Abstract
A two factor three level factorial design was used to investigate the effects of carbopol and cationic hydrophilic polymers which have a common use in buccal drug formulations. Statistical models with interaction terms were derived to evaluate influence of carbopol (X1) and chitosan (X2) on tablet disintegration (Y1) and dissolution (Y2), mechanical properties (Y3), swelling (Y4). Tablet disintegration studies were carried out using two different pH environments within buccal region pH limits and also two different commonly used dissolution methods for buccal tablets were also investigated to compare the effect of polymer type on dissolution. Polymer type and ratio affect the characteristics of the buccal tablets due to their different physicochemical behavior at buccal pH. Also significant variances between dissolution profiles for buccal tablets, using either USP Paddle or flow through cell methods were found. These results indicate that both polymer type and ratio as well as combination of them effects the drug behavior in different ways.
Keywords: Buccal tablet, Hydrophilic matrix, Effect of polymer, Carbopol, Chitosan
1. Introduction
Delivery of drugs through several transmucosal routes has received a great deal of attention in recent years. Absorption of drugs from the oral transmucosal routes provides a direct entrance into the systemic circulation, thus avoiding hepatic and gastrointestinal side effects. The buccal route has received great attention owing to its inimitable advantages like availability for controlled or immediate release, high patient acceptance and improved bioavailability (Sudhakar et al., 2006).
To attain an optimum buccal drug delivery, these systems must ensure enough adhesivity to attach to the mucus layer of the buccal mucosa. In order to meet the specifications, excipients used in buccal drug delivery system need to provide drug permeation enhancement and modified drug release profiles. Different polymers both alone or in combination are used to release the drug by mechanisms like erosion, swelling and hydration (Munasur et al., 2008).
Hydrophilic polymers are commonly used in buccal drug delivery systems as hydrophilic matrices due to their compatibility and suitability to the buccal region. Hydrophilic matrices are dispersions of drugs and other excipients incorporated in a hydrophilic polymer which swells upon water contact. The hydration of the matrix is affected by the polymer characteristics and influences the drug behavior (Timmins et al., 2016). Changes in the hydrated surface layer properties caused by pH variation also influence the performance of polymer and drug delivery system (Perez-Marcos et al., 1996).
By virtue of developing a hydrophilic matrix tablet deals a modest and effective approach to the buccal drug delivery, it needs a careful attention of the physicochemical properties of the active substance, polymer, and the excipients (De Robertis et al., 2015, Palmer et al., 2013). Formulation and the process variables optimization can be time-consuming. While developing a buccal tablet with direct-compression method, it is essential to find appropriate polymers with good adhesivity and drug release capability (Deshmukh et al., 2014).
Numerous polymer types with different solution–gel transitions have been examined to develop swellable matrices (Russo et al., 2016). Due to drug release rate influenced by the viscosity and thickness of the gel layer matrix, choice of right polymer with the suitable viscosity and disintegration rate is essential for designing a buccal tablet (Malakar and Nayak, 2013).
Buccal drug delivery requires the use of mucoadhesive polymers because these dosage forms are supposed to show enough adhesion to the mucosa and resist salivation and mechanical movements in the mouth for a long time periods. Mucoadhesive polymers can be classified as anionic such as carbopol (Singla et al., 2000), polycharbophil (Barua et al., 2016) and sodium carboxymethylcellulose (Kassem et al., 2014), cationic such as chitosan (Park et al., 2008) or non-ionic such as hydroxypropylmethyl cellulose (HPMC) (Nafee et al., 2004). Polymers are generally identified as macromolecular organic hydrocolloids that contain numerous hydrogen bond-forming groups, notably carboxyl, hydroxyl, amide and amine groups. High molecular weight, sufficient degree of polarity and flexibility of the polymer chains are considered vital in order to provide sufficient driving force for polymer-mucus adsorption and interpenetration (Nafee et al., 2004).
Carbopol is a high molecular weight, anionic based, cross-linked polymer of acrylic acid that forms hydrogel. Its hydration degree depends on the concentration. It has shown that acrylic polymers are influenced by pH changes and the presence of electrolytes. Also, its cross-linked network makes it an appropriate carrier for extended drug delivery systems (Singla et al., 2000). It has numerous benefits as a candidate for a modified-release tablet matrix, e.g. a good gel-forming capability and mucoadhesive property. Carbopol forms a gel at alkaline pH which affects the drug release. pH-dependent drug release can cause in vivo variability and to control the drug release other polymeric materials can be added to the composition of matrix tablets (Kranz et al., 2005).
Chitosan is a cationic, natural polysaccharide used in various types of formulations. It forms gel between pH 2 and 4 providing both fast disintegration and controlled release depending on the concentration and type. As a cationic polymer it forms a gel structure in acidic pH, it is altered from both synthetic high molecular weight polymers which are generally neutral or anionic. The combination of these two polymers in a formulation may have some advantages such as gel forming ability of carbopol is very useful in conjunction with chitosan as it enhances the disintegration time of the tablets. Also chitosan can be used as a permeation enhancer (Nigalaye et al., 1990, Park et al., 2008).
In this study, factorial design was used to investigate the effects of anionic and cationic hydrophilic polymers which have common use in buccal drug formulations. A two factors, three levels (32) full factorial design was used and nine experimental runs were performed. Statistical models with interaction terms were derived to evaluate influence of carbopol (X1) and chitosan (X2) on tablet disintegration (Y1), dissolution (Y2), mechanical properties (Y3) and swelling (Y4). In order to enlighten the properties of these two polymers those have various swelling capacity at different pH values, tablet disintegration studies were carried out using two different pH environments within buccal region pH limits. In addition, because of the lack of official monographs, two different commonly used dissolution methods for buccal tablets were also investigated to compare the effect of polymer type on dissolution. In this study, diclofenac sodium is used as a model drug which can is an option for both immediate and controlled release form and can be used by buccal route due to its gastrointestinal side effects.
2. Material and methods
2.1. Materials
Diclofenac sodium (DS) was a gift from Deva Pharm. Co. (Istanbul, Turkey) and mannitol was a gift from Eczacıbaşı Baxter (Turkey). The following materials were used as received: Carbopol 941 (B.F. Goodrich Chemical Company, USA), cellulose membrane (Travenol Lab. Inc., USA), chitosan (medium molecular weight) and HPMC (Viscosity of 4% solution 25 °C; 4000 cps) (Sigma-Aldrich, USA), Colloidal Silicon Dioxide (Aerosil 200) (Evonik, Germany), sucrallose (Kimetsan, Turkey).
2.2. Experimental design
A 32 full factorial design was used for the preparation of buccal tablets in which two factors were studied each at three levels (Abdelbary et al., 2009). The amount of carbopol (X1), and the amount of chitosan (X2) were selected as independent variables. The disintegration time in water and artificial saliva, swelling capacity, dissolution, peak detachment force and permeability were selected as dependent variables. The factor levels were chosen so as to their relative alteration was acceptable to have a computable effect on the response, together with the information that the designated levels are within practical use.
Design Expert 7.0. (Stat-Ease, Inc, USA) was used for the analysis of effect of each variable on the designated response. Quantitative and qualitative contribution of each variable on each of the response was analyzed. The significant response polynomial equations generated by Design Expert were used to validate the statistical design (Bolton, 2009). Response surface plots were generated to visualize simultaneous effect of each variable on each response parameter. The constant and regression coefficients were calculated also using the software. Calculated equations were used to predict the effect of polymer type and concentration on disintegration time, swelling, mechanical properties, permeation and dissolution rate of DS buccal tablets in the experimental region.
2.3. Preparation of buccal tablets
DS buccal tablets were prepared by direct-compression method (Shangraw, 1989). The composition of each tablet was 25 mg DS, 20 mg HPMC K4M, 1 mg of colloidal silicone dioxide (CSD) as a lubricant, 0.4 mg sucrallose and various concentrations of hydrophilic polymer and mannitol as a diluent to reach up to the total weight of any tablet to 100 mg (Table 1). Before pressing the tablets, the earlier sieved drug, diluent and other excipients were mixed for 5 min, then the lubricant was added and the final blend was mixed for further 2 min. 100 mg of blend was filled into the 8 mm die and compressed into flat-faced tablets using a single punch tablet machine (Korsch KO, Germany).
Table 1.
Tablet formulation design.
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
|---|---|---|---|---|---|---|---|---|---|
| DS | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Carbopol | 5 | 5 | 10 | 0 | 0 | 5 | 0 | 10 | 10 |
| Chitosan | 15 | 7.5 | 0 | 15 | 0 | 0 | 7.5 | 15 | 7.5 |
| HPMC | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| CSD | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sucrallose | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Mannitol | 33.6 | 41.1 | 43.6 | 38.6 | 53.6 | 48.6 | 46.1 | 28.6 | 36.1 |
| Total (mg) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
2.4. Tablet properties
Samples were tested for hardness and friability to determine any variability related to the preparation method. Hardness tester (Sotax, Switzerland) was used to determine tablet hardness. Ten tablets were chosen from the samples for each of the tableting runs and the average value was determined in Newton (N ± SD.). The friability of 20 tablets from each formulation was examined at 25 rpm for 4 min using a friability tester (Sotax, Switzerland). The friability is expressed in terms of loss of weight and is calculated in fraction of the original weight (Pharmacopeia, 2014).
2.5. Disintegration
Disintegration of buccal tablets is examined by using water and artificial saliva to evaluate the effect of media pH on disintegration. Disintegration time of each lot was determined in minutes using the USP disintegration test apparatus. To determine disintegration time, 1000 ml water (pH 5.9) or artificial saliva fluid (pH 6.75) (1.491 g KCl, 0.015 g MgCl2·6H2O, 0.06 g CaCl2·2H2O, 0.005 g NaF, 0.108 g NaH2PO4, 0.124 g Na2HPO4 and 1.157 g NaCl per liter of distilled water, pH adjusted to 6.75 with phosphoric acid) (Topcu et al, 2009) was placed inside the vessel. Tablet was placed on the sieve. The time that all the tablet particles pass through the sieve was calculated as a disintegration time of the buccal tablet. Six tablets were selected from the each formulation and the average value was determined (Pharmacopeia, 2014).
2.6. Swelling studies of buccal tablets
Buccal tablets were balanced separately (designated as W1) and placed individually in petri dishes, incubated in 15 ml of artificial saliva at 37 ± 1 °C, and observed for any physical changes. At regular 1-h time intervals until 8 h, tablets were taken from the petri dishes using cover slips and excess surface liquid was removed using the whatman filter paper. The swollen buccal tablets were then reweighed (W2), and the swelling index (SI) was calculated using the equation (Park and Munday, 2004). The experiments were achieved in triplicate, and average values were calculated using the equation shown below.
2.7. Ex vivo mucoadhesive strength
Fresh bovine buccal tissue was obtained from a local slaughterhouse. The mucosal membrane was detached by removing the underlying fat and loose tissues. The membrane was washed and placed in Krebs buffer solution (Hausenloy et al., 2002), and stored at 4 °C till further usage. Adhesion studies of intended formulations were performed using modified texture analyzer (Tinius Olsen, USA). The thawed mucosal tissue was cut into pieces and held using clips on a holder. Artificial saliva maintained at 37 °C was dropped on the tissue, so that the liquid is just in interaction with the surface of the mucosal membrane. A piece of buccal mucosa was tied to the bottom of the tester. The buccal tablet was attached to the tip of probe using cyanoacrylate adhesive. The probe was lowered until the tablet made contact with mucosal tissue. A constant force was applied for 30 s, after which the probe was withdrawn at a speed of 50 mm/min.
2.8. In vitro drug release
Dissolution tests were performed on buccal tablets using USP paddle and flow through cell methods respectively (Medina et al., 2014). Trials were carried out, in 6 tablets, using a phosphate buffer solution (pH 6.8) at 37 °C as the dissolution medium. The dissolution media were chosen considering literature and pharmacopeia data about media drug delivery systems applied to oral cavity. Samples are taken at predetermined time intervals and DS quantity was determined by UV spectrophotometer at 275 nm, which is the wavelength of maximal absorption. Two different dissolution apparatuses were used as follows:
The first method to study the dissolution from the buccal tablets was USP rotating paddle (Apparatus 2) (Caleva 7, England). The release medium composed of 900 ml of phosphate buffer pH 6.8. The test was carried out at 37 ± 0.2 °C, with a rotation speed of 50 rpm. Tablets were placed at the bottom of the vessel. Samples (5 ml) were withdrawn at predetermined time and same volume was replaced with fresh medium. The samples were filtered through whatman filter paper (0.45 μm), analyzed after suitable dilution and the time at which 85% of drug is dissolved was calculated using interpolation of the model equations.
The other method to obtain the dissolution profiles was an automated flow-through cell system (USP Apparatus 4) with 22.6 mm cells and a piston pump (Sotax CE7, Switzerland) with an auto-controlled UV/VIS spectrophotometer (Thermo, USA). In all trials laminar flow (using glass beads) was used. The dissolution medium, phosphate buffer pH 6.8 at 37.0 ± 0.5 C, was pumped at a flow rate of 2 ml/min which is the minimum rate limit for automated system. An open system was used, without recycling the dissolution media. The amount of DS dissolved was determined online at predetermined time intervals and the time at which 85% of drug is dissolved was calculated using interpolation of the model equations.
2.9. In vitro and ex vivo permeation of buccal tablets
Experimental study design was performed using modified horizontal diffusion cell (Bayrak et al., 2011). The temperature (37 °C) was maintained by using a water bath and a thermometer assembled to it. Buccal movements were simulated using magnetic stirrer. Cellulose membrane was used for in vitro permeation study. Study was conducted using 32 ml pH 6.8 phosphate buffer for receiver chamber. Buccal tablets were placed in the donor chamber in a position that tablet can attach to the cellulose membrane, then 2 ml pH 6.8 phosphate buffer was added.
For ex vivo study bovine buccal mucosa was obtained from a slaughterhouse. The tissue was stored in Krebs buffer at 4 °C upon collection. Permeation study was conducted using krebs buffer solution in both donor and receiver chamber and 95% O2-5% CO2 gas mixture was used to maintain the tissue viability.
Samples (2 ml) were withdrawn at predetermined time intervals and filtered through a 0.2-μm filter, and the permeated total drug through the buccal mucosa was then calculated by measuring the absorbance at 275 nm using a UV spectrophotometer. The medium taken from the receiver compartment was exchanged with an equivalent volume of prewarmed buffer (2 ml). The tests were carried out in triplicate (n = 3) and mean values were used to calculate the flux and permeability coefficient. The cumulative of DS permeated per unit area was calculated against time, and the slope of the linear portion of the plot was used as steady state flux (JSS). The permeability coefficient (Kp) was calculated with equation, in which CV is the total drug concentration of the formulation.
3. Results and discussion
3.1. Experimental design
Totally 9 formulations were suggested by the 32 factorial design for two independent variables: amount of Carbopol (X1, mg) and Chitosan (X2, mg) that were three different levels (high, medium and low). The effect of these factors on disintegration time (water and artificial saliva), swelling, dissolution (t85%(min)), peak detachment force and in vitro permeation (%) were examined as response parameters in the study. According to the 32 factorial design, various trial formulations of DS buccal tablets were prepared by direct compression method. Summary of the variables and observed responses are presented in Table 2. The Design Expert 7.0 software calculated suitable model equations after fitting these data.
Table 2.
Experimental design with response values for buccal tablet formulations.
| Code | Independent variables |
Responses |
||||||
|---|---|---|---|---|---|---|---|---|
| Carbopol (X1) | Chitosan (X2) | Dis.*** (water) (min) | Dis.* (Ar. Sal.) (min) | Swelling | t85%** (min) | Peak Det. For.* (N) | Permeation (%) | |
| F1 | 5 (0) | 15 (+1) | 210 ± 8 | 226 ± 6 | 4.59 ± 0.442 | 1206 ± 25 | 2.79 ± 0.05 | 7.29 ± 0.34 |
| F2 | 5 (0) | 7.5 (0) | 201 ± 4 | 212 ± 7 | 4.97 ± 0.223 | 2260 ± 126 | 1.21 ± 0.17 | 4.91 ± 0.03 |
| F3 | 10 (+1) | 0 (−1) | 203 ± 11 | 226 ± 3 | 7.11 ± 0.274 | 2191 ± 61 | 4.80 ± 0.56 | 1.82 ± 0.40 |
| F4 | 0 (−1) | 15 (+1) | 190 ± 2 | 186 ± 5 | 1.60 ± 0.12 | 14 ± 2 | 0.79 ± 0.15 | 12.77 ± 0.99 |
| F5 | 0 (−1) | 0 (−1) | 8 ± 1 | 4 ± 2 | 0.73 ± 0.114 | 9 ± 1 | 0.37 ± 0.08 | 9.26 ± 0.39 |
| F6 | 5 (0) | 0 (−1) | 170 ± 4 | 174 ± 5 | 5.69 ± 0.345 | 1462 ± 48 | 1.76 ± 0.14 | 3.82 ± 0.23 |
| F7 | 0 (−1) | 7.5 (0) | 92 ± 6 | 87 ± 6 | 1.81 ± 0.105 | 14 ± 1 | 0.47 ± 0.05 | 13.01 ± 0.20 |
| F8 | 10 (+1) | 15 (+1) | 204 ± 6 | 211 ± 4 | 7.91 ± 0.305 | 1328 ± 23 | 4.24 ± 0.16 | 1.69 ± 0.12 |
| F9 | 10 (+1) | 7.5 (0) | 178 ± 8 | 199 ± 5 | 7.85 ± 0.294 | 2245 ± 46 | 4.63 ± 0.13 | 1.73 ± 0.11 |
(+1) high values, (0) medium values and (−1) low values
Peak Det. For.* = Peak Detachment Force.
Dissolution** = USP Paddle Method Results.
Dis*** = Disintegration.
Mean ± SD.
The ANOVA results, as illustrated in Table 3, showed that all models were significant (p < .05). Model simplification was carried out by eliminating non-significant terms (p > .05) in equations (Nayak et al., 2011), giving:
Table 3.
Summary of the response parameters.
| Source | Sum of squares | df | Mean square | F | Prob > F | r2 |
|---|---|---|---|---|---|---|
| (a) Disintegration time in water (Model: 2FI) | ||||||
| Model | 30982.58 | 3 | 10327.53 | 8.60 | 0.0204* | 0.8376 |
| X1 | 14504.17 | 1 | 14504.17 | 12.07 | 0.0178* | |
| X2 | 8288.17 | 1 | 8288.17 | 6.90 | 0.4670 | |
| X1X2 | 8190.25 | 1 | 8190.25 | 6.82 | 0.4760 | |
| (b) Disintegration time in artificial saliva (Model: Quadratic) | ||||||
| Model | 44602.69 | 5 | 8920.54 | 39.97 | 0.0060* | 0.9852 |
| X1 | 21480.17 | 1 | 21480.17 | 96.25 | 0.0023* | |
| X2 | 7993.50 | 1 | 7993.50 | 35.82 | 0.0093* | |
| X1X2 | 9702.25 | 1 | 9702.25 | 43.47 | 0.0071* | |
| X12 | 5373.39 | 1 | 5373.39 | 24.08 | 0.0162* | |
| X22 | 53.39 | 1 | 53.39 | 0.24 | 0.6583 | |
| (c) Swelling capacity (Model: Linear) | ||||||
| Model | 58.54 | 2 | 29.27 | 76.76 | <0.0001* | 0.9624 |
| X1 | 58.48 | 1 | 58.48 | 153.38 | <0.0001* | |
| X2 | 0.05 | 1 | 0.05 | 0.14 | 0.7197 | |
| (c) 85% Dissolution time (min) (Model: Linear) | ||||||
| Model | 5673254 | 2 | 2836627 | 9.246711 | 0.0147* | 0.7550 |
| X1 | 5466422 | 1 | 5466422 | 17.8192 | 0.0056* | |
| X2 | 206832.7 | 1 | 206832.7 | 0.674224 | 0.4430 | |
| (d) Peak detachment force (Model: Linear) | ||||||
| Model | 24.29 | 2 | 2.12 | 32.98 | 0.0006* | 0.9166 |
| X1 | 24.16 | 1 | 24.16 | 65.61 | 0.0002* | |
| X2 | 0.13 | 1 | 0.13 | 0.36 | 0.5712 | |
| (e) Permeation% at 360 min (Model: Linear) | ||||||
| Model | 155.92 | 2 | 77.96 | 42.18 | 0.0003* | 0.9336 |
| X1 | 148.14 | 1 | 148.14 | 80.14 | 0.0001* | |
| X2 | 7.78 | 1 | 7.78 | 4.21 | 0.0861 | |
X1 and X2 represent amount of Carbopol (mg) and Chitosan (mg) and X1X2 is the interaction effect.
df indicates degree of freedom.
Significant factors.
The model equation relating Disintegration time in water (min) as response became: Disintegration (water) = +161.78 + 49.17 ∗ X1.
The model equation relating Disintegration time in artificial saliva (min) as response became: Disintegration (artificial saliva) = +200.56 + 59.83 ∗ X1 + 36.50 ∗ X2 − 49.25 ∗ X1 ∗ X2 − 51.83 ∗ X12.
The model equation relating Swelling index as response was found: Swelling index = +4.70 + 3.12 ∗ X1.
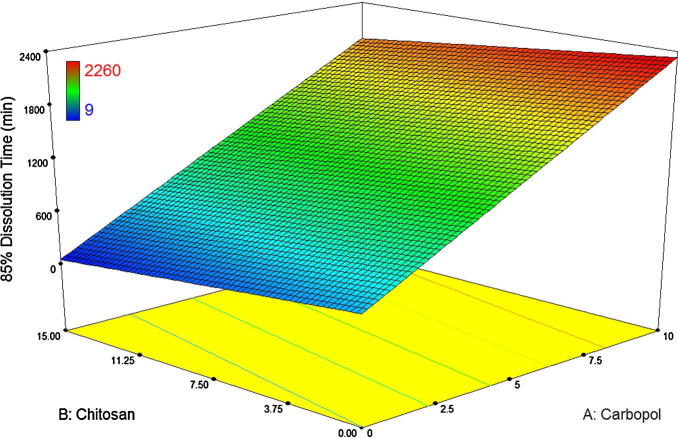
The model equation relating 85% Dissolution time (min) as response was found: Dissolution = +1182.11 + 954.50 ∗ X1.
The model equation relating Peak Detachment Force (N) as response was found: Peak Detachment Force = +2.34 + 2.01 ∗ X1.
The model equation relating Permeation at 360 min (%) as response was found: Permeation = +6.26 − 4.97 ∗ X1.
The data obviously indicates that the response values are strongly dependent on the selected independent variables. Table 3 displays the effects of the analysis of variance (ANOVA), which was performed to categorize insignificant factors. The high correlation coefficient values of disintegration time in artificial saliva and swelling capacity indicate a good fit. The equations can be used for the calculation of prediction of the response as a small error of variance was noticed in the replicates (Gohel et al., 2004).
3.2. Tablet properties
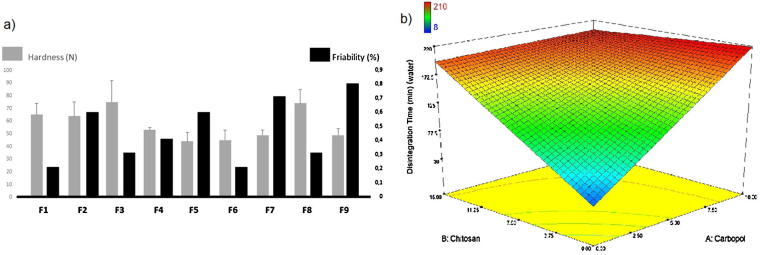
The hardness of all the tablet formulations were within the range of 44–75 N and the highest hardness values were found in F3 and F8 with mean of 75 ± 17 N and 74 ± 11 N respectively (Fig. 1a). The increase in hardness of might be based on the good binding properties of carbopol (Fayed et al., 2013).
Fig. 1.
Effect of formulation variables on hardness and friability (a) and disintegration time (b) of buccal tablets.
Friability test is a useful technique to obtain physical strength of the tablet. All the prepared formulations submitted with the pharmacopeial standards as none of the formulations had percentage loss in weights exceeding 1%, also; no tablet was cracked, split or broken in each formulation composition (Pharmacopeia, 2014) (Fig. 1a).
3.3. Disintegration
According to the compendial standards, buccal tablets should disintegrate within 4 hours in case of the implementation of the test for disintegration of conventional tablets and capsules (Pharmacopeia, 2014). Although all the formulations disintegrate within given time, the disintegration time of F1, F2, F3 and F8 formulations are close to 4 hours. (210, 201, 203 and 208 min, respectively). The least disintegration time, on an average of 8 min, was observed with F5 containing no chitosan and carbopol polymer.
The effect of carbopol and chitosan concentration on the disintegration times of the buccal tablet formulations are shown in Fig. 1b. High level of both polymer concentration increased disintegration time and the rate of disintegration of chitosan matrices (F4, F7) was faster than carbopol ones. The lack of gel forming ability of chitosan at disintegration medium pH 5.9 may be the cause of fast disintegration. Due to compression of particles in tablet press, the surface pores are sealed resulting in retardation of the water uptake between the compressed particles in tablet formulations, which prolongs disintegration (Betageri et al., 2001, Park et al., 2008). Higher disintegration times were observed with increase in the level of hydrophilic polymers in the buccal tablets of chitosan and carbopol. This indicates that higher polymer concentration had a negative effect on the disintegration of the tablets. This result shows that at greater polymer ratios, formation of a viscous gel layer by swollen polymers might have formed a thick barrier to the additional penetration of the disintegration medium and delayed the disintegration or leakage of tablet contents (Patel et al., 2007).
Moreover, the combination of these polymers slows down the release more than their alone forms. These observed differences could be attributed to ionic cross-linked hydrogels structure. Erosion of the network structure of matrices is prevented by ionic interactions, which exist between both polymer chains. It may support the claim of the interaction between ammonium ion in chitosan and carboxylate ion in carbopol and high concentration of this complex plays a preventive role in disintegration (Ahn et al., 2001).
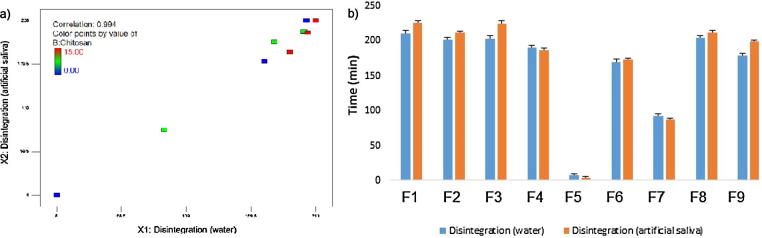
Disintegration time may vary according to different media composition (Anwar et al., 2005). In this study, water and artificial saliva was used to compare the effect of media type on disintegration properties. The extent of hydrogen and chemical bonding between carbopol and chitosan in the mixtures depending on the pH of the medium can cause difference in the solubility degree (Nafee et al., 2004). Chitosan and carbopol matrices are medium-dependent due to ionic nature of the polymer. Although the disintegration times in both mediums are well correlated (0.994), there are also differences due to charge and concentration of the polymers (Fig. 2a and b). The disintegration rate of chitosan matrices (F4, F7) in artificial saliva was not significantly different but numerically faster than those of in water. The cationic structure of chitosan at neutral pH might retards the rate of disintegration of the tablet in water (Betageri et al., 2001). In case of the carbopol matrices (F3, F6), the rate of disintegration was also influenced by the pH of the medium. As the pH increases, swelling of the polymer is greater, which results the formation of a gel layer and almost all the carboxyl groups will dissociate at pH 6.8 resulting in the formation of a swollen gel. But, the carboxyl groups of carbopol will not dissociate at pH 5.9 as well as at pH 6.8 resulting in a less viscous hydrogel (Bonacucina et al., 2004). Therefore, the rate of disintegration at the carbopol matrix in artificial saliva was slower than that of in water. Carbopol was swollen in both medium with a higher swelling index. Thus, the influence of medium to the disintegration of carbopol/chitosan matrices was found similar to carbopol matrices.
Fig. 2.
Comparison (b) and correlation (a) of disintegration time in artificial saliva and water.
3.4. Swelling studies of buccal tablets
The swelling degree of mucoadhesive polymers is a vital factor affecting dosage form behavior. For instance, adhesion occurs shortly after the beginning of swelling (Peh and Wong, 1999). The water uptake results in relaxation of the polymer chains leading to exposure of all polymer adhesion sites for bonding to occur. The higher swelling of the polymer, the faster beginning of diffusion and formation of adhesive bonds result more rapid start of adhesion. Also drug release from polymer matrix is mainly controlled by swelling. The drug is released from the swollen hydrophilic polymer system, which gradually erodes and finally completely dissolves (El-Samaligy, Yahia, and Basalious, 2004).
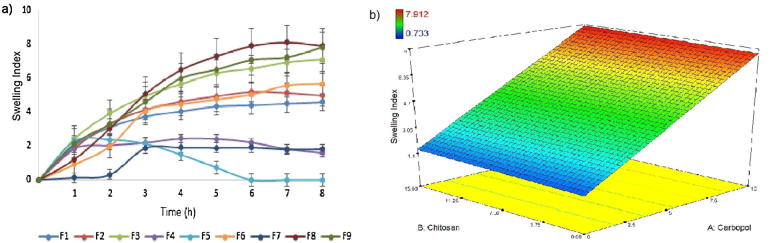
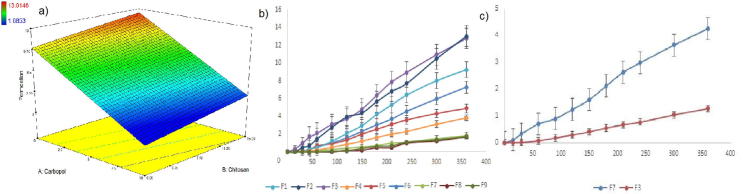
The interaction between polymer concentration and swelling degree was investigated in dynamic swelling studies (Fig. 3a). There is a tendency that higher hydrophilic polymer concentrations have a higher swelling ability. Fig. 3b, shows a direct correlation between carbopol and the chitosan concentration within the network and its swelling properties. The maximum swelling index was increased by the increased concentration of carbopol. The high molecular weight polymer in the formulation could enable the initial hydration of the hydrogels by generating an osmotic gradient. And the existence of carbopol within these hydrogels could help the protonation of amine groups from chitosan resulting in an electrostatic repulsion among polymeric chains (Nafee et al., 2004). Formulations F3, F8 and F9 have showed the highest swelling index because of the presence of highest carbopol concentration in the tablets.
Fig. 3.
Dynamic swelling results (a) and effect of polymer on swelling (b) of formulations.
Formulations without carbopol (F4, F5 and F7) exhibited the lowest swelling index. Low gel-forming ability of F4 and F7 formulations at neutral pH was stated to be responsible for the low swelling features of chitosan (Kristmundsdottir et al., 1995). Increasing chitosan ratio within the network improved the swelling degree. This might be attributed to the higher amount of chitosan within the network structure with a major amount of pendant groups. These groups ionize in this pH environment (pH 6.75 with an increase in electrostatic repulsions (Junginger, 1991). The erosion of the hydrogel was observed after the maximum swelling index of tablets.
3.5. Ex vivo mucoadhesive strength
The peak detachment force is assessed to be dependent on the formation of hydrogen bonds between the polymer functional groups and the musin. Physical entanglement is likewise associated to the peak detachment forces as it induces chain inter-locking due to the inter-diffusion of the polymer chains into the mucus glycoprotein (Boyapally et al., 2010).
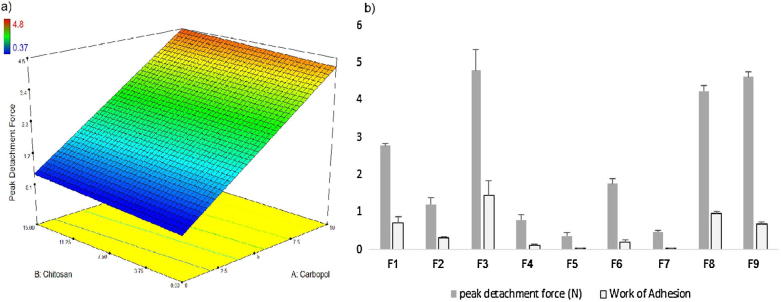
Fig. 4a illustrates that the ex vivo mucoadhesive strength was improved with increasing the hydrophilic polymer concentration after 30 s of contact time with bovine buccal mucosa. The increase in the mucoadhesion may be the result of the formation of a strong gel that enters deeply into the mucin molecules. The mucoadhesion forces demonstrate the superiority of the anionic polymer (carbopol), over the cationic (chitosan) ones. This classification is almost comparable to results found by Nafee et al (2004).
Fig. 4.
Peak detachment force (a) and Work of adhesion (b) values of formulations.
Carbopol showed high adhesive strength possibly owing to the formation of secondary mucoadhesion bonds with the mucin. The carbopol undergo fast swelling and interpenetration into the interfacial region while other polymers showed only superficial adhesion (Patel et al., 2007). In addition, the work of adhesion (area under the force/distance curve) of carbopol could be related to the interpenetration of the polymer chains into the mucus. Rapid swelling characteristics of this polymer increases the physical entanglement, and produces a broader force/distance curve (Park and Munday, 2002). Consequently work of adhesion values of carbopol based formulations is greater than the formulation containing chitosan (Fig. 4b).
3.6. In vitro drug release
The effect of polymer type and concentration on drug release was analyzed and results are illustrated in Fig. 5. Dissolution rate mainly increased with the decrease in carbopol concentration. The rate of release through matrix tablets is controlled by the rate and extent of polymer swelling. Thus, ionic strength and pH value of the dissolution medium affect the release rates. Matrix tablets are among the most widely used drug delivery system in the world. The drug release mechanism from hydrophilic polymeric matrices involves solvent penetration, hydration and swelling of the polymer, diffusion of the dissolved drug in the matrix, and erosion of the gel layer. At the start, the diffusion coefficient of the drug in the dehydrated hydrogel is very low, but it increases considerably as the gel imbibes water (Roy and Rohera, 2002). The osmotic pressure produced by the polymers causes the solvent movement until achievement of equilibrium between the internal and external chemical potentials (Peppas and Korsmeyer, 1987). Thus, increasing the percentage of carbopol content in the tablets produces a water-swollen gel-like phase that can significantly lower the penetration of dissolution medium into the tablets and so the dissolution rate (Varshosaz and Dehghan, 2002).
Fig. 5.
Effect of polymer type on dissolution of buccal tablets.
Fig. 6 displays the effect of chitosan on the release profile of DS. All of the DS was dissolved before 120 min when chitosan used without carbopol. The low solubility of chitosan in this pH may be the main factor of relatively rapid dissolution. So, it seems that using this polymer alone, this polymer is not a suitable candidate to sustain the DS release from these mucoadhesive tablets. In our formulations carbopol sustained the drug release due to its high swelling capacity in dissolution media. Although, polyacrylic acids are water soluble, selection of high molecular grades can tailor the release profile. Furthermore, combination of anionic polymer with cationic polymer produces a synergistic decrease in dissolution rate (Korsmeyer, Gurny, Doelker, Buri, and Peppas, 1983). With regard to the mixture of carbopol and chitosan, this fact is also confirmable, i.e. an increase in carbopol/chitosan ratio induces a decrease in the dissolution rate. In the event of an increase in carbopol/chitosan ratio, a significant decrease in the rate of release could be expected. The fact that carbopol is more hydrophilic and swellable than chitosan and promotes liquid entry and entrapment of drug molecules in the polymer network may be responsible for this (Costa and Lobo, 2001).
Fig. 6.
Drug release profiles of formulations.
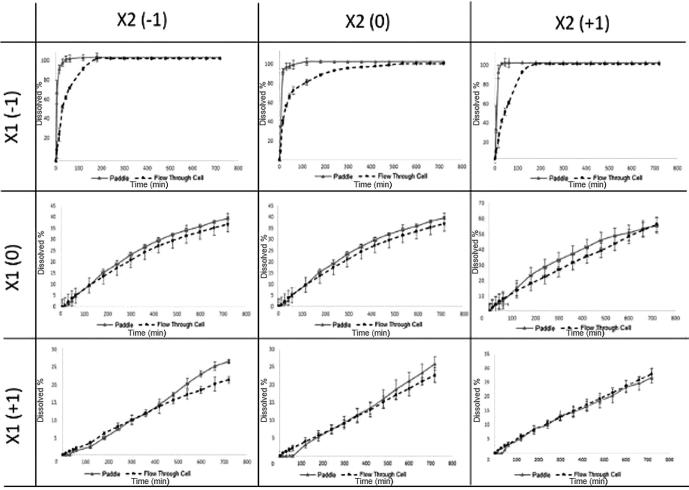
So as to compare the effect of dissolution apparatus on the dissolution of DS, USP paddle apparatus and USP flow through cell were used. The individual dissolution profiles are presented in Fig. 6. The drug release profiles for DS buccal tablets show that the overall release trends from the matrix system are sensitive both to type and concentration of polymer in the apparatus and moreover to the hydrodynamic conditions.
Dissolution testing is carried out with the equipment which has demonstrated suitability; however, there is no official dissolution apparatus for buccal drug delivery systems. Differences in dissolution behavior of tablets were observed in two different apparatus and release was greater in USP paddle apparatus. The lower drug release rate detected in USP flow through cell can be described by variances in hydrodynamic conditions that characterize these systems. Flow through cell has no stirring mechanism, so the dosage form and drug particles are exposed continuously to laminar flow, resulting a slow drug release rate. Instead in the paddle method the turbulent flow related to stirring mechanisms imparts variable degrees of physical abrasion of the solids, owing to nonhomogeneous shear rate of transfer over the surface of the particles, hence improving the drug release rate (Abdou, 1989).
To define the DS release kinetics from buccal tablets, dissolution profiles were fitted to several kinetics dissolution models: zero-order, first-order, Hixson-Crowell, Higuchi, and Weibull’s equations (Langenbucher et al., 1989, Polli et al., 1997, Yuksel et al., 2000, Savaşer et al., 2005). The regression analysis was done for all nine batches. Residual values were used to compare best fit of the experimental data to the predicted (r2 > 0.99 and minimum residual mean square, RMS and model parameters). The results are shown in Table 4. As can be seen, Weibull function fitted best to the dissolution data for products without carbopol. But in other products best fitted model was varied between zero-order, first-order and Higuchi models. Shifting dissolution apparatus changed the best fitted model probably as a consequence of different hydrodynamic conditions of the systems.
Table 4.
Kinetic results of formulations.
| F5 |
F7 |
F4 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apparatus 2 |
Apparatus 4 |
Apparatus 2 |
Apparatus 4 |
Apparatus 2 |
Apparatus 4 |
|||||||||||||
| Model parameters | r2 | RMS | Model parameters | r2 | RMS | Model parameters | r2 | RMS | Model parameters | r2 | RMS | Model parameters | r2 | RMS | Model parameters | r2 | RMS | |
| Weibull | a = 1.19 × 10−1 b = 2.37 × 10−1 Tlag = 4.99 |
0.9697 | 70.9 | a = 6.48 b = 8.71 × 10−1 Tlag = 1.33 |
0.9945 | 32.8 | a = 1.64 b = 6.45 × 10−1 Tlag = 4.42 |
0.9193* | 204.9 | a = 4.83 × 101 b = 9.94 × 10−1 Tlag = 0 |
0.9501 | 362.7 | a = 3.93 × 10−1 b = 4.20 × 10−1 Tlag = 4.99 |
0.9756 | 71.3 | a = 2.56 × 101 b = 8.05 × 10−1 Tlag = 2.88 |
0.9837 | 106.8375 |
| Higuchi | K = 7.89 | 0.8708 | 302.7 | K = 7.32 | 0.9566 | 206.7 | K = 7.48 | 0.6636 | 1031.1 | K = 5.83 | 0.8035 | 1677.4 | K = 7.43 | 0.9529 | 129.7 | K = 5.30 | 0.9847 | 107.6362 |
| First order | k = 2.18 × 10−4 | 0.9869* | 119.3 | k = 1.07 × 10−3 | 0.9956* | 32.8 | k = 8.47 × 10−4 | 0.9184 | 1014.1 | k = 3.36 × 10−3 | 0.9775* | 1413.8 | k = 9.30 × 10−4 | 0.9772* | 115.2 | k = 4.53 × 10−3 | 0.951 | 2210.026 |
| Hixon crowel | K = 1.39 × 10−4 Q0 = 9.56 × 101 Tlag = 4.34 |
0.9464 | 1060.9 | K = 6.52 × 10−4 Q0 = 8.03 × 101 Tlag = 2.20 × 10−1 |
0.9811 | 514.5 | K = 4.88 × 10−4 Q0 = 8.52 × 101 Tlag = 2.97 |
0.7653 | 3915.7 | K = 1.79 × 10−3 Q0 = 5.10 × 101 Tlag = 2.97 |
0.8996 | 11749 | K = 5.25 × 10−4 Q0 = 8.41 × 101 Tlag = 1.22 |
0.9287 | 249.9 | K = 2.33 × 10−3 Q0 = 4.12 × 101 Tlag = 2.76 |
0.9987* | 23.1798 |
| Zero order | k = 8.21 × 10−3 | 0.7824 | 5507.6 | k = 3.51 × 10−2 | 0.8388 | 4154.8 | k = 2.40 × 10−2 | 0.5225 | 17394.6 | k = 8.13 × 10−2 | 0.6391 | 33024 | k = 2.49 × 10−2 | 0.7349 | 8811 | k = 9.87 × 10−2 | 0.9077 | 3018.807 |
| F6 |
F2 |
F1 |
||||||||||||||||
| Weibull | a = 5.56 × 1014 b = 5.50 Tlag = 2.61 |
0.9885 | 40.1 | a = 7.41 × 105 b = 2.13 Tlag = 4.99 |
0.9296 | 19.9 | a = 2.92 × 1010 b = 3.89 Tlag = 4.62 |
0.9795 | 86.9 | a = 6.35 × 105 b = 2.15 Tlag = 4.99 |
0.9852 | 50.2 | a = 4.20 × 105 b = 2.18 Tlag = 4.99 |
0.994 | 25.7 | a = 4.41 × 105 b = 2.17 Tlag = 4.99 |
0.9962 | 36.8 |
| Higuchi | K = 9.75 × 10−3 | 0.9909 | 19.1 | K = 1.62 × 10−1 | 0.9900* | 8.4 | K = 4.26 × 10−2 | 0.9972* | 6.74 | K = 2.06 × 10−1 | 0.9960* | 9.22 | K = 3.30 × 10−1 | 0.9945* | 28.3 | K = 3.02 × 10−1 | 0.9803 | 91.1 |
| First order | k = 6.10 × 10−2 | 0.9989* | 2.37 | k = 2.74 × 10−2 | 0.9137 | 8.9 | k = 4.88 × 10−2 | 0.9817 | 41.6 | k = 2.74 × 10−2 | 0.9883 | 25.3 | k = 2.69 × 10−2 | 0.9901 | 48.4 | k = 2.67 × 10−2 | 0.9952 | 18.7 |
| Hixon crowel | K = 4.52 × 10−3 Q0 = 4.70 × 10−1 Tlag = 2.34 |
0.9979 | 14.8 | K = 3.27 × 10−3 Q0 = 1.59 Tlag = 1.55 |
0.9743 | 36.7 | K = 4.09 × 10−3 Q0 = 1.19 Tlag = 5.9 × 10−1 |
0.9759 | 121.4 | K = 3.50 × 10−3 Q0 = 2.14 Tlag = 1.51 |
0.9841 | 90.3 | K = 3.86 × 10−3 Q0 = 3.87 Tlag = 2.34 |
0.983 | 226.5 | K = 3.79 × 10−3 Q0 = 3.38 Tlag = 1.84 |
0.9984* | 28.1 |
| Zero order | k = 5.88 × 10−2 | 0.9928 | 15.5 | k = 4.26 × 10−2 | 0.965 | 69.2 | k = 5.79 × 10−2 | 0.962 | 243 | k = 5.33 × 10−2 | 0.9737 | 174.9 | k = 7.9 × 10−2 | 0.9633 | 611.9 | k = 7.59 × 10−2 | 0.9971 | 169.6 |
| F3 |
F9 |
F8 |
||||||||||||||||
| Weibull | a = 2.98 × 1020 b = 7.35 Tlag = 0 |
0.9932 | 3.10 | a = 1.04 × 106 b = 2.09 Tlag = 4.99 |
0.9988* | 1.59 | a = 2.84 × 1020 b = 7.34 Tlag = 0 |
0.9985* | 0.77 | a = 9.94 × 105 b = 2.07 Tlag = 4.99 |
0.9941 | 10.1 | a = 1.04 × 1018 b = 6.52 Tlag = 0 |
0.9783 | 9.75 | a = 8.89 × 105 b = 2.08 Tlag = 4.99 |
0.9991 | 2.62 |
| Higuchi | K = 3.28 × 10−4 | 0.951 | 48.2 | K = 1.02 × 10−1 | 0.977 | 17.4 | K = 3.19 × 10−4 | 0.9553 | 32.9 | K = 9.81 × 10−2 | 0.9563 | 35.1 | K = 1.41 × 10−3 | 0.9887 | 9.50 | K = 1.11 × 10−1 | 0.9752 | 29.6 |
| First order | k = 8.85 × 10−2 | 0.9904 | 10.3 | k = 2.78 × 10−2 | 0.9985 | 1.10 | k = 8.89 × 10−2 | 0.9924 | 5.74 | k = 2.69 × 10−2 | 0.997 | 2.45 | k = 7.41 × 10−2 | 0.9966* | 3.14 | k = 2.69 × 10−2 | 0.9995 | 0.57 |
| Hixon crowel | K = 4.53 × 10−3 Q0 = 1.70 × 10−2 Tlag = 9.55 × 10−1 |
0.9922 | 73.7 | K = 3.04 × 10−3 Q0 = 8.19 × 10−1 Tlag = 4.38 |
0.9978 | 1.59 | K = 4.48 × 10−3 Q0 = 1.67 × 10−2 Tlag = 1.65 |
0.9945 | 47.8 | K = 2.83 × 10−3 Q0 = 8.43 × 10−1 Tlag = 2.72 |
0.998 | 4.66 | K = 4.20 × 10−3 Q0 = 8.74 × 10−2 Tlag = 4.00 |
0.9958 | 6.79 | K = 2.96 × 10−3 Q0 = 9.45 × 10−1 Tlag = 8.40 × 10−1 |
0.9997* | 5.16 |
| Zero order | k = 3.88 × 10−2 | 0.9948* | 51.9 | k = 3.03 × 10−2 | 0.996 | 3.96 | k = 3.79 × 10−2 | 0.9976 | 28.5 | k = 2.69 × 10−2 | 0.9994* | 1.01 | k = 3.74 × 10−2 | 0.993 | 18.3 | k = 3.12 × 10−2 | 0.9988 | 15.5 |
K, k, a, b: constants.
Q0: start value of Q.
Tlag: lag time.
3.7. In vitro and ex vivo permeation of buccal tablets
The permeation parameters such as steady state flux, permeability coefficient were shown in Table 5. Permeation profiles of DS depended on mostly polymeric excipients used in formulations and diffusion barrier characteristics (Fig. 7a and b). Carbopol containing formulations showed less cumulative drug permeation percentage which may be referred to the low and slow release of the DS from the formulations. High swelling index of carbopol can also trigger swollen matrix structure resulting a decrease in drug release (Tønnesen and Karlsen, 2002).
Table 5.
In vitro (a) and ex vivo (b) permeation parameters of formulations.
| (a) | r2 | % drug | jss (mcg cm2/h) | Kp (permeability coefficient) | t (lag time) (min) |
|---|---|---|---|---|---|
| F1 | 0.9958 | 7.2884 | 364 | 0.0291 | 0.8767 |
| F2 | 0.9963 | 4.9109 | 306 | 0.0244 | 0.8723 |
| F3 | 0.9944 | 1.8249 | 87 | 0.0069 | 0.7552 |
| F4 | 0.9941 | 12.7722 | 578 | 0.0462 | 0.0872 |
| F5 | 0.9964 | 9.2642 | 458 | 0.0366 | 0.6507 |
| F6 | 0.9984 | 3.8244 | 184 | 0.0147 | 0.8227 |
| F7 | 0.9931 | 13.0146 | 600 | 0.0480 | 0.6801 |
| F8 | 0.9991 | 1.6853 | 134 | 0.0107 | 2.1431 |
| F9 | 0.9906 | 1.7275 | 106 | 0.0084 | 1.5599 |
| (b) | r2 | % drug | jss (mcg cm2/h) | Kp (permeability coefficient) | t (lag time) (min) |
| F7 | 0.9880 | 4.2574 | 200 | 0.0160 | 1.6501 |
| F3 | 0.9868 | 1.2748 | 90 | 0.0072 | 0.4268 |
Fig. 7.
(a) The effect on polymer type and concentration, in vitro (b) and ex vivo (c) permeation profiles of formulations in 6 h.
Chitosan formulations showed higher drug release, steady state flux and permeability coefficient values compared to carbopol ones. Chitosan has the characteristic of penetration enhancer. One mechanism of this property can be explained with the drug permeation by transporting the drug through the aqueous barrier towards the surface of the membrane on the occasion of the acceleration of the drug passage from the compound into the membrane (Madhav, Shakya, Shakya, and Singh, 2009).
After in vitro permeation studies, in order to enlighten the effect of bovine mucosa on permeation of DS from carbopol and chitosan two formulations were investigated. Formulations that have the highest permeability coefficient and steady state flux values were chosen for permeation studies carried out with bovine buccal mucosa. Permeation values from the bovine buccal mucosa were lower than that of the cellulose membrane on account of keratinized and lipophilic structure of bovine buccal mucosa hindering the diffusion of DS (Table 4, Fig. 7c). As a result of the higher swelling effect of carbopol, permeability values of F3 formulation was found lower than F7 formulation.
4. Conclusion
Polymer type and ratio affect the drug release from the buccal tablets due to their different swelling capacity. Chitosan seems to be a good option for immediate release of DS from buccal tablets based on in vitro and ex vivo studies. Carbopol based formulations showed best mucoadhesive performance. Formulations containing more than 5% carbopol ratio dissolved 22–56% within 12 h time. This finding shows that sustained release buccal tablet formulations must have appropriate ratios of carbopol. Disintegration times changed depending on the polymer’s charges in different pH values. Significant variances between dissolution profiles for buccal tablets, using either USP paddle or flow through cell methods were found. In the same manner, the release profiles and sometimes release kinetics altered when different dissolution methods were used. The total amount of DS released from the tablets was practically the same regardless of the system used. Keratinized and thicker structure of the bovine buccal mucosa has limited the permeation of DS.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelbary A., Elshafeey A., Zidan G. Comparative effects of different cellulosic-based directly compressed orodispersable tablets on oral bioavailability of famotidine. Carbohyd. Polym. 2009;77(4):799–806. [Google Scholar]
- Abdou, H.M., 1989. Dissolution, Bioavailability & Bioequivalence. Mack Pub Co.
- Ahn J.-S., Choi H.-K., Cho C.-S. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials. 2001;22(9):923–928. doi: 10.1016/s0142-9612(00)00256-8. [DOI] [PubMed] [Google Scholar]
- Anwar S., Fell J., Dickinson P. An investigation of the disintegration of tablets in biorelevant media. Int. J. Pharm. 2005;290(1):121–127. doi: 10.1016/j.ijpharm.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Barua S., Kim H., Jo K., Seo C.W., Park T.J., Lee K.B., Lee J. Drug delivery techniques for buccal route: formulation strategies and recent advances in dosage form design. J. Pharm. Invest. 2016:1–21. [Google Scholar]
- Bayrak Z., Tas C., Tasdemir U., Erol H., Ozkan C.K., Savaser A., Ozkan Y. Formulation of zolmitriptan sublingual tablets prepared by direct compression with different polymers: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2011;78(3):499–505. doi: 10.1016/j.ejpb.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Betageri G.V., Deshmukh D.V., Gupta R.B. Oral sustained-release bioadhesive tablet formulation of didanosine. Drug Develop. Indust. Pharm. 2001;27(2):129–136. doi: 10.1081/ddc-100000479. [DOI] [PubMed] [Google Scholar]
- Bolton S., Bon C. CRC Press; 2009. Pharmaceutical Statistics: Practical and Clinical Applications. [Google Scholar]
- Bonacucina G., Martelli S., Palmieri G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004;282(1):115–130. doi: 10.1016/j.ijpharm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Boyapally H., Nukala R.K., Bhujbal P., Douroumis D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf., B. 2010;77(2):227–233. doi: 10.1016/j.colsurfb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Costa P., Lobo J.M.S. Modeling and comparison of dissolution profiles. Euro. J. Pharm. Sci. 2001;13(2):123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- De Robertis S., Bonferoni M.C., Elviri L., Sandri G., Caramella C., Bettini R. Advances in oral controlled drug delivery: the role of drug–polymer and interpolymer non-covalent interactions. Expert Opin. Drug Deliv. 2015;12(3):441–453. doi: 10.1517/17425247.2015.966685. [DOI] [PubMed] [Google Scholar]
- Deshmukh V., Jadhav J., Sakarkar D. Formulation and in vitro evaluation of theophylline anhydrous bioadhesive tablets. Asian J. Pharm. (AJP) 2014;3(1) (Free full text articles from Asian J Pharm) [Google Scholar]
- El-Samaligy M., Yahia S., Basalious E. Formulation and evaluation of diclofenac sodium buccoadhesive discs. Int. J. Pharm. 2004;286(1):27–39. doi: 10.1016/j.ijpharm.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Fayed M.H., Mahrous G.M., Ibrahim M.A., Sakr A. Influence of Carbopol 71G-NF on the release of dextromethorphan hydrobromide from extended-release matrix tablets. Pharm. Develop. Technol. 2013;18(5):971–981. doi: 10.3109/10837450.2011.586037. [DOI] [PubMed] [Google Scholar]
- Gohel M., Patel M., Amin A., Agrawal R., Dave R., Bariya N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. Aaps Pharmscitech. 2004;5(3):10–15. doi: 10.1208/pt050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D.J., Maddock H.L., Baxter G.F., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc. Res. 2002;55(3):534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Junginger H. Mucoadhesive hydrogels. Pharmazeutische Industrie. 1991;53(11):1056–1065. [Google Scholar]
- Kassem M.A., ElMeshad A.N., Fares A.R. Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2014;463(1):68–80. doi: 10.1016/j.ijpharm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15(1):25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Kranz H., Guthmann C., Wagner T., Lipp R., Reinhard J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Euro. J. Pharm. Sci. 2005;26(1):47–53. doi: 10.1016/j.ejps.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Kristmundsdottir T., Ingvarsdottir K., Semundsdottir G. Chitosan matrix tablets: the influence of excipients on drug release. Drug Develop. Indust. Pharm. 1995;21(13):1591–1598. [Google Scholar]
- Langenbucher F., Benz D., Kürth W., Möller H., Otz M. Standardized flow-cell method as an alternative to existing pharmacopoeial dissolution testing. Pharmazeutische Industrie. 1989;51(11):1276–1281. [Google Scholar]
- Madhav N.S., Shakya A.K., Shakya P., Singh K. Orotransmucosal drug delivery systems: a review. J. Control. Rel. 2009;140(1):2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Malakar J., Nayak A. Floating bioadhesive matrix tablets of ondansetron HCl: optimization of hydrophilic polymer-blends. Asian J. Pharm. 2013;7(4):174. [Google Scholar]
- Medina J.R., Salazar D.K., Hurtado M., Cortes A.R., Domínguez-Ramírez A.M. Comparative in vitro dissolution study of carbamazepine immediate-release products using the USP paddles method and the flow-through cell system. Saudi Pharm. J. 2014;22(2):141–147. doi: 10.1016/j.jsps.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munasur A.P., Pillay V., Choonara Y.E., Mackraj I., Govender T. Comparing the mucoadhesivity and drug release mechanisms of various polymer-containing propranolol buccal tablets. Drug Develop. Indust. Pharm. 2008;34(2):189–198. doi: 10.1080/03639040701539842. [DOI] [PubMed] [Google Scholar]
- Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Develop. Indust. Pharm. 2004;30(9):985–993. doi: 10.1081/ddc-200037245. [DOI] [PubMed] [Google Scholar]
- Nayak A., Laha B., Sen K. Development of hydroxyapatite-ciprofloxacin bone-implants using «Quality by design». Acta Pharm. 2011;61(1):25–36. doi: 10.2478/v10007-011-0002-x. [DOI] [PubMed] [Google Scholar]
- Nigalaye A.G., Adusumilli P., Bolton S. Investigation of prolonged drug release from matrix formulations of chitosan. Drug Develop. Indust. Pharm. 1990;16(3):449–467. [Google Scholar]
- Palmer D., Levina M., Douroumis D., Maniruzzaman M., Morgan D.J., Farrell T.P., Nokhodchi A. Mechanism of synergistic interactions and its influence on drug release from extended release matrices manufactured using binary mixtures of polyethylene oxide and sodium carboxymethylcellulose. Colloids Surf., B. 2013;104:174–180. doi: 10.1016/j.colsurfb.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Park C.R., Munday D.L. Development and evaluation of a biphasic buccal adhesive tablet for nicotine replacement therapy. Int. J. Pharm. 2002;237(1):215–226. doi: 10.1016/s0378-5173(02)00041-8. [DOI] [PubMed] [Google Scholar]
- Park C.R., Munday D.L. Evaluation of selected polysaccharide excipients in buccoadhesive tablets for sustained release of nicotine. Drug Develop. Indust. Pharm. 2004;30(6):609–617. doi: 10.1081/ddc-120037492. [DOI] [PubMed] [Google Scholar]
- Park S.-H., Chun M.-K., Choi H.-K. Preparation of an extended-release matrix tablet using chitosan/Carbopol interpolymer complex. Int. J. Pharm. 2008;347(1):39–44. doi: 10.1016/j.ijpharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Patel V.M., Prajapati B.G., Patel M.M. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. Aaps Pharmscitech. 2007;8(1):E147–E154. doi: 10.1208/pt0801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh K.K., Wong C.F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999;2(2):53–61. [PubMed] [Google Scholar]
- Peppas N.A., Korsmeyer R. Dynamically swelling hydrogels in controlled release applications. Hydrog. Med. Pharm. 1987;3:109–136. [Google Scholar]
- Perez-Marcos B., Ford J.L., Armstrong D.J., Elliott P.N., Rostron C., Hogan J.E. Influence of pH on the release of propranolol hydrochloride from matrices containing hydroxypropylmethylcellulose K4M and carbopol 974. J. Pharm. Sci. 1996;85(3):330–334. doi: 10.1021/js950359z. [DOI] [PubMed] [Google Scholar]
- Pharmacopeia, United States, 2014. 38/National Formulary 33.
- Polli J.E., Rekhi G.S., Augsburger L.L., Shah V.P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J. Pharm. Sci. 1997;86(6):690–700. doi: 10.1021/js960473x. [DOI] [PubMed] [Google Scholar]
- Roy D.S., Rohera B.D. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Euro. J. Pharm. Sci. 2002;16(3):193–199. doi: 10.1016/s0928-0987(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Russo E., Selmin F., Baldassari S., Gennari C., Caviglioli G., Cilurzo F., Parodi B. A focus on mucoadhesive polymers and their application in buccal dosage forms. J. Drug Deliv. Sci. Technol. 2016;32:113–125. [Google Scholar]
- Savaşer A., Özkan Y., Işımer A. Preparation and in vitro evaluation of sustained release tablet formulations of diclofenac sodium. Il Farmaco. 2005;60(2):171–177. doi: 10.1016/j.farmac.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Shangraw R.F. Compressed tablets by direct compression. Pharm. Dosage Forms: Tablets. 1989;1:195–246. [Google Scholar]
- Singla A.K., Chawla M., Singh A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: a review. Drug Develop. Indust. Pharm. 2000;26(9):913–924. doi: 10.1081/ddc-100101318. [DOI] [PubMed] [Google Scholar]
- Sudhakar Y., Kuotsu K., Bandyopadhyay A. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J. Control. Rel. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Timmins P., Desai D., Chen W., Wray P., Brown J., Hanley S. Advances in mechanistic understanding of release rate control mechanisms of extended-release hydrophilic matrix tablets. Therap. Deliv. 2016;7(8):553–572. doi: 10.4155/tde-2016-0026. [DOI] [PubMed] [Google Scholar]
- Topcu F.T., Sahinkesen G., Yamanel K., Erdemir U., Oktay E.A., Ersahan S. Influence of different drinks on the colour stability of dental resin composites. Eur. J. Dent. 2009;3(1):50–56. [PMC free article] [PubMed] [Google Scholar]
- Tønnesen H.H., Karlsen J. Alginate in drug delivery systems. Drug Develop. Indust. Pharm. 2002;28(6):621–630. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- Varshosaz J., Dehghan Z. Development and characterization of buccoadhesive nifedipine tablets. Eur. J. Pharm. Biopharm. 2002;54(2):135–141. doi: 10.1016/s0939-6411(02)00078-4. [DOI] [PubMed] [Google Scholar]
- Yuksel N., Kanık A.E., Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and-independent methods. Int. J. Pharm. 2000;209(1):57–67. doi: 10.1016/s0378-5173(00)00554-8. [DOI] [PubMed] [Google Scholar]