Abstract
Background
Type 2 diabetes is a chronic condition that requires pharmacotherapy interventions. Metformin and gliclazide are widely used drugs in monotherapy. However, their complementary action made utilization of the combination of these drugs an appealing approach.
Aims
The study compared major therapeutic potentials of combined metformin/gliclazide treatment over metformin monotherapy based on the following parameters: oxidative stress, lipid profile, and hepatorenal functions.
Subjects and methods
This is a comparative study was conducted from March 2015 to March 2016. The study screened 80 type 2 diabetic patients, of which 40 patients underwent combined metformin + gliclazide therapy (500 mg BD + 80 mg OD, respectively). The other 40 were matched for age and duration of diabetes mellitus with the previous group and received metformin monotherapy (500 mg BD). The levels of fasting blood glucose (FBG), total glycated hemoglobin (HbA1c), lipid peroxidation, total antioxidant capacity, serum creatinine, aspartate and alanine transaminases, total cholesterol, triglycerides, high-density lipoproteins, and low-density lipoproteins were measured according to the standard methods.
Results
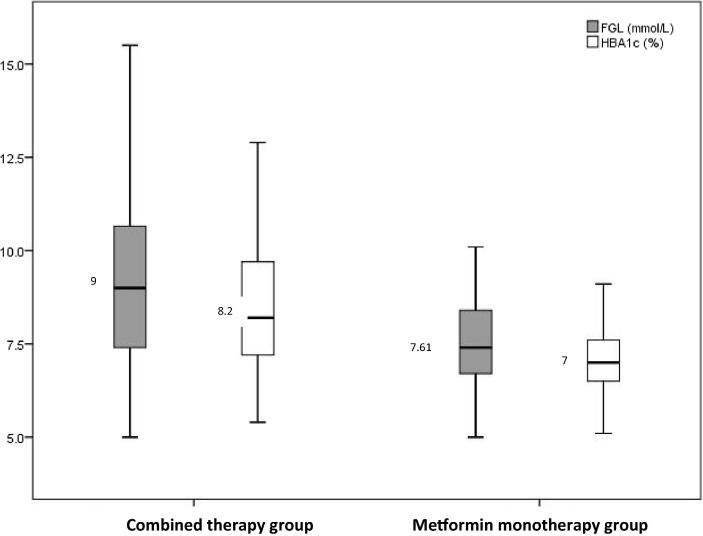
Oxidative stress, lipid profile, and hepatorenal functions were comparable in patients of both groups. However, patients on metformin treatment showed significantly lower levels of FBG [7.61 (6.70–8.89) mmol/L vs. 9.00 (7.30–10.68) mmol/L; P = .022] and HBA1c [7.00 (6.40–7.65)% vs. 8.20 (7.20–9.75)%; P < .001] compared to those on combined therapy.
Conclusion
Oxidative stress, lipids profile, and hepatorenal functions were not different in patients who were on combined metformin/gliclazide therapy and compared to those metformin alone. In contrast, glycemic control was poor in the diabetic patients undergoing combined therapy.
Keywords: Diabetes mellitus, Gliclazide, Glucose, Lipids, Metformin, Oxidative stress
1. Introduction
Diabetes mellitus (DM) compromises cardiovascular (Chawla et al., 2016), kidneys (Narres et al., 2016), liver (Morling et al., 2015), functions and affects the antioxidant capacity of the body (Grindel et al., 2016). Recent studies have reported that most of diabetic complications result from disturbed oxidative stress in different body organs (Asmat et al., 2016) as well as dyslipidemia (de Souza Bastos et al., 2016). Understanding the mechanisms of diabetic complications constitutes the cornerstone of the evaluation of drug regimens used for the treatment of DM (Zhang et al., 2013). Potential therapeutic benefits of anti-diabetic drugs are usually assessed by evaluating their effects on glycemic control (Goldstein et al., 2003), oxidative stress (Sun et al., 2014), lipid profile (Manik et al., 2013), and hepatorenal functions (Ahad et al., 2014).
In clinical practice, metformin and gliclazide are used either separately or in combination for the treatment of type 2 DM (T2DM). Metformin monotherapy was proved to improve metabolic disturbances (Chakraborty et al., 2011, Esteghamati et al., 2013) and attenuate oxidative stress (Kim et al., 2013) in diabetic patients. More recently, it has been proved that adding gliclazide to metformin-treated type 2 diabetic patients synergistically improved the fasting blood glucose (FBG) and lower glycosylated hemoglobin (HbA1c) level (Al-Gareeb et al., 2016). However, there is a lack of sufficient reports on the therapeutic benefits of combined metformin/gliclazide therapy on oxidative stress, lipid profile, and hepatorenal functions (Hong et al., 2013). Moreover, there is a scarcity of research dealing with the combination therapy of metformin/gliclazide for T2DM management. The present study aims at comparing the major therapeutic potentials of combined metformin/gliclazide treatment and metformin monotherapy when used for T2DM patients.
2. Subjects and methods
2.1. Subjects
The study recruited 80 T2DM patients from Al Basam Diabetes Center – Unaiza – Qassim – KSA during the period from March 2015 to March 2016. The control group consisted of 40 T2DM patients on metformin treatment (500 mg BD). The metformin monotherapy group was matched for age and duration of DM with a test group of 40 patients on combined metformin/gliclazide therapy (combined therapy group) (500 mg BD + 80 mg OD, respectively). The patients with other systemic diseases, major diabetic complications, treatment with beta blocker, steroids, thiazides, and/or insulin were excluded from the study. The study was approved by the Institutional Review Board (IRB) of College of Medicine, Qassim University.
2.2. Methods
Five ml of venous blood were taken from each patient during their regular follow-up in the general diabetes clinic. To determine HbA1c level, 1.5 ml of the blood sample was added to EDTA tube. The blood serum was separated from the remaining 3.5 ml to determine oxidative stress status, FBG level, hepatorenal function, and lipid profile.
HbA1c level was determined according to the standard technique (Bunn et al., 1976) using a specific glycated hemoglobin kit (Sigma–Aldrich, St. Louis, MO, USA). FBG level was determined in mmol/L with the standard oxidase methods (Barham and Trinder, 1972). The extent of lipid peroxidation was determined colorimetrically (Ohkawa et al., 1979) using lipid peroxide (LPD) kit (GenWay Biotech, Inc, USA). Total antioxidant capacity (TAC) was measured colorimetrically (Koracevic et al., 2001) using TAC kit (GenWay Biotech, Inc, USA). Total cholesterol was measured using enzymatic, liquid, colorimetric test – CHOD/PAP method with UDI diagnostics kits (CAT.NO EL24-1200, KSA) (Allain et al., 1974). Serum triglycerides were measured through enzymatic colorimetric GPO method with UDI diagnostics kits (CAT.NO EL59L-1000, KSA) (Fossati and Prencipe, 1982). Serum HDL was measured by enzymatic, colorimetric test – HDL precipitating reagent phosphotungstate method with UDI diagnostics kits (CAT.NO EL41-360, KSA) (Warnick and Albers, 1978). LDL was indirectly measured using the Friedewald equation (Nauck et al., 2002) based on the presence of total cholesterol, HDL, and triglyceride levels [LDL = total cholesterol – HDL – (triglycerides/5)]. Serum creatinine was determined according to the standard colorimetric method (Fabiny and Ertingshausen, 1971). Aspartate (AST) and alanine (ALT) transaminase (IU) were assessed colorimetrically (Reitman and Frankel, 1957).
2.3. Statistical analysis
Data were analyzed using SPSS for Windows (version 16.0, Chicago, SPSS Inc. USA). Normal distribution of variables was examined using Shapiro-Wilk test. The studied variables were described with median and 25–75 interquartile (Q1–Q3) and Boxplot charts. Significant statistical differences of studied variables were assessed among diabetic groups using Mann-Whitney U test. A P value of <.05 was considered significant.
3. Results
The duration of T2DM and age at the time of diagnosis of the diabetic patients undergoing metformin treatment (Median (Q1–Q3) = 50.00 (45.00–57.75) and 2.00 (1.00–5.00) years, respectively) were not significantly different as compared to those in the combined therapy group [Median (Q1–Q3) = 53.00 (45.25–60.00) and 4.00 (2.00–6.75) years, respectively; P > .05]. Patients on metformin treatment exhibited significantly lower levels of FBG [7.61 (6.70–8.89) mmol/L vs. 9.00 (7.30–10.68) mmol/L, P = .022] and HBA1c [7.00 (6.40–7.65)% vs. 8.20 (7.20–9.75)%, P < .001] compared to those on combined therapy (Table 1).
Table 1.
Comparison of glycemic control, lipid profile, hepatorenal functions and oxidative stress among diabetic patients on mono and combined anti-diabetic therapy.
| Diabetic patients on combined therapy N = 40Median (Q1–Q3) |
Diabetic patients on Metformin monotherapy N = 40Median (Q1–Q3) |
P | |
|---|---|---|---|
| Glycemic control | |||
| FBG (mmol/L) | 9.00 (7.30–10.68) | 7.61 (6.70–8.89) | .022* |
| HBA1c (%) | 8.20 (7.20–9.75) | 7.00 (6.40–7.65) | <.001* |
| Oxidative stress | |||
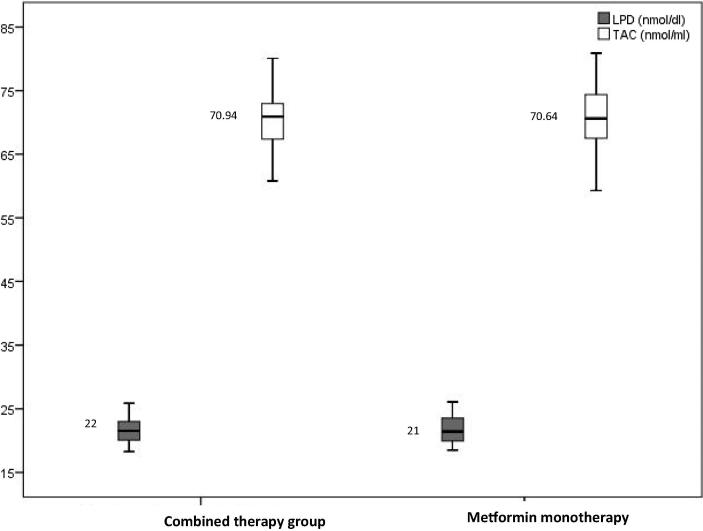
| LPD (nmol/dl) | 22.00 (20.00–23.00) | 21.00 (20.00–24.00) | .888 |
| TAC (nmol/ml) | 70.94 (67.00–73.02) | 70.64 (67.37–74.41) | .751 |
| Lipid profile | |||
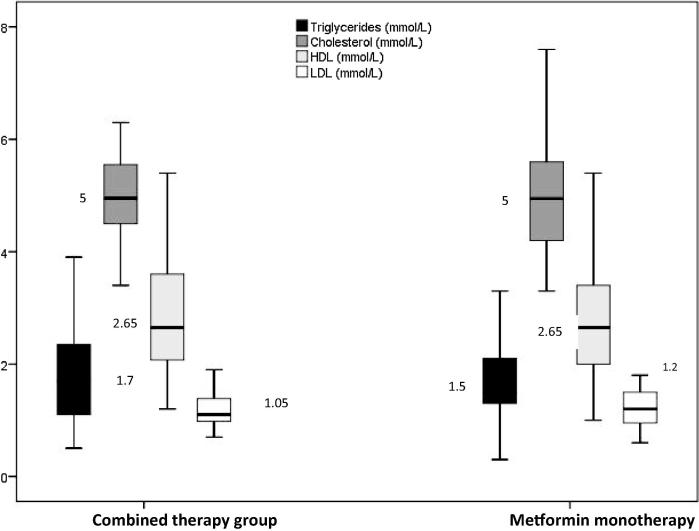
| Triglycerides (mmol/L) | 1.70 (1.13–2.58) | 1.50 (1.30–2.16) | .582 |
| Cholesterol (mmol/L) | 5.00 (4.50–5.60) | 5.00 (4.20–5.68) | .599 |
| HDL (mmol/L) | 2.65 (2.01–3.60) | 2.65 (2.00–3.40) | .630 |
| LDL (mmol/L) | 1.05 (0.94–1.38) | 1.20 (0.94–1.50) | .498 |
| Hepatorenal functions | |||
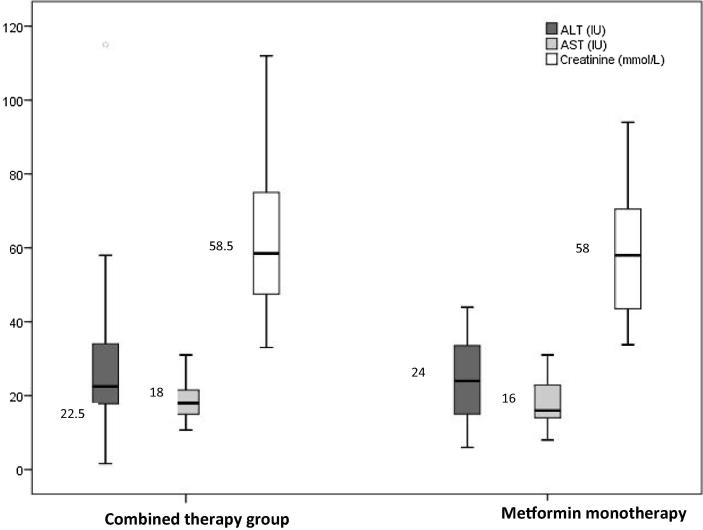
| ALT (IU) | 22.50 (17.70–34) | 24.00 (15.00–34.00) | .841 |
| AST (IU) | 18.00 (14.93–21.75) | 16.00 (14.00–23.50) | .898 |
| Creatinine (mmol/L) | 58.50 (47.25–75.00) | 58.00 (43.00–71.00) | .563 |
FBG: fasting blood glucose; HBA1c: haemoglobin A1c; LPD: lipid peroxidase; TAC: total antioxidant capacity; HDL: high density lipoproteins; LDL: low density lipoproteins; ALT: alanine transaminase; AST: aspartate transaminase.
Statistically significant.
Glycemic control (Fig. 1), oxidative stress (Fig. 2), lipid profile (Fig. 3), and hepatorenal functions (Fig. 4) were comparable in patients in mono and combined therapy groups (P > .05) (Table 1). As shown in Fig. 1, the FBG (7.61 mmol/L) and HBA1c (7%) levels of metformin monotherapy group were lower compared to the levels of those on combined therapy.
Fig. 1.
Boxplot showing indicators of glycemic control in patients on mono and combined anti-diabetic therapy.
Fig. 2.
Boxplot showing oxidative stress in patients on mono and combined anti-diabetic therapy.
Fig. 3.
Boxplot showing lipid profile in patients on mono and combined anti-diabetic therapy.
Fig. 4.
Boxplot showing hepatorenal functions in patients on mono and combined anti-diabetic therapy.
Fig. 2 showed improved results for LPD (21 nmol/dL) levels in patients undergoing metformin monotherapy. However, there was no significant difference in the TAC of the studied groups.
The triglycerides level in combined therapy group and LDL levels in monotherapy group were found to have significantly higher values (Fig. 3). However, there was no difference in the levels of HDL and cholesterol in both the groups.
AST level in the monotherapy group was lower compared to that of the combined therapy group (Fig. 4). In contrast, ALT level was higher in monotherapy group while creatinine level showed no improvements in both studied groups.
4. Discussion
The combined metformin/gliclazide therapy is commonly used for treating T2DM; however, the therapeutic benefits of this combination on oxidative stress, lipid profile, and hepatorenal functions have not been thoroughly studied before. In the present study, it is evident that the above-mentioned parameters were comparable in both studied groups. Glycemic control was poor in the diabetic patients undergoing combined therapy compared to those on metformin monotherapy.
Nowadays, oxidative stress in diabetic patients is the cornerstone for the pathogenesis of all complications in diabetes (Asmat et al., 2016). Increased free radical production greatly affects glycemic control, lipid profile, and hepatorenal function in diabetic patients. The observed insignificant differences between monotherapy and combined therapy groups in this study is consistent with the results of previous studies (Meişoğullari and Tuerkeli, 2008), which demonstrated that separate (not combined) administration of metformin or gliclazide greatly improved oxidative stress status in diabetic patients. Previous reports proved that administration of gliclazide or metformin decreased oxidative stress as evidenced by decreased catalase (CAT), glutathione S- transferase (GST), erythrocytes glutathione peroxidase (Gpx) levels, and malondialdehyde (MDA) levels with no significant differences between gliclazide or metformin-treated groups (Meişoğullari and Tuerkeli, 2008). Moreover, Chen and his colleagues also recommended the use of combined gliclazide/metformin therapy over metformin monotherapy for better oxidative stress status in T2DM patients as evidenced by improved MDA and superoxide dismutase (SOD) levels (Chen et al., 2010). Contrary to the results of this study, Hassan and Abd-Allah demonstrated that combined metformin/gliclazide treatment greatly improved lipid profile as evidenced by decreased total cholesterol and significantly increased HDL level compared with the control group (Hassan and Abd-Allah, 2015). The comparable effects of mono vs. combined therapies regarding lipid profile as proved in this study is parallel with a previous study that similar efficacy of both drugs on lipid profile (Tessier et al., 1999).
With respect to the hepatorenal function, this study revealed no significant differences in both studied groups. Our results were consistent with a large study involving 600 T2DM patients conducted by Su et al. They concluded that no statistical differences were observed before and after 24 weeks of metformin treatment (Su et al., 2014). The present results regarding glycemic control contradict to the findings of at least two recent reports that proved better glycemic control in patients with combined gliclazide/metformin therapy {FormattingCitation}. In clinical practice, diabetic patients are offered combined therapy only when monotherapy fails. This indicates that patients on combined therapy are likely to suffer from a higher degree of insulin resistance and are expected to respond poorly to anti-diabetic treatment. It should be noted that some studies revealed no significant differences on glycemic control between mono and combined therapy (Chen et al., 2010), and so further investigations are recommended to clarify this point.
In conclusion, the combined metformin/gliclazide treatment seems to be beneficial to safeguarde against important complications of DM. This is because oxidative stress, lipids profile, and hepatorenal functions of poorly controlled diabetic subjects on combined therapy are still comparable with that of the patients on monotherapy.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahad A., Mujeeb M., Ahsan H., Siddiqui W.A. 10. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie. 2014;106:101–110. doi: 10.1016/j.biochi.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Al-Gareeb A.I., Alrubai H.F., Suliaman S.M. Effects of gliclazide add on metformin on serum omentin-1 levels in patients with type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2016;20:195–198. doi: 10.4103/2230-8210.176355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. 28. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress – a concise review. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham D., Trinder P. 22. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- Bunn H.F., Haney D.N., Kamin S., Gabbay K.H., Gallop P.M. 21. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Invest. 1976;57:1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A., Chowdhury S., Bhattacharyya M. R: 36. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2011;93:56–62. doi: 10.1016/j.diabres.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Chawla A., Chawla R., Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian. J. Endocrinol. Metab. 2016;20:546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liao Y., Zeng T., Yu F., Li H., Feng Y. Effects of metformin plus gliclazide compared with metformin alone on circulating endothelial progenitor cell in type 2 diabetic patients. Endocrine. 2010;38:266–275. doi: 10.1007/s12020-010-9383-8. [DOI] [PubMed] [Google Scholar]
- de Souza Bastos A., Graves D.T., de Melo Loureiro A.P., Júnior C.R., Corbi S.C.T., Frizzera F., Scarel-Caminaga R.M., Câmara N.O., Andriankaja O.M., Hiyane M.I., Orrico S.R.P. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J. Diabetes Complicat. 2016;30:1593–1599. doi: 10.1016/j.jdiacomp.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteghamati A., Eskandari D., Mirmiranpour H., Noshad S., Mousavizadeh M., Hedayati M., Nakhjavani M. R: 38. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin. Nutr. 2013;32:179–185. doi: 10.1016/j.clnu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Fabiny D.L., Ertingshausen G. 25. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- Fossati P., Prencipe L. 27. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Goldstein B.J., Pans M., Rubin C.J. 8. Multicenter, randomized, double-masked, parallel-group assessment of simultaneous glipizide/metformin as second-line pharmacologic treatment for patients with type 2 diabetes mellitus that is inadequately controlled by a sulfonylurea. Clin. Ther. 2003;25:890–903. doi: 10.1016/s0149-2918(03)80112-1. [DOI] [PubMed] [Google Scholar]
- Grindel A., Guggenberger B., Eichberger L., Pöppelmeyer C., Gschaider M., Tosevska A., Mare G., Briskey D., Brath H., Wagner K.-H. 2. Oxidative stress, DNA damage and DNA repair in female patients with diabetes mellitus type 2. PLoS One. 2016;11:e0162082. doi: 10.1371/journal.pone.0162082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.H., Abd-Allah G.M. Effects of metformin plus gliclazide versus metformin plus glimepiride on cardiovascular risk factors in patients with type 2 diabetes mellitus. Pak. J. Pharm. Sci. 2015;28:1723–1730. [PubMed] [Google Scholar]
- Hong J., Zhang Y., Lai S., Lv A., Su Q., Dong Y., Zhou Z., Tang W., Zhao J., Cui L., Zou D., Wang D., Li H., Liu C., Wu G., Shen J., Zhu D., Wang W., Shen W., Ning G., Investigators S.P.R.E.A.D.-D.I.M.C.A.D. 14. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–1311. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S., Shin J.-A., Lee S.-H., Kim E.-S., Cho J.-H., Son H.-Y., Yoon K.-H. 16. A comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol. Ther. 2013;15:810–816. doi: 10.1089/dia.2013.0038. [DOI] [PubMed] [Google Scholar]
- Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. 24.p2. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manik S., Gauttam V., Kalia A.N. 9, Anti-diabetic and antihyperlipidemic effect of allopolyherbal formulation in OGTT and STZ-induced diabetic rat model. Indian J. Exp. Biol. 2013;51:702–708. [PubMed] [Google Scholar]
- Memi̇şoğullari R., Tuerkeli M. Effect of metformin or gliclazide on lipid peroxidation and antioxidant levels in patients with diabetes mellitus. Turkish J. 2008;38:545–548. [Google Scholar]
- Morling, J.R., Fallowfield, J.A., Guha, I.N., Williamson, R.M., Ali, M., Glancy, S., Strachan, M.W.J., Price, J.F., 2015. Clinically significant chronic liver disease in people with Type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Qjm 2374, hcv191. doi: 10.1093/qjmed/hcv191. [DOI] [PMC free article] [PubMed]
- Narres M., Claessen H., Droste S., Kvitkina T., Koch M., Kuss O., Icks A. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: a systematic review. PLoS One. 2016;11:e0147329. doi: 10.1371/journal.pone.0147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M., Warnick G.R., Rifai N. 30. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. 22. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Su Y., Su Y.-L., Lv L.-F., Wang L.-M., Li Q.-Z., Zhao Z.-G. A randomized controlled clinical trial of vildagliptin plus metformin combination therapy in patients with type II diabetes mellitus. Exp. Ther. Med. 2014;7:799–803. doi: 10.3892/etm.2014.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Chen Y., Li X., Tai G., Fan Y., Zhou Y. 11. Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Funct. 2014;5:845. doi: 10.1039/c3fo60326a. [DOI] [PubMed] [Google Scholar]
- Tessier D., Maheux P., Khalil A., Fülöp T. Effects of gliclazide versus metformin on the clinical profile and lipid peroxidation markers in type 2 diabetes. Metabolism. 1999;48:897–903. doi: 10.1016/s0026-0495(99)90226-3. [DOI] [PubMed] [Google Scholar]
- Warnick G.R., Albers J.J. 29. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J. Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- Zhang F., Xiang H., Fan Y., Ganchuluun T., Kong W., Ouyang Q., Sun J., Cao B., Jiang H., Nie S. The effects of sulfonylureas plus metformin on lipids, blood pressure, and adverse events in type 2 diabetes: a meta-analysis of randomized controlled trials. Endocrine. 2013;44:648–658. doi: 10.1007/s12020-013-9970-6. [DOI] [PubMed] [Google Scholar]