Abstract
Objective
The purpose of this study was to study the antimicrobial activity of chitosan nanoparticles (CSNPs) on Pseudomonas aeruginosa with special emphasis on their sensitivity to pH and the effect of pH on their activity.
Methodology
Antimicrobial activity of CSNPs against Pseudomonas aeruginosa at different pH was tested using broth dilution method. Further assessment of antivirulence activity and sensitization of CSNPs on Pseudomonas aeruginosa were examined.
Results
Significant antimicrobial effects of CSNPs against Pseudomonas aeruginosa were detected at slightly acidic pH 5, whereas the activity was abolished at a pH of greater than 7. The antivirulence activity of CSNPs was then investigated and treatment with CSNPs (1000 ppm) resulted in a significant reduction or even complete inhibition of pyocyanin production by P. aeruginosa compared with untreated P. aeruginosa indicating the antivirulence activity of CSNPs. CSNPs also sensitized P. aeruginosa to the lytic effects of sodium dodecyl sulfate (SDS); such sensitization was not blocked by washing chitosan-treated cells prior to SDS exposure revealing that CSNPs disturb the outer membrane leading to irreversible sensitivity to detergent even at low concentration (100 ppm).
Conclusions
These findings highlight CSNPs as potentially useful as indirect antimicrobial agents for a variety of applications.
Keywords: Chitosan nanoparticles, Pseudomonas aeruginosa, pH, Antimicrobial, Virulence
1. Introduction
Pseudomonas aeruginosa is a ubiquitous opportunistic Gram-negative pathogen with a large genome (6.3 Mb) that confers environmental versatility (Stover et al., 2000). P. aeruginosa is adapted to cause disease in different hosts and exhibits antibiotic resistance. P. aeruginosa infection causes a high rate of morbidity and mortality. Its virulence factors include motility and the production of abundant exoproteins such as protease, phospholipases and elastase that allow tissue invasion and systemic dissemination. Persistence infections caused by P. aeruginosa result from a combination of the aggressive characteristics of multidrug resistance, biofilm production and other virulence factors (O'Toole and Kolter, 1998, O'Toole et al., 2000, Drenkard and Ausubel, 2002, Ma et al., 2003, Henderson et al., 2004, Cascales, 2008). The emergence of antibiotic-resistant microorganisms is a great public health concern, requiring solutions potentially from the biotechnology and nanotechnology industries.
Chitosan has broad spectrum antimicrobial activity to which Gram-negative bacteria, Gram-positive bacteria and fungi are highly susceptible (Goy et al., 2009). Several concepts have been proposed to explain the antimicrobial mechanism of action of chitosan (Zheng and Zhu 2003). One such concept, the intracellular leakage theory, is widely accepted and is based on a significant feature of chitosan, namely its positively-charged amino group at C-2 below its pK at pH 6.3 (Neidhardt et al., 1987, Goosen, 1996, Nikaido, 1996). Via this mechanism, positively-charged chitosan binds to the negatively-charged bacterial surface leading to alterations in membrane permeability, which result in leakage of intracellular elements and ultimately cell death. Although chitosan use as an antimicrobial agent has been restricted to neutral pH, a recent study showed that this need not be the case as other mechanisms are involved (Chen et al., 1980, Vogel and Jähnig, 1986, Pautsch and Schulz, 1998, Brinkman et al., 2000). To investigate this further, the antimicrobial and antivirulence activities of CSNPs against P. aeruginosa at different pH ranges need to be tested.
This study aimed to elucidate the effect of variable pH ranges on the antimicrobial activity of CSNPs. Furthermore, the antivirulence and sensitizing activity of CSNPs on P. aeruginosa were investigated using a range of both direct and indirect techniques.
2. Materials and methods
2.1. Preparation and characterization of CSNPs
CSNPs were prepared by the ionic gelatin method as previously mentioned (Dong et al., 2013). The preparation procedures were carried out as follows: (i) chitosan (Cserhati, Forgacs et al.) solution was prepared by dissolving chitosan in acetic acid (0.5% v/v) to obtain chitosan concentrations of 2.5 mg/ml. The mixture was then stirred overnight at room temperature using a magnetic stirrer to obtain a clear solution. Then, 1 M of NaOH solution was used to adjust the pH of the resulting solution (Antony et al., 2004). Sodium tripolyphosphate (TPP solution was prepared by dissolving TPP in pure water to obtain a concentration of 2 mg/ml. (iii) CSNPs were prepared by adding TPP solution drop-wise to the CS solution under probe sonication and then continuous stirring for 30 min at room temperature.
2.1.1. Particle size, polydispersity and zeta potential measurements
The particle size, polydispersity and zeta potential of the prepared nanoparticles was measured by photon correlation spectroscopy. These measurements were obtained using a Zetasizer Nano ZS (Malvern Instruments, UK). Light scattering was monitored at 25 °C at a 90° angle. The particle size values given are the averages of five measurements and are expressed as the z-average. All measurements were performed in triplicate. The zeta potential of the CSNPs was determined after dilution with distilled water.
2.2. Bacterial strains
Pseudomonas aeruginosa PAO1(ATCC 27853) wild-type strain was used as the parent strain. All strains were grown in Mueller–Hinton broth (MHB) or Luria broth (LB) for 18 h at 37 °C.
2.3. Antimicrobial activity of CSNPs
The antimicrobial activity of CS was assessed against P. aeruginosa PAO1 wild-type strain treated with or without CSNPs using an ELx800Absorbance Reader.
2.3.1. In vitro antimicrobial activity assay and pH sensitivity assay
The antimicrobial activities of the CSNPs used in this study were determined using the agar diffusion and broth microdilution method according to the Clinical and Laboratory Standards Institute protocol (Institute 2007), with some adjustments. The bacteria were cultured in LB broth for 18 h at 37 °C. After bacterial growth, inoculums in LB at different pH (5, 6, 7, 8 and 9) were prepared for each colony and adjusted to the 0.5 McFarland scale. Then, 50 µl of CSNPs at a concentration of 1 mg/ml (1000 ppm) was added to 96-well plates. Next, 50 µl of microbial suspension was added to the microwells that were assigned to evaluate the antimicrobial activities of the CSNPs. At the time of inoculation, the final concentration of microbial cells was about 5 × 105 colony-forming units (CFU)/ml for test microrganisms. Microwells containing inoculated MHB without CSNPs were included as a positive control for microbial growth, whereas microwells containing MHB mixed with CSNPs not inoculated with any of the test microbes were included as a negative control. The inoculated plates were incubated at 37 °C for 24 h. The experiments were performed in triplicate. For agar diffusion, cultures adjusted to the 0.5 McFarland scale were also added to top-soft agar to be spotted with CSNPs (1000 ppm; 5, 10 and 50 µl), using azithromycin (10 mg) as a control, to detect the zone of inhibition.
2.3.2. Virulence factor (pyocyanin) production
Pyocyanin production by P. aeruginosa was determined as previously described (Essar et al., 1990; Klein et al., 2012). Briefly, cultures inoculated with a starting OD600 of 0.02 were grown in the absence or presence of CSNPs in LB medium at 37 °C, with shaking at 200 rpm, and a humidity of 75% for 16 h. For pyocyanin determination, cultures were extracted with chloroform and re-extracted with 0.2 M HCl. The OD520 was determined and normalized to the cell growth measured at OD600. For each sample, cultivation and extraction were performed at least in triplicate and 0.2 M HCl was used as a control.
2.3.3. Permeability assays
CSNP-mediated sensitization of P. aeruginosa to the lytic action of SDS detergent was investigated as previously reported (Helander et al., 2001). CSNPs were used directly for the optical monitoring of bacteriolysis, omitting the centrifugation step and re-suspension in buffer. Cells were exposed to 100 ppm of CSNPs for 10 min at room temperature, followed by washing in buffer and exposure to 0.2% SDS. Turbidity was then measured at OD600, using buffer as a control.
3. Results
3.1. Characterization of CSNPs
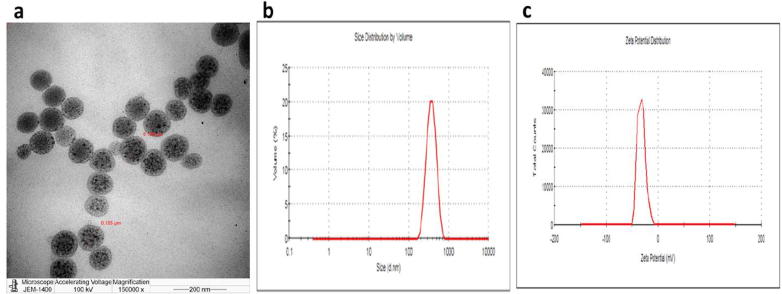
The prepared CSNPs were subjected to particle size analysis, as particle size is crucial for the function of nanoparticles. It has been confirmed that smaller particle size results in a larger surface area, providing high nanoparticle absorption. The CSNPs were found to be 105–106 nm in size (Fig. 1A). Moreover, the Poly Dispersity Index of the prepared CSPNs was 0.14, and the zeta potential was found to be −32.2 ± 0.92 mV (Fig. 1B and C).
Fig. 1.
characterization of CSNPs. (a) TEM micrscopical analysis of CSNPs, (b) Particle size of the prepared CNPs, and (c) Zeta potential of the prepared CNPs.
3.2. Antimicrobial activity and pH sensitivity
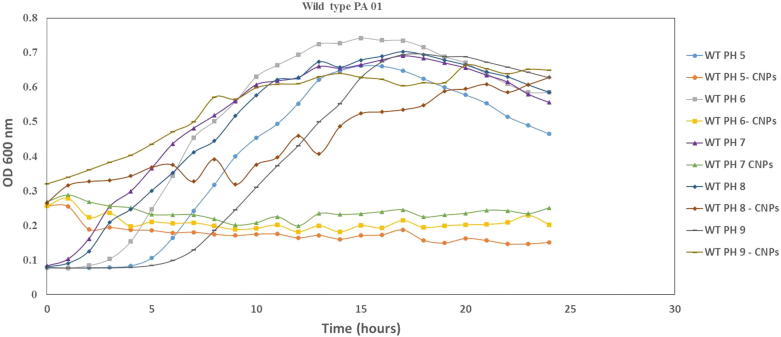
The antimicrobial activity of CSNPs against P. aeruginosa was investigated. As shown in Fig. 2, when CSNPs (1000 ppm in 5, 10 and 50-µl volumes) were applied to P. aeruginosa a zone of inhibition comparable to the azithromycin control was observed indicating the bactericidal activity of CSNPs. To determine whether the antimicrobial activity of CSNPs is effected by pH, different inocula of P. aeruginosa were grown in LB at different pH (5, 6, 7, 8 and 9). Fig. 3 shows that CSNP-treated cultures at pH values ranging from 5 to 7 strongly affected the growth of P. aeruginosa compared with untreated cells. Moreover, an increase in the pH from 7.5 to 9 abolished CSNP activity as both the treated and untreated cultures showed the same growth curves at this pH range. Knowing that neutral pH maintains CSNP activity in a similar manner to acidic pH 5, all further experiments were carried out at neutral pH.
Fig. 2.
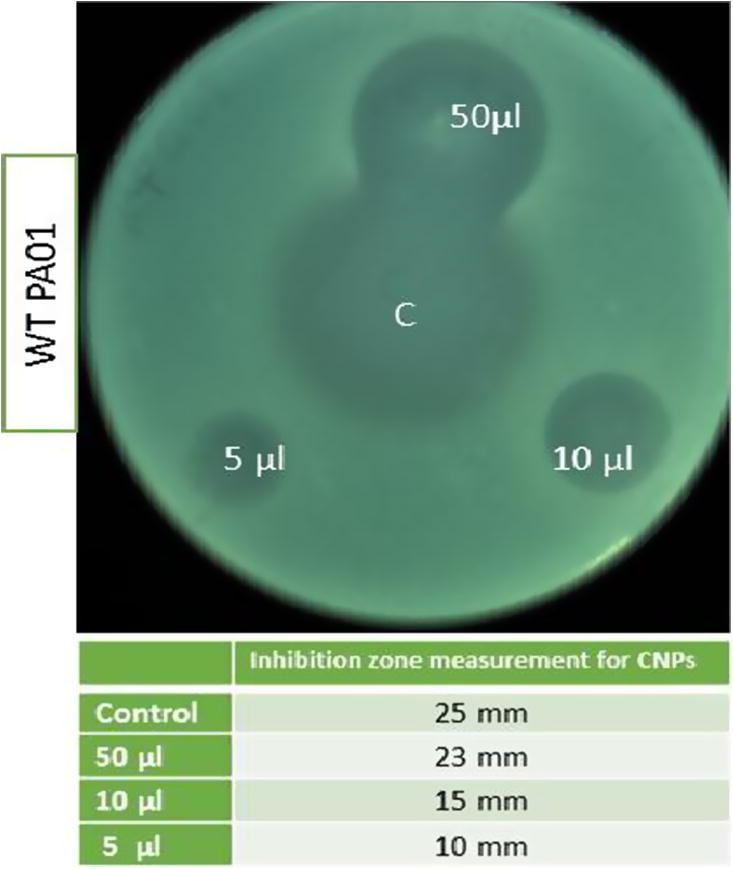
Antimicrobial activity detection of CSNPs by agar diffusion method. CSNPs spotted on agar inoculated with P. aeruginosa adjusted to 0.5 McFarland scale. Various volumes of CSNPs used as 5, 10 and 50 µl of final concentration of 1000 ppm. Plate was incubated at 37°C for 18 h. Azithromycin 10 mg/ml were used as control.
Fig. 3.
Growth curve of the relation between antimicrobial activity of CSNPs and pH. Different inoculum of P. aeruginosa grown in LB at different PHs (5, 6, 7, 8, and 9) were prepared for each colony and adjusted to 0.5 McFarland scale. 50 μl of CSNPs at a concentration of 1000 ppm was added to 96 well plates and read at 37°C for 24 h. comparing the CSNPs untreated cells to treated cells shown the importance of pH 5–7 for the activity of CSNPs. whereas, above pH 7 abolish its activity.
3.3. Antivirulence activity
The bacterial culture supernatants from both CSNP-treated cells and control cells were investigated for the presence of the major virulence factor pyocyanin. As shown in Fig. 4, CSNP treatment significantly inhibited pyocyanin production by P. aeruginosa compared with untreated control cells (p = 0.001942). This confirmed that CSNPs exert not only antimicrobial activity but also antivirulence activity.
Fig. 4.
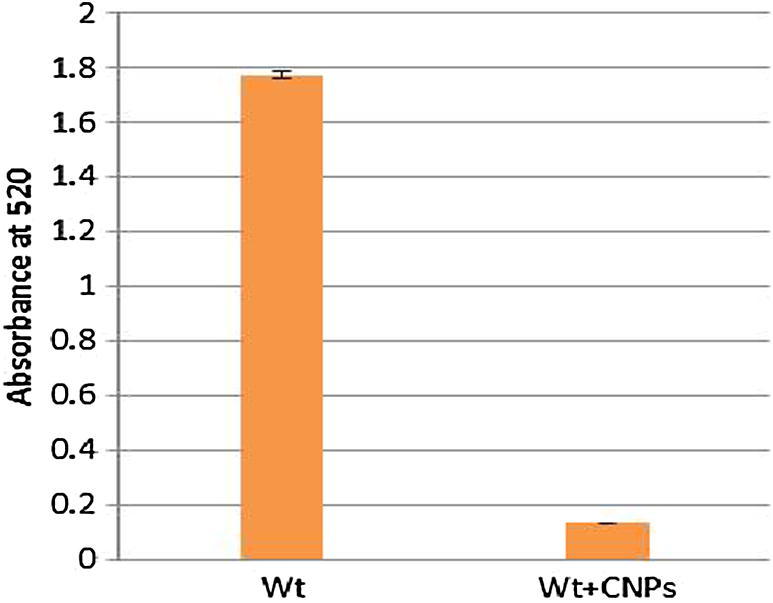
Anti-virulence activity assay. Pyocyanin production detected by measuring the supernatant of treated P. aeruginosa with CSNPs after extraction with chloroform and re-extraction with 0.2 M HCl that was used as a control.
3.4. Sensitizing activity of CSNPs on P. aeruginosa to the lytic effects of SDS
The CSNP-mediated sensitization of P. aeruginosa to the lytic action of SDS was investigated. As shown in Fig. 5, a low concentration of CSNPs (100 ppm) significantly promoted the lysis of P. aeruginosa when treated with 0.2% SDS (p = 0.01164). Since the cells had been washed twice in buffer after exposure to CSNPs for 10 min at room temperature, these CSNP-induced permeability effects were irreversible.
Fig. 5.
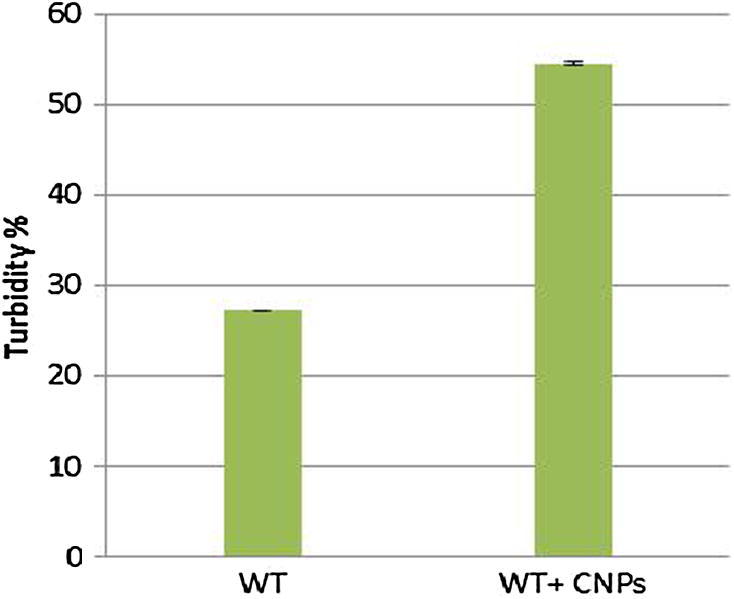
Sensitization of P. aeruginosa to the lytic action of the detergent as SDS by CSNPs. Cells were exposed to 100 ppm CNPs for 10 min at room temperature, followed by washing in buffer and exposure to 0.2% SDS. Turbidity was then measured at OD600 using buffer as control.
4. Discussion
Chitosan is known to exert antimicrobial activity from previous studies and the mechanism of action has been investigated (Neidhardt et al., 1987, Goosen, 1996, Nikaido, 1996, Zheng and Zhu, 2003, Goy et al., 2009, Jayakumar et al., 2010). Recent advances in the field of nanotechnology have enabled the formulation of chitosan polymers as nanoparticles either loaded with specific drugs or used as empty nanoparticles (Jayakumar et al., 2010, Kong et al., 2010). In this study, we attempted to prepare CSNPs using the previously reported ionic gelatin method (Dong et al., 2013). The prepared CSNPs were nano sized (105–106 nm) with a PDI of 14, indicating a narrow size distribution and high homogeneity (Hathout and Nasr 2013). Regarding the zeta potential, the surface charge of the particles affects their stability (Gershanik and Benita 2000) and the zeta potential of the CSNPs was −32.2. This indicated high electrostatic repulsion forces between the particles leading to the avoidance of particle coalescence.
This study demonstrated the antimicrobial activity of CSNPs on P. aeruginosa and their sensitivity to pH. CSNPs (1000 ppm) exerted significant antimicrobial effects at slightly acidic pH 5, whereas their activity was abolished at a pH of greater than 7.5. Ensuring a suitable pH range is important in maintaining the protonation of CSNPs, a key feature for CSNP activity (Goy et al., 2009). Moreover, the experimental results presented in this work provide evidence of the antivirulence activities of CSNPs, as reflected by a significant reduction in virulence factor pyocyanin levels in CSNP-treated cells compared with untreated wild-type P. aeruginosa.
In addition to the direct antimicrobial and antivirulence activities, the ability of CSNPs to sensitize P. aeruginosa was investigated and chitosan was found to disrupt the permeability of the outer membrane sensitizing cells to various types of reagents, as previously reported (Zheng and Zhu, 2003, Goy et al., 2009). CSNPs increased the sensitivity of treated P. aeruginosa to the inhibitory action of anionic detergents such as SDS. This effect was observed at chitosan concentrations (100 ppm) well below that needed to achieve substantial killing of P. aeruginosa. Such sensitization was not blocked by washing CSNP-treated cells prior to SDS exposure, illustrating that CSNPs disturbed the outer membrane leading to irreversible sensitivity to detergent.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group NO (RGP- 1438-003). We also thank Kate Fox, DPhil, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Antony J.M., van Marle G., Opii W., Butterfield D.A., Mallet F., Yong V.W., Wallace J.L., Deacon R.M., Warren K., Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004;7(10):1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- Brinkman F.S., Bains M., Hancock R.E. The amino terminus of pseudomonas aeruginosaouter membrane protein OprF forms channels in lipid bilayer membranes: correlation with a three-dimensional model. J. Bacteriol. 2000;182(18):5251–5255. doi: 10.1128/jb.182.18.5251-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E. The type VI secretion toolkit. EMBO Rep. 2008;9(8):735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proceed. Nat. Acad. Sci. 1980;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Ng W.K., Shen S., Kim S., Tan R.B. Scalable ionic gelation synthesis of chitosan nanoparticles for drug delivery in static mixers. Carbohyd. Poly. 2013;94(2):940–945. doi: 10.1016/j.carbpol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Drenkard E., Ausubel F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416(6882):740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- Gershanik T., Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Europ. J. Pharmaceut. Biopharmaceut. 2000;50(1):179–188. doi: 10.1016/s0939-6411(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Goosen M.F. CRC Press; 1996. Applications of Chitan and Chitosan. [Google Scholar]
- Goy R.C., Britto D.d., Assis O.B. A review of the antimicrobial activity of chitosan. Polímeros. 2009;19(3):241–247. [Google Scholar]
- Hathout R.M., Nasr M. Transdermal delivery of betahistine hydrochloride using microemulsions: physical characterization, biophysical assessment, confocal imaging and permeation studies. Colloids Surfaces B: Biointerfaces. 2013;110:254–260. doi: 10.1016/j.colsurfb.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Helander I., Nurmiaho-Lassila E.-L., Ahvenainen R., Rhoades J., Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Internat. J. Food Microbiol. 2001;71(2):235–244. doi: 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- Henderson I.R., Navarro-Garcia F., Desvaux M., Fernandez R.C., Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol. Molecul. Biol. Rev. 2004;68(4):692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute, C. a. L. S. (2007). Performance Standards for Antimicrobial Susceptibility Testing. Seventh Informational Supplement CLSI document M100-S17,. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA, Clinical and Laboratory Standards Institute.
- Jayakumar R., Menon D., Manzoor K., Nair S., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydrate Poly. 2010;82(2):227–232. [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Internat. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Ma, Q., Y. Zhai, J. C. Schneider, T. M. Ramseier and M. H. Saier Jr (2003). “Protein secretion systems of< i> Pseudomonas aeruginosa</i> and< i> P. fluorescens</i>.” Biochimica et Biophysica Acta (BBA)-Biomembranes 1611(1): 223-233. [DOI] [PubMed]
- Neidhardt F.C., Ingraham J.L., Low K.B., Magasanik B., Schaechter M., Umbarger H. American Society for Microbiology Washington; DC: 1987. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. [Google Scholar]
- Nikaido H. Outer membrane. Escherichia coli Salmonella. 1996:29–47. [Google Scholar]
- O'Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54(1):49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- O'Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Molecul. Microbiol. 1998;28(3):449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Pautsch A., Schulz G.E. Structure of the outer membrane protein A transmembrane domain. Nat. Struct. Mole. Biol. 1998;5(11):1013–1017. doi: 10.1038/2983. [DOI] [PubMed] [Google Scholar]
- Stover C., Pham X., Erwin A., Mizoguchi S., Warrener P., Hickey M., Brinkman F., Hufnagle W., Kowalik D., Lagrou M. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of< i> Escherichia coli</i> derived from raman spectroscopy and prediction methods. J. Mole. Biol. 1986;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Zheng L.-Y., Zhu J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohyd. Poly. 2003;54(4):527–530. [Google Scholar]