Abstract
The antimicrobial, antioxidant, and anticancer activities of ethanolic extract of Laurencia catarinensis, L. majuscula and Padina pavonica were determined. The highest antibacterial activity; 23.40 ± 0.58 mm (00.98 µg/ml) and 22.60 ± 2.10 mm (03.90 µg/ml) were obtained against Klebsiella pneumonia by Laurencia catarinensis and Padina pavonica, respectively. However, Padina pavonica showed excellent antibacterial activity against Bacillus subtilis (21.7 ± 1.5 mm; 1.95 µg/ml), Staphylococcus aureus (21.7 ± 0.58 mm; 1.95 µg/ml), Streptococcus pyogenes (20.7 ± 1.2 mm; 1.95 µg/ml) and Acinetobacter baumannii (20.1 ± 1.2 mm; 3.9 µg/ml). Moreover, the highest antifungal activity; 24.7 ± 2.0 mm (0.98 µg/ml), 23.7 ± 1.5 mm (0.98 µg/ml), 23.6 ± 1.5 mm (0.98 µg/ml) was obtained by Padina pavonica against Candida tropicalis, C. albicans and Aspergillus fumigatus, respectively. The algal extracts showed DPPH radical scavenging activity in a concentration–dependent manner with maximum scavenging activity (77.6%, IC50 = 5.59 µg/ml and 77.07%, IC50 = 14.3 µg/ml) was provided by Padina pavonica and Laurenica majuscula, respectively. The in vitro antitumor activity revealed that the IC50 values of Padina pavonica were 58.9, 115.0, 54.5, 59.0, 101.0, 101.0, and 97.6 µg/ml; Laurencia catarinensis were 55.2, 96.8, 104.0, 78.7, 117.0, 217.0, 169.0 µg/ml; and Laurencia. majuscula were 115.0, 221.0, 225.0, 200.0, 338.0, 242.0, and 189.0 µg/ml; respectively against A-549 (Lung carcinoma), Caco-2 (Intestinal carcinoma), HCT-116 (Colon carcinoma), Hela (Cervical carcinoma), HEp-2 (Larynx carcinoma), HepG-2 (Hepatocellular carcinoma), and MCF-7 (Breast carcinoma) cell lines.
Keywords: HCT-116 (Colon carcinoma), Hela (Cervical carcinoma), HEp-2 (Larynx carcinoma), HepG-2 (Hepatocellular carcinoma), MCF-7 (Breast carcinoma), Acinetobacter baumannii, Bacillus subtilis
0. Introduction
Marine algae have long been used as food and medicine in many Asian countries including Japan, China, Thailand and Korea. Natural products of marine algae are in great demand due to their prolific biological activities that might represent useful leads in the discovery of novel bioactive compounds and new pharmaceutical agents (Blunden, 2001, Iwamoto et al., 2001). Consumption of the marine algae is thought to ameliorate some inflammatory disorders, breast cancer and high cholesterol level (Fitton and Helen, 2003). Numerous novel compounds have been isolated, during the last few decades, from marine organisms and many of these substances have been proved to possess remarkable biological activities (El Gamal, 2010, Proksch et al., 2002, Faulkner, 2002, Faulkner, 2001).
Different compounds isolated from marine algae have shown antimicrobial activities and are used in pharmaceutical industries (Rajasulochana et al., 2009, El-Fatemy, 2008, Venkateswarlu et al., 2007, Tüney et al., 2006, Ely et al., 2004, Lima-Filho et al., 2002). Antioxidant activity is important in various pharmacological activities such as anti-aging, anti-inflammatory, and anti-cancer activities (Lee et al., 2004, Middleton et al., 2000). Antioxidant activity is claimed to be present in most of the nutraceuticals and cosmeceuticals. However, numerous synthetic antioxidants are produced, but are quite unsafe and their toxicity is of concern (Madhavi et al., 1995). On the other hand, Natural products with antioxidant activity are used for human consumption because of their safety. Different compounds with cytostatic, antiviral, antihelmintic, antioxidant, antifungal and antibacterial activities have been detected in green, brown and red algae (Newman et al., 2003, Lindequest and Schweder, 2001).
One of the most life-threatening in developed and developing countries is cancer. Natural anticancer compounds are able to control the growth of cancer cells with no or minor side effects. Accordingly, identification of new effective cancer chemopreventive agents has become an important worldwide strategy in cancer prevention. Different compounds isolated from marine algae were found to have antiproliferative activity in cancer cell lines in vitro, as well as inhibitory activity of tumor growth in mice (Yang et al., 2008, Ye et al., 2008, Rocha de Souza et al., 2007, Kwon and Nam, 2006). The current study was carried out to determine the antimicrobial, antioxidant and anticancer activities of Laurencia catarinensis, L. majuscula and Padina pavonica.
1. Material and methods
1.1. Algal samples collection, extraction and screening
1.1.1. Algal species collections
The algal species used in this study; namely, Laurencia catarinensis, Laurencia majuscula and Padina pavonica were collected from Alharra, Umluj, Red Seashore, Kingdom of Saudi Arabia. Algal species were identified according to Aleem, 1978, Aleem, 1993, Bold and Wynne, 1978, Coppejans et al., 2009. Samples collected were air-dried in shade, reduced to fine powder, packed in tightly closed containers and stored for phytochemical and biological studies.
1.1.2. Algal extraction
Dry powder of each alga under investigation were separately (600 g) was extracted by percolation in 95% ethanol (Awaad et al., in press) at room temperature for two days. The ethanol extracts were separately filtered and the residues were re-percolated for five times for each alga. The total ethanol extracts were separately concentrated under reduced pressure at a temperature not exceeding 35 °C
1.1.3. Phytochemical screening
Powdered samples from the of the investigated alga were subjected to phytochemical screening for their different constituents such as; carbohydrates and/or glycosides, flavonoides, tannins, sterols and/or triterpenes, proteins and/or amino acids, alkaloids and/or nitrogenous bases, saponins, anthraquinones, cardinolides and oxidase enzyme (Khan et al., 2011).
1.2. Antimicrobial activity
1.2.1. Test organisms
Different clinically isolated microorganisms including 10 bacterial strains; Gram-negative bacteria, Acinetobacter baumannii (RCMB 0100282-9), Escherichia coli (RCMB 010056), Klebsiella pneumonia (RCMB 0010093), Proteous mirablilis (RCMB 0100254-2) and Pseudomonas aeruginosa (RCMB 0100243-5), Gram-positive bacteria, Bacillus subtilis (RCMB 0100169-3), Staphylococcus aureus, Staphylococcus epidermidis (RCMB 010027), Streptococcus pyogenes (RCMB 0100174-2) and Streptococcus sanguinis (RCMB 0100171-3); and 10 fungal strains including Aspergillus fumigatus (RCMB 02568), Aspergillus niger (RCMB 02724), Candida albicans (RCMB 05036), C. tropicalis (RCMB 05239), Cryptococcus neoformans (RCMB 05642), Geotricum candidum (RCMB 05097), Microsporum canis (RCMB 0834), Penicillium expansum (RCMB 01924), Syncephalastrum racemosum (RCMB 05922) and Trichophyton mentagrophytes (RCMB 0925) were identified by in the Microbiology Laboratory, Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt and used as test organisms.
1.2.2. Antimicrobial assay
The antibacterial and antifungal activities of ethanolic extract of Laurencia catarinensis, L. majuscula and Padina pavonica were determined using the well diffusion method (Zain et al., 2012). Petri plates containing 20 ml of, nutrient (for bacteria) or malt extract (for fungi), agar medium were seeded with 1–3 day cultures of microbial inoculums. Wells (6 mm in diameter) were cut off from agar and 50 µl of algal extracts were tested in a concentration of 100 mg/ml and incubated at 37 °C for 24–48 h (bacterial strains) and for 3–5 days (fungal strains). The antibacterial and antifungal activities were determined by measurement of the diameter of the inhibition zone around the well.
1.2.3. Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) was determined by micro-dilution method using serially diluted (2 folds) algal extracts (Zain et al., 2012). The MIC of Laurencia catarinensis, L. majuscula and Padina pavonica extracts were determined by dilution of concentrations from 0.0 to 100 mg/ml. Equal volume of each extract and nutrient broth were mixed in a test tube. Specifically 0.1 ml of standardized inoculum (1–2 × 107 cfu/ml) was added in each tube. The tubes were incubated at 37 °C for 24–48 h and/or 3–5 days. Two control tubes, containing the growth medium, saline and the inoculum were maintained for each test batch. The lowest concentration (highest dilution) of the algal extract that produced no visible microbial growth (no turbidity) when compared with the control tubes were regarded as MIC.
1.3. Antioxidant activity (DPPH (1-diphenyl-2-picrylhydrazyl) radical-scavenging assay)
The antioxidant activity of Laurencia catarinensis, L. majuscula and Padina pavonica extract was determined using the DPPH free radical scavenging assay according to the method described by Yen and Duh (1994). The assay was carried out in triplicate and the mean value was recorded.
Freshly prepared (0.004%w/v) methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was prepared and stored at 10 °C in the dark. A methanol solution of the test compound was prepared. A 40 µL aliquot of the methanol solution was added to 3 ml of DPPH solution, under light protection. Absorbance measurements were recorded immediately with a UV–visible spectrophotometer (Milton Roy, Spectronic 1201). The decrease in absorbance at 515 nm was determined continuously, with data being recorded at 1 min intervals until the absorbance stabilized (16 min). The absorbance of the DPPH radical without antioxidant (control) and the reference compound ascorbic acid were also measured. The percentage inhibition (PI) (scavenging activity) of the DPPH radical was calculated according to the formula (Yen and Duh, 1994):
where AC = Absorbance of the control at t = 0 min and AT = absorbance of the sample + DPPH at t = 16 min.
1.4. Antitumor activity
The cell lines A-549 (Lung carcinoma), Caco-2 (Colorectal carcinoma), HCT-116 (Colon carcinoma), Hela (Cervical carcinoma), HEp-2 (Larynx carcinoma), HepG-2 (Hepatocellular carcinoma), and MCF-7 (Breast carcinoma) were used for determination of antitumor activity of Laurencia catarinensis, L. majuscula and Padina pavonica. The tumor cell lines were suspended in medium at concentration 5 × 104 cell/well in Corning® 96-well tissue culture plates and then incubated for 24 hr. The tested algal extracts were then added into 96-well plates (six replicates) to achieve seven concentrations for each extract. Six vehicle controls with media or 0.5% DMSO were run for each 96 well plate as a control. After incubation for 24 h, the numbers of viable cells were determined by the MTT assay method.
Briefly, the media was removed from the 96 well plate and replaced with 100 µl of fresh culture RPMI 1640 medium without phenol red, then 10 µl of the 12 mM MTT (Sigma) stock solution (5 mg of MTT in 1 mL of PBS) was added to each well including the untreated controls. The 96 well plates were then incubated at 37 °C and 5% CO2 for 4 hours. An 85 µl aliquot of the media was removed from the wells, and 50 µl of DMSO was added to each well and mixed thoroughly with the pipette and incubated at 37 °C for 10 min. Then, the optical density was measured at 590 nm with the microplate reader (SunRise, TECAN, Inc, USA) to determine the number of viable cells.
The percentage of viability was calculated as:
where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells.
The relation between surviving cells and extract concentration is plotted to get the survival curve of each tumor cell line after treatment with the specified extract. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots of the dose response curve for each concentration using Graphpad Prism software (San Diego, CA. USA) (Kameyama et al., 2005).
1.5. Statistical analysis
All values were expressed as mean ± S.D. Comparisons between means were carried out using a one-way ANOVA test followed by the Tukey HSD test using SPSS, version 14 (SPSS, Chicago, IL). Differences at p50.05 were considered statistically significant.
2. Results and discussion
2.1. Preliminary phytochemical screening
Preliminary phytochemical screening of the three alga under investigations (Laurencia catarinensis, L. majuscula and Padina pavonica methanolic) contains; unsaturated sterols and/or triterpenoides, Flavonoids, Carbohydrates or glycosides, Proteins and/or amino acids, Tannins and Coumarin.
2.2. Antimicrobial activity
The antibacterial and antifungal activities of Laurencia catarinensis, L. majuscula and Padina pavonica methanolic extracts were determined using well-diffusion method. All the investigated algal extracts showed antibacterial and antifungal activities (Tables 1 and 2).
Table 1.
Antibacterial activity of L. catarinensis, L. majuscula and Padina pavonica against clinically isolated bacteria.
| Bacteria | Sample |
|||||||
|---|---|---|---|---|---|---|---|---|
|
L. catarinensis |
L. majuscule |
Padina pavonica |
Standard antibiotic |
|||||
| Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
|
| Gram negative | Gentamycin | |||||||
|
Acinetobacter baumannii (RCMB 0100282-9) |
20.70 ± 1.50 | 03.90 | 18.30 ± 2.10 | 07.81 | 20.10 ± 1.20 | 03.90 | 23.40 ± 1.20 | 00.98 |
| Escherichia coli (RCMB 010056) | 21.20 ± 2.10 | 01.95 | 16.30 ± 2.10 | 31.25 | 18.20 ± 0.63 | 07.81 | 20.30 ± 0.85 | 03.90 |
| Klebsiella pneumoniae (RCMB 0010093) | 23.40 ± 0.58 | 00.98 | 20.50 ± 1.20 | 01.95 | 22.60 ± 2.10 | 03.90 | 27.20 ± 2.10 | 00.49 |
| Proteous mirablilis (RCMB 0100254-2) | 00.00 | ND | 00.00 | ND | 00.00 | ND | 21.20 ± 1.20 | 01.95 |
|
Pseudomonas aeruginosa (RCMB 0100243-5) |
21.30 ± 0.63 | 01.95 | 17.20 ± 1.50 | 15.63 | 19.60 ± 0.63 | 03.90 | 20.60 ± 1.50 | 01.95 |
| Gram positive | Ampicillin | |||||||
|
Bacillus substilis (RCMB 0100169-3) |
17.39 ± 2.10 | 15.57 | 14.70 ± 1.50 | 62.50 | 21.70 ± 1.50 | 01.95 | 22.30 ± 0.63 | 01.95 |
|
Staphylococcus aureus (RCMB 010027) |
20.49 ± 1.20 | 03.90 | 17.10 ± 1.00 | 15.63 | 21.70 ± 0.58 | 01.95 | 22.00 ± 1.00 | 01.95 |
|
Staphylococcus epidermidis (RCMB 010024) |
15.70 ± 0.58 | >1000 | 14.30 ± 1.50 | 62.50 | 18.30 ± 0.58 | 07.81 | 23.00 ± 1.20 | 00.98 |
|
Streptococcus pyogenes (RCMB 0100174-2) |
16.20 ± 1.50 | 5000 | 17.70 ± 0.58 | 15.63 | 20.70 ± 1.20 | 01.95 | 22.70 ± 0.58 | 00.98 |
|
Streptococcus sanguis (RCMB 0100171-3) |
00.00 | ND | 00.00 | ND | 00.00 | ND | 21.70 ± 1.50 | 01.95 |
ND, not determined. These are the mean of three determinations.
Table 2.
Antifungal activity of L. catarinensis, L. majuscula and Padina pavonica against clinically isolated fungi.
| Sample | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fungi |
L. catarinensis |
L. majuscule |
Padina pavonica |
Standard Antibiotic (Amphotericin B) |
||||
| Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
Inhibition zone (mm) | MIC (µg/ml) |
|
|
Aspergillus fumigatus (RCMB 02568) |
19.50 ± 1.20 | 03.90 | 22.40 ± 1.00 | 01.95 | 23.6 ± 1.50 | 00.98 | 25.70 ± 1.50 | 0.49 |
|
Aspergillus niger (RCMB 02724) |
16.30 ± 0.58 | 31.25 | 18.60 ± 1.50 | 03.90 | 20.3 ± 0.58 | 03.90 | 20.44 ± 0.36 | 03.90 |
| Candida albicans (RCMB 05036) | 15.20 ± 0.51 | 62.50 | 21.30 ± 1.50 | 03.90 | 23.7 ± 1.50 | 00.98 | 21.30 ± 1.50 | 01.95 |
| Candida trobicalis (RCMBA 05239) | 19.10 ± 0.32 | 03.90 | 23.10 ± 1.30 | 00.98 | 24.2 ± 2.00 | 00.98 | 23.70 ± 2.00 | 00.98 |
| Cryptococcus neoformans (RCMB 05642) | 00.00 | ND | 00.00 | ND | 00.00 | ND | 21.00 ± 1.44 | 01.95 |
| Geotricum candidum (RCMB 05097) | 20.10 ± 0.58 | 03.90 | 20.30 ± 1.50 | 03.90 | 21.30 ± 1.50 | 01.95 | 20.31 ± 1.50 | 03.90 |
| Microsporum canis (RCMB 0834) | 00.00 | ND | 00.00 | ND | 00.00 | ND | 23.30 ± 1.50 | 00.98 |
|
Penicillium expansum (RCMB 01924) |
14.60 ± 1.50 | >1000 | 16.10 ± 1.70 | 31.25 | 21.70 ± 2.00 | 01.95 | 21.70 ± 2.00 | 01.95 |
|
Syncephalastrum racemosum (RCMB 05922) |
00.00 | ND | 00.00 | ND | 00.00 | ND | 24.30 ± 1.20 | 0.98 |
|
Trichophyton mentagrophytes (RCMB 0925) |
11.80 ± 1.21 | >1000 | 00.00 | ND | 00.00 | ND | 21.30 ± 1.50 | 01.95 |
ND, not determined. These are the mean of three determinations.
The antibacterial activity of Laurencia catarinensis, L. majuscula and Padina pavonica revealed that the highest activities; 23.40 ± 0.58 mm (00.98 µg/ml) and 22.60 ± 2.10 mm (03.90 µg/ml) were obtained against Klebsiella pneumonia by Laurencia catarinensis and Padina pavonica, respectively (Table 1). The extract of Padina pavonica revealed significant antibacterial activity against Bacillus subtilis (21.7 ± 1.5 mm; 1.95 µg/ml), Staphylococcus aureus (21.7 ± 0.58 mm; 1.95 µg/ml), Streptococcus pyogenes (20.7 ± 1.2 mm; 1.95 µg/ml) and Acinetobacter baumannii (20.1 ± 1.2 mm; 3.9 µg/ml). The antibacterial activity of Laurencia catarinensis was obtained against Pseudomonas aeruginosa (21.3 ± 0.63 mm; 1.95 µg/ml), Escherichia coli (21.2 ± 2.1 mm; 1.95 µg/ml), Acinetobacter baumannii (20.7 ± 1.5 mm; 3.9 µg/ml) and Staphylococcus aureus (20.49 ± 1.2 mm; 3.9 µg/ml). The highest antibacterial activity obtained by L. majuscule was 20.5 ± 1.2 mm (1.95 µg/ml) against Klebsiella, 18.30 ± 2.10 (7.81 µg/ml) against Acinetobacter baumannii and 17.7 ± 0.58 (15.63 µg/ml) against Streptococcus pyogenes (Table 1).
On the other hand, the antifungal activity of the three algal extracts showed that the highest activity; 24.7 ± 2.0 mm (0.98 µg/ml), 23.7 ± 1.5 mm (0.98 µg/ml), 23.6 ± 1.5 mm (0.98 µg/ml) was obtained by Padina pavonica against Candida tropicalis, C. albicans and Aspergillus fumigatus, respectively (Table 2).
2.2.1. Antioxidant activity
The free radicals are involved in several diseases including cancer, AIDS and neurodegenerative diseases. The scavenging activity of antioxidants is very useful for the control of those diseases. The DPPH assay is most commonly used method for screening antioxidant activity and it is a sensitive method to determine the antioxidant activity of different plant, fungal, or algal extracts (Suresh et al., 2008, Koleva et al., 2002).
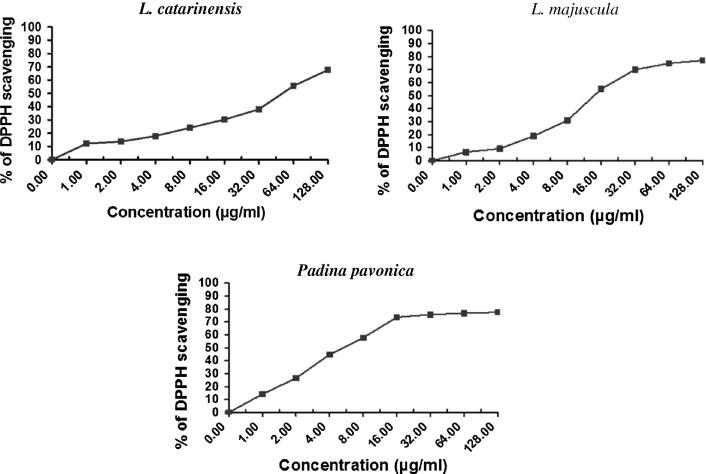
The ethanolic extract of Laurencia catarinensis, L. majuscula and Padina mavonica showed DPPH radical scavenging activity in a concentration–dependent manner (Table 3, Fig. 1). The maximum scavenging activity (77.6%, IC50 = 5.59 µg/ml and 77.07%, IC50 = 14.3 µg/ml) was provided by Padina pavonica and Laurenica majuscula, respectively (Table 3). However, the scavenging activity of Laurencia catarinensis was 67.65% (IC50 = 53.8 µg/ml).
Table 3.
The scavenging activity of DPPH radicals of L. catarinensis, L. majuscula and Padina pavonica.
| Concentration (µg/ml) | DPPH scavenging (%) |
||
|---|---|---|---|
| L. catarinensis | L. majuscula | Padina pavonica | |
| 000 | 00.00 | 00.00 | 00.00 |
| 001 | 12.17 ± 1.50 | 06.67 ± 1.32 | 14.13 ± 1.41 |
| 002 | 13.65 ± 1.11 | 09.20 ± 1.21 | 26.53 ± 1.44 |
| 004 | 17.83 ± 1.71 | 18.93 ± 1.54 | 44.80 ± 1.62 |
| 008 | 24.09 ± 1.32 | 30.93 ± 1.33 | 57.87 ± 1.57 |
| 016 | 30.26 ± 1.91 | 55.07 ± 1.38 | 73.60 ± 1.75 |
| 032 | 38.00 ± 1.22 | 70.00 ± 1.30 | 75.73 ± 1.51 |
| 064 | 55.57 ± 1.58 | 74.80 ± 1.27 | 76.67 ± 1.14 |
| 128 | 67.65 ± 1.30 | 77.07 ± 1.12 | 77.60 ± 1.09 |
| IC50 | 53.80 ± 1.22 | 14.30 ± 1.35 | 05.59 ± 1.55 |
These are the mean of three determinations.
Fig. 1.
The scavenging activity of DPPH radicals of Laurencia catarinensis, L. majuscula and Padina pavonica.
2.2.2. Antitumor activity
Marine Algae when uttered strikes about its healing property (Dziwornu et al., 2017) due its unique bioactive compounds present in it. The compounds present in it paves way for the synthesis of new drug molecules in treating various diseases (Alves et al., 2016).
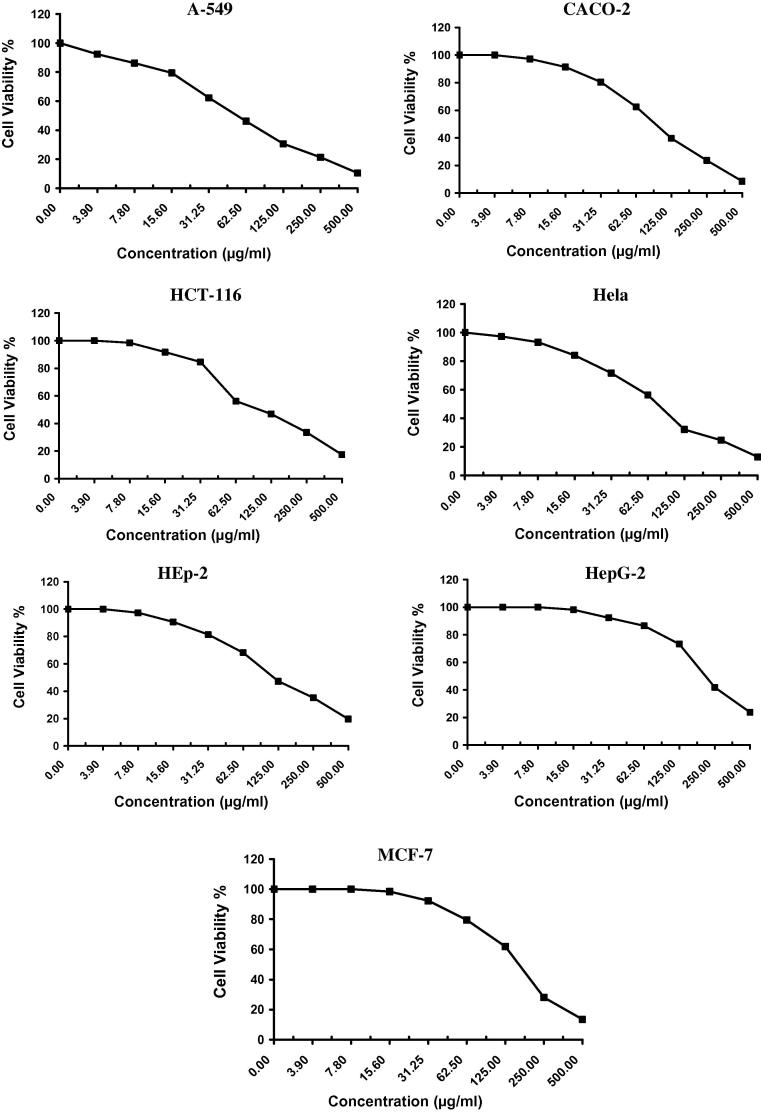
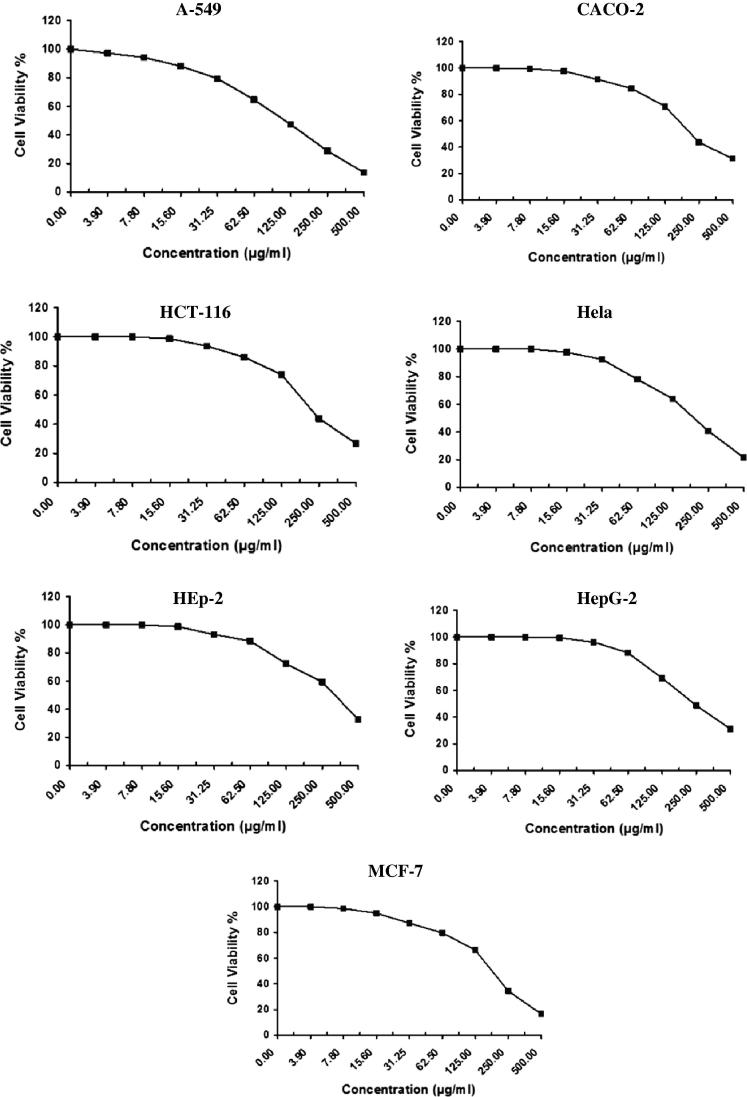
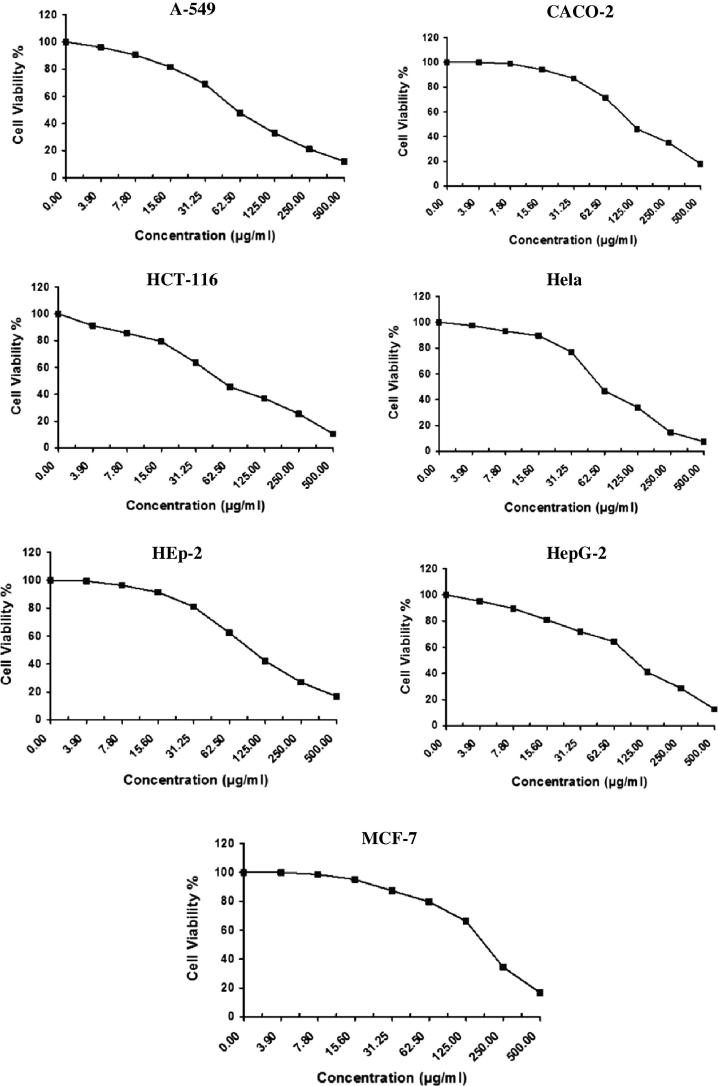
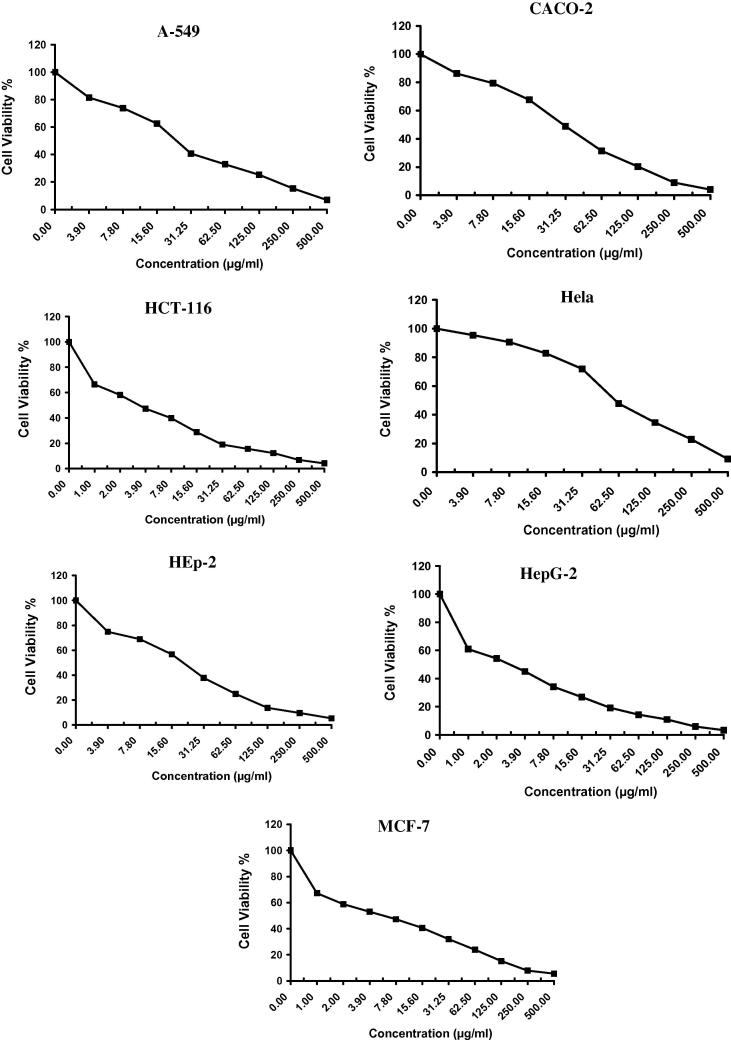
The in vitro antitumor activity of algal species like Laurencia catarinensis, L. majuscula and padina pavonica extract was evaluated on A-549 (Lung carcinoma), CACO (Intestinal carcinoma), HCT-116 (Colon carcinoma), Hela (Cervical carcinoma), HEp-2 (Larynx carcinoma), HepG-2 (Hepatocellular carcinoma), and MCF-7 (Breast carcinoma) cell lines by using MTT assay method which is reliable to assess the in vitro cytotoxicity of the anticancer compounds (Allely et al., 1998). The obtained results exhibited direct cytotoxic effect of the investigated algal extracts on the cell lines in a concentration dependent manner (Fig. 2, Fig. 3, Fig. 4). The results indicated that the extract of Padina pavonica has the lowest percentage of viability and shows significant antitumor activity followed by Laurencia catarinensis and L. majuscula (Table 1).
Fig. 2.
The cytotoxic effect of L. catarinensis on A-549, CACO, HCT-116, Hela, HepG-2, and MCF-7 cell lines.
Fig. 3.
The cytotoxic effect of L. majuscula on A-549, CACO, HCT-116, Hela, HepG-2, and MCF-7 cell lines.
Fig. 4.
The cytotoxic effect of Padina pavonica on A-549, CACO, HCT-116, Hela, HepG-2, and MCF-7 cell lines.
The IC50 values of Padina pavonica were 58.9, 115.0, 54.5, 59.0, 101.0, 101.0, and 97.6 µg/ml; Laurencia catarinensis were 55.2, 96.8, 104.0, 78.7, 117.0, 217.0, 169.0 µg/ml; and L. majuscula were 115.0, 221.0, 225.0, 200.0, 338.0, 242.0, and 189.0 µg/ml; respectively against A-549 (Lung carcinoma), CACO (Intestinal carcinoma), HCT-116 (Colon carcinoma), Hela (Cervical carcinoma), HEp-2 (Larynx carcinoma), HepG-2 (Hepatocellular carcinoma), and MCF-7 (Breast carcinoma) (Table 4). Standard reference Vinblastine Sulphate showed various effect on the same sell lines (Table 4 and Fig. 5).
Table 4.
The IC50 values of L. catarinensis, L. majuscula and Padina pavonica extracts on cell lines.
| Algal extract | ||||
|---|---|---|---|---|
| Cell line | IC50 (µg/ml) |
|||
| L. catarinensis | L. majuscula | Padina pavonica | Vinblastine Sulphate | |
| A-549 (Lung carcinoma) | 055.2 ± 0.7 | 115.0 ± 0.4 | 058.9 ± 0.1 | 24.6 ± 0.7 |
| CACO-2 (Colorectal carcinoma) | 096.8 ± 0.3 | 221.0 ± 0.6 | 115.0 ± 0.9 | 30.3 ± 1.4 |
| HCT-116 (Colon carcinoma) | 104.0 ± 0.4 | 225.0 ± 0.2 | 054.5 ± 0.3 | 3.5 ± 0.2 |
| Hela (Cervical carcinoma) | 078.7 ± 0.5 | 200.0 ± 0.3 | 059.0 ± 0.1 | 59.7 ± 2.1 |
| HEp-2 (Larynx carcinoma) | 117.0 ± 0.2 | 338.0 ± 0.5 | 101.0 ± 0.2 | 21.2 ± 0.9 |
| HepG-2 (Hepatocellular carcinoma) |
217.0 ± 0.3 | 242.0 ± 0.2 | 101.0 ± 0.4 | 2.93 ± 0.3 |
| MCF-7 (Breast carcinoma) | 169.0 ± 0.1 | 189.0 ± 0.1 | 097.6 ± 0.3 | 5.9 ± 0.4 |
These are the mean of three determinations.
Fig. 5.
The cytotoxic effect of Vinblastine Sulfate as Reference Standard on A-549, CACO, HCT-116, Hela, HepG-2, and MCF-7 cell lines.
L. catarinensis effect (55.2 ± 0.7 µg/ml) on A-549 (Lung carcinoma) showed activity the closest to Vinblastine Sulphate (24.6 ± 0.7 µg/ml) followed by Padina pavonica (58.9 ± 0.1 µg/ml), While Padina pavonica effect (59.0 ± 0.1 µg/ml) was equal to the standard (59.0 ± 0.7 µg/ml) when it tested on HCT-116 (Colon carcinoma).
In general Padina pavonica reported to have the best anticancer activities on the 7 tested cell lines followed by L. catarinensis and L. catarinensis respectively (Table 4 & Fig. 2, Fig. 3, Fig. 4, Fig. 5)
Footnotes
Peer review under responsibility of King Saud University.
References
- Aleem A.A. Contributions to the study of the marine algae of the Red Sea: III-Marine Algae from Obhor, in the vicinity of Jeddah, Saudi Arabia. Bull. Fac. Sci. K.A.U. Jeddah. 1978:99–118. [Google Scholar]
- Aleem, A.A., 1993. The marine Algae of Alexandria, Egypt. In: Aleem (Ed.) Faculty of Science, University of Alexandria, Egypt.
- Allely M.C., Seudero D.A., Monks A. Feasibility of drug screening with panels of human tumor cell lines using micro culture tetrazolium assay. Can. Res. 1998;58:589–601. [PubMed] [Google Scholar]
- Awaad, Amani S., AL-Mudhayyif, Hind A., Al-Othman, Monerah R., Zain, Mohamed E., El-Meligy, Reham M., 2017. Amhezole, a novel fungal secondary metabolite from Aspergillus terreus for treatment of microbial mouth infection. Phytother. Res. <https://doi.org/10.1002/ptr.5760> (in press). [DOI] [PubMed]
- Alves C., Pinteus S., Horta A., Pedrosa R. High cytotoxicity and anti-proliferative activity of algae extracts on an in vitro model of human hepatocellular carcinoma. Springerplus. 2016;5(1):1339. doi: 10.1186/s40064-016-2938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunden G. Biologically active compounds from marine organisms. Phytother. Res. 2001;15:89. doi: 10.1002/ptr.982. [DOI] [PubMed] [Google Scholar]
- Bold H.C., Wynne M.J. Prentice-Hall; Englewood Cliffs, New Jersey: 1978. Introduction to the Algae. Structure and Reproduction. xiv+706 p. [Google Scholar]
- Coppejans, E., Leliaert, F., Dargent, O., Gunasekara, R., Clerck, O., 2009. University of Ruhuna, Dept. of Botany; Matora, Srilanka. Srilanka Seaweeds Methodologies and Field Guide to the Dominant Species. pp. 1–265.
- Dziwornu G.A., Caira M.R., Mare J.A., Edkins A.L., Bolton J.J., Beukes D.R., Sunassee S.N. Isolation, characterization and antiproliferative activity of new metabolites from the South African Endemic Red Algal Species Laurencia alfredensis. Molecules. 2017;22(4) doi: 10.3390/molecules22040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gamal A.A. Biological importance of marine algae. Saudi Pharm. J. 2010;18:1–25. doi: 10.1016/j.jsps.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fatemy, A.S., 2008. Study of the effective of some brown algal species extractions (order: Dictyotales) against pathogenic fungi. M.Sc. Thesis, Botany Department, Faculty of Science, Garyounis University, Libya.
- Ely R., Supriya T., Naik C.G. Antimicrobial activity of marine organisms collected off the coast of south East India. J. Exp. Biol. and Ecol. 2004;309:121–127. [Google Scholar]
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- Fitton J., Helen Brown marine algae: A survey of therapeutic potentials. Altern. Complement. Ther. 2003:29–33. [Google Scholar]
- Khan F.Al., Iqbal H., Shahid F., Majed A., Muhammad A., Inayat U.R. Phytochemical screening of some Pakistanian Medicinal Plants. Middle-East J. Sci. Res. 2011;8(3):575–578. [Google Scholar]
- Iwamoto C., Yamada T., Ito Y., Minoura K., Numata A. Cytotoxic cytochalasans from a Penicillium species separated from a marine alga. Tetrahedron. 2001;57:2904–2997. [Google Scholar]
- Kameyama Y., Yamashita K., Kobayashi K., Hosokawa M., Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+ C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet. Genom. 2005;15:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- Koleva I.I., Van Beek T.A., Linseen J.P.H., de Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Kwon M.J., Nam T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006;79:1956–1962. doi: 10.1016/j.lfs.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Lee J., Koo N., Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Safety. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Lima-Filho J.V., Carvalho A., Freitas S.M. Antimicrobial activity of extracts of six macroalgae from the Northeastern Brazilian Coast. Braz. J. Microbiol. 2002;33:311–313. [Google Scholar]
- Lindequest U., Schweder T. Marine biotechnology. In: Rehm H.J., Reed G., editors. vol. 10. Wliey-VHC; Weinheim, Germany: 2001. pp. 441–484. (Biotechnology). [Google Scholar]
- Madhavi D.L., Deshpande S.S., Salunkhe D.K. Dekker; New York: 1995. Food Antioxidants; p. 267. [Google Scholar]
- Middleton E., Kandaswamy C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Proksch P., Edrada R.A., Ebel R. Drugs from the seas-current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- Rajasulochana R., Dhamotharan P., Krishnamoorthy S., Murugesan Antibacterial activity of the extracts of marine red and brown algae. J. Am. Sci. 2009;5(3):20–25. [Google Scholar]
- Rocha de Souza M.C., Marques C.T., Dore C.M.G., Ferreira da Silva F.R., Rocha H.A.O., Leite E.L. Antioxidant activities of sulphated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007;19:153–160. doi: 10.1007/s10811-006-9121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh P.K., Sucheta S., Sudarshana V.D., Selvamani P., Latha S. Antioxidant activity in some selected Indian medicinal plants. Afr. J. Biotech. 2008;7:1826–1828. [Google Scholar]
- Tüney I., Çadircl B.H., Ünal D., Sukatar A. Antimicrobial activity of the extracts of marine algae from coast of Urla (Izmir, Turkey) Turk. J. Biol. 2006;30:171–175. [Google Scholar]
- Venkateswarlu S., Panchagnula G.K., Gottumukkala A.L., Subbaraju G.V. Synthesis, structural revision, and biological activities of 4′-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron. 2007;63(29):6909–6914. [Google Scholar]
- Yang C., Chung D., Shin I.S., Lee H.Y., Kim J.C., Lee Y.J. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Ye H., Wang K., Zhou C., Liu J., Zeng X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008;111:428–432. doi: 10.1016/j.foodchem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Yen G.C., Duh P.D. Scavenging effect of methanolic extracts of peanut hulls on free radical and active oxygen species. J. Agric. Food Chem. 1994;42:629–632. [Google Scholar]
- Zain M.E., Awaad A.S., Al-Outhman M.R., El-Meligy R.M. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology. 2012;2(1):106–113. [Google Scholar]