Abstract
Two novel quinazoline derivatives named as; 3-[(4-hydroxy-3-methoxy-benzylidene)-amino]-2-p-tolyl-3H-quinazolin-4-one (5) and 2-p-Tolyl-3-[3,4,5-trimethoxy-benzylidene-amino]-3H-quinazolin-4-one (6) in addition to one acetamide derivative named as 2-(2-Hydroxycarbonylphenylamino)-N-(4-aminosulphonylphenyl) 11 were synthesized, and evaluated for their anti-ulcerogenic & Anti-Ulcerative colitis activities.
All of the three compounds showed curative activity against acetic acid induced ulcer model at a dose of 50 mg/kg, they produced 65%, 85% & 57.74% curative ratio for compounds 5, 6 & 11 respectively. The effect of the tested compounds 5, 6 & 11 at dose 50 mg/kg were significantly (P < 0.01) more effective than dexamesathone (0.1 mg/kg) in reducing all parameters.
Compounds showed curative activity of for peptic ulcer (induced by absolute alcohol (at a dose of 50 mg/kg, it produced Curative of control ulcer 56.00%, 61.70% & 87.1% for compounds 5, 6 & 11 respectively at dose 50 mg/kg, while the standard drug (Omeprazole 20 mg/kg) produced 33.3%. In both tests, the activity of our target compounds were higher than the standard drugs used for treatment of peptic ulcer and ulcerative colitis. No side effects were reported on liver and kidney functions upon prolonged oral administration of this compounds.
Keywords: Liver functions, Ulcers, Dexamesathone, Quinazoline, Acetamide, Ulcerative colitis
1. Introduction
Ulcers in the gastrointestinal tract could be divided into two common types according to location; ulcerative colitis (lower) and peptic ulcer (upper). Ulcerative colitis (UC) is an inflammatory bowel disease that primarily affecting the colonic mucosa. In its most limited form it may be restricted to the distal rectum, while in its most extended form, the entire colon is involved. UC can occur in both sexes and in any age group but most often begins in people between 15 and 30 years of age. The exact causes of UC are still not clear but different factors have been postulated as possible etiologic agents. They are genetic factors, infective agents, immunological basis, smoking, medications and pathological factors (Awaad et al., 2013c, Nagao-Kitamoto and Kamada, 2017, Ruan et al., 2017).
Medicinal chemistry plays an important role in development of drug for cure; maintain and improve health of human being. It is also equally important to design chemical entities to prevent the growth of micro-organism, which come in contact with human being in day-to-day life (Katke et al., 2011, Kim et al., 2017).
Different heterocyclic compounds are made to synthesize by large number of efforts and their derivatives were found to possess antitumor, anti-diabetic, antimicrobial, anticonvulsant and anthelmintic activities. Quinazoline containing compounds have diverse medicinal applications. One of these many capabilities is their analgesic and anti-inflammatory activity (Ghorab, 2017, Park et al., 2016, Patel et al., 2012).
Furthermore, several new ethyl 1-methyl-5-(substituted 3,4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates 2, substituted at 2 and, alternatively at, 6, 7 or 8 positions of the quinazolinone nucleus, were synthesized. The compounds were screened for their analgesic and anti-inflammatory activities, acute toxicity and ulcerogenic effect. Substitution in the benzene moiety of the quinazolinone ring did not show any advantage for the analgesic activity, whereas it improved in some cases the anti-inflammatory activity. Some compounds showed appreciable anti-inflammatory activity and, at the same time, very low ulcerogenic index (Junek et al., 2009, Maggio et al., 2001).
Acetamide and various N-substituted chloroacetamide derivatives showed many biological activities such as antibacterial, antifungal (Katke et al., 2011), antioxidant (Autore et al., 2010), anti-cancer (Ghorab et al., 2011) and anthelmintic (Sawant and Kawade, 2011).
In this regard we thought to synthesize some novel Schiff bases from the quinazoline backbone and our target acetamides backbone to test their ulcerative colitis activities.
2. Experimental
2.1. Synthesis of quinazoline derivatives
2.1.1. Synthesis of 2-(4-Methylbenzamido)benzoic acid (2)
4-Methylbenzoyl chloride (8.50 g, 0.05 mol) was added dropwise to a stirred solution of anthranilic (6.85 g, 0.05 mol) and triethylamine (2 ml) in dichloromethane (70 ml) and the reaction mixture was stirred at room temperature for 2 h. The separated solid was filtered, washed several times with water, dried and crystallized from ethanol.
2.1.2. Synthesis of 2-p-Tolyl-4H-3,1-benzoxazin-4-one (3)
A mixture of 2-(4-methylbenzamido)benzoic acid (7.65 g, 0.03 mol) and acetic anhydride (7.5 g, 0.07 mol) was heated under reflux for 5 h. The solvent was removed under reduced pressure. The residue was triturated with water. The separated solid was collected by filtration, washed with water, dried and crystallized from ethanol.
2.1.3. Synthesis of 2-(4-Methylphenyl)-3-amino-3H-quinazolin-4-one (4)
The title compound was prepared by three methods:
Method A: A mixture of 2-(4-methylphenyl)-4H-3,1-benzoxazin-4-one 3 (1.18 g, 0.005 mol) and 98% hydrazine hydrate (0.6 g, 0.018 mol), in ethanol (10 ml) was heated under reflux for 10 h. The reaction mixture was cooled and the separated solid was filtered and dried. The solid obtained was separated on a column using chloroform as eluent to afford compound 6 in 15% yield.
Method B: A mixture of the benzoxazin-4-one 3 (1.18 g, 0.003 mol) and 98% hydrazine hydrate (0.6 g, 0.018 mol), in n-butanol (10 ml) was heated under reflux for 10 h. The reaction mixture was cooled and the separated solid was filtered and dried. The solid obtained was separated on a column using chloroform as eluent to afford compound 6 in 30% yield.
Method C: A mixture of benzoxazin-4-one 3 (1.18 g, 0.003 mol) and 98% hydrazine hydrate (0.6 g, 0.018 mol) was heated under reflux for 3 h. On cooling, the separated solid was filtered, washed with water and crystallized from ethanol.
The product obtained by these methods has the same physical constants.
2.1.4. Synthesis of 3-Substituted (arylideneamino)-2-p-tolyl-3H-quinazolin-4-one (5 & 6)
General procedure: A mixture of 2-(4-methylphenyl)-3-amino-3H-quinazolin-4-one 4 (2.51 g, 0.01 mol) and the appropriate aldehyde (0.01 mol) in acetic acid (20 ml) was heated under reflux for 2 h. On cooling, the separated solid was filtered, washed with water and crystallized from acetic acid to afford compounds 5 and 6.
5: Yield, 85%; m.p. 192–194 °C; IR, υ (cm−1): 3385 (OH), 3045 (Ar—CH), 2941 (CH), 1668 (C O). 1H NMR (CDCl3) ppm: δ 2.35 (s, 3H, CH3), 3.69(s, 3H, OCH3), 5.29 (s, 1H, OH), 7.30–7.76 (m, 11H, Ar—H), 8.36 (s, 1H, N CH). 13C NMR: 24.8 (CH3), 55.8 (OCH3), 114.9, 117.5, 120.2, 122.5, 122.9, 125.1, 126.3, 127.4, 128.0, 129.2, 133.8, 139.5, 143.7, 148.1, 151.8, 152.3, 159.8 (Ar—C), 167.2 (CO). MS m/z (Rel. Int.) 385 (M+, 100). Anal. (C23H19N3O3, 385.41) C, 71.67 (71.44); H, 4.97 (5.15); N, 10.90 (10.72).
6: Yield, 67%; m.p. 236–238 °C; IR, υ (cm−1): 3047 (Ar—CH), 2946 (CH), 1665 (C O). 1H NMR (CDCl3): δ 2.34 (s, 3H, CH3), 3.69 (s, 9H, 3OCH3), 7.30–7.76 (m, 10H, Ar—H), 8.39 (s, 1H, N CH). 13C NMR: 24.5 (CH3), 56.1 (2OCH3), 55.6 (OCH3), 108.6, 120.4, 122.9, 125.6, 126.2, 127.5, 128.2, 128.9, 129.4, 133.7, 139.1, 141.7, 143.5, 150.3, 150.9, 152.2, 159.5 (Ar—C), 164.7 (CO). MS m/z (Rel. Int.) 429 (M+, 100). Anal. (C25H23N3O4, 429.46) C, 69.92 (70.11); H, 5.40 (4.66); N, 9.78 (9.64).
2.2. Synthesis of acetamide derivatives
2.2.1. Synthesis of 2-Chloro-N-(4-aminosulphonylphenyl) acetamide (9)
2-Chloroacetyl chloride (1.12 g, 0.01 mol) was added drop wise with vigorous stirring to a cold suspension of sulfanilamide (1.72 g, 0.01 mol) in 10 ml dichloromethane containing 2 drops triethylamine. Stirring was continued for 1 h and the separated solid was filtered, washed with ether, dried and crystallized from aqueous-ethanol.
2.2.2. Synthesis of 2-(2-Hydroxycarbonylphenylamino)-N-(4-aminosulphonylphenyl)acetamide (11)
An equimolar proportion of both 2-chloro-N-(4-aminosulphonylphenyl)acetamide (9) (0.248 g, 0.001 mol) and anthranilic was boiled in ethanol containing catalytic amount of triethylamine in presence of potassium iodide (0.32 g) for 5 h. On cooling, the separated solid was filtered, washed with water, dried and crystallized from ethanol to afford compound 5 in Yield of 52%; m.p. 217–219 °C; 1H NMR (DMSOd6): 2.52 (s, 1H, NH, D2O exchange.), 3.26 (s, 2H, CH2), 4.21 (s, 2H, NH2, D2O exchange.), 7.30 (s, 1H, NH, D2O exchange.), 7.67–7.92 (m, 8H, Ar—H), 10.55 (s, 1H, OH, D2O exchange). 13C NMR: δ 54.2 (CH2), 108.1, 114.5, 118.3, 122.7, 126.7, 132.1, 133.8, 136.4, 141.4, 150.6 (Ar—C), 167.6, 169.0 (2C O). MS (EI): m/z [M+, %]. Anal. (C15H15N3O5S) C, H, N.
2.3. Pharmacological activities
2.3.1. Animals
Swiss albino mice of both sex (26–30 g) and male Wistar rats (180–200 g) were purchased from animal house of King Saud University, KSA. Animals were housed in standard polypropylene cages with wire mesh top and maintained under standard conditions (temperature 23 ± 1.0 °C, humidity 55 ± 10%, 12 h light/12 h dark cycle). They fed with a standard pellet diet with water ad libitum and were allowed to adapt to the laboratory environment for one week before experimentation.
2.3.2. Determination of median lethal dose (LD50)
The oral median lethal dose (LD50) of the target compound was determined as described by Lorke (1983). Swiss albino mice in groups of six, received one of 50, 100, 500, or 1000 mg/kg doses of the target compound. Control animals were received the vehicle and kept under the same conditions. Signs of acute toxicity and number of deaths per dose within 24 h were recorded.
2.3.3. Antiulcerogenic activity
Evaluation of the anti-ulcerogenic activity was carried out using absolute ethanol-induced ulcer model as described by Bighetti et al. (2005). Eighty sex male Wistar rats were divided into 5 groups each of 6 rats. Rats of groups 1 and 2 received the vehicle (5 mL/kg) and served as normal control and ulcer control groups. Group 3 administered dexamesathone at dose 0.1 mg/kg and served as Reference Drug group. Induction of peptic ulcer was carried out using absolute ethanol-induced ulcer method described by El-Meligy et al. (2017) animals received an oral dose of absolute ethanol (1 ml/200 g). One hour after inducing ulcer, groups 4–6 received orally the first dose of each synthesized compounds (5, 6 & 11) separately. Rest of the doses were given to the animal orally once daily for 3 consecutive days. At the end of the excrement animals were sacrificed, by ether inhalation, the stomachs were rapidly removed, opened along their greater curvature and gently rinsed under running tap water.
Lesion scores were quantified by the scoring system (0−5) (El-Meligy et al., 2017). Ulcer indices (mm) were calculated as the sum of the total length of long ulcers and petechial lesions in each group of rats divided by its number. The percent of protection was determined according to the formula: % Protection of control ulcer = Control UI – Test UI/Control UI × 100.
2.3.4. Anti-ulcerative colitis activity
Eighty sex male Wistar rats were divided into 5 groups each of 6 rats. Rats of groups 1 and 2 received the vehicle (5 mL/kg) and served as normal control and ulcer control groups. Group 3 administered dexamethasone (0.1 mg/kg) and served as Reference Drug group. Ulcerative colitis was induced by slowly infusion of 2 mL (4%, v/v) acetic acid in saline into the colon through the catheter (El-Meligy et al., 2011). Two hours after inducing colitis, groups 4–6 received orally the first dose of each synthesized compounds (5, 6 & 11) separately. Rest of the doses was given to the animal orally once daily for 5 consecutive days. At the end of the excrement animals were sacrificed, colonic segments (8 cm in length and 3 cm proximal to the anus) were excised, opened and were used for macroscopic scoring (Awaad et al., 2013b).
2.3.4.1. Assessment of colonic lesions
The colon specimens were weighted and wet weight/length ratio was calculated for all the rats. The specimens were examined under a dissecting microscope and the mucosal lesions were quantified by the scoring system (0−5) given by (Awaad et al., 2013a) after some modifications.
The lesion scores were: 0 = no damage, 1 = Local edema and inflammation without ulcers; 2 = One ulcer without inflammation; 3 = one to two ulcers with inflammation & lesion diameter <1 cm; 4 = More than two ulcers with lesion diameter 1–2 cm; 5 = Sever ulceration with lesion diameter >2 cm.
Ulcer area was measured using plane glass square. Each cell on the glass square was 1 mm2 in area and the number of cells was counted and the ulcer area was determined for each colon. Ulcer index was measured by summing the lesion score and the ulcer area for each colon specimen (Awaad et al., 2013b).
2.3.5. Effect on liver and kidney functions
Male Wister rats were divided into 2 equal groups each of 10 rats. The 1st group was left as a control and administrated the vehicle orally, while the 2nd group was orally administrated the synthesized compound in a dose of 100 mg/kg for 15 days. After the examination period, 6 h after the last dose blood samples were collected from the orbital plexus of rats. Samples were left to clot at room temperature for 30 min then centrifuged at 1000 rpm for 20 min.
The collected sera were used for determination of the activity of both (AST) aspirate aminotransferase and (ALT) alanine aminotransferase as liver markers. In addition, levels of blood urea, serum creatinine were also estimated as kidney markers (Awaad et al., 2013b).
2.3.5.1. Statistical analysis
All values were expressed as mean ± S.D. Statistical analysis was done by using SPSS 10. Statistical significance of differences between two means was assessed by unpaired Student's’ test. Differences at p < 0.05, 0.01, and 0.001 were considered statistically significant.
3. Results and discussion
3.1. Synthetic compounds
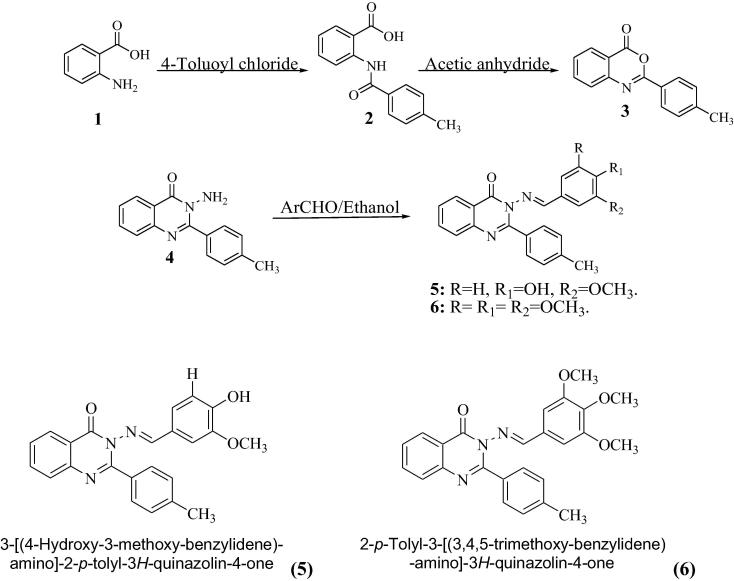
Anthranilic acid 1 was reacted with 4-toluoyl chloride in dichloromethane containing catalytic amount of triethylamine at room temperature. The solvent was evaporated under reduced pressure. The separated acid amide 2 solid product was then filtered, washed with water and crystallized from ethanol. The later amide derivative was then refluxed with acetic anhydride to yield the benzoxazone derivative 3 which was treated boiled with hydrazine hydrate in presence of absolute ethanol to afford the substituted 3-amino-3H-quinazolin-4-one derivative 4. The amino functionality was then treated with two selected aldehyde derivatives namely vanillin and 3,4,5-trimethoxy-benzaldehyde to afford the respective arylidene derivatives named as; 3-[(4-hydroxy-3-methoxy-benzylidene)-amino]-2-p-tolyl-3H-quinazolin-4-one (5) and 2-p-Tolyl-3-[3,4,5-trimethoxy-benzylidene-amino]-3H-quinazolin-4-one (6) (see Scheme 1).
Scheme 1.
Synthesis of quinazoline derivatives.
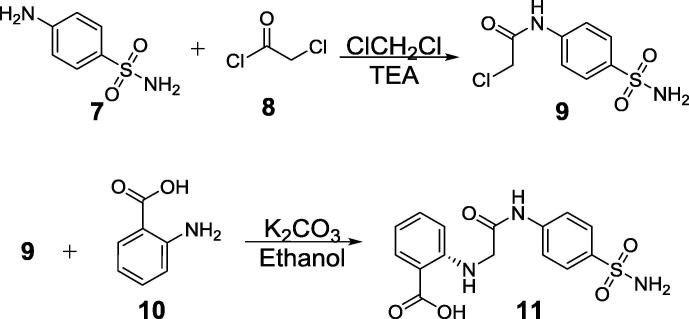
2-Chloroacetyl chloride reacted to sulfanilamide at room temperature in 10 ml dichloromethane containing 2 drops triethylamine. Stirring was continued for 1 h and the separated solid was filtered, washed with ether, dried and crystallized from aqueous-ethanol. 2-Chloro-N-(4-aminosulphonylphenyl)acetamide was obtained and boiled with equimolar proportion anthranilic in ethanol containing catalytic amount of triethylamine in presence of potassium iodide. On cooling, crystallized from ethanol we got compound (11) which was identified as; 2-(2-Hydroxycarbonylphenylamino)-N-(4-aminosulphonylphenyl)acetamide (see Scheme 2).
Scheme 2.
Synthesis of acetamide derivative.
3.2. Pharmacological activities
3.2.1. Determination of median lethal dose (LD50)
The target compounds (5, 6 & 11) in doses up to 1000 mg/kg did not produce any behavioral changes and mortality in mice. Therefore, it can be categorized as highly safe since substances possessing LD50 higher than 5000 mg/kg are nontoxic (Soliman et al., 2012).
3.2.2. Anti-ulcerogenic activity
The present results showed that the target compounds possessed a potent anti-ulcerogenic activity. It produced percent Curative of control ulcer 56.00%, 61.70% & 87.1% for compounds 5, 6 & 11 respectively at dose 50 mg/kg, while the standard drug (Omeprazole 20 mg/kg) produced 33.3% (Table 1). The target compound was significantly more effective than the standard in reducing ulcer index and ulcer score.
Table 1.
Anti-ulcerogenic effect of synthesized drugs on absolute alcohol-induced ulcer in rats.
| Groups | Dose mg/kg | Score | No of ulcers | Ulcer index | % Curative ratio |
|---|---|---|---|---|---|
| Control ulcer | – | 3.40 | 9.20 ± 0.84 | 17.00 ± 0.71 | 0.0 |
| Omeprazole | 20 | 3.20 | 7.60* ± 3.05 | 8.00* ± 2.24 | 33.30 |
| Compound 5 | 50 | 2.20 | 7.60** ± 2.07 | 6.60* ± 3.21 | 56.00 |
| Compound 6 | 50 | 2.00 | 4.80*** ± 2.05 | 4.60** ± 3.78 | 61.70 |
| Compound 11 | 50 | 1.20 | 3.00*@ ± 0.71 | 2.2*@ ± 0.76 | 87.1 |
Data are expressed as mean ± SD, n = 6.
Significantly different from control ulcer at p < 0.01.
Significantly different from control ulcer at p < 0.05.
Significantly different from omeprazole at p < 0.01
3.2.2.1. Anti-ulcerative colitis
The model of acetic acid induced colitis shares many of the histologic features of ulcerative colitis in human beings including mucosal edema and sub-mucosal ulceration. In rats of control group, no abnormal changes were observed suggesting that handling procedure had no interference with the experimental outputs. Macroscopic damage parameters of the colon of control colitis rats, 2 days after rectal infusion of acetic acid revealed dark brown lesions, mucosal hyperemia, edema, erosion, and ulceration. Control colitis rats showed lesion score, ulcer area and ulcer index values of 4.7 ± 0.89, 179.50 ± 1.21 mm2 and 183.5 ± 2.26, respectively (Table 2). The inflammatory changes of the intestinal tract were associated with a significant increase of wet weight/length of the colon specimens as an indicator of inflammation.
Table 2.
Effect of synthesized compounds on acetic acid induced-colitis in rats.
| Groups | Lesion score (0–5) | Ulcer area (mm2) | Ulcer index | Wet W/L (g/8 cm) | % Curative |
|---|---|---|---|---|---|
| Normal control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.41 ± 0.05 | – |
| Control colitis | 4.00 ± 0.89 | 179.50 ± 1.21 | 183.5 ± 2.26 | 0.83 ± 0.07 | – |
| Dexamethasone (0.1 mg/kg) | 2.93* ± 0.75 | 89.20* ± 1.47 | 93.00* ± 2.00 | 0.56* ± 0.05 | 58.8 |
| Compound 5 (50 mg/kg) | 2.33* ± 0.52 | 87.17*@ ± 1.17 | 89.50*@ ± 1.22 | 0.72*@ ± 0.03 | 65 |
| Compound 6 (50 mg/kg) | 2.17* ± 0.75 | 68.00*@ ± 1.55 | 70.17*@ ± 1.72 | 0.62* ± 0.04 | 85 |
| Target compound 11 (50 mg/kg) | 2.50* ± 0.52 | 15.31*@ ± 1.51 | 17.00*@ ± 1.90 | 0.81*@ ± 0.07 | 57.74 |
Significantly different from control colitis at p < 0.01.
Significantly different from dexamesathone at p < 0.01.
The Curative effect of the tested compounds 5, 6 & 11 at dose 50 mg/kg on acetic acid-induced colitis in rats is shown in table 1.The tested compounds 5, 6 & 11 administrated orally to rats showed a potent anti-ulcerative colitis activity. The extract at all tested doses induced a significant (p < 0.01) decrease in ulcer score, ulcer area, ulcer index and weight/length of the colon specimens.
The percent Curative ratio of control colitis were 65%, 85% & 57.74% for compounds 5, 6 & 11 respectively; however the percent Curative for dexamesathone (0.1 mg/kg) was 58.8%.
The effect of the tested compounds 5, 6 at dose 50 mg/kg were significantly (P < 0.01) more effective than dexamesathone (0.1 mg/kg) in reducing all parameters.
3.2.3. Effect on liver and kidney functions
Both liver and kidney functions were not affected as there is no significant difference between control and test group in all experiments, at the 0.05 level of probability (Table 3). These results showed that, the compounds didn’t reveal hepatotoxic manifestation. In addition, no apparent nephrotoxic manifestations were recorded. So all compounds are safe for use.
Table 3.
Effect of synthesized compounds on liver and kidney functions of rats.
| Groups | ALT(U/l) | AST(U/l) | Blood Urea (mg/dl) | Creatinine (mg/dl) |
|---|---|---|---|---|
| Control | 42.49 ± 0.37 | 60.77 ± 0.37 | 70.50 ± 1.36 | 0.88 ± 0.02 |
| Compound 5 (50 mg/kg) | 38.67 ± 0.22 | 63.23 ± 0.39 | 65.50 ± 1.9 | 0.85 ± 0.02 |
| Compound 6 (50 mg/kg) | 38.67 ± 0.22 | 63.23 ± 0.39 | 65.50 ± 1.9 | 0.85 ± 0.02 |
| Compound 11 (50 mg/kg) | 39.70 ± 0.22 | 66.28 ± 0.40 | 68.60 ± 1.9 | 0.86 ± 0.03 |
Data are expressed as mean ± SD, n = 10.
Acknowledgment
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Autore G., Caruso A., Marzocco S., Nicolaus B., Palladino C., Pinto A., Popolo A., Sinicropi M.S., Tommonaro G., Saturnino C. Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules. 2010;15:2028. doi: 10.3390/molecules15032028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaad A.S., Al-Jaber N.A., Moses J.E., El-Meligy R.M., Zain M.E. Antiulcerogenic activities of the extracts and isolated flavonoids of Euphorbia Cuneata Vahl. Phytother. Res. 2013;27:126–130. doi: 10.1002/ptr.4872. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., El-Meligy R.M., Al-Jaber N.A., Al-Muteeri H.S., Zain M.E., Alqasoumi S.I., Alafeefy A.M., Donia A.E.R.M. Anti-ulcerative colitis activity of compounds from Euphorbia Granuleta Forssk. Phytother. Res. 2013;27:1729–1734. doi: 10.1002/ptr.4985. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., El-Meligy R.M., Soliman G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013;17:101–124. [Google Scholar]
- Bighetti A.E., Antonio M.A., Kohn L.K., Rehder V.L., Foglio M.A., Possenti A., Vilela L., Carvalho J.E. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania Laevigata Schultz Bip. Phytomedicine. 2005;12:72–77. doi: 10.1016/j.phymed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- El-Meligy Reham M., Awaad Amani S., Soliman Gamal A., Kenawy Sanaa A., Alqasoumi Saleh I. Prophylactic and curative anti-ulcerogenic activity and the possible mechanisms of action of some desert plants. Saudi Pharm. J. 2017;25:387–396. doi: 10.1016/j.jsps.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Meligy Reham M., Awaad Amani S., Soliman Gamal A., Bacha Abir B., Alafeefy Ahmed M., Kenawy Sanaa A. Prophylactic and curative anti-ulcerative colitis activity and the possible mechanisms of action of some desert plants. J. Enzyme Inhib. Med. Chem. 2011;30(2):250–258. doi: 10.3109/14756366.2014.915394. [DOI] [PubMed] [Google Scholar]
- Ghorab Mostafa M., El-Gaby Mohamed S.A., Alsaid Mansour S., Elshaier Yaseen A.M.M., Soliman Aiten M., El-Senduny Fardous F., Badria Farid A. Novel thiourea derivatives bearing sulfonamide moiety as anticancer agents through COX-2 inhibition. Anticancer Agents Med. Chem. 2017;32(1):893–907. doi: 10.2174/1871520617666170327153735. [DOI] [PubMed] [Google Scholar]
- Ghorab K., El-Massry T., Shibamoto S. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian corn silk (Zea mays L.) J. Agric. Food Chem. 2011;55(2007):9124–9127. doi: 10.1021/jf071646e. [DOI] [PubMed] [Google Scholar]
- Junek R., Kverka M., Jandera A., Panajotova V., Šatinský D., Macháček M., Kuchař M. Antileukotrienic phenethylamido derivatives of arylalkanoic acids in the treatment of ulcerative colitis. Europ. J. Med. Chem. 2009;44:332–344. doi: 10.1016/j.ejmech.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Katke S.A., Amrutkar S.V., Bhor R.J., Khairnar M.V. Synthesis of biologically active 2-chloro-N-alkyl/aryl acetamide derivatives. Inter. J. Pharma. Sci. Res. 2011;2:148–156. [Google Scholar]
- Kim B., Ratnayake R., Lee H., Shi G., Zeller S.L., Li C., Luesch H., Hong J. Synthesis and biological evaluation of largazole zinc-binding group analogs. Bioorg. Med. Chem. 2017 doi: 10.1016/j.bmc.2017.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Maggio B., Daidone G., Raffa D., Plescia S., Mantione L., Cutuli V.M.C., Mangano N.G., Caruso A. Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted 3,4-dihydro-4-oxoquinazolin-3-Yl)-1h-pyrazole-4-acetates. Europ. J. Med. Chem. 2001;36:737–742. doi: 10.1016/s0223-5234(01)01259-4. [DOI] [PubMed] [Google Scholar]
- Nagao-Kitamoto H., Kamada N. Host-microbial cross-talk in inflammatory bowel disease. Immune. Netw. 2017;17:1–12. doi: 10.4110/in.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.Y., Kim J.I., Leem D.G., Shin J.S., Kim K.T., Choi S.Y., Lee M.H., Choi J.H., Lee Y.S., Lee K.T. Resveratrol analogue (E)-8-Acetoxy-2-[2-(3,4-diacetoxyphenyl)ethenyl]-quinazoline induces apoptosis via fas-mediated pathway In Hl-60 human leukemia cells. Oncol. Rep. 2016;36:3577–3587. doi: 10.3892/or.2016.5168. [DOI] [PubMed] [Google Scholar]
- Patel P., Pillai J., Darji N., Patel B. Recent advance in anti inflammatory activity of benzothiazole derivatives. Int. J. Drug Res. Technol. 2012;2:170–176. [Google Scholar]
- Ruan J., Chen Y., Zhou Y. Development and validation of a questionnaire to assess the quality of life in patients with inflammatory bowel disease in mainland China. Inflam. Bowel Diseas. 2017;23:431–439. doi: 10.1097/MIB.0000000000001024. [DOI] [PubMed] [Google Scholar]
- Sawant R., Kawade D. Synthesis and biological evaluation of some novel 2-phenyl benzimidazole-1-acetamide derivatives as potential anthelmintic agents. Acta Pharm. 2011;61:353–361. doi: 10.2478/v10007-011-0029-z. [DOI] [PubMed] [Google Scholar]
- Soliman G.A., Donia Ael R., Awaad A.S., Alqasoumi S.I., Yusufoglu H. Effect of emex spinosa, leptadenia pyrotechnica, haloxylon salicornicum and ochradenus baccatus extracts on the reproductive organs of adult male rats. Pharm. Biol. 2012;50:105–112. doi: 10.3109/13880209.2011.601465. [DOI] [PubMed] [Google Scholar]