Abstract
Evidence regarding carcinogenic mechanisms serves a critical role in International Agency for Research on Cancer (IARC) Monograph evaluations. Three recent IARC Working Groups pioneered inclusion of the US Environmental Protection Agency (EPA) ToxCast program high-throughput screening (HTS) data to supplement other mechanistic evidence. In Monograph V110, HTS profiles were compared between perfluorooctanoic acid (PFOA) and prototypical activators across multiple nuclear receptors. For Monograph V112 -113, HTS assays were mapped to 10 key characteristics of carcinogens identified by an IARC expert group, and systematically considered as an additional mechanistic data stream. Both individual assay results and ToxPi-based rankings informed mechanistic evaluations. Activation of multiple nuclear receptors in HTS assays showed that PFOA targets peroxisome proliferator activated and other receptors. ToxCast assays substantially covered 5 of 10 key characteristics, corroborating literature evidence of “induces oxidative stress” and “alters cell proliferation, cell death or nutrient supply” and filling gaps for “modulates receptor-mediated effects.” Thus, ToxCast HTS data were useful both in evaluating specific mechanistic hypotheses and in the overall evaluation of mechanistic evidence. However, additional HTS assays are needed to provide more comprehensive coverage of the 10 key characteristics of carcinogens that form the basis of current IARC mechanistic evaluations.
Keywords: Carcinogenicity, high throughput screening, in vitro, mechanisms
1. Introduction
The Monographs Programme of the International Agency for Research on Cancer (IARC) identifies potential causes of human cancer through evaluation of chemical, physical and biological agents for evidence of carcinogenicity. In the 45 years since its inception, well over 100 Monograph Volumes have been published on over 980 agents (Pearce et al., 2015). The process for characterizing the evidence for causation is described in the Preamble and the Instructions to Authors for each Monograph Volume (International Agency for Research on Cancer Monograph Working Group, 2006; International Agency for Research on Cancer, 2016). These documents undergo periodic review by independent groups of world-renowned experts. The evaluations are performed by experts vetted for real and perceived conflicts of interest. The process follows an established transparent methodology for reviewing the evidence from studies of cancer in humans and in experimental animals, and mechanistic and other data (International Agency for Research on Cancer Monograph Working Group, 2006; International Agency for Research on Cancer, 2016).
Following the systematic gathering and review of the publicly available scientific evidence, experts reach consensus hazard classifications based on evaluating the scientific evidence that the agent being reviewed causes cancer in humans. Classifications include 5 categories, ranging from Group 1 (carcinogenic to humans) to Group 4 (probably not carcinogenic to humans), with most agents evaluated to date assigned to Group 3 (not classifiable as to its carcinogenicity to humans). IARC evaluations are used worldwide by national and international health agencies as an authoritative source to support a wide range of subsequent activities ranging from research, to risk assessment, to preventative actions.
The cancer hazard classification methodology of IARC entails the evaluation and integration of three evidence streams: 1) human epidemiologic data on cancer, 2) experimental animal cancer bioassay data, and 3) mechanistic and other relevant data. The overall evaluation procedure (Figure 1) is described in the IARC Monographs Preamble that guides Working Group evaluations (International Agency for Research on Cancer Monograph Working Group, 2006). When human cancer evidence is less than sufficient, strong mechanistic data can play a pivotal role in the overall classification of an agent. Indeed, there are multiple examples of both upgrades and downgrades of the initial classification based on evidence of cancer in humans and experimental animals alone. For instance, d-limonene and saccharine and its salts were downgraded to Group 3 (Volume 73, 1999). On the other hand, ethylene oxide was classified as a Group 1 agent based on strong evidence for genotoxicity, including in exposed humans (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2008). Also, mechanistic evidence was used to classify several classes of agents, rather than individual compounds (Lauby-Secretan et al., 2016), such as the evaluation of dioxin-like polychlorinated biphenyls (PCBs) as “carcinogenic to humans” (Group 1) and polybrominated biphenyls (PBBs) as “probably carcinogenic to humans” (Group 2A).
Figure 1.
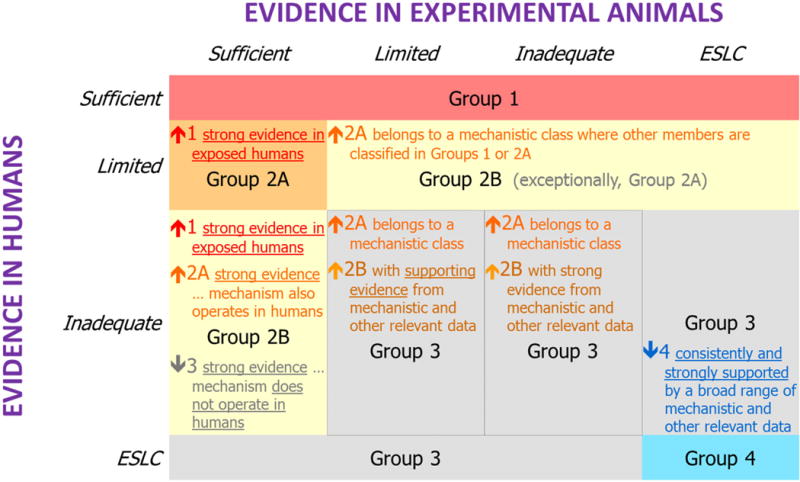
Framework for IARC Monographs evaluations of carcinogenicity, integrating cancer studies in humans, cancer studies in experimental animals, and mechanistic and other relevant data. The arrows indicate the potential for mechanistic and other relevant data to modify the cancer classification based solely on human and experimental animal evidence. ESLC = Evidence Suggesting Lack of Carcinogenicity. From http://monographs.iarc.fr
In evaluating the mechanistic data as “strong, moderate, or weak”, the Working Group considers whether the mechanistic events been established, the results are consistent in different experimental systems and the overall database is coherent (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2008). The Working Group also considers whether the mechanism has been challenged experimentally, through studies demonstrating that suppression of key mechanistic processes leads to suppression of tumour development. The mechanistic and other relevant evidence considered can be voluminous and diverse, and the abundance of information often presents a formidable challenge to ensure inclusiveness of scientific review and evidence integration. To address this challenge, recent IARC Monographs have implemented a systematic method for identifying, organizing, and summarizing mechanistic data based on 10 key characteristics of known human carcinogens (Table 1). Established human carcinogens commonly exhibit one or more of these key characteristics of carcinogens, as described separately by Smith et al. (Smith et al., 2016). Data on these characteristics can provide evidence pertinent to the evaluation of carcinogenicity, and can aid in interpreting the relevance and importance of findings of cancer in animals and in humans, with examples of their application by recent IARC Monographs Working Groups covering various classifications ranging from Group 1 (Loomis et al., 2015; Guyton et al., 2015; Grosse et al., 2016) to Group 2A or 2B (Loomis et al., 2015; Guyton et al., 2015; Guyton et al., 2016; Grosse et al., 2016) to Group 3 (Loomis et al., 2016). These examples illustrate that the key characteristics provide the basis for a structured approach to examining the breadth of mechanistic data that avoids the “looking under the lamppost” problem common to hazard identification approaches based on individual mechanistic hypotheses.
Table 1.
Key characteristics of carcinogens identified by IARC expert review of all Group 1 agents (Smith et al., 2016).
| Characteristic | Examples of relevant evidence |
|---|---|
| 1. Is electrophilic or can be metabolically activated | Parent compound or metabolite with an electrophilic structure (e.g., epoxide, quinone, etc), formation of DNA and protein adducts. |
| 2. Is genotoxic | DNA damage (DNA strand breaks, DNA- protein cross-links, unscheduled DNA synthesis), intercalation, gene mutations, cytogenetic changes (e.g., chromosome aberrations, micronuclei). |
| 3. Alters DNA repair or causes genomic instability | Alterations of DNA replication or repair (e.g., topoisomerase II, base-excision or double-strand break repair) |
| 4. Induces Epigenetic Alterations | DNA methylation, histone modification, microRNA expression |
| 5. Induces oxidative stress | Oxygen radicals, oxidative stress, oxidative damage to macromolecules (e.g., DNA, lipids) |
| 6. Induces chronic inflammation | Elevated white blood cells, myeloperoxidase activity, altered cytokine and/or chemokine production |
| 7. Is immunosuppressive | Decreased immunosurveillance, immune system dysfunction |
| 8. Modulates receptor-mediated effects | Receptor in/activation (e.g., ER, PPAR, AhR) or modulation of endogenous ligands (including hormones) |
| 9. Causes immortalization | Inhibition of senescence, cell transformation |
| 10. Alters cell proliferation, cell death or nutrient supply | Increased proliferation, decreased apoptosis, changes in growth factors, energetics and signaling pathways related to cellular replication or cell cycle control, angiogenesis |
In parallel, there has been an explosion of interest in and availability of high-throughput and high-content data to replace, reduce, and/or refine traditional animal experiments for use in the identification of chemically-induced health hazards (Collins et al., 2008; Tice et al., 2013). A recent IARC Advisory Group recognized the importance of using in vitro high-throughput and high-content data, particularly in the evaluation of mechanisms of carcinogenesis (Straif et al., 2014). One key advantage of high-throughput in vitro screening data is in filling in some of the data gaps that may exist in traditional evidence, in particular in addressing the potential for imbalance in the extent of study across different mechanisms. This concern is noted in the IARC Preamble (International Agency for Research on Cancer Monograph Working Group, 2006), which cautions that an “uneven level of experimental support for different mechanisms may reflect that disproportionate resources have been focused on investigating a favored mechanism.” Because high-throughput in vitro screening approaches enable screening of a large number of compounds across a diverse set of biological targets, they can potentially provide a more unbiased picture as to the level of experimental support across multiple carcinogenic mechanisms.
Several recent IARC Monograph Working Groups have incorporated analyses of data from ToxCast and Tox21 programs in their evaluations of mechanistic data (Loomis et al., 2015; Guyton et al., 2015; Benbrahim-Tallaa et al., 2014). In this article, we describe three case studies that were part of evaluations in Monographs 110, 112 and 113 to illustrate how high-throughput in vitro screening data were utilized in cancer hazard evaluations (Table 2). In each case, these data were used by the IARC Monographs Working Groups to answer specific questions related to the mechanisms of carcinogenesis.
Table 2.
Summary of Case Study Scenarios for Use of ToxCast data in IARC Evaluations of Mechanisms of Carcinogenicity.
| Case Study 1 | Case Study 2 | Case Study 3 | |
|---|---|---|---|
| IARC Monograph | Volume 110 | Volume 112 | Volume 113 |
| Chemical(s) | PFOA | Malathion; Z-Tetrachlorvinphos; Parathion; Diazinon |
2,4-D, DDT and related compounds; Lindane |
| Background/Motivation | It has been hypothesized that rodent tumors caused by PFOA are not relevant to humans because they act through PPAR activation. |
|
|
| Key Questions |
|
|
|
2. Methods
2.1. Case Study 1
PFOA and its ammonium salt are among the compounds that have been tested in a large number of assays as part of the ToxCast and Tox21 programs. Specifically, data on 821 in vitro assay endpoints across 1,860 chemicals were available through the iCSS Dashboard v0.5 as of June 1, 2014 (http://actor.epa.gov/dashboard/). The molecular signatures of PFOA (CAS#335-67-1) and its ammonium salt (CAS# 3825-26-1) were downloaded as well as data on several prototypical nuclear receptor activators: rifampicin (CAS#13292-46-1) for PXR, phenobarbital (CAS#57-30-7) for CAR, and DEHP (CAS#117-81-7) and MEHP (CAS#4376-20-9) for PPARs.
The data obtained included assignments for each chemical as “active” or “inactive” within each assay endpoint based on analysis of the full concentration-response profile, documented in previous publications (Sipes et al., 2013) and available online (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data). For chemicals active in a given assay, half-maximal activity concentration (AC50) values were extracted from the database, ranging from low nM to ~200 μM. The authors note that methodological refinements introduce periodic updates to activity calls and AC50 values in the database, although previous versions are maintained at the public download site to allow for exact replication of particular applications. Also, the data used for these analyses were not subject to further evaluations of the effects of cell stress and cytotoxicity on in vitro assay activity that were implemented into ToxCast data dashboards more recently (Judson et al., 2016).
Of the 821 assays, 166 were excluded because all compounds were not tested in them, 2 assays were excluded because they did not involve receptor-mediated effects (APR_MitoticArrest_72h_up, NVS_GPCR_hTXA2). For analysis, the 616 assays for which all of the six compounds were inactive were not considered further because there was no difference among the compounds for these assays. Thirty-seven (37) in vitro assay endpoints remained for the comparative analysis (about 4.5% of the total number of assay endpoints in the dashboard at that time). Most of these assays were designed for human enzymes and transcription factors, including: cell-free enzymatic and ligand-binding high-throughput screening assays [labeled “NVS”; (Sipes et al., 2013)], cell-based nuclear receptors and transcription factor response element assays [labeled “ATG”; (Martin et al., 2010)], and Tox21 robotic platform high-throughput assays spanning nuclear receptor and oxidative stress pathways predominantly [labeled “Tox21”; (Attene-Ramos et al., 2013)]. For ease of interpretation, the data on 37 in vitro assay endpoints were sub-divided into six groups by the molecular targets as follows: estrogen receptor, PPAR, PXR, aromatase, enzyme, and other (see Supplemental Materials).
2.2. Case Studies 2 and 3
Among the first set of compounds tested by the ToxCast program were pesticide active chemicals that were screened across hundreds of high-throughput cell-free assays and cell-based in vitro assays, in multiple human and rodent primary cells and cell lines (Richard et al. 2016). The availability of these data were noted by the IARC Advisory Group as the opportunity to enhance the use of the novel data streams in the evaluation of mechanistic and other evidence by the working groups (Straif et al., 2014). Accordingly, recent IARC reviews of a number of pesticides (Table 2) included ToxCast and Tox21 screening data (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2016b, 2017).
Two key issues need to be addressed in using these data to augment the usual evaluation of mechanistic data. First, not all assays are potentially informative as to carcinogenicity. Therefore, the first step was to “map” the available assays to the 10 key characteristics of carcinogens (Table 1; Smith et al., 2016). Second, those assays that can be mapped to key characteristics may not cover the range of relevant endpoints. Therefore, rather than using absolute number of “hits,” which may be misleading because of poor assay coverage, a relative ranking approach was used whereby the compounds of interest were ranked among those that have been evaluated both by IARC and in ToxCast/Tox21. The underlying hypothesis is therefore that, in the absence of other data, compounds that were ranked higher have a greater potential to be associated with a key characteristic of carcinogens relative to other chemicals. A high ranking may be useful to either corroborate or indicate a discordance with other mechanistic data, or it could indicate a “data gap” that could be further investigated (hypothesis generation). However, a low ranking cannot be interpreted as evidence against a key characteristic, because it may instead reflect poor assay coverage. In the absence of a computational model (e.g., Judson et al. 2015 [PMID: 26272952] for estrogen activity), this relative ranking approach is consistent with other uses of ToxCast/Tox21 data for prioritization and screening (Filer et al. 2014 [PMID: 25460227]; Reif et al. 2010 [PMID 20826373]; Pham et al. 2016 [PMID: 26969370]). Details as to these methods are described below.
2.2.1. Mapping of assays to key characteristics of carcinogens
To enable the use of the information from ToxCast and Tox21 screening data in the context of the 10 key characteristics of carcinogens (Table 1; Smith et al., 2016), in vitro assay descriptions were used to match each assay to one or more of the key characteristics. Assay summaries and annotations were downloaded from http://actor.epa.gov/dashboard/ on October 24, 2014 using iCSS Dashboard v0.5. The database consisting of 821 in vitro assay endpoints was used for the matching exercise, performed independently by 3 experts with knowledge in mechanistic toxicology (authors of this manuscript). This exercise aimed to apply expert judgment to establish whether the outcome of each in vitro assay may serve as an indication that a chemical will interact with, or have an effect on, targets relevant to each key characteristic of carcinogens. All inconsistent assignments were discussed to resolve minor disagreements among the experts. Following review by a second group of three experts (external peer reviewers acknowledged in this manuscript), a final consensus mapping, including sub-categorizations, was adopted as shown in Table 3. Complete lists of assignments of in vitro assay endpoints to the key characteristics are publicly available from http://monographs.iarc.fr/ENG/Monographs/vol112/112-Section4-Spreadsheet.xlsx for Monograph 112, and from http://monographs.iarc.fr/ENG/Monographs/vol113/113-Section4-Spreadsheet.xlsx for Monograph 113. In addition, output files from ToxPi analyses conducted by the working groups are also available online from http://monographs.iarc.fr/ENG/Monographs/vol112/112-Suppl-ToxPi-Files.zip (for Monograph 112), and from http://monographs.iarc.fr/ENG/Monographs/vol113/113-Suppl-ToxPi-Files.zip (for Monograph 113).
Table 3.
Coverage of ToxCast/Tox21 Assays Mapped to 10 Key Characteristics of Known Human Carcinogens.
| Key Characteristic | ToxCast/Tox21 Assay Mapping |
|---|---|
| 1. Is electrophilic or can be metabolically activated | 31 assays:
|
| 2. Is genotoxic | 9 assays:*
|
| 3. Alters DNA repair or causes genomic instability | No assays |
| 4. Induces epigenetic alterations | 11 assays:
|
| 5. Induces oxidative stress | 18 assays:
|
| 6. Induces chronic inflammation | 45 assays:
|
| 7. Is immunosuppressive | No assays |
| 8. Modulates receptor-mediated effects | 92 assays:
|
| 9. Causes immortalization | No assays |
| 10. Alters cell proliferation, cell death or nutrient supply | 68 assays:
|
These assays may have limited specificity as an indicator of genotoxicity, and were included in Case Study 2, but not 3 (Monograph Volume 113)
2.2.2. Data Extraction
Most of the 8 agents reviewed in IARC Monographs Volumes 112 (Guyton et al., 2015) and 113 (Loomis et al., 2015) were among the 1860 chemicals tested across the panel of ToxCast/Tox21 assays as of March 3, 2015. Additionally, five important metabolites and/or isomers of these compounds were tested in the assay battery, and thus the analyses encompassed 13 chemicals in total. Specifically, for Volume 112, the analyses included 7 chemicals: 3 of the 5 agents evaluated (diazinon, malathion and parathion), one isomer (of tetrachlorvinphos) and three metabolites (diazoxon, malaoxon and paraoxon). For Volume 113, the analyses included the three agents evaluated (2,4-dichlorophenoxyacetic acid (2,4-D), lindane, dichlorodiphenyltrichloroethane (DDT)) and 3 metabolites of DDT. For each chemical, the results of the in vitro assays that represent each key characteristic can be compared across a larger compendium of substances tested in the ToxCast program. For the purposes of these case studies, comparisons were to chemicals that were screened in ToxCast/Tox21 and had been previously evaluated in the IARC Monographs. The total number of comparison chemicals was 178 for Monograph Volume 112 (excluding 7 chemicals, diazinon, malathion and parathion, their oxon metabolites, and z-tetrachlorvinphos) and 181 for Monograph Volume 113 (excluding 6 chemicals, 2,4-D, lindane, DDT and 3 DDT metabolites). The total number of comparison chemicals were greater for Monograph Volume 113 because the Monograph Volume 112 evaluations were also included.
As described for Case Study 1, the data obtained included assignments for each chemical as “active” or “inactive” within each assay endpoint based on analysis of the full concentration-response profile. In the analysis by the Working Groups, each “active” was given a value of 1, and each “inactive” was given a value of 0. Thus, by assigning all active compounds a value of 1, the “potency” estimates from the concentration-response data were not explicitly utilized for all subsequent analyses. However, both active and inactive assay endpoints were included across in all analyses. These were not subject to further evaluations of the effects of cell stress and cytotoxicity on in vitro assay activity that were implemented into ToxCast data dashboards more recently (Judson et al., 2016).
2.2.3. Ranking and Visualization
To integrate data across individual in vitro assay endpoints into a cumulative score for each key characteristic of carcinogens, the Toxicological Prioritization Index (ToxPi) approach (Judson et al., 2010) and associated software (Reif et al., 2013) were used. ToxPi provides a dimensionless index score that integrates multiple, different in vitro assay results and displays them visually. Within each subset of endpoints (“slice”), data are transformed into ToxPi slice-wise scores for all compounds using methods detailed in publications describing applications of the approach and in documentation associated with the software package (Grimm et al., 2016; Auerbach et al., 2016; Sirenko et al., 2013; Rager et al., 2016). Briefly, within each individual slice for a given chemical, the distance from the origin represents the relative chemical-elicited activity of the component in vitro assay endpoints. As described in methods, binary hit calls for each chemical-assay pair were used, so in this application, slices extending farther from the origin were associated with “active” calls in more assay endpoints. The overall score for a chemical, visualized as a radial ToxPi profile, is the aggregation of all slice-wise scores. The overall scores were also arrayed as a cumulative distribution to compare ranks for all chemicals. The mappings of all in vitro assay endpoints to ToxPi slices and description of each assay endpoint’s target and/or model system (e.g., cell type, species, detection technology, etc.) are available as Supplemental Material. In these analyses, the higher ToxPi score provides an estimate of the potential for a chemical to be associated with a key characteristic of carcinogens relative to all other chemicals that were used for that particular comparison (i.e., have been evaluated in the IARC Monographs and had been screened in ToxCast). Thus, all ToxPi results (Figure 3 and Figure 4) consider the same, full set of reference chemicals to set slice lengths that are summed into overall profile scores. For complete details, the full source code is freely-available with (Auerbach et al., 2016) and can be used with the Supplemental Files cited in Section 2.2.1.
Figure 3.
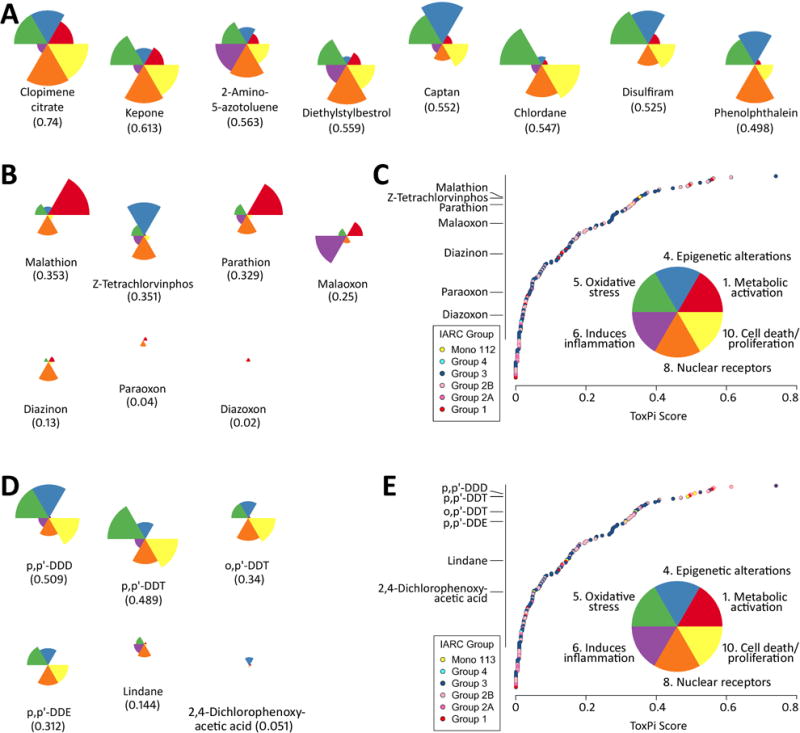
ToxPi ranking of several pesticides against other IARC-evaluated chemicals screened in Tox21/ToxCast assays mapped to six key characteristics of carcinogens. ToxPi profiles of the individual compounds are shown as pie charts with each slice representing assays for each key characteristic. (A) Top 8 compounds in both comparisons. (B and D) Pesticides and their metabolites considered in volumes 112 and 113, respectively. (C and E) Global ranking of the chemicals according to their cumulative ToxPi score, i.e. each data point represents the sum of individual key characteristic scores shown in the inset to each chart.
Figure 4.
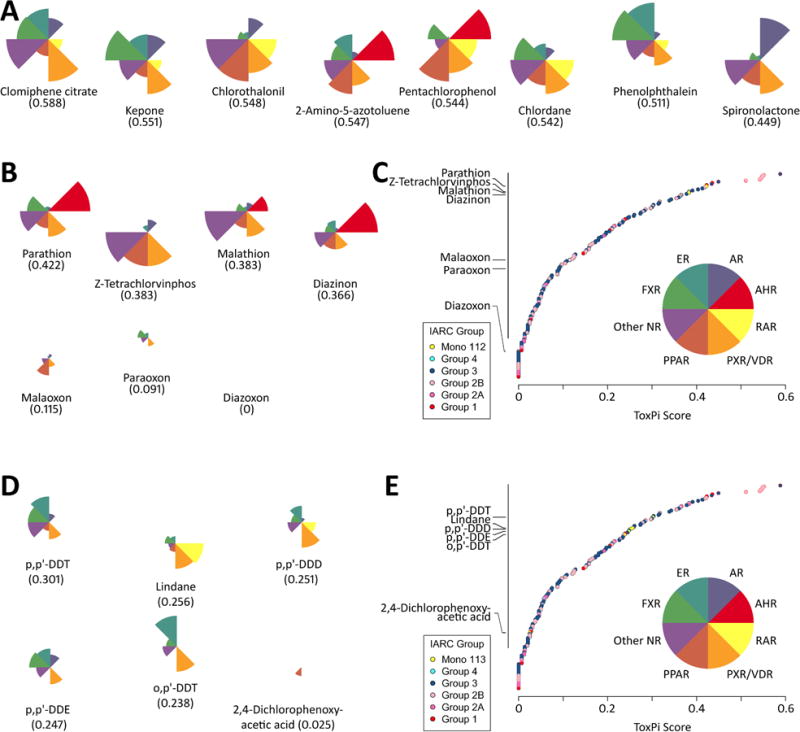
ToxPi rankings using ToxCast assay endpoints mapped to the key characteristic of modulates receptor-mediated effects. ToxPi profiles of the individual compounds are shown as pie charts with each slice representing assays related to different nuclear receptor families. (A) Top 8 compounds in both comparisons. (B and D) Pesticides and their metabolites considered in volumes 112 and 113, respectively. (C and E) Global ranking of the chemicals according to their cumulative ToxPi score, i.e. each data point represents the sum of nuclear receptor family scores shown in the inset to each chart.
3. Results
3.1. Case Study 1: PFOA-associated modulation of receptor-mediated effects
Perfluorooctanoic acid (PFOA) is a synthetic perfluorinated carboxylic acid and fluorosurfactant that has been used in many industrial and commercial products (Perez et al., 2013; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2016a). PFOA is persistent in the environment worldwide and it has been detected at low concentrations in the general population (Ahrens and Bundschuh, 2014; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2016a). PFOA was classified by the IARC Monographs Working Group as possibly carcinogenic to humans (Group 2B) on the basis of limited evidence in humans that PFOA causes testicular and renal cancer (Benbrahim-Tallaa et al., 2014). There was limited evidence in experimental animals of carcinogenicity. The mechanistic data were not strong, and did not support an up- or down-grade of the overall evaluation. A key mechanistic question was the extent to which PFOA carcinogenicity in rodent studies can be attributed to a single mechanism that would lack relevance for human health hazard assessment. It has been hypothesized that a number of rodent tumors caused by PFOA are the result of PPARα activation, a lack of human cancer health hazard relevance of the PFOA-associated tumors has been proposed on the basis of this major mechanism being operative (Klaunig et al., 2003; Klaunig et al., 2012; Corton et al., 2014). In order to address this hypothesis, a comparative analysis of ToxCast/Tox21 in vitro bioactivity profiling data of PFOA and several prototypical nuclear receptor activating compounds was performed for assays that broadly covered activation of PPARα and other nuclear receptors.
The overall results of the analysis of ToxCast in vitro assays related to modulation of receptor-mediated effects for PFOA are shown in Figure 2. PFOA or its ammonium salt were active in four out of five ER assays, unlike the comparison compounds, each of which were active in only one (DEHP and MEHP) or zero (rifampicin and phenobarbital) assays. The consistent activity in several ER assays is in concert with reports of PFOA effects on reproductive hormones and tissues (Biegel et al., 1995; Zhao et al., 2010b; Zhao et al., 2010a). PFOA and/or its ammonium salt were active in all seven PPAR and PXR assays, whereas DEHP or MEHP were active in five of these seven assays, and rifampicin and phenobarbital showed activity in one of seven. PFOA and the ammonium salt were each active in one androgen receptor assay, albeit different assays. Activity was seen in half of the ten other enzyme assays with PFOA or its ammonium salt, whereas the comparison compounds were active in at most two. Finally, for the remaining “other” assays, PFOA or its ammonium salt were active in six assays, with the comparison compounds active in no more than three. While for about half of the assays PFOA and its ammonium salt exhibited concordant effects, in other assays they were less consistent; still, the Working Group concluded that, in vitro toxicity assays provided additional support for PFOA exhibiting a broad range of receptor-mediated effects and lack of preference for PPAR receptors. It should be noted however, that observed activity of PFOA and its ammonium salt in many, albeit not all, in vitro assays evaluated herein may be also attributed to their surfactant properties, a limitation of in vitro screening described in (Sipes et al., 2013).
Figure 2.
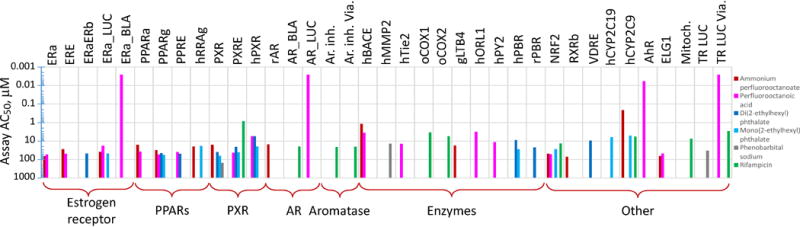
Results of Case Study 1: Comparison of ToxCast AC50s (in microM) for nuclear-receptor-related assays between PFOA (including its ammonium salt) and selected “prototypical” compounds DEHP (including its metabolite MEHP), phenobarbital, and rifampicin. Longer bar indicates greater potency (lower AC50). Assays for which all compounds were negative are not displayed. See Supplemental Materials for the assay endpoints corresponding to the abbreviations shown.
3.2. Case Study 2: Diazinon, malathion, parathion and z-tetrachlorvinphos
The carcinogenicity of the organophosphate pesticides tetrachlorvinphos, parathion, malathion, diazinon was evaluated by IARC in 2015 (Guyton et al., 2015). The insecticides tetrachlorvinphos and parathion were classified as “possibly carcinogenic to humans” (Group 2B) with inadequate evidence in humans and sufficient evidence in experimental animals. The mechanistic data were not strong, and therefore did not change the overall evaluations of tetrachlorvinphos and parathion. The insecticides malathion and diazinon were classified as “probably carcinogenic to humans” (Group 2A) with limited evidence in humans and sufficient (for malathion) and limited (for diazinon) evidence in experimental animals. For both diazinon and malathion, strong mechanistic evidence provided independent support of the Group 2A classification. Strong evidence for two key characteristics of carcinogens (is genotoxic, induces oxidative stress) was found, with malathion additionally showing three others (induces chronic inflammation, modulates receptor-mediated effects, alters cell proliferation).
Data were lacking on many key characteristics of carcinogens, likely reflective of the limited investigation of mechanisms other than genotoxicity for these chemicals. These four chemicals and three of the oxon metabolites were part of the library of compounds screened in ToxCast/Tox21 in vitro assays. Therefore, analyses of these toxicity screening data were undertaken using the ToxPi approach in conjunction with the 6 of 10 key characteristics of carcinogens with successfully mapped ToxCast/Tox21 assay endpoints, as detailed in methods (Figure 3). Figure 3A shows the top 8 agents overall, for which the most common key characteristics with “active” assay endpoints were “alters cell proliferation/death”, “modulates receptor-mediated effects” and “induces oxidative stress”. When compared to other 178 IARC-classified agents with similar complements of in vitro data and across 6 key characteristics that were mapped onto ToxCast/Tox21 in vitro assay endpoints, we observed that malathion, z-tetrachlorvinphos, parathion and malaoxon were in the top quartile of the agents (Figure 3B and C). Interestingly, parathion and malathion showed a very similar ToxPi profile, with the largest signal in aromatase inhibition assays followed by nuclear receptor activation. Receptor-mediated and oxidative stress effects in ToxCast/Tox21 in vitro assays were concordant with other mechanistic evidence; therefore, a more detailed examination of the types of nuclear receptors impacted by these agents was performed (Figure 4). As demonstrated in Figure 4A, the top nuclear receptor active compounds are agents that act on a number of receptors, most notably AHR, PPAR and ER. All four pesticides, but not their metabolites, were clustered in the top quartile of this analysis (Figure 4B and C), showing activity for AHR (parathion and diazinon), PXR (all four), and other nuclear receptors (z-tetrachlorvinphos and malathion).
3.3. Case Study 3: DDT, lindane and 2,4-D
The carcinogenicity of the insecticides lindane and DDT, and the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) was evaluated by IARC in 2015 (Loomis et al., 2015). Lindane was classified as “carcinogenic to humans” (Group 1) based on sufficient evidence for cancer in humans. The insecticide DDT was classified as “probably carcinogenic to humans” (Group 2A) with limited evidence for carcinogenicity in humans and sufficient evidence in experimental animals. 2,4-D was classified as “possibly carcinogenic to humans” (Group 2B) with inadequate evidence in humans and limited evidence in experimental animals.
A considerable amount of data on the mechanisms of carcinogenesis was available for each of these agents, and, in the case of DDT, for a number of its related compounds (o,p′-DDT, p,p′-DDE, and p,p′-DDE). The overall assignment of “strong/moderate/weak” evidence (see Section 1) is shown in Table 4. Also shown for each key characteristic are each compound’s ranking and the active fraction of the associated ToxCast assays.
Table 4.
Summary of evidence for key characteristics of carcinogens for 2.4,-D, DDT and lindane.
| 2,4-D | DDT (p,p′-DDT, o,p′-DDT, p,p′-DDE, or p,p′-DDD) |
Lindane | ||||
|---|---|---|---|---|---|---|
| Key Characteristic | Overall evidence | ToxCast/Tox21 | Overall evidence | ToxCast/Tox21 | Overall evidence | ToxCast/Tox21 |
| 1. Is electrophilic or can be metabolically activated | Inadequate data | Rank: 62/81 (1 out of 31 assays) |
Inadequate data | Rank: 62 or 81/81 (0 or 1 out of 31 assays) |
Inadequate data | Rank: 29/81 (1 out of 31 assays) |
| 2. Is genotoxic | Weak | N/A | Moderate | N/A | Moderate | N/A |
| 3. Alters DNA repair or causes genomic instability | Inadequate data | N/A | Inadequate data | N/A | Inadequate data | N/A |
| 4. Induces epigenetic alterations | Inadequate data | Rank: 44/56 (1 out of 11 assays) |
Inadequate data | Rank: 2, 12, or 21/56 (2 or 4 out of 11 assays) |
Inadequate data | Rank: 56/56 (0 out of 11 assays) |
| 5. Induces oxidative stress | Strong – can operate in humans |
Rank: 96/96 (0 out of 18 assays) |
Strong – can operate in humans |
Rank: 5, 6, 23, or 47/96 (4 to 9 out of 18 assays) |
Strong – can operate in humans |
Rank: 65/96 (2 out of 18 assays) |
| 6. Induces chronic inflammation | Inadequate data | Rank: 60/60 (0 out of 45 assays) |
Moderate | Rank: 16 or 60/60 (0 or 1 out of 45 assays) |
Weak | Rank: 16/60 (1 out of 45 assays) |
| 7. Is immunosuppressive | Moderate | N/A | Strong – can operate in humans | N/A | Strong – can operate in humans | N/A |
| 8. Modulates receptor-mediated effects | Weak | Rank: 79/165 (1 out of 92 assays) |
Strong – can operate in humans |
Rank: 30,41,43, or 46/165 (15 to 21 out of 92 assays) |
Moderate | Rank: 40/165 (11 out of 92 assays) |
| 9. Causes immortalization | Inadequate data | N/A | Inadequate data | N/A | Inadequate data | N/A |
| 10. Alters cell proliferation, cell death or nutrient supply | Weak | Rank: 96/118 (1 out of 68 assays) |
Moderate | Rank: 8, 11, 15, or 28/118 (17 to 27 out of 68 assays) |
Weak | Rank: 92/118 (2 out of 68 assays) |
Notes: Bold rows indicant key characteristics with “Strong” overall mechanistic evidence, where “overall evidence” refers to both in vitro and in vivo (human and experimental animal) data outside of ToxCast/Tox21. “N/A” denotes no ToxCast/Tox21 data available on those key characteristics. Rankings based on ToxPi score compared to 181 other chemicals in ToxCast/Tox21 also evaluated by IARC; denominators of rankings differ due to ties. Materials presented above for DDT and lindane represent the views of the authors, not IARC.
With respect to the overall mechanistic evaluation, 2,4-D had strong evidence for only one key characteristic (induces oxidative stress) (see Table 4), and the overall mechanistic evidence did not change the overall evaluation. For lindane, the overall mechanistic data also did not change the overall classification, because the human data were sufficient (see Figure 1). For DDT, the strong overall mechanistic evidence provided independent support for the overall classification. For DDT (and related compounds) and lindane, the overall results of the analysis of the mechanistic evidence revealed strong support for multiple key characteristics (see Table 4). DDT and lindane both had strong overall mechanistic evidence for two key characteristics (is immunosuppressive and induces oxidative stress), with DDT additionally showing strong overall mechanistic evidence for a third (modulates receptor-mediated effects).
ToxCast/Tox21 provided important additional mechanistic insight for a number of key characteristics. Although no assays were mapped to the characteristic “is immunosuppressive”, the DDT assay endpoint activity provided additional support for the two other key characteristics evaluated as having “strong evidence” (modulates receptor-mediated effects, induces oxidative stress). On the other hand, lindane was largely inactive in ToxCast/Tox21 in vitro assay endpoints mapped to “induces oxidative stress”. The greatest consistency between the ToxCast/Tox21 assay results and other sources of mechanistic data providing strong evidence of key characteristics was seen for “modulates receptor-mediated effects”.
More detailed insights can be obtained using the ToxPi approach for ranking and visualizing aggregate ToxCast/Tox21 in vitro assay endpoint activities (Figures 3 and 4). In the analysis of the data from ToxCast/Tox21 in vitro assays that mapped to 6 key characteristics, DDT and related compounds were among the top in terms of their overall score (Figure 3D and E). Consistently, they showed similarity in four key characteristics (alters cell proliferation, cell death or nutrient supply, modulates receptor-mediated effects and induces oxidative stress) and clustered closely together. Lindane had much less activity, with correspondingly low rankings, across most of the six key characteristics and 2,4-D showed almost no activity and very low rankings, so for these compounds the ToxCast/Tox21 data are less informative for the overall mechanistic evaluation.
ToxPi analyses of the in vitro assays for “modulates receptor-mediated effects” revealed an appreciable degree of similarity in both the ToxPi scores and the ToxPi shapes among the four DDT-related compounds (Figure 4). Moreover, although the ToxPi score for lindane was similar to that of several of the DDT-related compounds, its shape was distinctly different, with lindane showing much less activity related to ER, FXR, and other nuclear receptors, and much more activity for RAR. 2,4-D had very little activity, as evidenced by both the ToxPi score and its shape. It is also interesting to note that among tested compounds evaluated by IARC, the DDT-related compounds and lindane all ranked in the top quartile while 2,4-D was in the lower quartile demonstrating the relative activity difference across a wide battery of receptor-based assay endpoints.
The ToxCast/Tox21 in vitro assays did not substantively add to the overall evaluation of 7 remaining key characteristics of carcinogens: four had no mapped assay endpoints (genotoxic, alters DNA repair or causes genomic instability, immunosuppression, and immortalization)), and very little activity was seen across all the compounds for the others (electrophilic or can be metabolically activated, induces epigenetic alterations, and induces chronic inflammation).
4. Discussion
These three case studies provide a demonstration of several potential applications of in vitro toxicity screening data in the evaluation of carcinogenic mechanisms, including detailed mechanistic insight, corroborating mechanistic evidence from literature studies, as well as filling gaps in information on the potential of evaluated chemicals to interact with targets associated with the 10 key characteristics of carcinogens. Because such screening data are available across a wide range of chemicals, they are particularly well suited for making comparisons across chemicals and across endpoints. Such comparisons can be useful both for testing specific hypothesis as well as in supplementing or filling gaps in traditional mechanistic data.
For instance, in the PFOA case study, ToxCast/Tox21 data supported the conclusion that multiple molecular mechanisms are likely to be operative. As a result, there was no change in the conclusions on cancer in experimental animals based on any single mechanism being operative, nor was a downgrade of the overall classification supported by the mechanistic data. The evidence of multiple molecular mechanisms was seen in the activity across in vitro assays probing different families of receptors, with similar potencies to other prototypical nuclear receptor modulators; however, no conclusions can be drawn from ToxCast data alone on the causality of the involvement of other nuclear receptors or potency of PFOA towards their activation in vivo. PFOA and its ammonium salt had similar responses in each group of in vitro assays, and were more promiscuous than prototypical specific activators of nuclear receptors CAR, PXR, or PPARs. This outcome is consistent with observations that multiple nuclear receptors are activated by PFOA in vivo in rodents (Elcombe et al., 2010; Rosen et al., 2010). Additionally, similar results are found when comparing PFOA to other PPARα agonists clofibrate, bezafibrate, fenofibrate and gemfibrozil (see Supplementary Materials), though interestingly fenofibrate, but not the other fibrates, showed activity across multiple non-PPAR assays. The consistency in vitro and in vivo data for multiple pathway targets, even in the liver, is further corroborated by phenotypic outcomes in both human and experimental animals. For instance, limited data are available indicating liver toxicity in non-human primates (Butenhoff et al., 2002) and serum levels of PFOA have been positively associated with alanine aminotransferase, one marker of liver injury, in one study in humans (Gallo et al., 2012). Additionally, liver toxicity observed in rodents has been associated with both PPARα-dependent and independent mechanisms. For instance, cytotoxicity, cell proliferation, liver hypertrophy and activation of other pathways have been observed in studies of PFOA in rodents, indicating that additional mechanisms may also contribute to liver effects (Filgo et al., 2015; Yan et al., 2015; Rosen et al., 2010).
The results of the PFOA case study suggest that a more holistic approach looking at the potential involvement of multiple mechanistic events may be a more appropriate direction. Thus, the in vitro toxicity data was subsequently re-examined after development of the “10 key characteristics of carcinogens” (Smith et al., 2016), providing a broader and more systematic approach for mechanistic evaluation. By matching in vitro toxicity screening assays to key characteristics, additional insights could be obtained on the bioactivity profile for each of the evaluated compounds and their metabolites or structurally similar analogues specifically for the purpose of evaluating their potential to interact with or affect mechanisms involved in carcinogenesis. In addition, for each chemical, the results of the in vitro assays that represent each key characteristic can be compared to the results for a larger compendium of substances with similar in vitro data.
Particularly for receptor-mediated effects, the case studies of pesticides in ToxCast/Tox21 data provided additional support and confidence for the overall evaluation of mechanistic evidence that includes other types of mechanistic data. First, the availability of the broad compendium of assays on many key characteristics provided a more global dataset amenable to comparisons among agents under evaluation and the larger set of IARC-evaluated chemicals. Such data increased confidence in drawing conclusions from the mechanistic data as the Working Group was in a better position to conclude that data gaps were minimized because all chemicals were tested across the same assays and concentration ranges in the same laboratories. Second, most of the pesticides under evaluation had key metabolites or structural analogues tested across the same panel of assays. This is informative in consideration of the epidemiological studies, where exposures may be complex (Brouwer et al., 2016). For example, there was a remarkable similarity in the activity among the different isomers and metabolites of DDT that were tested; however, metabolites for lindane and 2,4-D were not tested. Overall, these data support a conclusion that the in vivo activity of DDT cannot be attributed to any specific DDT-related compound on this basis.
As the DDT example illustrates, the presence of bioactivity alone does not definitively imply that a particular key characteristic is involved in carcinogenesis by the compound, as the assay data are considered along with other in vivo and in vitro information. Conversely, because the available assays do not cover the full spectrum of targets that may be associated with these mechanisms, and because metabolic capacity in many of the assays is limited, the absence of bioactivity does not necessarily mean the particular key characteristic is not involved in carcinogenesis. For instance, the oxon metabolites of the insecticides malathion, diazinon and parathion were largely inactive in the in vitro assays. This observation was attributed to the high reactivity and short half-life of these metabolites. Other chemical properties (e.g., vapor pressure, molecular weight, solubility, etc), as well as factors such as the need for metabolism, can be inherent limitations to in vitro testing and are important considerations for the relevance of the data from in vitro studies to carcinogenicity evaluations.
Such limitations are illustrated in the example of 2,4-D, which was essentially negative in all assays but had strong evidence for one of the key characteristics based on other available evidence. The coverage of the available HTS assays was limited for most of the key characteristics, especially those that are usually well-addressed using traditional studies available to IARC Working Groups from the publicly available publications. For example, the compendium of HTS data mapped to the “induces oxidative stress” key characteristic comprised assays querying only a few related proteins for transcription factor activity, phosphorylation or gene expression. Most of these assays were negative for 2,4-D, DDT and lindane. At the same time, traditional mechanistic evidence databases comprise more typical endpoints such as formation of hydroxyl radicals, lipid and protein oxidation, increased activity of the antioxidant enzymes, and protective effects of antioxidants.
Overall, it is important to note that “mapping” of in vitro toxicity assays to the key characteristics of carcinogens clearly delineates some of the gaps in assay availability in the context of evaluating carcinogenicity. Historically, genotoxicity has been the primary key characteristic assessed in the mechanistic literature and used in assay batteries supporting cancer hazard evaluations; however, additional mechanisms are gaining greater recognition (Chappell et al., 2016; Smith et al., 2016). With regards to the ToxCast and Tox21 research programs, endpoints relevant to receptor-mediated effects and cell-based alterations, including oxidative stress, cytotoxicity and proliferation, have the greatest coverage given the availability of commercial in vitro assays. However, suitable in vitro assays were not identified from the ToxCast library for several of the 10 key characteristics of carcinogens. Thus, although the assay battery offers advantages for making comparisons across chemicals and across endpoints, it is generally clear that coverage of a diversity of mechanistic events relevant to carcinogenicity is fairly poor. As in vitro assays are further developed to interrogate a broader range of biological pathways, there may also be more sophisticated, computational pathway-based approaches to mapping them to key characteristics. Until there is better assay coverage, and a standardized methodology for integrating the traditional mechanistic evidence with the data from high-throughput screening platform, it will be difficult to evaluate how predictive high throughput assay data alone can inform conclusions about potential human carcinogenicity. Likewise, different sets of the 10 key characteristics may be of varying degree of relevance depending on the target organ, as well as on the timing of their alteration along the process leading to a very complex adverse outcome such as cancer.
In addition, there are a number of methodological approaches that could help improve the informativeness of the HTS data for mechanistic evaluations. As noted in Methods, the generation and analysis of HTS data is an active research area. As data grow and analysis methods mature, updates to activity calls and AC50 values in the database will hopefully present a more precise portrait of underlying biology. For example, data obtained for the applications described here were obtained prior to publication of a strategy to post-filter results at concentrations exceeding a putative cytotoxic interference threshold (Judson et al., 2016). The appropriateness of accounting for cytotoxicity across molecular targets linked to cell cycle and cellular stress can also be argued. Although analytical strategies cannot perfectly account for all factors that might interfere with intended assay readouts, such refinements may be incorporated in future applications to augment confidence in overall interpretation. Additionally, formal multivariate clustering or similarity analyses could be developed that would provide more information than the ToxPi score and rank alone in making comparisons across compounds. This can already be accomplished through visual comparison of ToxPi shape, but a formal method applied to large-scale comparisons across many compounds would add a beneficial layer of interpretation. Incorporation of additional in vitro assay summary values, such as efficacy or potency information or relevant chemical properties (Grimm et al., 2016), rather than just an active/inactive designation, could provide richer quantitative information on carcinogenic mechanisms.
In sum, we have demonstrated the application of HTS data in the evaluation of carcinogenicity by IARC through three case studies. While in vitro HTS data alone, even when mapped to key characteristics, are not yet sufficient to draw conclusions about cancer hazard, we anticipate that future IARC Working Groups will continue to refine the methods used to analyze and interpret HTS data for evaluating mechanisms of chemical carcinogenesis. We also hope that this demonstration will spur the development of additional HTS assays that provide greater coverage across the 10 key characteristics of carcinogens, leading to a substantial improvement in IARC’s ability to identify human carcinogens.
Supplementary Material
Acknowledgments
The authors would like to thank all other members of Working Groups, and of the IARC secretariat, for IARC Monographs Volumes 110, 112, and 113 for their comments and insights during the development of the approaches described in this manuscript. We also acknowledge Drs. Kevin Crofton and Keith Houck of US EPA National Center for Computational Toxicology for critical review of the mapping of in vitro assays to key characteristics. This research was supported in part by the National Institutes of Health, National Institute for Environmental Health Sciences (P42 ES005948).
Footnotes
Disclaimer: The views are those of the authors and do not necessarily reflect views or policies of US EPA.
Conflict of interest statement: The authors declare that they have no potential conflicts of interest.
References
- Ahrens L, Bundschuh M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ Toxicol Chem. 2014;33:1921–1929. doi: 10.1002/etc.2663. http://dx.doi.org/10.1002/etc.2663. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Miller N, Huang R, et al. The Tox21 robotic platform for the assessment of environmental chemicals–from vision to reality. Drug Discov Today. 2013;18:716–723. doi: 10.1016/j.drudis.2013.05.015. http://dx.doi.org/10.1016/j.drudis.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach S, Filer D, Reif D, et al. Prioritizing Environmental Chemicals for Obesity and Diabetes Outcomes Research: A Screening Approach Using ToxCast High Throughput Data. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510456. http://dx.doi.org/10.1289/ehp.1510456. [DOI] [PMC free article] [PubMed]
- Benbrahim-Tallaa L, Lauby-Secretan B, Loomis D, et al. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014;15:924–925. doi: 10.1016/s1470-2045(14)70316-x. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Liu RC, Hurtt ME, et al. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol. 1995;134:18–25. doi: 10.1006/taap.1995.1164. http://dx.doi.org/10.1006/taap.1995.1164. [DOI] [PubMed] [Google Scholar]
- Brouwer M, Schinasi L, Beane Freeman LE, et al. Assessment of occupational exposure to pesticides in a pooled analysis of agricultural cohorts within the AGRICOH consortium. Occup Environ Med. 2016;73:359–367. doi: 10.1136/oemed-2015-103319. http://dx.doi.org/10.1136/oemed-2015-103319. [DOI] [PubMed] [Google Scholar]
- Butenhoff J, Costa G, Elcombe C, et al. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci. 2002;69:244–257. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- Chappell G, Pogribny IP, Guyton KZ, et al. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat Res Rev Mutat Res. 2016;768:27–45. doi: 10.1016/j.mrrev.2016.03.004. http://dx.doi.org/10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319:906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC, Cunningham ML, Hummer BT, et al. Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARalpha) as a case study. Crit Rev Toxicol. 2014;44:1–49. doi: 10.3109/10408444.2013.835784. http://dx.doi.org/10.3109/10408444.2013.835784. [DOI] [PubMed] [Google Scholar]
- Elcombe CR, Elcombe BM, Foster JR, et al. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARalpha and CAR/PXR. Arch Toxicol. 2010;84:787–798. doi: 10.1007/s00204-010-0572-2. http://dx.doi.org/10.1007/s00204-010-0572-2. [DOI] [PubMed] [Google Scholar]
- Filgo AJ, Quist EM, Hoenerhoff MJ, et al. Perfluorooctanoic Acid (PFOA)-induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARalpha Is Not Required. Toxicol Pathol. 2015;43:558–568. doi: 10.1177/0192623314558463. http://dx.doi.org/10.1177/0192623314558463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 2012;120:655–660. doi: 10.1289/ehp.1104436. http://dx.doi.org/10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Iwata Y, Sirenko O, et al. A chemical–biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives. Green Chem. 2016;18:4407–4419. doi: 10.1039/c6gc01147k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, et al. Carcinogenicity of some industrial chemicals. Lancet Oncol. 2016;17:419–420. doi: 10.1016/S1470-2045(16)00137-6. http://dx.doi.org/10.1016/s1470-2045(16)00137-6. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Loomis D, Grosse Y, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–491. doi: 10.1016/S1470-2045(15)70134-8. http://dx.doi.org/10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Loomis D, Grosse Y, et al. Carcinogenicity of pentachlorophenol and some related compounds. Lancet Oncol. 2016;17:1637–1638. doi: 10.1016/S1470-2045(16)30513-7. http://dx.doi.org/10.1016/s1470-2045(16)30513-7. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide) IARC Monogr Eval Carcinog Risks Hum. 2008;97:3–471. [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 110. Perfluorooctanoic Acid, Tetrafluoroethylene, Dichloromethane, 1,2-Dichloropropane, and 1,3-Propane Sultone. IARC Monogr Eval Carcinog Risks Hum. 2016a;110:1–74. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 113. 2,4-Dichlorophenoxyacetic acid (2,4-D) and Some Organochlorine Insecticides. IARC Monogr Eval Carcinog Risks Hum. 2016b;113 [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 112. Some Organophosphate Insecticides and Herbicides. IARC Monogr Eval Carcinog Risks Hum. 2017;112 [Google Scholar]
- International Agency for Research on Cancer Monograph Working Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Preamble. World Health Organization; 2006. http://monographs.iarc.fr/ENG/Preamble/CurrentPreamble.pdf. [Google Scholar]
- Judson R, Houck K, Martin M, et al. Editor’s Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space. Toxicol Sci. 2016;152:323–339. doi: 10.1093/toxsci/kfw092. http://dx.doi.org/10.1093/toxsci/kfw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RS, Martin MT, Reif DM, et al. Analysis of eight oil spill dispersants using rapid, in vitro tests for endocrine and other biological activity. Environ Sci Technol. 2010;44:5979–5985. doi: 10.1021/es102150z. http://dx.doi.org/10.1021/es102150z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, et al. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33:655–780. doi: 10.1080/713608372. http://dx.doi.org/10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Hocevar BA, Kamendulis LM. Mode of Action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and Human Relevance. Reprod Toxicol. 2012;33:410–418. doi: 10.1016/j.reprotox.2011.10.014. http://dx.doi.org/10.1016/j.reprotox.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Baan R, et al. Use of mechanistic data in the IARC evaluations of the carcinogenicity of polychlorinated biphenyls and related compounds. Environ Sci Pollut Res Int. 2016;23:2220–2229. doi: 10.1007/s11356-015-4829-4. http://dx.doi.org/10.1007/s11356-015-4829-4. [DOI] [PubMed] [Google Scholar]
- Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16:891–892. doi: 10.1016/S1470-2045(15)00081-9. http://dx.doi.org/10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- Loomis D, Guyton KZ, Grosse Y, et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016;17:877–878. doi: 10.1016/S1470-2045(16)30239-X. http://dx.doi.org/10.1016/s1470-2045(16)30239-x. [DOI] [PubMed] [Google Scholar]
- Martin MT, Dix DJ, Judson RS, et al. Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA’s ToxCast program. Chem Res Toxicol. 2010;23:578–590. doi: 10.1021/tx900325g. [DOI] [PubMed] [Google Scholar]
- Pearce NE, Blair A, Vineis P, et al. IARC Monographs: 40 Years of Evaluating Carcinogenic Hazards to Humans. Environ Health Perspect. 2015 doi: 10.1289/ehp.1409149. http://dx.doi.org/10.1289/ehp.1409149. [DOI] [PMC free article] [PubMed]
- Perez F, Nadal M, Navarro-Ortega A, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. http://dx.doi.org/10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rager JE, Strynar MJ, Liang S, et al. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ Int. 2016;88:269–280. doi: 10.1016/j.envint.2015.12.008. http://dx.doi.org/10.1016/j.envint.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Reif DM, Sypa M, Lock EF, et al. ToxPi GUI: an interactive visualization tool for transparent integration of data from diverse sources of evidence. Bioinformatics. 2013;29:402–403. doi: 10.1093/bioinformatics/bts686. http://dx.doi.org/10.1093/bioinformatics/bts686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Schmid JR, Corton JC, et al. Gene Expression Profiling in Wild-Type and PPARalpha-Null Mice Exposed to Perfluorooctane Sulfonate Reveals PPARalpha-Independent Effects. PPAR Res 2010. 2010 doi: 10.1155/2010/794739. http://dx.doi.org/10.1155/2010/794739. [DOI] [PMC free article] [PubMed]
- Sipes NS, Martin MT, Kothiya P, et al. Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol. 2013;26:878–895. doi: 10.1021/tx400021f. http://dx.doi.org/10.1021/tx400021f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O, Cromwell EF, Crittenden C, et al. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol. 2013;273:500–507. doi: 10.1016/j.taap.2013.09.017. http://dx.doi.org/10.1016/j.taap.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Guyton KZ, Gibbons CF, et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ Health Perspect. 2016;124:713–721. doi: 10.1289/ehp.1509912. http://dx.doi.org/10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif K, Loomis D, Guyton K, et al. Future priorities for the IARC Monographs. Lancet Oncol. 2014;15:683–684. [Google Scholar]
- Tice RR, Austin CP, Kavlock RJ, et al. Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect. 2013;121:756–765. doi: 10.1289/ehp.1205784. http://dx.doi.org/10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Wang J, Dai J. Activation of sterol regulatory element-binding proteins in mice exposed to perfluorooctanoic acid for 28 days. Arch Toxicol. 2015;89:1569–1578. doi: 10.1007/s00204-014-1322-7. http://dx.doi.org/10.1007/s00204-014-1322-7. [DOI] [PubMed] [Google Scholar]
- Zhao B, Hu GX, Chu Y, et al. Inhibition of human and rat 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 activities by perfluoroalkylated substances. Chem Biol Interact. 2010a;188:38–43. doi: 10.1016/j.cbi.2010.07.001. http://dx.doi.org/10.1016/j.cbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan YS, Haslam SZ, et al. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci. 2010b;115:214–224. doi: 10.1093/toxsci/kfq030. http://dx.doi.org/10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


