola R, Garbassi F, Humphrey P, Johnson D. J. Colloid Interface Sci. 1990;137:11–24. [Google Scholar]
- 44.Chaudhury MK, Whitesides GM. Langmuir. 1991;7:1013–1025. [Google Scholar]
- 45.Seiler K, Fan ZHH, Fluri K, Harrison DJ. Analytical Chemistry. 1994;66:3485–3491. [Google Scholar]
- 46.Wu J, Xu K, Landers JP, Weber SG. Anal. Chem. 2013;85:3095–3103. doi: 10.1021/ac302676q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skoog DA, Holler FJ, Crouch SR. Principles of Instrumental Analysis. Thomson Brooks/Cole; Belmont, CA: 2007. [Google Scholar]