Abstract
The development of nanoparticle-based drug formulations has yielded the opportunities to address and treat challenging diseases. Nanoparticles vary in size but are generally ranging from 100 to 500 nm. Through the manipulation of size, surface characteristics and material used, the nanoparticles can be developed into smart systems, encasing therapeutic and imaging agents as well as bearing stealth property. Further, these systems can deliver drug to specific tissues and provide controlled release therapy. This targeted and sustained drug delivery decreases the drug related toxicity and increase patient’s compliance with less frequent dosing. Nanotechnology has proven beneficial in the treatment of cancer, AIDS and many other disease, also providing advancement in diagnostic testing.
1. Introduction
Comparing current practice of medicine to that of the last century, one cannot help but to notice innumerable advancements to address previously incurable diseases (Sheingold and Hahn, 2014). Numerous new medications have been developed to effectively treat complicated conditions, but at the same time some of them produce severe side effects that the benefit does not always outweigh the risk (Liebler and Guengerich, 2005). On the other hand, some drugs have been proven to be very effective in vitro but cannot withstand the endogenous enzymes found within the gastrointestinal (GI) tract (if taken orally), deeming them nearly worthless in vivo (Rostami-Hodjegan and Tucker, 2007). While incredible progress has been made in identifying drug targets, designing and making better drug molecules; there is still room to improve the drug delivery systems and targeting (Tiwari et al., 2012).
Within past few decades, nanotechnology, in particular manufacturing of nanoparticles has found an unprecedented attention in broad areas of science (Bhattacharyya et al., 2009). A PubMed search (“nanoparticles”) reveals, last year alone (2016), there were “19,338” articles published related to various aspects of nanoparticle technology (PubMed, 2017). The clever use of nanoparticles has revolutionized how drugs are formulated and delivered. Nanotechnology is a multi-disciplinary scientific field applying engineering and manufacturing principles at the molecular level (Emerich and Thanos, 2006). By applying nanotechnology to medicine, nanoparticles have been created to mimic or alter biological processes (Singh and Lillard, 2009). Nanoparticles are solid, colloidal particles with size range from 10 nm to <1000 nm; however, for nanomedical application, the preferential size is less than 200 nm (Biswas et al., 2014). One of the most significant areas of study has been in the creation of nanoparticle drug delivery systems. This succinct review will focus on the desirable characteristics for successful nanoparticle based drug delivery systems as well as the various disease states in which these nanoparticle systems have shown promise.
2. Necessity for nanoparticle-based drug formulations
There are various reasons why using nanoparticles for therapeutic and diagnostic agents, as well as advancement of drug delivery, is important and much needed. One of them is that, traditional drugs available now for oral or injectable administration are not always manufactured as the optimal formulation for each product. Products containing proteins or nucleic acids require a more innovative type of carrier system to enhance their efficacy and protect them from unwanted degradation (Vo et al., 2012). It is notable that the efficiency of most drug delivery systems is directly related to particle size (excluding intravenous and solution). Due to their small size and large surface area, drug nanoparticles show increase solubility and thus enhanced bioavailability, additional ability to cross the blood brain barrier (BBB), enter the pulmonary system and be absorbed through the tight junctions of endothelial cells of the skin (Kohane, 2007). Specifically, nanoparticles made from natural and synthetic polymers (biodegradable and non-biodegradable) have received more attention because they can be customized for targeted delivery of drugs, improve bioavailability, and provide a controlled release of medication from a single dose; through adaptation the system can prevent endogenous enzymes from degrading the drug (Zhang and Saltzman, 2013). Secondly, the development of new drug delivery systems is providing another advantage for pharmaceutical sales to branch out. Innovative drug delivery is driving the pharmaceutical companies to develop new formulations of existing drugs. While these new formulations will be beneficial to the patients, it will also create a powerful market force, driving the development of even more effective delivery methods (Emerich and Thanos, 2007).
Furthermore, not only will the companies thrive to develop new formulations for their own “intellectual property,” but will have motivation due to patent expirations (Osakwe and Rizvi, 2016). The benefit of pharmaceutical companies taking advantage of this new technology is that nanotechnology gives new life to those drugs those were previously considered unmarketable due to low solubility and bioavailability, and high toxicity and marked side effects (Onoue et al., 2014). Finally, we would like to highlight a very recent article from Prof. Robert Langer’s group, at the Massachusetts Institute of Technology (Kakkar et al., 2017), with an up-to-date survey of the types of polymeric systems used in the drug delivery.
3. Characteristics of nanoparticle drug formulations
Before defining exactly what an ideal nanoparticle-based drug delivery system is made of, understanding how the body handles the exogenous particulate matter is warranted. Nanoparticles can enter the human body, via three main route, direct injection, inhalation and oral intake. Once, they enter systemic circulation, particle-protein interaction is the first phenomenon taking place before distribution into various organs (Mu et al., 2014, Prado-Gotor and Grueso, 2011). Absorption from the blood capillaries allows the lymphatic system to further distribute and eliminate the particles. This system has three main functions, two of which pertain to drug delivery. The first, fluid recovery, involves the filtering of fluids by the lymphatic system from blood capillaries. The second encompasses immunity, and may be the most relevant to this topic. As the system recovers excess fluid, it also picks up foreign cells and chemicals from the tissues. As the fluids are filtered back into the blood, the lymph nodes detect any foreign matter passing through (Park et al., 2016). If something is recognized as foreign, macrophages will engulf and clear it from the body. This tends to be the struggle with nanoparticle based drug delivery; however, clearance can be influenced by the size and surface characteristics of particles, which will be elaborated on in following subsections (Alexis et al., 2008).
3.1. Size of particle
The shape and size of nanoparticles affects how cell in the body “see” them and thus dictate their distribution, toxicity, and targeting ability. Most importantly, nanoparticles can cross the BBB providing sustained delivery of medication for diseases that were previously difficult to treat (McMillan et al., 2011). Not only is it possible to reach new targets, but this technique can be manipulated to control drug distribution. It has been reported that 100 nm nanoparticles exhibited a 2.5-fold greater uptake compared to 1 μm diameter particles and a 6-fold great uptake than a 10 μm particles (Desai et al., 1997).
It has been discussed how important the nanoparticle drug delivery systems are, but these systems would be on no use if the drug is not released or released effectively (Chavanpatil et al., 2007). As particles size get smaller, their surface area to volume ratio gets larger. This would imply that more of the drug is closer to the surface of the particle compared to a larger molecule. Being at or near the surface would lead to faster drug release (Buzea et al., 2007). It would be beneficial to create nanoparticle systems that have a large surface area to volume ratio; however, toxicity must always be monitored. As mentioned earlier, the size of the nanoparticle determines biological fate. Remember that the vascular and lymph systems are responsible for the filtering and clearance of foreign matter and chemicals. This is yet another factor that must be engineered into the ideal nanoparticle system. It has been shown that particles 200 nm or larger tend to activate the lymphatic system and are removed from circulation quicker (Prokop et al., 2008). Thus, from the literature evaluation and discussion so far, it is clear as though the optimum size for a nanoparticle is approximately 100 nm. At this size, the particle could pass through the BBB, sufficient amount of drug delivery due to high surface area to volume ratio, and avoiding immediate clearance by the lymphatic system.
3.2. Surface properties
It has been noted how size can influence the performance of nanoparticle-based drug formulations; however, manipulation of surface characteristics is another opportunity to generate the ideal system (Bantz et al., 2014). In order to create an optimum nanoparticle drug delivery system, the incorporation of appropriate targeting ligands, surface curvature and reactivity is important to address the prevention of aggregation, stability, and receptor binding and subsequent pharmacological effects of the drug (Khanbabaie and Jahanshahi, 2012).
First, clearance of nanosystems must be addressed. Since nanoparticles can be recognized by the lymphatic system, they are subject to the body’s natural immune response to foreign matter. The more hydrophobic a nanoparticle is, the more likely it is to be cleared due to higher binding of blood components (Kou, 2013). As hydrophobic nanoparticles are cleared easily, it seems logical to assume that making their surface hydrophilic would increase their time in circulation. In fact, coating the nanoparticles with polymers or surfactants or creating copolymers like polyethylene glycol (PEG), diminishes the opsonization), polyethylene oxide, polyethylene glycol (prevents hepatic and splenic localization), polyoxamer, poloxamine, and polysorbate 80 has been proven valuable (Araujo et al., 1999, Labhasetwar et al., 1998). PEG is hydrophilic and relatively inert polymer that when incorporated in the nanoparticle surface, hinders the binding of plasma proteins (opsonization), and thus preventing substantial loss of the given dose. PEGylated nanoparticles are often referred as “stealth” nanoparticles, because without opsonization, they remain undetected by the reticuloendothelial system (RES) (Li and Huang, 2010, Angra et al., 2011). By creating polymer complexes, clearance issues have been addressed, but aggregation is still a concern with small particles due to large surface area. Particularly, nanoparticles such as dendrimers, quantum dots, and micelles are especially prone to aggregation. Several strategies have been employed to prevent aggregation and call for particles coating with capping agents and altering the zeta potential (surface charges) (Li and Kaner, 2006).
Overall, these methods and theories can be summarized into one idea: the size of the particle must be large enough to avoid leakage into blood capillaries, but not too large to become susceptible to macrophage clearance. By manipulating the surface, the extent of aggregation and clearance can be controlled (Sykes et al., 2016).
3.3. Drug loading and release
The size and surface properties of nanoparticles have been explored to optimize bioavailability, decrease clearance, and increase stability. By controlling these characteristics, it is possible to get the drug to tissues in the body that may have been inaccessible before. However, there is no significance of this practice if the drug cannot then be released from the nanoparticle matrix. The release of drug from the nanoparticle-based formulation depends on may factors including, pH, temperature, drug solubility, desorption of the surface-bound or adsorbed drug, drug diffusion through the nanoparticle matrix, nanoparticle matrix swelling and erosion, and the combination of erosion and diffusion processes (Son et al., 2017, Mura et al., 2013, Siepmann and Göpferich, 2001).
Depending on the type of nanoparticle being used, the release of drug will differ. The prepared polymeric nanoparticles can be called nanocapsules or nanospheres based on their composition. Nanospheres are homogeneous system such that the polymer chains arrange in a similar fashion to surfactants in micelle formation (phase-separated from the bulk solution). While, nanocapsules are heterogeneous system, such that the drug is inside a reservoir composed of the polymer (like vesicle) (Mora-Huertas et al., 2010).
In relation to nanospheres, which are matrix system where the drug is physically and uniformly dispersed, drug is released by erosion of the matrix. There is a rapid burst of drug release related to weakly bound drug to the large surface area of the nanoparticle followed by a sustained release (Lee and Yeo, 2015). On the other hand, if nanocapsules are used, the release is controlled by drug diffusion through the polymeric layer and thus, drug diffusibility through that polymer is definitely a determining factor of its deliverability. If there are ionic interactions between the drug and polymer, they will form complexes which inhibit the release of drug from the capsule. This can be avoided by adding other auxiliary agents such as polyethylene oxide-propylene oxide (PEO-PPO). This will decrease the interactions between the drug and capsule matrix allowing for greater release of drug to target tissues (Calvo et al., 1997).
4. Targeted drug delivery
After recognizing the importance of nanoparticle manipulation to achieve a successful drug delivery system, the next logical step is the development of targeted drug delivery. The nanoparticles can breach the inflamed or damaged tissue due to larger epithelial junctions. This penetration can occur passively or actively. Active targeting is when the drug carrier system is conjugated to a tissue or cell-specific ligand, while passive targeting is when nanoparticle reaches the target organ due to the leaky junctions (Varshosaz and Farzan, 2015).
An ideal nanoparticle drug delivery system (Fig. 1) should be able to reach, recognized, bind and deliver its load to specific pathologic tissues, and minimize or avoids drug induced damage to healthy tissues. Thus, coating specific targeting ligand(s) on the surface of nanoparticles is the most common strategy. These targeting ligands could be in the form of small molecules, peptides, antibodies, designed proteins, and nucleic acid aptamers (Liu et al., 2009, Friedman et al., 2013).
Fig. 1.
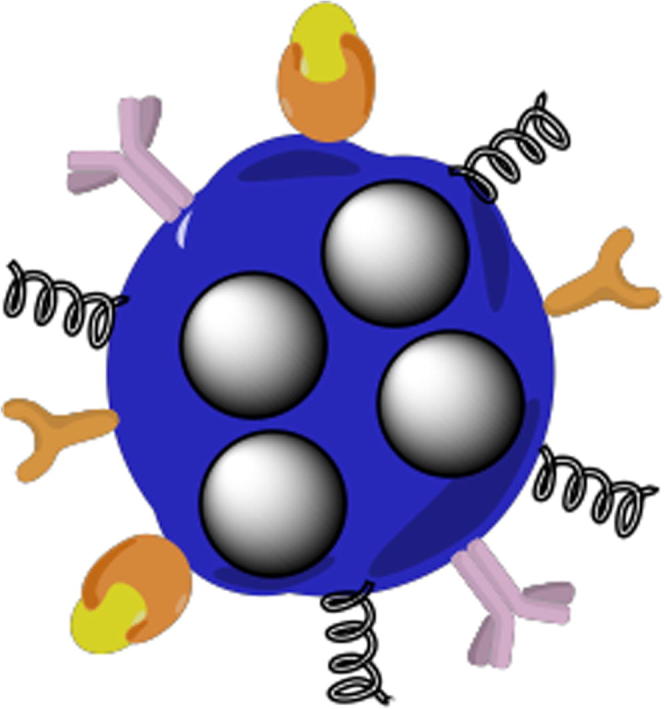
Representation of a smart multifunctional drug loaded nanoparticle, decorated with various moieties for targeting, imaging and stealth properties.
Small organic molecules are the most commonly employed targeting agents due to relative ease of preparation, stability, and control of conjugation chemistry with. These targeting ligands may not have desired specificity and affinity. Biotin (vitamin H), due to very high affinity for streptavidin has been widely used for conjugation with nanoparticles (Pramanik et al., 2016). Folic acid (vitamin B9) has excessive affinity for endogenous folate receptor and thus has been investigated for targeting many types of cancers where folate receptors are highly expressed (Zhao et al., 2008). Similarly, many other task specific carbohydrates, short peptides, antibodies and small molecules have been designed and employed (Friedman et al., 2013).
Another useful discovery to aid in the targeted delivery of drugs is liposomes. Since they mimic the cell membrane, one can design specific lipid monomer to tailor physicochemical properties such as size and charge and can also incorporate surface targeting ligands as discussed above (Kelly et al., 2011). This system over additional advantage, since liposomal composition is similar to the targeted cell membrane, an enhanced lipid-lipid exchange occurs. This speeds up the convective flux of lipophilic drugs from the liposomal lipid layer into the targeted cell membrane (Singh and Lillard, 2009, Kelly et al., 2011, Pattni et al., 2015).
5. Application of nanoparticle technology
5.1. Cancer therapy
The type of therapy used to treat cancer patients today, has saved lives of many individuals; however, the side effects of treatment are harsh, affecting the entire body due to non-specificity of the chemotherapeutic agents. Cancer is a very complicated biological phenomenon, and can be considered a disease of many diseases. One of the hallmark of cancerous cells is that they divide and multiply rapidly and out of control (Hanahan and Weinberg, 2011). Current chemotherapy is mainly aimed at destroying all rapidly dividing cells. The downside of this therapy is that the body’s other rapidly proliferating cells, such as in the hair follicles and intestinal epithelium are also killed off, leaving the patient to cope with life altering side effects (Baudino, 2015). The development of nanoparticles has provided a new avenue for chemotherapy. With smartly designed nanoparticles, targeted drug delivery at the tumor site or a certain group of cells do largely avoid the toxic effects to other normal tissues and organs (Shen et al., 2016, Huang, et al., 2015). There have been several systems tested to provide this type of therapy.
Micelles and liposomes offer another option for delivery of chemotherapeutic agents. Additionally, micelles are also a great way to make insoluble drugs soluble due to their hydrophobic core and hydrophilic shell. If the micelle’s surface is further PEGylated, it increases the ability of the nanocarriers to get through fenestrated vasculature of tumors and inflamed tissue through passive transport, thus resulting in higher drug concentration in tumors. As of now, several polymeric micelles containing anticancer drugs, NK012, NK105, NK911, NC-6004, and SP1049C are under clinical trials (Oerlemans et al., 2010) and one such system, Genexol-PM (paclitaxel) is approved for breast cancer patients (Zhang et al., 2014).
Dendrimers are highly branched macromolecules with many functional groups available for the attachment of drug, targeting and imaging agents and their absorption, distribution, metabolism and elimination (ADME) profile is dependent upon various structural feature (Somani and Dufes, 2014, Kaminskas et al., 2011). A polyfunctional dendrimer system has been reported for successful localization (Folic acid), imaging (fluorescein) and delivery of the anticancer drug methotrexate in vitro (Quintana et al., 2002). Nanoparticle therapeutics based on dendrimers can improve the therapeutic index of cytotoxic drugs by employing biocompatible components, and the surface derivatization with PEGylation, acetylation, glycosylation, and various amino acids (Baker, 2009, Cheng et al., 2011).
While there are several other forms of nanoparticles that have shown promise in cancer treatment, one of the most recent system is the carbon nanotubes. Carbon nanotubes (CNTs) is an allotropic form of carbon with cylindrical framework and deepening on number of sheets in concentric cylinders, they can be classified as single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs) (Rastogi et al., 2014, Sanginario et al., 2017). Since Carbon nanotubes have very hydrophobic hollow interior, water insoluble drugs can easily be loaded them. The large surface area allows for outer surface functionalization and can be done specifically for a particular cancer receptor as well as contrast agents (Dinesh et al., 2016).
Finally, Buckminsterfullerene C60 (spherical molecule) and its derivatives are evaluated for the treatment of cancer (Murugesan et al., 2007, Chen et al., 2012). Fullerene C60 can bind up to six electrons, and thus act as an excellent scavenger of reactive oxygen species (ROS) as well (Prylutska et al., 2008). It has been reported that fullerene nanocrystals (Nano-C60) can enhance the cytotoxicity of chemotherapeutic agents and thus a Nano-C60 adjunct chemotherapy can be further evaluated (Zhang et al., 2009). Prylutska et al., (2015) conducted another study using the complex of Fullerene C60 with Doxorubicin and noted the tumor volumes of the treated rats (C60 + Dox) to be 1.4 times lower compared to the control group (untreated rats). Furthermore, the mechanism of action of C60 + Dox complex is thought to be via its direct action on tumor cells as well as immunomodulating effect.
5.2. Diagnostic testing
The use of nanoparticle for diagnostic purposes is an area that currently unavailable for clinical application, but heavily explored in academia (Kolluru et al., 2013). Since current technology for diagnostic testing is hindered by the inadequacies of fluorescent markers including fading of fluorescence after single use, color matching, and restricted use of dyes due to a bleeding effect, fluorescent nanoparticles provide researchers with the answer to overcome these disadvantages (Wolfbeis, 2015).
One important breakthrough was the discovery of quantum dots which can be custom-made in many sharply defined colors. Their absorption spectrum ranges from UV to a wavelength within visible spectrum and provide high quantum yield, tunable emission spectrum and photostability. Size of the nanodot determines where in the spectra that individual particle falls. Larger particles have longer wavelengths and emission is narrow (Emerich and Thanos, 2006, Li and Zhu, 2013, Michalet et al., 2005). Tagging of the quantum dots has several advantages. First, they are excitable using white light. Secondly, they can be linked to biomolecules that can spend consider amount of time in the living system to probe various bio-mechanisms. This technology further allows one to monitor many biological events simultaneously by tagging various biological molecules with nanodots of a specific color (Datta and Jaitawat, 2006).
Recently, theranostic nanoparticles, nanoparticles that can be used for treatment as well as diagnoses have gain much attention (Janib et al., 2010). This strategy has been realized in many classes of nanoparticles including, drug conjugates, dendrimers, surfactant aggregates (micelles and vesicles), core-shell particles, and carbon nanotubes. By combing both drug and imaging agent in one smart formulation various, it is possible to monitor the pathway and localization of these nanoparticle at the target site as well drug action to assess therapeutic response (Bhojani et al., 2010).
5.3. HIV and AIDS treatment
Infection with human immunodeficiency virus (HIV), if not addressed can lead to acquired immune deficiency syndrome (AIDS) is a devastating disease where an individual’s immune system is almost destroyed (Moss, 2013). When treatment was first developed for this disease, it was painstakingly involved, where most patients could be taking 30–40 pills a day. In the past decade, there have been advancements in therapeutics to reduce the pill count down to just a few each day (Bartlett and Moore, 1998). Research has shown a way to make this therapy even more effective by creating polymeric nanoparticles that deliver antiretroviral (ARV) drugs intracellularly as well as to the brain (Mamo et al., 2010). This technology can also be used in adjunct with vaccinations to prevent HIV infections (Khalil, 2011).
Antiretroviral drugs that are used to treat HIV, can be categories depending on the stages of HIV life cycle they work most suitably on. In order to prevent the development of resistance and aggressively counter the HIV progression, a combination of multiple drugs (three or more) are used, this known as highly effective antiretroviral therapy (HAART) (Crabtree-Ramírez et al., 2010). Nanotechnology has played a pivotal role in delivering the antiretroviral drugs and improving compliance (Jayant, 2016). Antiretroviral drugs must be able to cross the mucosal epithelial barrier when taken orally or other non-parental routes (suppository and patches, etc.). Lymphoid tissues are major sites for HIV to infect and thrive. A number of reports have demonstrated that nanoparticle loaded with antiretroviral drugs were able to target monocytes and macrophages in vitro (Shah and Amiji, 2006, Mallipeddi and Rohan, 2010). A prime example of superiority and success of nanoparticle system for sustained and targeted drug delivery was reported by Destache et al. (2009). The investigators used poly(lactic-co-glycolic acid) (PLGA) to prepare nanoparticles entrapping three antiretroviral drugs, ritonavir, lopinavir, and efavirenz. The nanoparticle system yielded sustained drug release for over 4 weeks (28 days), while free drugs were eliminated within 48 h (2 days). The Central nervous system (CNS) is another site for HIV to inoculate and thrive resulting in serious HIV-associated neurocognitive disorder (HAND) (Spudich and Ances, 2011). Nanoparticles are a known to be able to cross BBB by endocytosis/phagocytosis and many reports exist showing successful delivery of anti-HIV medications (Rao et al., 2009, Nowacek et al., 2010).
5.4. Nutraceutical delivery
Nutraceuticals are food derived, standardized components with noticeable health benefits. They are commonly consumed as complement to various allopathic treatments as well as to provide extra health benefits and decrease risks of several chronic illnesses (Aggarwal et al., 2009). Similar to the case of any other drug, the bioavailability and thus efficacy of orally consumed nutraceuticals is affected by food matrices interactions, aqueous solubility, degradation/metabolism, and epithelial permeability (McClements et al., 2015). Most nutraceuticals are lipophilic molecules, such as fat-soluble vitamins (A, D, E and K), polyunsaturated lipids and other phytochemicals. Nanotechnology again offers comprehensive assistance and most of the investigations have been aimed at improving the dissolution mechanisms of nutraceuticals via nanoparticle formulations (Acosta, 2009, McClements, 2015).
A large number of nutraceuticals, posse anti-inflammatory, antioxidative, antiapoptotic, and antiangiogenic activities, among those, the most prominent and studied is curcumin (diferuloylmethane). It is practically water-insoluble and has very poor bioavailability, thus various methods have been implemented to address this issue, such as liposomes, phospholipid vesicles, and polymer-based nano-formulation (Mohanty et al., 2010, Carvalho and Takeuchi et al., 2015). A 9-fold higher oral bioavailability of curcumin was observed when compared to curcumin co-administered with piperine (absorption enhancer) (Shaikh et al., 2009.). Another study of colloidal nanoparticles of curcumin dubbed, Theracurmin when compared to curcumin powder, exhibited 40-fold higher area under the curve (AUC) in rats and 27-fold higher in healthy human volunteers as well as inhibitory actions against alcohol intoxication (Sasaki et al., 2011).
Resveratrol is an important non-flavonoid polyphenol, naturally occurs in several plants but most abundantly found in Vitis vinifera, labrusca, and muscadine grapes (Celotti and Ferrarini, 1996.). It is known for antioxidant, cardioprotective, anti-inflammatory and anticancer activities (Summerlin et al., 2015). Resveratrol has low solubility, with decent bioavailability, however, it is rapidly metabolized and eliminated (Walle, 2011, Kapetanovic et al., 2011) from the body. There are two geometric isomers of resveratrol (cis- and trans), however the more abundant and bioactive trans-resveratrol, is photosensitive, converters to cis-resveratrol in the presence of light (Trela and Waterhouse, 1996). Many nanoformulations of resveratrol to improve the pharmacokinetic profile and bioavailability have been reported. These include polymeric nanoparticles (da Rocha Lindner and Bonfanti Santos, 2015, Sanna et al., 2013), Zein based nanoparticles (Penalva et al., 2015), nanoemulsions (Sessa et al., 2013), liposomes (Catania et al., 2013), cyclodextrins (Venuti et al., 2014), and dual nanoencapsulation methods (Soo et al., 2016). Recently, neuroprotective effects of resveratrol were evaluated by preparing solid lipid nanoparticles decorated with apolipoprotein E for LDL receptor recognition on the blood-brain barrier (Neves et al., 2015).
6. Conclusion
Nanotechnology is truly a multidisciplinary science where chemists, physicist, biologists and pharmaceutical scientist all have played major roles to develop novel treatment and diagnosing modalities. It is evident through this review that application of nontechnology in drug delivery and medicine has paved new pathways and opened many doors for providing customizable and safer treatment option. The treatment of cancer and HIV/AIDS, non-invasive imaging as well as nutraceutical delivery have all progressed with the application of nanotechnology. Ultimately, through the manipulation of molecular size and surface properties, researchers are able to deliver drugs for longer period of time with less frequent dosing (sustained release) and with greater precision and penetration in difficult to access tissues.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Syed A.A. Rizvi, Email: srizvi@nova.edu.
Ayman M. Saleh, Email: salehay@ksau-hs.edu.sa.
References
- Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009;14(1):3–15. [Google Scholar]
- Aggarwal B.B., Van Kuiken M.E. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. (Maywood) 2009;234(8):825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis F., Pridgen E. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angra P.K., Rizvi S.A.A. Novel approach for preparing nontoxic stealth microspheres for drug delivery. Eur. J. Chem. 2011;2:125–129. [Google Scholar]
- Araujo L., Lobenberg R. Influence of the surfactant concentration on the body distribution of nanoparticles. J. Drug Target. 1999;6:373–385. doi: 10.3109/10611869908996844. [DOI] [PubMed] [Google Scholar]
- Baker J.R., Jr. Dendrimer-based nanoparticles for cancer therapy. Hematol. Am. Soc. Hematol. Educ. Program. 2009:708–719. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- Bantz C., Koshkina O. The surface properties of nanoparticles determine the agglomeration state and the size of the particles under physiological conditions. Zellner, R., ed. Beilstein J. Nanotechnol. 2014;5:1774–1786. doi: 10.3762/bjnano.5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, J.G., Moore, R.D., 1998. Improving HIV therapy. Sci. Am. 279(1), 84–7, 89. [DOI] [PubMed]
- Baudino T.A. Targeted cancer therapy: the next generation of cancer treatment. Curr. Drug Discov. Technol. 2015;12(1):3–20. doi: 10.2174/1570163812666150602144310. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D., Singh S. Nanotechnology, big things from a tiny world: a review. Int. J. u- and e-Serv, Sci. Technol. 2009;2(3):29–38. [Google Scholar]
- Bhojani M.S., Van Dort M. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol. Pharm. 2010;7(6):1921–1929. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, A.K., Islam, M.R., et al., 2014. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci.: Nanosci. Nanotechnol. 5, 043001.
- Buzea, C., Pacheco, I.I., et al., 2007. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2(4), MR17-71. [DOI] [PubMed]
- Calvo P., Remuñan-López C. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997;14:1431–6143. doi: 10.1023/a:1012128907225. [DOI] [PubMed] [Google Scholar]
- Carvalho D.d.M., Takeuchi K.P. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. (Campinas) 2015;35(1):115–119. [Google Scholar]
- Catania A., Barrajón-Catalán E. Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res. Treat. 2013;1:55–65. doi: 10.1007/s10549-013-2667-y. [DOI] [PubMed] [Google Scholar]
- Celotti E., Ferrarini R. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J. Chromatogr. A. 1996;730(1–2):47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- Chavanpatil M.D., Khdair A. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J. Pharm. Sci. 2007;96(12):3379–3389. doi: 10.1002/jps.20961. [DOI] [PubMed] [Google Scholar]
- Chen Z., Mao R. Fullerenes for cancer diagnosis and therapy: preparation, biological and clinical perspectives. Curr. Drug Metab. 2012;13(8):1035–1045. doi: 10.2174/138920012802850128. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Zhao L. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem. Soc. Rev. 2011;40(5):2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- Crabtree-Ramírez B., Villasís-Keever A. Effectiveness of highly active antiretroviral therapy (HAART) among HIV-infected patients in Mexico. AIDS Res. Hum. Retroviruses. 2010;26(4):373–378. doi: 10.1089/aid.2009.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha Lindner G., Bonfanti Santos D. Improved neuroprotective effects of resveratrol-loaded polysorbate 80-coated poly(lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine (Lond) 2015;10(7):1127–1138. doi: 10.2217/nnm.14.165. [DOI] [PubMed] [Google Scholar]
- Datta R., Jaitawat S. Nanotechnology - the new frontier of medicine. Med. J. Armed Forces India. 2006;62(3):263–268. doi: 10.1016/S0377-1237(06)80016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M.P., Labhasetwar V. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- Destache C.J., Belgum T. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect. Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh B., Bianco A. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale. 2016;8(44):18596–18611. doi: 10.1039/c6nr06728j. [DOI] [PubMed] [Google Scholar]
- Emerich D.F., Thanos C. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006;23:171–184. doi: 10.1016/j.bioeng.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Emerich D.F., Thanos C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007;15(3):163–183. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- Friedman A.D., Claypool S.E. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013;19(35):6315–6329. doi: 10.2174/13816128113199990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Huang, B., Abraham, W.D., et al., 2015. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Trans. Med. 7, 291ra94. [DOI] [PMC free article] [PubMed]
- Janib S.M., Moses A.S. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayant R., Nair M. Nanotechnology for the treatment of NeuroAIDS. J. Nanomed. Res. 2016;3(1):00047. [Google Scholar]
- Kakkar A., Traverso G. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017;1(8):0063. doi: 10.1038/s41570-017-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskas L.M., Boyd B.J. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine. 2011;6(6):1063–1084. doi: 10.2217/nnm.11.67. [DOI] [PubMed] [Google Scholar]
- Kapetanovic I.M., Muzzio M. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Jefferies C. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv. 2011 doi: 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Carraro E. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin., Drug Deliv. 2011;8(1):95–112. doi: 10.1517/17425247.2011.543673. [DOI] [PubMed] [Google Scholar]
- Khanbabaie R., Jahanshahi M. Revolutionary impact of nanodrug delivery on neuroscience. Curr. Neuropharmacol. 2012;10(4):370–392. doi: 10.2174/157015912804143513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007;96(2):203–209. doi: 10.1002/bit.21301. [DOI] [PubMed] [Google Scholar]
- Kolluru L.P., Rizvi S.A.A. Formulation development of albumin based theragnostic nanoparticles as a potential tumor targeting and delivery system. J. Drug Target. 2013;21:77–86. doi: 10.3109/1061186X.2012.729214. [DOI] [PubMed] [Google Scholar]
- Kou L., Sun J. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J. Pharm. Sci. 2013;8:1–10. [Google Scholar]
- Labhasetwar V., Song C. Arterial uptake of biodegradable nanoparticles: effect of surface modifications. J. Pharm. Sci. 1998;87:1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci. 2015;125:75–84. doi: 10.1016/j.ces.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Kaner R.B. Shape and aggregation control of nanoparticles: not shaken. Not Stirred. J. Am. Chem. Soc. 2006;128(3):968–975. doi: 10.1021/ja056609n. [DOI] [PubMed] [Google Scholar]
- Li J., Zhu J.J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst. 2013;138:2506–2515. doi: 10.1039/c3an36705c. [DOI] [PubMed] [Google Scholar]
- Li S.-D., Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Control Rel. 2010;145(3):178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler D.C., Guengerich F.P. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discov. 2005;4(5):410–420. doi: 10.1038/nrd1720. [DOI] [PubMed] [Google Scholar]
- Liu R., Kay B.K. Nanoparticle delivery: targeting and nonspecific binding. MRS Bull. 2009;34(6):432–440. [Google Scholar]
- Mallipeddi R., Rohan L.C. Progress in antiretroviral drug delivery using nanotechnology. Int. J. Nanomed. 2010;5:533–547. [PMC free article] [PubMed] [Google Scholar]
- Mamo T., Moseman E.A. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5(2):269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements D.J., Li F. The nutraceutical bioavailability classification scheme: classifying nutraceuticals according to factors limiting their oral bioavailability. Ann. Rev. Food Sci. Technol. 2015;6:299–327. doi: 10.1146/annurev-food-032814-014043. [DOI] [PubMed] [Google Scholar]
- McClements D.J. Nanoscale nutrient delivery systems for food applications: improving bioactive dispersibility, stability, and bioavailability. J. Food Sci. 2015;80(7):N1602–N1611. doi: 10.1111/1750-3841.12919. [DOI] [PubMed] [Google Scholar]
- McMillan J., Batrakova E. Cell delivery of therapeutic nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011;104:563–601. doi: 10.1016/B978-0-12-416020-0.00014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X., Pinaud F.F. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709) doi: 10.1126/science.1104274. 538 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty C., Sahoo S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31(25):6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- Mora-Huertas C.E., Fessi H. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010;385(1–2):113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Moss, J.A., 2013. HIV/AIDS review. Radiol. Technol. 84(3), 247–267. [PubMed]
- Mu Q., Jiang G. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014;114(15):7740–7781. doi: 10.1021/cr400295a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura S., Nicolas J. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- Murugesan S., Mousa S.A. Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor-promoted angiogenesis. FEBS Lett. 2007;581:1157–1160. doi: 10.1016/j.febslet.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves A.R., Queiroz J.F. S Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2015;14:27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek A.S., McMillan J. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. J. Neuroimmune. Pharmacol. 2010;5(4):592–601. doi: 10.1007/s11481-010-9198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans C., Bult W. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm. Res. 2010;27:2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue S., Yamada S. Nanodrugs: pharmacokinetics and safety. Int. J. Nanomed. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakwe O., Rizvi S.A.A. Academic Press; Cambridge, MA: 2016. Social Aspects of Drug Discovery, Development and Commercialization. [Google Scholar]
- Park H.S., Nam S.H. Clear-cut observation of clearance of sustainable upconverting nanoparticles from lymphatic system of small living mice. Sci. Rep. 2016;6:27407. doi: 10.1038/srep27407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni B.S., Chupin V.V. New developments in liposomal drug delivery. Chem. Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- Penalva R., Esparza I. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J. Agric. Food Chem. 2015;63(23):5603–5611. doi: 10.1021/jf505694e. [DOI] [PubMed] [Google Scholar]
- Prado-Gotor R., Grueso E. A kinetic study of the interaction of DNA with gold nanoparticles: mechanistic aspects of the interaction. Phys. Chem. Chem. Phys. 2011;13(4):1479–1489. doi: 10.1039/c0cp00901f. [DOI] [PubMed] [Google Scholar]
- Pramanik A.K., Siddikuzzaman, Biotin decorated gold nanoparticles for targeted delivery of a smart-linked anticancer active copper complex: in vitro and in vivo studies. Bioconjug. Chem. 2016;27(12):2874–2885. doi: 10.1021/acs.bioconjchem.6b00537. [DOI] [PubMed] [Google Scholar]
- Prokop A., Davidson J.M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008;97(9):3518–3590. doi: 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prylutska S.V., Grynyuk I.I. Anti-oxidant properties of C60 fullerenes in vitro. Fuller Nanotub. Carbon Nanostruct. 2008;16:698705. [Google Scholar]
- Prylutska S.V., Skivka L.M. Complex of C60 fullerene with doxorubicin as a promising agent in antitumor therapy. Nanosc. Res. Lett. 2015;10(1):499. doi: 10.1186/s11671-015-1206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PubMed, 2017 <https://www.ncbi.nlm.nih.gov/pubmed>.
- Quintana A., Raczka E., Piehler L. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharmaceut. Res. 2002;19:1310–1316. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- Rastogi V., Yadav P. Carbon nanotubes: an emerging drug carrier for targeting cancer cells. J. Drug Deliv. 2014;2014:670815. doi: 10.1155/2014/670815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K.S., Ghorpade A. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009;6(8):771–784. doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami-Hodjegan A., Tucker G.T. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat. Rev. Drug Discov. 2007;6:140–148. doi: 10.1038/nrd2173. [DOI] [PubMed] [Google Scholar]
- Sanginario A., Miccoli B. Carbon nanotubes as an effective opportunity for cancer diagnosis and treatment. Biosensors. 2017;7(1):9. doi: 10.3390/bios7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V., Siddiqui I.A. Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly(d, l-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 2013;10(10):3871–4381. doi: 10.1021/mp400342f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Sunagawa Y. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011;34(5):660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- Sessa M., Balestrieri M.L. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2013;147:42–50. doi: 10.1016/j.foodchem.2013.09.088. [DOI] [PubMed] [Google Scholar]
- Shah L.K., Amiji M.M. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm. Res. 2006;23:2638–2645. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- Shaikh J., Ankola D.D. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009;37(3–4):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Sheingold, B.H., Hahn, J.A., 2014. The history of healthcare quality: the first 100 years 1860–1960. IJANS. 1, 18–22.
- Shen B., Ma Y. Smart multifunctional magnetic nanoparticle-based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. ACS Appl. Mater Interf. 2016;8(37):24502–24508. doi: 10.1021/acsami.6b09772. [DOI] [PubMed] [Google Scholar]
- Siepmann J., Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001;48(2–3):229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- Singh R., Lillard J.W., Jr Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani S., Dufes C. Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine. 2014;9(15):2403–2414. doi: 10.2217/nnm.14.130. [DOI] [PubMed] [Google Scholar]
- Son G.H., Lee B.J. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharmaceut. Invest. 2017 [Google Scholar]
- Soo E., Thakur S. Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J. Colloid. Interface Sci. 2016;462:368–374. doi: 10.1016/j.jcis.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Spudich S.S., Ances B.M. Central nervous system complications of HIV infection. Top Antivir. Med. 2011;19:48–57. [PMC free article] [PubMed] [Google Scholar]
- Summerlin N., Soo E. Resveratrol nanoformulations: challenges and opportunities. Int. J. Pharm. 2015;479(2):282–290. doi: 10.1016/j.ijpharm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Sykes E.A., Dai Q. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA. 2016;113:E1142–E1151. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari G., Tiwari R. Drug delivery systems: an updated review. Int. J. Pharm. Invest. 2012;2(1):2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela B.C., Waterhouse A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996;44:1253–1257. [Google Scholar]
- Varshosaz J., Farzan M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World J. Gastroenterol. 2015;21(42):12022–12041. doi: 10.3748/wjg.v21.i42.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti V., Cannava C. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B Biointerf. 2014;115:22–28. doi: 10.1016/j.colsurfb.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Vo T.N., Kasper F.K. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012;64(12):1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann. N.Y. Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wolfbeis, O.S., 2015. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 44(14), 4743–4468. [DOI] [PubMed]
- Zhang J., Saltzman M. Engineering biodegradable nanoparticles for drug and gene delivery. Chem. Eng. Prog. 2013;109(3):25–30. [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yang W. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy. 2009;5(8):1107–1117. doi: 10.4161/auto.5.8.9842. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang Y. Nanomicellar carriers for targeted delivery of anticancer agents. Ther. Deliv. 2014;5(1):53–68. doi: 10.4155/tde.13.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li H. Targeted drug delivery via folate receptors. Expert Opin. Drug Deliv. 2008;5(3):309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]


