Abstract
The interaction of trolox with ammonia, alkylamines of different classes, and amino derivatives of heterocyclic compounds, including nitroxyl radicals and alkaloids, led to the production of ammonium salts called ion conjugates (ICs). Five ICs were characterised by X-ray diffraction. This is the first time a wide range of ICs were made from trolox with amines, and ESI-MS data demonstrated they have the potential to generate pseudomolecular [(A−B+) + H]+ ions. For all obtained trolox ICs, a significant increase (1–3 orders of magnitude) in water solubility was achieved while retaining high antioxidant activity. ICs synthesised from two biologically active fragments may be used to create polyfunctional agents with varying solubility and bioavailability.
Keywords: Trolox, Amines, Ion conjugates, Antioxidants, Mass-spectrometry
1. Introduction
Modification of lead molecules with pronounced biological activity is one of the most efficient ways in synthetic organic and medicinal chemistry to develop novel medicinal agents (Christiaans and Timmerman, 1996, Šebestik et al., 2011). Conjugation of two or more pharmacologically active molecules with different essential properties can be used to synthesise new, biologically active compounds (Šebestik et al., 2011, Hadjipavlou-Litina et al., 2010, Anbharasi et al., 2010). As a rule, hybrid compounds generally have better pharmacological activity when compared to their precursors, and in some cases they demonstrate other types of activities. To illustrate this point, it is common knowledge that many conjugates of various classes of biologically active compounds with nitroxyl radicals have considerable antitumor and antioxidant activity as well as a significant decrease in their general toxicity and an increase in their selective cytotoxicity (Grigor’ev et al., 2014).
Molecules with high antioxidant activity, including substances containing a pharmacophoric chromane core such as α-tocopherol, trolox, dihydroquercetin (taxifolin), rutin, etc., are used today in directed chemical transformations for the synthesis of compounds with high pharmacological potential (Grigor’ev et al., 2014, Nepovimova et al., 2015). For example, hybrid compounds containing trimethyl chromane fragments have been shown to be efficient multifunctional agents with antitumor (Nakagawa-Goto et al., 2007, Arya et al., 1998) anti-inflammatory (Goto et al., 2002), cardioprotective (Koufaki et al., 2003) and neuroprotective properties (Koufaki et al., 2009).
Trolox (6-hydroxy-2,5,7,8-tetra methyl chromane-2-carboxylic acid) 1 is one of the most widely known antioxidants (Scott et al., 1974) and is used as an antioxidant platform for the synthesis of polyfunctional hybrids containing different pharmacologically active fragments covalently bonded to base compounds either directly or through a corresponding linker. Based on trolox, a whole series of hybrid compounds with various types of biological activities was synthesised (Stvolinsky et al., 2010, Koufaki et al., 2004). Trolox conjugated to tacrine seems to be a promising polyfunctional drug with cholinergic, antioxidant, neuroprotective and hepatoprotective properties that can cure Alzheimer's disease. It is a strong inhibitor of acetylcholine esterase and butyrylcholinesterase, which demonstrates its antioxidative properties and that it can penetrate through hemo-encephalitic barriers. Its hepatotoxicity is significantly lower than tacrine (Xie et al., 2015). Moreover, notable hybrid systems with NO-emitting fragments were obtained from a trolox base (Lopez et al., 2005). Recently, we have shown that trolox conjugates with nitroxyl radicals have antitumor activity in addition to their antioxidative properties (Zakharova et al., 2016).
Most covalently bonded trolox conjugates are hydrophobic compounds, and that limits the scope of their use in medicine as water soluble drugs. Formation of salts of trolox conjugates is a simple and accessible way to increase the aqueous solubility of the active pharmaceutical ingredient (API). Many drugs are pharmaceutical salts, composed of pharmacologically active amines and pharmaceutically acceptable acids (Serajuddin, 2007, Stahl and Wermuth, 2002).
Data on the biological properties of trolox ion hybrids are extremely limited. Cytotoxicity of the 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline (EQ) salt of trolox was less than that of EQ and is used as an antioxidant in various food products (Błaszczyk and Skolimowski, 2007). The 1,4-dihydroxy-2,2,6,6-tetramethylpiperidine salt of trolox is a promising antioxidant and radioprotector (Metodiewa et al., 1996).
Using the Twin Drug Approach (Contreras and Sipp, 2008), we synthesised three types of trolox dimers with different types of binding (covalent-covalent, ionic-covalent, ionic-ionic) in which the monomers were connected through an ethylenediamine linker (Yushkova et al., 2015). A similar approach using ionic liquids was used to prepare pharmacologically active conjugates with different types of binding (Egorova et al., 2015).
The aim of this research was to obtain a series of biologically active, antioxidative trolox ion conjugates (ICs) with different amines and study their water solubility and antioxidative properties.
2. Material and methods
2.1. General techniques
Analytical and spectroscopic studies were performed in the Chemical Service Center for collective use at the Siberian Branch of Russian Academy of Sciences (SB RAS). NMR spectra were recorded on Bruker AV-400 and Bruker Drx-500 spectrometers, operating at 400 and 500 MHz for protons and at 100 and 125 MHz for carbons, respectively. Chemical shifts (ppm) were referenced to solvent peaks: δH 3.31 and δC 49.1 for CD3OD, δH 2.50 for DMSO-d6. IR spectra were recorded on a Vector-22 infrared spectrometer. UV-spectra were recorded on a Cary-50 Varian spectrometer. EPR spectra were recorded on a Bruker ESP-300 spectrometer (X-band, microwave power 265 mW, modulation frequency 100 kHz, modulation amplitude 0.01 mT) equipped with a dual-resonator. Elemental analyses were performed in a EURO EA 3000 Elemental Analyzer. Melting points were measured on a Metler Toledo FP 90 Central Processor with a heating rate of 5 °C per minute in the temperature range 50–300 °C with an accuracy of ±0.3 °C. All samples were dried using a vacuum pump Becool 3CFM at ∼20 °C. High resolution mass spectra were obtained on an Agilent 1200 liquid chromatograph with a diode-array detector and a Bruker micrOTOF-Q hybrid quadrupole-TOF mass spectrometer with a direct mode of sample introduction. Mass detection was performed by electrospray ionisation mass spectrometry (ESI-MS) at atmospheric pressure. Positive and negative ions were scanned in the range m/z 80–3000 amu, with a capillary voltage (Vcap) of 2500 V, nebuliser pressure of 1.0 bar, temperature of the dry gas at 140 °C, flow rate of the dry gas at 4 L/min. Samples 2a–2q were dissolved in THF or MeOH for analysis at a concentration of 0.5 mg/mL.
All solvents and chemicals were of analytical grade and were used without further purification. Trolox was from Acros Organics, the amines (b–f, j, k, n, and o) were from ICN Biomedicals and Sigma-Aldrich, the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was from Sigma-Aldrich. The amines (g, h, and i) and the alkaloid (p) were obtained from Pilot Plant of Novosibirsk Institute of Organic Chemistry SB RAS, and the alkaloids (l, m, and q) were obtained from the S. Yu. Yunusov Institute of the Chemistry of Plant Substances (ICPS ASRU).
2.2. General procedure for preparation of compounds 2a and 2b
For preparation of the compound 2a, NH3 (2b, CH3NH2) in excess was bubbled through a solution of trolox (1, 30 mg, 0.12 mmol) in 0.5 mL THF (NH3/CH3NH2 is liberated from a solution of ammonia hydrochloride /methylamine hydrochloride on treatment with sodium hydroxide). After removing the solvent, the raw product was ground in Et2O and, as a result, a powdery substance was obtained. The precipitate was filtered off, washed with THF, and dried in vacuo.
2.3. General procedure for preparation of compounds 2c–2q
A solution containing the appropriate amine (c–q, 0.12 mmol) in 0.3 mL THF was added to a solution of trolox (1, 30 mg, 0.12 mmol) in 0.5 mL of THF with stirring. After 16–20 h, the reaction mixture was evaporated and the viscous residue was triturated in Et2O to form a solid powder. The powder was filtered off, washed with EtOAc, and dried in vacuo.
2.3.1. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate ammonium (2a)
White powder, yield 82%, mp 208.7–210.0 °C. IR (KBr) νmax 1395 and 1557 (COO−), 1497 (N—H) cm−1; UV (MeOH) λmax (lgε) 292 (3.44); 1H NMR (400 MHz, DMSO-d6): δ 2.44–2.41 (m, 2H, CH2-4), 2.29–2.24 (m, 1H, CH2-3), 1.56–1.48 (m, 1H, CH2-3); 2.03 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H) − methyl groups of the phenolic moiety, 1.36 (s, 3H, CH3-2). 13C NMR (125 MHz, CD3OD): δ 179.60 (C9); 146.04, 144.06, (C6, C8a); 122.39, 121.25, 120.01, 116.78 (C5, C7, C8, C4a); 77.61 (C2); 30.45, 20.68 (C3, C4); 23.72 (CH3-2); 10.97, 10.46, 9.99 – methyl groups of the phenolic moiety; HRESIMS m/z (pos): 268.154 C14H22NO4 [M + H]+ (calcd. 268.155).
2.3.2. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate methylammonium (2b)
White powder, yield 85%, mp 191.0–191.4 °C. UV (MeOH) λmax (lgε) 293 (3.65); IR (KBr) νmax 1398 and 1634 (COO−), 1545 (N—H) cm−1; 1H NMR (400 MHz, DMSO‑d6): δ 2.44–2.40 (m, 2H, CH2-4); 2.29–2.24 (m, 1H, CH2-3); 1.54–1.46 (m, 1H, CH2-3); 2.03 (s, 3H), 1.99 (s, 3H), 1.95 (s, 3H) − methyl groups of the phenolic moiety; 2.25 (s, 3H, CH3NH3+), 1.35 (s, 3H, CH3-2); HRESIMS m/z (pos): 282.170 C15H24NO4 [M + H]+ (calcd. 282.169).
2.3.3. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 2-aminoethylanammonium (2c)
White powder, yield 99%, mp 155.4–156.5 °C. UV (MeOH) λmax (lgε) 292 (3.60); IR (KBr) νmax 1400 and 1605 (COO−), 1528 (N—H) cm−1; 1H NMR (400 MHz, DMSO‑d6): δ 2.65 (s, 4H, CH2-groups of ethylenediamine fragment), 2.44–2.40 (m, 2H, CH2-4), 2.30–2.24 (m, 1H, CH2-3), 1.54–1.46 (m, 1H, CH2-3); 2.03 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H) − methyl groups of the phenolic moiety; 1.35 (s, 3H, CH3-2); HRESIMS m/z (pos): 311.196 C16H27N2O4 [M + H]+ (calcd. 311.197).
2.3.4. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 2-methylpropane-2-ammonium (2d)
White powder, yield 95%, mp 199.6–200.0 °C. UV (MeOH) λmax (lgε) 292 (3.57); IR (KBr) νmax 1400 and 1607 (COO−), 1543 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 2.69–2.55 (m, 2H, CH2-4), 2.44–2.38 (m, 1H, CH2-3), 1.78–1.71 (m, 1H, CH2-3); 2.17 (s, 3H), 2.15 (s, 3H), 2.07 (s, 3H) − methyl groups of the phenolic moiety; 1.52 (s, 3H, CH3-2), 1.34 (s, 9H, methyl groups of tert-butylamine fragment). Anal. Calcd. for C18H29NO4: C, 66.8; H, 9.0; N, 4.3. Found: C, 66.9; H, 9.0; N, 4.3.
2.3.5. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 2-hydroxyethylanammonium (2e)
White powder, yield 76%, mp 169.5–171.1 °C. UV (MeOH) λmax (lgε) 292 (3.60); IR (KBr) νmax 1404 and 1570 (COO−), 1537 (N—H) cm−1; 1H NMR (400 MHz, DMSO‑d6): δ 3.49 (t, 2H, J = 5.3 Hz), 2.73 (t, 2H, J = 5.3 Hz) CH2-groups of ethanolamine fragment; 2.44–2.41 (m, 2H, CH2-4), 2.30–2.24 (m, 1H, CH2-3), 1.56–1.48 (m, 1H, CH2-3); 2.03 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H) − methyl groups of the phenolic moiety; 1.37 (s, 3H, CH3-2); HRESIMS m/z (pos): 312.181 C16H26NO5 [M + H]+ (calcd. 312.178).
2.3.6. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 1,3-dihydroxy-2-(hydroxymethyl) propan-2-ammonium (2f)
Beige powder, yield 97%, mp 185.2 °C (dec.). UV (MeOHλmax (lgε) 292 (3.46), 270 (3.58); IR (KBr) νmax 1408 and 1604 (COO−), 1576 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 3.67 (s, 6H, CH2-groups of Tris fragment), 2.59–2.65 (m, 2H, CH2-4), 2.44–2.38 (m, 1H, CH2-3), 1.79–1.72 (m, 1H, CH2-3); 2.17 (s, 3H), 2.15 (s, 3H), 2.08 (s, 3H) − methyl groups of the phenolic moiety, 1.53 (s, 3H, CH3-2); HRESIMS m/z (pos): 372.199 C18H30NO7 [M + H]+ (calcd. 372.202).
2.3.7. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 1-oxyl-2,2,6,6-tetramethylpiperidine-4-ammonium (2g)
Orange powder, yield 70%, mp 128.1–132.3 °C. UV (MeOH) λmax (lgε) 292 (3.61); IR (KBr) νmax 1398 and 1607 (COO−), 1543 (N—H) cm−1; EPR/G: aN = 16.0; HRESIMS m/z (pos): 422.283 C23H38N2O5 [M + H]+ (calcd. 422.278).
2.3.8. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate 1-oxyl-2,2,5,5-tetramethylpirrolidin-4-ammonium (2h)
Yellow powder, yield 75%, mp 137.4 °C (dec.). UV (MeOH) λmax (lgε) 292 (3.67); IR (KBr) νmax 1398 and 1618 (COO−), 1524 (N—H) cm−1; EPR/G: aN = 14.9; HRESIMS m/z (pos): 408.260 C22H36N2O5 [M + H]+ (calcd. 408.262).
2.3.9. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, 2,2,6,6-tetramethylpiperidine-4-ammonium (2i)
Light yellow powder, yield 84%, mp 119.6–121.2 °C. UV (MeOH) λmax (lgε) 293 (3.64); IR (KBr) νmax 1394 and 1626 (COO−), 1574 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 3.38–3.30 (m, 1H, H-4/ of piperidine fragment), 2.69–2.55 (m, 2H, CH2-4); 2.44–2.38 (m, 1H), 1.78–1.71 (m, 1H) CH2-3; 1.92 (dd, 2H, J1 = 12.8 Hz, J2 = 3.7 Hz, CH2 fragment of piperidine); 2.17 (s, 3H), 2.15 (s, 3H), 2.07 (s, 3H) methyl groups of the phenolic moiety); 1.52 (s, 3H, CH3-2); 1.34 (s, 6H), 1.31 (s, 6H) − piperidine methyl groups. Anal. Calcd. for C23H38N2O4: C, 67.9; H, 9.4; N, 6.9. Found C, 67.7; H, 9.3; N, 6.2.
2.3.10. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate diethylammonium (2j)
White powder, yield 97%, mp 210.5–211.9 °C. UV (MeOH) λmax (lgε) 293 (3.57); IR (KBr) νmax 1393 and 1631 (COO−), 1558 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 2.92 (q, 4H, J = 7.4 Hz, CH2-groups of diethylamine fragment), 2.68–2.55 (m, 2H, CH2-4); 2.45–2.39 (m, 1H), 1.78–1.70 (m, 1H) CH2-3; 2.17 (s, 3H), 2.15 (s, 3H), 2.07 (s, 3H) methyl groups of the phenolic moiety; 1.53 (s, 3H, CH3-2); 1.24 (t, 6H, J = 7.4 Hz methyl groups of diethylamine fragment); HRESIMS m/z (pos): 324.214 C18H30NO4 [M + H]+ (calcd. 324.217).
2.3.11. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, di(2-hydroxyethyl) ammonium (2k)
White powder, yield 95%, mp 131.8–137.1 °C. UV (MeOH) λmax (lgε) 292 nm (3.51); IR (KBr) νmax 1402 and 1629 (COO−), 1562 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 3.76–3.73 (m, 4H) and 2.99–2.97 (m, 4H) CH2-groups of diethanolamine fragment; 2.69–2.56 (m, 2H, CH2-4); 2.45–2.39 (m, 1H) and 1.78–1.71 (m, 1H) CH2-3; 2.17 (s, 3H), 2.15 (s, 3H), 2.08 (s, 3H) – methyl groups of the phenolic moiety; 1.53 (s, 3H, CH3-2); HRESIMS m/z (pos): 356.207 C18H30NO6, [M + H]+ (calcd. 356.207).
2.3.12. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, 1,2,3,4,5,6-hexahydro-1,5-methano-8H-pyridinium-[1,2-α][1,5]-diazotsin-8-one (2l)
White powder, yield 78%, mp 150.3 °C (dec.). UV (MeOH) λmax (lgε) 299 (3.98); IR (KBr) νmax 1400 and 1649 (COO−), 1545 (N—H) cm−1; 1H NMR (400 MHz, DMSO-d6): δ 7.32 (dd, 1H, J1 = 9.6 Hz, J2 = 6.9 Hz, H′-10), 6.20 (dd, 1H, J1 = 9.0 Hz, J2 = 1.4 Hz, H′-9), 6.05 (dd, 1H, J1 = 6.9 Hz, J2 = 1.3 Hz, H′-11), 3.84–3.66 (m, 2H, H′-6), 2.97–2.77 (m, 6H, H′-1, H′-2, H′-4, H′-5), 2.46–2.36 (m, 1H, CH2-3), 2.29–2.24 (m, 2H, CH2-4); 2.05 (s, 3H), 2.02 (s, 3H), 1.98 (s, 3H) methyl groups of the phenolic moiety, 1.81 (t, 2H, J = 2.9 Hz, H′-7), 1.76–1.66 (m, 1H), 1.47 (s, 3H, CH3-2); HRESIMS m/z (pos): 463.205 C25H32N2O5Na [M + Na]+ (calcd. 463.220).
2.3.13. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, 3-(3,4-dimethoxybenzoate)-8-ammoniybicyclo[3.2.1]octane (2m)
White powder, yield 98%, mp 215.2–215.6 °C. UV (MeOH) λmax (lgε) 292 (4.04), 265 (4.16); IR (KBr) νmax 1396 and 1634 (COO−), 1589 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 7.67 (dd, 1H, J1 = 8.5 Hz, J2 = 2.0 Hz, H′-6), 7.56 (1H, d, J = 2.0 Hz, H′-2), 7.08 (1H, d, J = 8.5 Hz, H′-5), 3.93 (s, 3H) and 3.91 (s, 3H), 3′-OCH3, 4′-OCH3, 2.64–2.60 (m, 2H, CH2-4); 2.47–2.41 (m, 1H) and 1.78–1.70 (m, 1H) CH2-3; 2.18 (s, 3H), 2.15 (s, 3H), 2.08 (s, 3H) methyl groups of the phenolic moiety, 1.54 (s, 3H, CH3-2); HRESIMS m/z (pos): 542.276 C30H40NO8 [M + H]+ (calcd. 542.275).
2.3.14. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, triethylammonium (2n)
White powder, yield 96%, mp 58.6 °C (dec.). UV (MeOH) λmax (lgε) 292 (3.57); IR (KBr) νmax 1395 and 1593 (COO−), 1580 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 3.10 (q, 6H, J = 7.3 Hz, CH2-groups of triethylamine fragment), 2.63–2.60 (m, 2H, CH2-4), 2.46–2.40 (m, 1H) and 1.80–1.73 (m, 1H) CH2-3, 2.16 (s, 3H), 2.15 (s, 3H), 2.07 (s, 3H) – methyl groups of the phenolic moiety, 1.55 (s, 3H, CH3-2), 1.26 (t, 9H, J = 7.3 Hz, methyl groups of triethylamine frafment); HRESIMS m/z (pos): 352.248 C20H34NO4 [M + H]+ (calcd. 352.248).
2.3.15. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, tri(2-hydroxyethyl) ammonium (2o)
White powder, yield 94%, mp 109.6 °C (dec.). UV (MeOH) λmax (lgε) 292 (3.51); IR (KBr) νmax 1402 and 1580 (COO−), 1560 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 3.80–3.77 (m, 6H) and 3.13–3.11 (m, 6H) CH2-groups of triethanolamine fragment, 2.64–2.60 (m, 2H) CH2-4, 2.45–2.39 (m, 1H) and 1.80–1.73 (m, 1H) CH2-3; 2.17 (s, 3H), 2.15 (s, 3H), 2.08 (s, 3H) methyl groups of the phenolic moiety; 1.54 (3H, s, CH3-2); HRESIMS m/z (neg): 398.219 C20H32NO7 [M−H]− (calcd. 398.218).
2.3.16. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, 4-(N-acetylantranoiloxy)-8,9-dihydroxy-1α,14α,16β-trimethoxy-N-ethylammonium-18-norakonan (2p)
White powder, yield 95%, mp 175.2 °C (dec.). UV (MeOH) λmax (lgε) 296 (3.96), 254 (4.13); IR (KBr) νmax 1379 and 1589 (COO−), 1528 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 8.67 (d, 1H, J = 8.4 Hz), 7.94 (dd, 1H, J1 = 8.1 Hz, J2 = 1.5 Hz), 7.53–7.493 (m, 1H), 7.06–7.03 (m, 1H) hydrogen atoms from benzene ring; 3.41 (s, 3H), 3.33 (s, 3H), 3.30 (s, 3H) OCH3 groups; 2.24 (s, 3H) – CH3 group of N-acetylate, 2.17 (s, 3H), 2.16 (s, 3H), 2.08 (s, 3H) methyl groups of the phenolic moiety; 1.62 (s, 3H, CH3-2); HRESIMS m/z (neg): 833.430C46H61N2O12 [M−H]− (calcd. 833.422).
2.3.17. 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylate, 1-(1H-indol-3-yl)-N,N-dimetilmetanammonium (2q)
White powder, yield 67%, mp 155. °C (dec.). UV (MeOH) λmax (lgε) 288 (3.92); IR (KBr) νmax 1400 and 1587 (COO−), 1547 (N—H) cm−1; 1H NMR (400 MHz, CD3OD): δ 7.68 (dd, 1H, J1 = 7.8 Hz, J2 = 1.0 Hz, H′-4), 7.46 (s, 1H, H′-6), 7.44 (dd,1H, J1 = 8.0 Hz, J2 0.9 = Hz, H′-1), 7.22–7.12 (m, 2H, H-2, H-3), 4.33 (s, 2H, 9′-CH2), 2.69 (s, 6H, N(CH3)2), 2.64–2.60 (m, 2H, CH2-4), 1.78–1.70 (m, 1H), 2.47–2.41 (1H, m) CH2-3; 2.18 (s, 3H), 2.15 (s, 3H), 2.08 (s, 3H) methyl groups of the phenolic moiety; 1.54 (s, 3H, CH3-2); HRESIMS m/z (pos): 425.242 C25H33N2O4 [M + H]+ (calcd. 425.243).
2.4. Structural analysis of 2a, 2b, 2c, 2e, and 2l by X-ray diffraction
X-ray crystal structure analysis was performed on a Bruker Kappa Apex II CCD diffractometer (graphite monochromator, λ(Mo-Kα) = 0.71073 Å, ω,φ-scanning). The absorption was taken into account empirically by the program SADABS (Bruker/Siemens area detector absorption and other Corrections) (Sheldrick and Madison, 2002). The structure was solved by direct methods. The positions of the atoms and the temperature parameters were specified by the anisotropic approximation. The hydrogen atoms of the hydroxyl and ammonium groups in 2a, 2b, and 2c were refined isotropically, while in 2e and 2 l they were treated like all other hydrogen atoms and refined in a riding model. All calculations were performed using the programs SHELX-97.
Crystallographic data for structural analysis of the trolox salts has been deposited with the Cambridge Crystallographic Data Centre: CCDC 1055443 (2a); CCDC 1055444 (2b); CCDC 1055445 (2c); CCDC 1055446 (2e); CCDC 1055447 (2l). These data may be obtained free of charge from the Cambridge Crystallographic Data Center through www.ccdc.cam.ac.uk/data_request/cif.
2.5. Solubility of the compounds 1, 2a–q, 3g, and 3h
Solubility of the compounds 1, 2a–q, 3 g, and 3 h was determined according to the (State pharmacopeia of the Russian Federation, 2008). Synthesis of substances 1, 2a–q is described in the Sections 2.2 and 2.3; compounds 3g and 3h were synthesized previously (Yushkova et al., 2013). Compounds 1, 2a–q, 3g, and 3h (2–5 mg) were pounded into powder, then 0.1 mL of distilled water was added, and samples were shaken for 10 min. Water was added in this manner until the substances dissolved completely. The errors were ±15% for the range 0.1–10.0 g/L; ±10% for the range 10.0–100.0 g/L.
2.6. DPPH radical scavenging activity
The free radical scavenging activity of compounds 1, 2a–q, 3g, and 3h was measured by reduction of the stable free radical DPPH following the method of (Kamkar et al., 2010). Solutions of the samples (0.1 mL, in methanol) in the concentration range 0.05–0.65 mg/ml were added to 4 mL of DPPH solution in methanol (70 μM), and maintained for 10 min at 20–25 °C. Absorbance at 517 nm was measured for the prepared solutions and the DPPH solution (A and A0, respectively). The antioxidative activity of compounds 1, 2a–2q, 3g, and 3h was expressed as the concentration required to quench the stable radical DPPH by 50% (IC50, μM). Error was ±10%. (n = 3).
3. Results and discussion
3.1. Synthesis and characterisation of trolox ammonium salts
In order to synthesise the trolox ammonium salts 2a–2l (Scheme 1), we chose amines from different groups of compounds. Ammonia (a) and alkylamines (b–f, j, k, n, and o) are often used in the production of pharmaceutical salts (Berge et al., 1977), and heterocyclic compounds containing amino groups (g–i) are very promising in the design of new types of pharmacologically active compounds. Of special interest are nitroxyl radicals, which are widely used to synthesise spin-labelled, biologically active natural compounds (Grigor’ev et al., 2014). Finally, we used compounds of particular interest that belonged to a group of pharmacologically active plant alkaloids. Compounds in this group such as cytisine (l), convolvine (m), lappaconitine (p), and donaxine (q) have psychoactive, bronchiolitis, local anesthetic, antiarrhythmic, vasodilating and other types of pharmacological activities. Today, some of these compounds are used in the production of medicinal agents (Fattorusso and Taglialatela-Scafati, 2008).
Scheme 1.
Synthesis of trolox ICs 2a–2q.
Trolox ICs were produced either by passing gaseous amines (a, b) through a trolox solution in THF or adding the amine solutions in THF (c–q) to trolox solutions in THF at 20–25 °C (Scheme 1).
In IR spectra of the obtained trolox ICs 2a–2q, the band for stretching vibrations of the trolox carboxyl group at 1711 cm−1 was lacking; instead, there were bands for the asymmetric and symmetric stretching vibrations of carboxylate anions at 1557–1649 cm−1 and 1379–1408 cm−1, respectively, as well as bending vibrations for the N—H bonds of ammonium groups at 1497–1589 cm−1 (Socrales, 1994). These signals confirmed that compounds 2a–2q have ionic structures. Evidence of the ionic structure was also found in the 13C NMR spectrum of compound 2a, which had the characteristic downfield shifts of a carboxylate and nodal C-2 carbon atoms at 3.5 ppm and 1.2 ppm, respectively. These peaks are typical for carbon atoms of carboxylic acids when salts are generated (Kalinowski et al., 1998). Thus, the data obtained by IR- and 13C NMR-spectroscopy explicitly demonstrated the formation of an ionic bond between the trolox carboxyl group and the corresponding amine.
In UV spectra of the obtained compounds 2a–2q, maximums of absorption were observed in the range of 288–299 nm, which is typical for trolox derivatives (λmax = 292 nm) (Gorecki et al., 2014) and verified the existence of a chromane core. In UV spectra of ICs with convolvine 2m and lappaconitine 2p, additional aromatic ring absorption bands were observed at 265 and 254 nm, respectively.
The data on molecular masses and molecular formulas of the trolox ICs were obtained by atmospheric pressure ESI-MS and direct infusion.
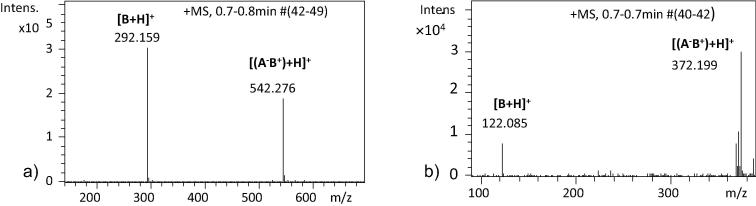
Solutions of the ICs 2a–2c, 2e–2h, 2j, 2m, 2n, and 2q in THF were analysed in positive ionisation mode and exhibited intense peaks for the pseudomolecular ion [(A−B+) + H]+ and less intense peaks for the ICs 2k and 2l (where A− is a deprotonated trolox molecule and B+ is a protonated amine). As an example, mass spectra of the ICs 2m and 2f with intense pseudomolecular ion peaks are shown in Fig. 1a and b. For solutions of the synthesised ICs 2a, 2b, and 2f–2h in methanol, pseudomolecular ions [(A−B+) + H]+ were observable.
Fig. 1.
Positive ion ESI mass spectra of the ICs 2m (a) and 2f (b) in THF.
For the ICs 2o and 2p in THF, we obtained mass spectra with intense pseudomolecular ion peaks [(A−B+) − H]− in negative ionisation.
It is noteworthy that protic or aprotic solvents (e.g. methanol, water and acetonitrile) were used for ESI-MS (Cech and Enke, 2001). This method was used to study organic ammonium salts, but pseudomolecular ions of the cation type [(A−B+) + H]+ have not been observed before now (Kalariya et al., 2014, Wang and Cole, 1996). There are only few examples where pseudomolecular ions of the anion type [(A−B+) − H]− have been observed by ESI-MS analysis, which include the ICs of retinoid amine derivatives with dicarboxylic acids (Um et al., 2004).
The intensity of the pseudomolecular ion peaks for the ICs 2f, 2g, 2h, and 2p in methanol was considerably lower than in THF. For example, the peak intensity of the protonated conjugate 2f was higher in THF than in methanol (100 and 56%, respectively). This phenomenon is likely influenced by the nature of the solvent and the degree to which salts are dissociated.
For solutions of the ICs 2d and 2i in THF and MeOH, the corresponding pseudomolecular ions [(A−B+) + H]+ or [(A−B+) – H]− were not detected. Data for the molecular formulas of the ICs 2d and 2i were obtained by elemental analysis.
In 1H NMR spectra of the ICs 2a–2f and 2i–2q, all typical signals for hydrogen atoms of trolox and amine fragments were obtained. Methyl groups in the phenol fragment of trolox were observed in the range of 1.95–2.18 ppm, methyl group in the second position of the pyran ring were observed in the range of 1.35–1.62 ppm, 3-CH2 groups were found at 1.46–1.80 ppm and 2.24–2.47 ppm, and the 4-CH2 group was found at 2.24–2.69 ppm. 1H NMR spectra of compounds 2g and 2h had extremely broad signals due to the presence of nitroxyl radicals in these molecules. Very often in order to confirm the structure of spin-labelled compounds by high resolution NMR they are reduced to their corresponding hydroxylamines or amines (Yushkova et al., 2013, Kosheleva et al., 2014). The diamagnetic analogue of the nitroxyl radical 2g is the secondary amine 2i whose NMR spectrum corresponded to its structure and, as a consequence, confirmed the structure of the corresponding nitroxyl radical 2g.
Values of the hyperfine interaction (HFI) constants, aN, in EPR spectra of the spin-labelled ICs 2g and 2h were equal to 16.0 and 14.9 G, respectively, and corresponded to HFI constants for nitroxyl radicals of the piperidine 2g and pyrrolidine 2h types (Yushkova et al., 2013).
3.2. X-Ray structure determination
Compounds 2a, 2b, 2c, 2e, and 2 l were crystallised and their structures were characterised by X-ray diffraction analysis. Crystal data and refinement results are summarised in Table 1.
Table 1.
Crystal data and structure refinement details.
| Compound | 2a | 2b | 2c | 2e | 2 l |
|---|---|---|---|---|---|
| Chemical formula | C14H21NO4 | C15H23NO4 | C16H26N2O4 | C16H25NO5 | C25H32N2O5.25 |
| Formula weight [g mol−1] | 267.32 | 281.34 | 310.39 | 311.37 | 444.53 |
| Temperature [K] | 296(2) | 296(2) | 296(2) | 200(2) | 200(2) |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/n | P-1 | P21/c | P21/c | P212121 |
| Crystal colour | Colourless | Colourless | Colourless | Colourless | Colourless |
| a [Å] | 6.1753(2) | 6.3672(3) | 14.187(2) | 14.016(3) | 13.9403(4) |
| b [Å] | 10.2741(5) | 9.8169(5) | 25.474(4) | 25.527(5) | 15.7776(4) |
| c [Å] | 21.6500(10) | 12.6802(6) | 8.8259(12) | 8.7038(15) | 20.9671(5) |
| α [°] | 90.00 | 105.088(2) | 90.00 | 90.00 | 90.00 |
| β [°] | 93.484(2) | 99.153(2) | 92.745(6) | 95.460(7) | 90.00 |
| γ [°] | 90.00 | 103.935(2) | 90.00 | 90.00 | 90.00 |
| Volume [Å3] | 1371.06(10) | 721.65(6) | 3186.0(8) | 3100.0(10) | 4611.6(2) |
| Z | 4 | 2 | 8 | 8 | 8 |
| Dcalc [g cm−3] | 1.295 | 1.295 | 1.294 | 1.334 | 1.281 |
| Absorption coefficient [mm−1] | 0.094 | 0.093 | 0.093 | 0.099 | 0.090 |
| F(0 0 0) | 576 | 304 | 1344 | 1344 | 1904 |
| θ range [°] | 1.88–27.23 | 1.71–27.55 | 1.44–25.16 | 1.46–25.40 | 1.62–26.08 |
| Index ranges | –7 ≤ h ≤ 7, –13 ≤ k ≤ 13, –27 ≤ l ≤ 27 | –8 ≤ h ≤ 8, –12 ≤ k ≤ 12, –16 ≤ l ≤ 16 | –16 ≤ h ≤ 13, –30 ≤ k ≤ 30, –10 ≤ l ≤ 10 | –16 ≤ h ≤ 16, –30 ≤ k ≤ 30, –9 ≤ l ≤ 10 | –17 ≤ h ≤ 15, –19 ≤ k ≤ 19, –24 ≤ l ≤ 25 |
| Reflections collected | 26837 | 17850 | 36693 | 40384 | 36621 |
| Independent reflections | 3048 [R(int) = 0.0496] | 3326 [R(int) = 0.0363] | 5645 [R(int) = 0.1337] | 5641 [R(int) = 0.0864] | 8781 [R(int) = 0.0459] |
| Goodness-of-fit on F2 | 0.952 | 1.010 | 0.994 | 1.111 | 1.033 |
| Final R indices [I > 2σ(I)] | R1 = 0.0427, wR2 = 0.1223 | R1 = 0.0427, wR2 = 0.1239 | R1 = 0.0965, wR2 = 0.2362 | R1 = 0.1381, wR2 = 0.4131 | R1 = 0.0521, wR2 = 0.1643 |
| R indices (all data) | R1 = 0.0594, wR2 = 0.1532 | R1 = 0.0519, wR2 = 0.1391 | R1 = 0.1765, wR2 = 0.2829 | R1 = 0.1814, wR2 = 0.4400 | R1 = 0.0688, wR2 = 0.1844 |
| Largest diff. peak and hole [eÅ−3] | 0.401, −0.347 | 0.326, −0.209 | 0.451, −0.349 | 0.928, –0.908 | 0.484, –0.237 |
The Cambridge Structural Database does not contain information on the structure of the trolox anion with a COO– group and the structure of a protonated cytisine with an NH2+. In the ICs 2a, 2b, 2c, 2e, and 2 l, the difference in the C—O bond lengths of the carboxylate anion did not exceed 0.03 Å, which is typical for a deprotonated carboxyl group, whereas, the difference between average C—O and C O bond lengths in the carboxyl group was approximately 0.13 Å (Allen et al., 1987). Structures of the ICs 2b and 2l are illustrated in Fig. 2.
Fig. 2.
Structures of the ICs 2b and 2l (the disordered cytisine cation is not shown).
The data obtained confirmed that the dihydropyran cycle in the examined compounds was close to the half-chair form with an axial carboxylate group. In the ICs 2a and 2b, the C1 atom deviated from the plane more than atom C2 (0.49, 0.48 and 0.24, 0.20 Å, respectively). By contrast, in the ICs 2c, 2d, and 2 l, deviation of these atoms did not go beyond the range of 0.18–0.35 and 0.40–0.56 Å (0.31–0.32 and 0.40–0.42 Å in trolox polymorphs (Burton et al., 1985)). Another distinction was related to the orientation of the carboxylate group. In the ICs 2c, 2e, and 2l and trolox 1, polymorphs of the OCCO torsion angle did not exceed 6.1°, while in the ICs 2a and 2b it was equal to 22.8 and 26.8°, respectively. Hydrogen bonds are highly likely to be the cause of these differences. In compounds 2a and 2b, each oxygen atom of the carboxylate group plays a role as an acceptor of two hydrogen bonds, whereas in 2c, 2e, and 2l, one out of the two carboxylate oxygen atoms forms only one hydrogen bond. The conformations of heterocycles (envelope with a deviation in atom C35 by 0.76 Å and chair) in the cytisine cation of compound 2l were similar to those in a neutral cytosine (Freer et al., 1987). We note that the C—NH2+ bonds to 1.474(4) and 1.488(3) in the cytisine cation (ordered) of 2l compound were longer than the 1.445–1.461 Å found in the initial cytisine (Barlow and Johnson, 1989).
Numerous O—H⋯O and N—H⋯O hydrogen bonds were observed by crystallography, and the crystal 2c also had N—H…N hydrogen bonds. These hydrogen bonds formed the 3D-architecture of crystals 2a, 2c, and 2e and the 2D-architecture of crystals 2b and 2l.
In summary, data from IR-, UV-, NMR- and EPR-spectroscopy, mass-spectrometry, and X-ray and elemental analysis on the obtained compounds all strongly support the ionic structure of the trolox conjugates 2a–2q.
3.3. Water solubility and antioxidant activity of trolox ammonium salts 2a-2q
In publications on trolox, authors often use the term “water-soluble analogue of α-tocopherol” (Wu et al., 1991), which indicates better water solubility than α-tocopherol. According to this classification of drug solubility (Jouyban, 2010), trolox belongs to a group of very slightly soluble compounds (1.0–0.1 g/L), while α-tocopherol is a practically insoluble compound (<0.1 g/L) (Takagi et al., 2006). Thus, both of these compounds are poorly soluble in water.
The water solubility of the synthesised ICs 2a–2q (Table 2) varied over a wide concentration range (1–330 g/L), which was 1–3 orders of magnitude higher than trolox.
Table 2.
Water solubility and antioxidant activity of compounds 1, 2a–2q, 3g, and 3h.
| Entry | Water solubilitya, g/L | DPPH scavenging (bIC50, μM) | Entry | Water solubilitya, g/L | DPPH scavenging (bIC50, μM) |
|---|---|---|---|---|---|
| 1 | 0.1c | 58 | 2j | 46 | 55 |
| 2a | 2 | 62 | 2k | 310 | 68 |
| 2b | 120 | 60 | 2l | 59 | 65 |
| 2c | 35 | 65 | 2m | 2 | 63 |
| 2d | 67 | 63 | 2n | 3 | 63 |
| 2e | 150 | 54 | 2o | 330 | 61 |
| 2f | 78 | 68 | 2p | 7 | 65 |
| 2g | 1 | 76 | 2q | 4 | 57 |
| 2h | 2 | 58 | 3g | 0.2 | 63 |
| 2i | 170 | 66 | 3h | 0.3 | 69 |
Water solubility was determined by the gravimetric method (The State Pharmacopoeia of the Russian Federation, 2008).
Concentration at which there was a 50% loss in the initial level of DPPH free radical scavenging.
According to (Arellano et al., 2011), the solubility of trolox in water is <0.2 g/L.
The most soluble ICs were the ethanolamine derivatives 2e, 2k, and 2o and the most water soluble compound was the triethanolamine derivative 2o (3300-fold increase in solubility). The trolox ICs with nitroxyl radicals, 2g and 2h, had a 10 and 20–fold increase in solubility, respectively. Trolox conjugates with alkaloids, 2l, 2m, 2p, and 2q, exhibited a 20–590-fold increase in solubility relative to trolox. Alkaloids per se are highly active pharmacological compounds that are widely used in medicine; however, they are poorly soluble in water and are mainly used in their salt forms (e.g. hydrochlorides or hydrobromides) (Fattorusso and Taglialatela-Scafati, 2008). Increasing the solubility of compounds usually increases their bioavailability (Rath et al., 2013, Newa et al., 2008, Skyner et al., 2015). Solubility is one of the key physicochemical parameters of a new molecule that needs to be assessed and understood very early on in drug discovery and drug candidate selection process. Membrane permeability is another key property in the drug design pipeline, which also needs to be estimated. (Bennion et al., 2017, Kawabata et al., 2011). This will be the subject of further research for the compounds we are studying. Thus the synthesis of water-soluble trolox ion hybrids is a useful approach for the production of novel medicinal agents with synergistic effects relative to their fragment components.
And earlier study (Yushkova et al., 2013) found that the interaction of trolox with piperidine and pyrrolidine nitroxyl radicals resulted in the synthesis of spin-labelled amides of trolox 3g and 3h (Fig. 3), which are per se covalent hybrid systems. As expected, the covalent conjugates were significantly less water soluble than their ion analogues, 2g and 2h (see Table 2). Thus synthesis of the water soluble, spin-labelled ICs 2g and 2h may be a new way to create pharmacologically active compounds and imaging agents for magnetic resonance methods.
Fig. 3.
Structure of covalent trolox conjugates 3g and 3h.
To estimate the antioxidative activity of the ICs 2a–q and the covalent trolox conjugates 3g and 3h, spectrophotometric methods with the stable DPPH radical were applied (Kamkar et al., 2010). Quantitative analysis of hydrogen transfer from the antioxidants to DPPH was an easy, simple, and efficient way to estimate their antioxidative properties.
This technique is based on detecting a decrease in the absorption intensity of the stable DPPH radical at 517 nm in the absence or presence of an antioxidant. The inhibition rate of DPPH (P,%) was calculated according to the formula:
where A0 and A are the absorbance of DPPH at 517 nm before and after adding the sample of interest.
Antioxidative properties of the synthesised compounds are presented in the form of IC50 values (Table 2), which are comparable to similar data published previously (Koufaki et al., 2001). The whole series of ICs 2a–2q and the covalent conjugates 3g and 3h preserved their high antioxidant activity, which is typical of trolox compounds modified at position 2 of the chromane core when the phenol group is still present. It should be noted that antioxidant activity is an important characteristic of the biological activity of these compounds. The obtained trolox ICs 2a–2q contain various biologically active fragments that are highly likely to be useful in studies of other types of biological activity.
4. Conclusions
Interaction between the antioxidant trolox and ammonia, a series of alkylamines, amino derivatives of heterocyclic compounds (including nitroxyl radicals and alkaloids) led to the production of ICs in high yield. The structures of all synthesised compounds were confirmed by MS-spectrometry, IR, UV, EPR, 1H and 13C NMR spectroscopy. The five trolox-amine ICs were characterised by X-ray diffraction. The conditions needed to generate the pseudomolecular ions [(A−B+) + H]+ and [(A−B+) – H]− was optimised by atmospheric pressure ESI-MS. A significant increase in water solubility (1–3 orders of magnitude) was observed for all synthesised trolox-amine ICs, and their antioxidative activities remained high. We anticipate that the ICs containing two APIs will have new biological properties. These results support ICs as a promising general approach to create polyfunctional pharmacological salts with a broad range of solubility and bioavailability that could be used for complex therapies and the creation of target drug delivery systems.
The aim of this research was to promote further investigation into the biological activity of this novel group of water soluble hybrid antioxidants. From our point of view, expansion of this approach to other molecules with pronounced biological activity, such as lead molecules and current drugs, will simultaneously carry out two important tasks: to increase water solubility and to synthesise new polyfunctional pharmacological agents. Apart from this, the synthesis of water soluble spin-labelled compounds may also be used to create new types of imaging agents for applications in medicinal magnetic resonance tomography. Given the high potential for this methodology, it deserves significant attention and further development.
Acknowledgements
We thank the Collective Service Center of Siberian Branch of the Russian Academy of Sciences for spectral and structural measurements.
Acknowledgments
Conflict of interest
Authors declare no financial/commercial conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2017.10.008.
Contributor Information
Yuliya V. Yushkova, Email: yushkova@nioch.nsc.ru.
Elena I. Chernyak, Email: chernyak@nioch.nsc.ru.
Yuriy V. Gatilov, Email: gatilov@nioch.nsc.ru.
Vladimir G. Vasil'ev, Email: vgvasil@nioch.nsc.ru.
Sergey V. Morozov, Email: moroz@nioch.nsc.ru.
Igor A. Grigor'ev, Email: grig@nioch.nsc.ru.
Appendix A. Supplementary material
References
- Allen F.H., Kennard O., Watson D.G., Brammer L., Orpen A.G., Taylor R.J. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. Chem. Soc. Perkin Trans. II. 1987;12:1–19. [Google Scholar]
- Anbharasi V., Cao N., Feng S.S. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol succinate and folic acid as a prodrug for targeted chemotherapy. J. Biomed. Mat. Res. A. 2010;94(3):730–743. doi: 10.1002/jbm.a.32734. [DOI] [PubMed] [Google Scholar]
- Arellano J.B., Li H., González-Pérez S., Gutiérrez J., Melo T.B., Vacha F., Naqvi K.R. Trolox, a water-soluble analogue of α-tocopherol, photoprotects the surface-exposed regions of the photosystem II reaction center in vitro. Is this physiologically relevant? Biochemistry. 2011;50(39):8291–8301. doi: 10.1021/bi201195u. [DOI] [PubMed] [Google Scholar]
- Arya P., Alibhai N., Qin H., Burton G.W. Design and synthesis of analogs of vitamin E: antiproliferative activity against human breast adenocarcinoma cells. Bioorg. Med. Chem. Lett. 1998;8(18):2433–2438. doi: 10.1016/s0960-894x(98)00438-7. [DOI] [PubMed] [Google Scholar]
- Barlow R.B., Johnson O. Relations between structure and nicotine-like activity: X-ray crystal structure analysis of (-)-cytisine and (-)-lobeline hydrochloride and a comparison with (-)-nicotine and other nicotine-like compounds. Br. J. Pharmacol. 1989;98(3):799–808. doi: 10.1111/j.1476-5381.1989.tb14608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion B.J., Be N.A., McNerney M.W., Lao V., Carlson E.M., Valdez C.A., Malfatti M.A., Enright H.A., Nguyen T.H., Lightstone F.C., Carpenter T.S. Predicting a drug’s membrane permeability: a computational model validated with in vitro permeability assay data. J. Phys. Chem. B. 2017;121:5228–5237. doi: 10.1021/acs.jpcb.7b02914. [DOI] [PubMed] [Google Scholar]
- Berge S.M., Bighley L.D., Monkhouse D.R. Pharmaceutical salts. J. Pharm. Sci. 1977;66(1):1–19. doi: 10.1002/jps.2600660104. [DOI] [PubMed] [Google Scholar]
- Błaszczyk A., Skolimowski J. Apoptosis and cytotoxicity caused by ethoxyquin salts in human lymphocytes in vitro. Food Chem. 2007;105(3):1159–1163. [Google Scholar]
- Burton G.W., Doba T., Gabe E.J., Hughes L., Lee F.L., Prasad L., Ingold K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985;107(24):7053–7065. [Google Scholar]
- Cech N.B., Enke C.G. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom. Rev. 2001;20(6):362–387. doi: 10.1002/mas.10008. [DOI] [PubMed] [Google Scholar]
- Christiaans J.A.M., Timmerman H. Cardiovascular hybrid drugs: combination of more than one pharmacological property in one single molecule. Eur. J. Pharm. Sci. 1996;4(1):1–22. [Google Scholar]
- Contreras J.M., Sipp W. In: The Practice of Medicinal Chemistry. Wermuth C.G., editor. Prestwick Chemical Inc.; Illkirch: 2008. Homo and heterodimer ligands: the twin drug approach; pp. 380–414. [Google Scholar]
- Egorova K.S., Seitkalieva M.M., Posvyatenko A.V., Khrustalev V.N., Ananikov V.P. Cytotoxic activity of salicylic acid-containing drug models with ionic and covalent binding. ACS Med. Chem. Lett. 2015;6(11):1099–1104. doi: 10.1021/acsmedchemlett.5b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorusso E., Taglialatela-Scafati O., editors. Modern Alkaloids. Structure Isolation, Synthesis and Biology. Wiley-VCH; Weinheim: 2008. [Google Scholar]
- Freer A.A., Robins D.J., Sheldrake G.N. Structures of (-)-cytisine and (-)-N-methylcytisine: tricyclic quinolizidine alkaloids. Acta Crystallogr. Sec. C: Cryst. Struc. Commun. 1987;C43:1119–1122. [Google Scholar]
- Gorecki M., Suszczyńska A., Woźnica M., Baj A., Wolniak M., Cyrański M.K., Witkowskib S., Frelek J. Chromane helicity rule – scope and challenges based on an ECD study of various trolox derivatives. Org. Biomol. Chem. 2014;12(14):2235–2254. doi: 10.1039/c3ob42376j. [DOI] [PubMed] [Google Scholar]
- Goto Y., Watanabe N., Kogawa N., Tsuchiya M., Takahashi O., Uchi H., Furue M., Hayashi H. CX-659S: a novel diaminouracil derivative that has antioxidative and acute anti-inflammatory activities. Eur. J. Pharmacol. 2002;438(3):189–196. doi: 10.1016/s0014-2999(02)01340-7. [DOI] [PubMed] [Google Scholar]
- Grigor’ev I.A., Tkacheva N.I., Morozov S.V. Conjugates of natural compounds with nitroxyl radicals as a basis for creation of pharmacological agents of new generation. Curr. Med. Chem. 2014;21(24):2839–2852. doi: 10.2174/0929867321666140304153104. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou-Litina D., Magoulas G.E., Bariamis S.E., Drainas D., Avgoustakis K., Papaioannou D. Does conjugation of antioxidants improve their antioxidative/anti-inflammatory potential? Bioorg. Med. Chem. 2010;18(23):8204–8217. doi: 10.1016/j.bmc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Jouyban A. CRS Press; New York: 2010. Handbook of Solubility Data for Pharmaceuticals. [Google Scholar]
- Kalariya P.D., Raju B., Borkar R.M., Namdev D., Gananadhamu S., Nandekar P.P., Sangamwar A.T., Srinivas R. Characterization of forced degradation products of ketorolac tromethamine using LC/ESI/Q/TOF/MS/MS and in silico toxicity prediction. J. Mass Spectrom. 2014;49(5):380–391. doi: 10.1002/jms.3351. [DOI] [PubMed] [Google Scholar]
- Kalinowski, H-O., Berger, S., Braun, S. 1998. Carbon-C13 NMR Spectroscopy (Ch. 3). John Wiley & Sons, Chichester.
- Kamkar A., Javan A.J., Asadi F., Kamalinejad M. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food. Chem. Toxicol. 2010;48(7):1796–1800. doi: 10.1016/j.fct.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Kawabata Y., Wada K., Nakatani M., Yamada S., Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharmaceut. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Kosheleva N.V., Chernyak E.I., Polienko Yu.F., Morozov S.V., Grigor'ev I.A. Use of the Mannich reaction to synthesize spin-labeled derivatives of the natural flavonoid dihydroquercetin. Chem. Nat. Comp. 2014;50(2):261–265. [Google Scholar]
- Koufaki M., Calogeropoulou T., Detsi A., Roditis A., Kourounakis A.P., Papazafiri P., Tsiakitzis K., Gaitanaki C., Beis I., Kourounakis P.N. Novel potent inhibitors of lipid peroxidation with protective effects against reperfusion arrhythmias. J. Med. Chem. 2001;44(24):4300–4303. doi: 10.1021/jm010962w. [DOI] [PubMed] [Google Scholar]
- Koufaki M., Calogeropoulou T., Rekka E., Chryselis M., Papazafiri P., Gaitanaki C., Makriyannis A. Bifunctional agents for reperfusion arrhythmias: novel hybrid vitamin E/class I antiarrhythmics. Bioorg. Med. Chem. 2003;11(23):5209–5219. doi: 10.1016/j.bmc.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Koufaki M., Detsi A., Theodorou E., Kiziridi C., Calogeropoulou T., Vassilopoulos A., Kourounakis A.P., Rekka E., Kourounakis P.N., Gaitanaki C., Papazafiri P. Synthesis of chroman analogues of lipoic acid and evaluation of their activity against reperfusion arrhythmias. Bioorg. Med. Chem. 2004;12:4835–4841. doi: 10.1016/j.bmc.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Koufaki M., Kiziridi Ch., Alexi X., Alexi M.N. Design and synthesis of novel neuroprotective 1,2-dithiolane/chroman hybrids. Bioorg. Med. Chem. 2009;17(17):6432–6441. doi: 10.1016/j.bmc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Lopez G.V., Batthyany C., Blanco F., Botti H., Trostchansky A., Migliaro E., Radi R., Gonzalez M., Cerecettoa H., Rubbob H. Design, synthesis, and biological characterization of potential antiatherogenic nitric oxide releasing tocopherol analogs. Bioorg. Med. Chem. 2005;13(20):5787–5796. doi: 10.1016/j.bmc.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Metodiewa D., Skolimowski J., Karolczak S. Tempace and troxyl-novel synthesized 2,2,6,6-tetramethylpiperidine derivatives as antioxidants and radioprotectors. Biochem. Mol. Biol. Int. 1996;40(6):1211–1219. doi: 10.1080/15216549600201853. [DOI] [PubMed] [Google Scholar]
- Nakagawa-Goto K., Yamada K., Nakamura S., Chen T.H., Chiang P.C., Bastow K.F., Wang S.C., Spohn B., Hung M.C., Lee F.Yu., Lee F.C., Lee K.H. Antitumor agents. 258. Syntheses and evaluation of dietary antioxidant – taxoid conjugates as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2007;17(18):5204–5209. doi: 10.1016/j.bmcl.2007.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepovimova E., Korabecny J., Dolezal R., Babkova K., Ondrejicek A., Jun D., Sepsova V., Horova A., Hrabinova M., Soukup O., Bukum N., Jost P., Muckova L., Kassa J., Malinak D., Andrs M., Kuca K. Tacrine-trolox hybrids: a novel class of centrally active, nonhepatotoxic multi-target-directed ligands exerting anticholinesterase and antioxidant activities with low in vivo toxicity. J. Med. Chem. 2015;58(22):8985–9003. doi: 10.1021/acs.jmedchem.5b01325. [DOI] [PubMed] [Google Scholar]
- Newa M.N., Bhandari K.H., Kim J.O., Im J.S., Kim J.A., Yoo B.K., Woo J.S., Choi H.J., Yong C.S. Enhancement of solubility, dissolution and bioavailability of ibuprofen in solid dispersion systems. Chem. Pharm. Bul. 2008;56(4):569–574. doi: 10.1248/cpb.56.569. [DOI] [PubMed] [Google Scholar]
- Rath K.S., McCann G.A., Cohn D.E., Rivera B.K., Kuppusamy P., Selvendiran K. Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs. J. Ovarian Res. 2013;6(1):35. doi: 10.1186/1757-2215-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.W., Cort W.M., Harley H., Parrish D.R., Saucy G. 6-Hydroxychroman-2-carboxylic acids: Novel antioxidants. J. Am. Oil Chem. Soc. 1974;51:200–203. [Google Scholar]
- Šebestik J., Marques S.M., Fale P.L., Santos S., Arduino D.M., Cardoso S.M., Oliveira C.R., Serralheiro M.L.M., Santos M.A. Bifunctional phenolic-choline conjugates as anti-oxidants and acetylcholinesterase inhibitors. J. Enzyme. Inhib. Med. Chem. 2011;26(4):485–497. doi: 10.3109/14756366.2010.529806. [DOI] [PubMed] [Google Scholar]
- Serajuddin T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007;59(7):603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Skyner R.E., McDonagh J.L., Groom C.R., Mourika T., Mitchell J.B.O. A review of methods for the calculation of solution free energies and the modeling of systems in solution. Phys. Chem. Chem. Phys. 2015;17:6174–6191. doi: 10.1039/c5cp00288e. [DOI] [PubMed] [Google Scholar]
- Socrales, G., 1994. Infrared Characteristic Group Frequencies: Tables and Charts. Wiley, New York.
- Sheldrick, G.M., 2002. SADABS. Madison, Bruker AXS Inc., Wisconsin, USA.
- Stahl P.H., Wermuth C.G. Wiley-VCH; Weinheim: 2002. Handbook of Pharmaceutical Salts: Properties, Selection, and Use. [Google Scholar]
- State pharmacopeia of the Russian Federation, 2008. Federal Scientific Center for Evaluation of Medical Products, Moscow.
- Stvolinsky S.L., Bulygina E.R., Fedorova T.N., Meguro K., Sato T., Tyulina O.V., Abe H., Boldyrev A.A. Biological activity of novel synthetic derivatives of carnosine. Cell Mol. Neurobiol. 2010;30(3):395–404. doi: 10.1007/s10571-009-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Ramachandran Ch., Bermejo M., Yamashita S., Yu L.X., Amidon G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharmaceutics. 2006;3(6):631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- Um S.J., Kwon Y.J., Han H.S., Park S.H., Park M.S., Rho Y.S., Sin H.S. Synthesis and biological activity of novel retinamide and retinoate derivatives. Chem. Pharm. Bull. 2004;52(5):501–506. doi: 10.1248/cpb.52.501. [DOI] [PubMed] [Google Scholar]
- Wang G., Cole R.B. Effects of solvent and counterion on ion pairing and observed charge states of diquaternary ammonium salts in electrospray ionization mass spectrometry. J. Am. Soc. Mass. Spectrom. 1996;7(10):1050–1058. doi: 10.1016/1044-0305(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Wu T.W., Hashimoto N., Au J.X., Wu J., Micle D.A., Carey D. Trolox protects rat hepatocytes against oxyradical damage and the ischemic rat liver from reperfusion injury. Hepatology. 1991;13(3):575–580. [PubMed] [Google Scholar]
- Xie S.S., Lan J.S., Wang X.B., Jiang N., Dong G., Li Z.R., Wang K.D.G., Guo P.P., Kong L.Y. Multifunctional tacrine-trolox hybrids for the treatment of Alzheimer's disease with cholinergic, antioxidant, neuroprotective and hepatoprotective properties. Eur. J. Med. Chem. 2015;93:42–50. doi: 10.1016/j.ejmech.2015.01.058. [DOI] [PubMed] [Google Scholar]
- Yushkova Yu.V., Chernyak E.I., Morozov S.V., Grigor'ev I.A. Antioxidants based on covalently and ionically bound trolox conjugates. Chem. Nat. Comp. 2015;51(6):1070–1073. [Google Scholar]
- Yushkova Yu.V., Chernyak E.I., Polienko Yu.F., Gatilov Yu.V., Morozov S.V., Grigor'ev I.A. Synthesis of spin-labeled amides of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) Chem. Nat. Compd. 2013;49(2):253–257. [Google Scholar]
- Zakharova O.D., Frolova T.S., Yushkova Y.V., Chernyak E.I., Pokrovsky A.G., Pokrovsky M.A., Morozov S.V., Sinitsina O.I., Grigor'ev I.A., Nevinsky G.A. Antioxidant and antitumor activity of trolox, trolox succinate, and α-tocopheryl succinate conjugates with nitroxides. Eur. J. Med. Chem. 2016;122:127–137. doi: 10.1016/j.ejmech.2016.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.