Abstract
The products of erythrocyte lyses, haemoglobin (Hb) and haem, are recognized as neurotoxins and the main contributors to delayed cerebral oedema and tissue damage after intracerebral haemorrhage (ICH). Finding a means to efficiently promote absorption of the haemolytic products (Hb and haem) around the bleeding area in the brain through stimulating the function of the body's own garbage cleaning system is a novel clinical challenge and critical for functional recovery after ICH. In this review, available information of the brain injury mechanisms underlying ICH and endogenous haematoma scavenging system is provided. Meanwhile, potential intervention strategies are discussed. Intracerebral blood itself has ‘toxic’ effects beyond its volume effect after ICH. Haptoglobin–Hb–CD163 as well as haemopexin–haem–LRP1 is believed to be the most important endogenous scavenging pathway which participates in blood components resolution following ICH. PPARγ–Nrf2 activates the aforementioned clearance pathway and then accelerates haematoma clearance. Meanwhile, the scavenger receptors as novel targets for therapeutic interventions to treat ICH are also highlighted.
Keywords: haematoma resolution, haematoma scavenge, scavenger receptors, intracerebral haemorrhage, neural recovery
| • Introduction |
| • Current understanding of the mechanisms underlying ICH‐induced brain injury |
| • Phagocytosis in haematoma resolution |
| • Potential endogenous haematoma scavenger receptors after ICH |
| • CD36 |
| • CD47 |
| • SRA |
| • Hp‐Hb‐CD163 |
| • Hx‐Heme‐LRP1 |
| • The upstream regulatory mechanism and intervention strategy for scavenger receptors after ICH |
| ‐ Nuclear factor erythroid 2‐related factor 2 (Nrf2) |
| ‐ Peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) |
| • Potential treatment options/strategies for haematoma resolution via CD36 |
| ‐ PPAR‐γ agonists |
| ‐ Nrf2 agonists |
| • The agonists for the other scavenger receptors |
| ‐ CD163 agonists |
| ‐ Haptoglobin(Hp) and haemopexin(Hx) agonists |
| ‐ LRP1 agonists |
| ‐ SRA agonists |
| ‐ CD47 agonists |
| ‐ Iron chelators |
| • Conclusion |
| • Acknowledgements |
| • Conflict of interest |
Introduction
Extravasated blood and subsequent intrahaematoma haemolytic products trigger a series of adverse events after intracerebral haemorrhage (ICH), leading to secondary brain injury, oedema and severe neurological deficits or death. Haematomas are the primary cause of neurological deficits associated with ICH. Although the haematoma in human's brain gradually resolves within months, full restoration of neurological function can be slow and often incomplete, leaving survivors with devastating neurological deficits. Unless haematoma is cleared, the reservoirs of blood continue to inflict injury to neurovascular structures and blunt the brain repair processes 1. However, only a few evidence‐based targeted treatments are used for ICH management, and interventions focus primarily on supportive care and comorbidity prevention. Effective haematoma clearance and/or facilitating haematoma absorption result in the removal of all the toxic components, which is a goal and a novel therapeutic strategy for ICH, as haematoma removal/resolution can relieve mechanical compression, limit inflammatory injury and promote the recovery of neuronal function 2, 3, 4.
Endogenous garbage cleaning system also known as scavenger receptors plays important roles in the regulation of haematoma resolution in ICH 5. This review seeks to understand how the endogenous garbage cleaning system or scavenger receptor system in the brain works together to remove blood from the brain and reduce brain damage, then find a medical measure to speed up this process.
Current understanding of the mechanisms underlying ICH‐induced brain injury
Brain injury due to ICH initially occurs within the first few hours as a result of mass effect due to haematoma formation. But many patients continue to deteriorate clinically despite no signs of rehaemorrhage or haematoma expansion. This continued insult after primary haemorrhage is believed to be mediated by direct toxicity and inflammatory responses induced by the components and metabolic products of late‐stage haematomas and aggravates neurological deficits 6. In other words, intracerebral blood itself has ‘toxic’ effects beyond its mass effect 7. Oxidative stress caused by components of the lysed erythrocytes contributes to the brain injury after ICH 8. Hb, haem and iron released after red blood cell lysis aggravate ICH‐induced severe brain oedema and direct neuronal damage(Fig. 1). To offset this process, phagocytic cells, including the brain's microglia and haematogenous macrophages, phagocytose and then remove extravasated erythrocytes before lysis and subsequent toxicity occurs 3. So the better understanding of phagocytosis through corresponding scavenger receptors is beneficial to explore removal of blood from the ICH‐affected brain, thus limiting/preventing haemolysis from occurring 5. The potential endogenous scavenger receptors following ICH were illustrated in Figure 2.
Figure 1.
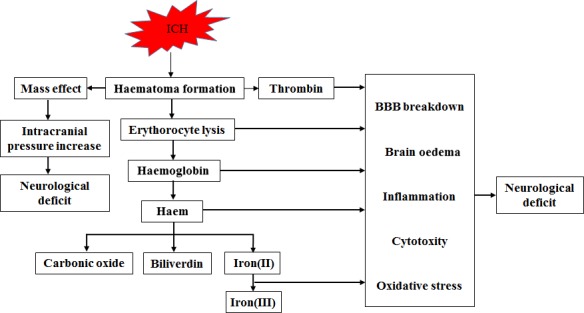
The major factors contributing to brain injury after ICH (including mass effect, thrombin and blood components).
Figure 2.
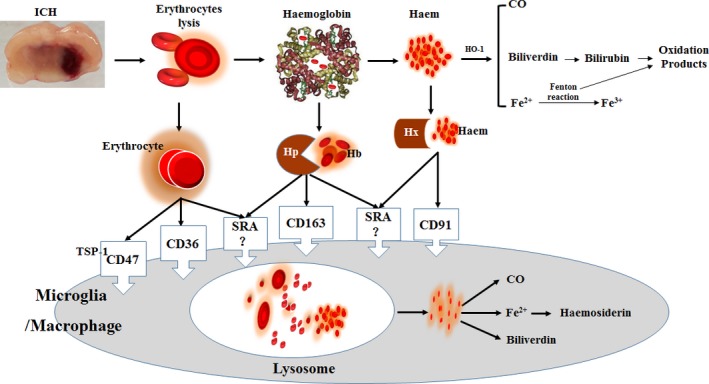
The potential endogenous scavenger receptors (such as CD36, CD47, SRA, Hp–Hb–CD163 and Hx–haem–CD91) following ICH.
Phagocytosis in haematoma resolution
Microglia/macrophages (MMΦ) represent the primary phagocytic system and the first line of defence against brain injuries that mediates the clean‐up of haematoma. Thus, the efficacy of phagocytic function by microglia/MMΦ is an essential step in limiting ICH‐mediated damage 1. The resident microglia and peripheral macrophages are rapidly mobilized to the lesions and initiate the release of mediators and recruitment of other immune cells 9. Microglia is a key factor to remove the haematoma and clear debris, but it is a source of ongoing inflammation. Activated microglia/MMΦ may play a potentially detrimental neurotoxic role by eliciting the expression of pro‐inflammatory and initiating neuroprotective properties. Although overactivation of microglia/MMΦ amplifies inflammatory neuronal damage, anti‐inflammatory agents have failed to show clinical benefits in many stroke trials so far. The undesired effects may result from a broad suppression of microglia/ MMΦ which deprive the normal defensive functions of brain. It is worth noting that acute inflammation serves many protective functions, whereas chronic inflammation is more likely to exacerbate injury. So experimental stroke therapies should be shifted from blanketed microglia/ MMΦ suppression towards a more nuanced adjustment of the balance between protective and toxic microglia/MMΦ phenotypes 10. Phagocyte activation inevitably release pro‐inflammatory mediators and free radicals during haematoma resolution which is toxic to neighbouring cells, leading to secondary brain injury, but promotion of phagocytosis in a timely and efficient manner which may limit the toxic effects of persistent blood products on surrounding tissue and this manner may be important for recovery after ICH 2. The process of the haematoma resolution probably be related to the concomitant acute brain swelling. Recent studies found that enhancing microglia/MMΦ‐mediated phagocytosis speeds up haematoma clearance and then improves functional outcome after ICH 1. Scavenger receptors, expressed on microglia/astrocytes or endothelial cells, play important roles in the regulation of phagocytosis in microglia/MMΦ.
Potential endogenous haematoma scavenger receptors after ICH
As shown in Figure 2, potential endogenous scavenger receptors, as a major subset of innate pattern recognition receptors, are mainly functioned in endocytosis and exogenous invaders 11. They play a crucial role in maintenance of cerebral homeostasis and phagocytic regulation. This section summarizes the newly recognized functions of scavenger receptors in haematoma clearance following ICH.
CD36
CD36 is a well‐recognized integral microglia/macrophage cell membrane protein and a type II scavenger receptor which is expressed on the surface of macrophages and monocytes and plays an important role in mediating the recognition and phagocytosis. Cells lacking phagocytic abilities acquire phagocytic functions following transfection with CD36 12. The low levels of CD36 present may control adhesion of erythrocytes and may have a signal transduction role in platelets and monocytes. CD36 and thrombospondin (TSP) appear to be involved in several cell adhesion processes, including thrombin‐induced platelet aggregation, adhesion of platelets to monocytes and so on 13.
CD47
CD47 is an integrin‐associated transmembrane protein expressed in a variety of cells types including microglia/ MMΦ, oligodendrocytes and erythrocytes. CD47, a well‐known ‘don’t eat me' signal, controls erythrocyte lifespan positively through inhibition of phagocytosis via signal regulatory protein (SIRPα) on normal/healthy erythrocytes, and it revealed an important role in the clearance of aged erythrocytes 14. CD47 on erythrocytes and other cells can function as a regulator of target cell phagocytosis 15. As a switch for erythrophagocytosis, CD47 undergoes a conformational change during ageing, which causes thrombospondin (TSP‐1) binding and recognition of CD47 as an ‘eat me’ signal by SIRPα. The conformational status of CD47 can be changed through oxidative stress, and binding of TSP‐1 to apoptotic cells enhanced phagocytosis without inducing the secretion of pro‐inflammatory cytokines 14. CD47 expression was increased in the perihaematomal white and grey matter after ICH, and deferoxamine treatment attenuated brain CD47 expression after ICH 16. Higher microglial activation at day 3 after experimental ICH was found after CD47 knockout blood injection, and CD47 has a key role in haematoma clearance after ICH 17. The present results of CD47 in ICH are confusing and are contradictory to its phagocytic property in pathological conditions, and the exact role of CD47 in erythrocyte clearance after ICH still needs to be further study.
SRA
Scavenger receptor A (SRA), also known as the macrophage scavenger receptor and cluster of differentiation 204 (CD204), plays roles in lipid metabolism, atherogenesis and a number of metabolic processes. SRA is reported to be host protective in some disease states, but there is also compelling evidence that SRA plays a role in the pathophysiology of other diseases 18, 19. On the one hand, SRA is clearly beneficial and host protective in some models of disease. SRA on microglial cells mediates the binding of β amyloid fibrils and is responsible for preventing the accumulation of amyloid in the brain. The decrease in SRA activity could contribute to the progression of neurodegeneration 20. SRA was highly expressed in erythrocyte lysate‐treated microglia. Genetic SRA ablation increased microglia activation and cytokine production, and sensitized mice to ICH‐induced neuron injury 21. SRA down‐regulated inflammatory response expression in microglia by suppressing TLR4‐induced activation 22. SRA mediates activation of inflammatory signalling and apoptosis in ischaemic stroke, both of which contribute to cerebral injury. The published data have given rise to an intriguing dilemma. As well as the other markers of microglia/MMΦ activation, SRA is a two‐edged sword in health and disease 18, 19. Oxidized erythrocytes were internalized via SRA on macrophages and then sent to lysosomes for scavenging. The exact role of SRA in ICH is dramatic and unclear, as a scavenger receptor, and it should participate in haematoma resolution through microglia activation and maybe produce undesirable inflammatory response, but the results of SRA in ICH are confusing 21, 22.
Hp–Hb–CD163
CD163 is a phagocytic marker and a haemoglobin scavenger receptor, of which expression is thought to be exclusive to perivascular (PVM) and monocyte—macrophage system. It is a glycoprotein belonging to class B of the scavenger receptor cysteine‐rich superfamily. It functions as a membrane‐bound scavenger receptor for clearing extracellular haptoglobin–haemoglobin (Hp‐Hb) complexes 23. Both in vitro and in vivo investigations have shown that ROS is highly produced after exposing Hb to cell culture or injecting Hb into mouse striatum 24, 25. Haptoglobin(Hp) is the primary Hb‐binding protein in human plasma, which attenuates the adverse biochemical and physiological effects of extracellular Hb. The cellular receptor target of Hp is the monocyte/MMΦ scavenger receptor, CD163. Excessive Hb up‐regulated expression of Hp and the Hb–Hp receptor CD163 in neurons in vivo and in vitro 26. Free Hb binds to Hp and once Hp‐Hb complex is endocytosed by CD163, which mediated delivery of Hb to the macrophage may fuel an anti‐inflammatory response because haem metabolites have potent anti‐inflammatory effects 27.
Hx–Haem–LRP1
With chronic haemolysis following ICH, Hp is depleted and Hb readily distributes to tissues where it might be exposed to oxidative conditions. In such conditions, haem can be released from ferric Hb. The free haem is highly toxic which can accelerate tissue damage by promoting peroxidative reactions and activation of inflammatory cascades 28. The haem scavenger protein–haemopexin (Hx) contributes to haematoma removal as well as Hp‐Hb after ICH 29, and Hx is another plasma glycoprotein able to bind haem with high affinity. Hx sequesters haem in an inert, non‐toxic form and transports it to the liver for catabolism and excretion 30. Hp and Hx have been characterized as a sequential defence system with Hp as the primary protector and Hx as a backup when Hp has been depleted during severe ICH. The linear relationship between Hx concentration and protection defined a highly efficient backup scavenger system during conditions of large excess of free Hb 31. The haem–Hx complex is endocytosed by cells expressing the low‐density lipoprotein receptor‐related protein‐1 (LRP1)/CD91 receptor 32, 33. LRP1 is a transmembrane receptor expressed on several cell types including macrophages, hepatocytes, neurons, vascular endothelial cells, pericytes, smooth muscle cells and astrocytes. Half of the BBB clearance is mediated by brain endothelial LRP1 in various model systems 34. As the only known endocytic receptor for Hx–haem complexes, LRP1 combined function of Hx may mediate localized haem clearance in the brain during cerebral haemorrhage. Upon binding of haem–Hx to LRP1, the complex becomes internalized via endocytosis into cells, and inside the cell, the haem–Hx complex is dissociated by lysosomal activity. Haem is catabolized by haem oxygenases into biliverdin, carbon monoxide and iron. Activation of LRP1 scavenging system in humans has favourable effects after subarachnoid haemorrhage (SAH) 35. Recently, it is confirmed that the activation of the LRP1 system is beneficial in experimental ICH 33. It should be proved in clinic through above‐mentioned findings.
The upstream regulatory mechanism and intervention strategy for scavenger receptors after ICH
Nuclear factor erythroid 2‐related factor 2 (Nrf2)
Nrf2 itself is a ubiquitous pleiotropic transcription factor and a pivotal mediator in redox homeostasis and inflammatory disorders, within the regulated region of many cytoprotective and antioxidant target genes which encode for critical mediators of cellular defence functions 36. In unstressed conditions, Nrf2 is retained in the cytoplasm by its inhibitor kelch‐like ECH‐associated protein 1 (Keap1), upon activation by oxidative and electrophilic stress, and Nrf2 disassociates from Keap1, transactivates the antioxidant response element (ARE) and then promotes the related cytoprotective pathways. So Nrf2 is a key regulator of cellular resistance against oxidants 37. In addition, the transcriptional activity of Nrf2 is essential for the clearance of phosphorylated tau via the selective autophagy 38. Also, knockout of Nrf2 reduced the efficiency of macrophage accumulation and impaired clearance of myelin debris and phosphorylated tau 39. Nrf2 controls the expression of CD36, which may represent a key component in attaining brain clean‐up after stroke or ICH. Removal of RBC by microglia and/or MMΦ may have a multiple indirect effect on oxidative stress, as it could reduce the accumulation of haemoglobin–haem–free iron and consequently the formation of free radicals. Furthermore, activated Nrf2 up‐regulated the levels of Hp in blood plasma and in ICH‐affected brain in animals as well as the expression of CD163 40. In conclusion, Nrf2 in microglia/MMΦ plays a pivotal role in regulating the phagocytic functions of these cells, and that in an experimental model of ICH, Nrf2 appears to be essential to haematoma clearance 1. The potential role of Nrf2 following ICH is illustrated in Figure 3.
Figure 3.
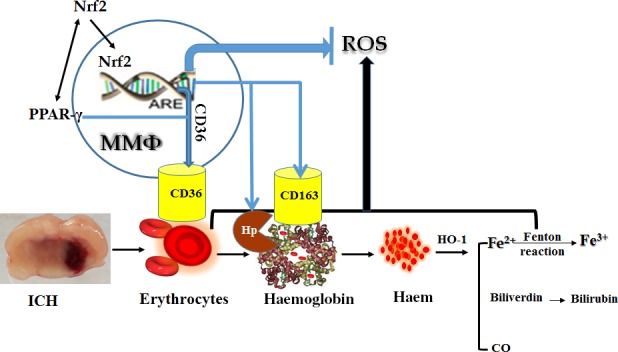
The potential role of Nrf2 and the interaction of PPAR‐γ with Nrf2 following ICH.
Peroxisome proliferator‐activated receptor‐γ (PPAR‐γ)
The peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) is a ligand‐activated transcription factor belonging to the nuclear hormone receptor superfamily and controlling reproduction, metabolism, development and immune response. PPAR‐γ expressed not only in adipocytes, but also in vascular tissues, such as vascular smooth muscle cells (VSMCs) and endothelial cells, and in macrophages 41. PPAR‐γ and its agonists have a protective role in several neurological diseases via reducing inflammation, decreasing oxidative damage and attenuating cell death 42. PPAR‐γ is protective not only to neurons, astrocytes, oligodendrocytes, endothelia, but also to microglia/MMΦ both in vitro and in vivo. PPAR‐γ agonists reduce the ability of inflammatory stimuli to activate the alveolar macrophage while simultaneously stimulating phagocytosis of both opsonized and unopsonized particles, via the Fc‐γ and CD36 receptors, respectively 43. PPAR‐γ ligands have also been shown to up‐regulate the expression of CD36 and then promote microglia/MMΦ‐mediated clearance of toxic cellular debris 44. PPAR‐γ agonists not only increased microglia‐mediated phagocytosis of RBC, but also reduced the production of H2O2 during the process of engulfment 2, 44. So enhancement of phagocytosis by PPAR‐γ agonists inevitably results in the inflammatory response and the dose‐dependent neurotoxicity.
Some studies showed PPAR‐γ had an interaction with Nrf2. Endogenous PPAR‐γ ligands activate Nrf2 expression, meanwhile Nrf2 regulated PPAR‐γ expression 45. Nrf2 controls CD36 expression independently of PPAR‐γ. Expression of Nrf2 was reduced by knockdown of PPAR‐γ, whereas PPAR‐γ was reduced by knockdown of Nrf2, thereby demonstrating two‐way positive interactions. PPAR‐γ agonists up‐regulate Nrf2, and knockdown of PPAR‐γ reduced the mRNA expression for Nrf2 46. This indicates a tight, positive, two‐way reinforcing transcriptional interaction between PPAR‐γ and Nrf2 that may improve endothelial function 46. The interaction of PPAR‐γ with Nrf2 following ICH was illustrated in Figure 3. Potential exogenous pharmacological/molecular manipulations direct at haematoma resolution after ICH (as illustrated in Fig. 4).
Figure 4.
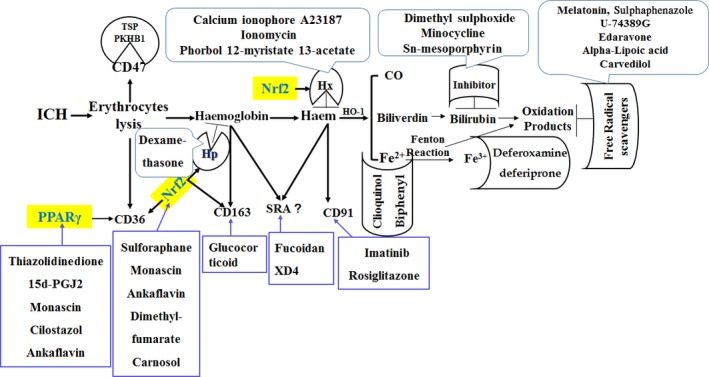
Summary of current potential exogenous pharmacological/molecular manipulations direct at haematoma resolution after ICH.
Potential treatment options/strategies for haematoma resolution via CD36
PPAR‐γ agonists
PPAR‐γ agonist‐induced up‐regulation of CD36 in macrophages enhances the ability of microglia to phagocytose red blood cells (in vitro assay), helps to improve haematoma resolution and reduces a mouse ICH‐induced deficit. In rat primary microglia in culture, PPAR‐γ agonists not only increased microglia‐mediated phagocytosis of RBC, but also reduced the production of H2O2 during the process of engulfment 2. PPAR‐γ agonists could represent a potential treatment strategy for ICH 3.
Thiazolidinediones are potent and selective activators of PPAR‐γ 47. The derivatives of the parent compound thiazolidinedione such as rosiglitazone and pioglitazone are generally well tolerated in AD and aMCI patients 48. The Safety of Pioglitazone for Hematoma Resolution In ICH (SHRINC) Study 49, a prospective, randomized, blinded, placebo‐controlled, dose‐escalation safety trial, was recently completed, and its results showed that pioglitazone should be a potential therapy for ICH in future. The cyclopentanone prostaglandins (e.g. 15d–PGJ2) and monascin as well as thiazolidinediones are PPAR‐γ agonists, which have been proven to act as potent and safe pro‐survival factors for primary neurons subjected to either excitotoxic insult, oxygen–glucose deprivation (OGD) or H2O2‐induced oxidative stress 44, 47, 50 and then attenuate ROS generation. These PPAR‐γ agonists are proposed to act as endogenous PPAR‐γ ligands demonstrate rather limited selectivity towards PPAR‐γ with some of its biological activation of Nrf2. Monascin regulated PPAR‐γ and Nrf‐2 to improve lung oxidative inflammation 51. Cilostazol, a potent phosphodiesterase type III inhibitor, has been used as a vasodilating antiplatelet drug for the treatment of ischaemic symptoms in chronic peripheral arterial obstruction for preventing recurrence of cerebral infarction 52. Cilostazol stimulates PPAR‐γ transcriptional activity in human endothelial cells and may offer an effective therapeutic window along with complementary effects for individuals at high risk of type 2 diabetes by improving insulin sensitivity with anti‐inflammatory effects 53. Ankaflavin exerted PPAR‐γ agonist activity by the up‐regulation of the signalling pathway of Nrf2 54.
The existing evidence showed that dose‐dependent neurotoxicity of the 15d–PGJ2 in cerebellar granule cells, primary cortical neurons and spinal cord motor neurons which were believed to be associated with induction of apoptosis and not likely associated with the activation of PPAR‐γ 55. On the other hand, the clinically relevant, more selective PPAR‐γ agonist, such as rosiglitazone, was linked to peripheral oedema, increase in body weight, and cardiomyopathies and heart failure 56. It is likely that PPAR‐γ agonist treatment for ICH will be short term, potentially avoiding these side‐effects, although this needs further testing.
Nrf2 agonists
Nrf2 as a second important transcription factor involved in the induction of the scavenger receptor CD36 and antioxidant stress genes in atherosclerosis 57. Nrf2 transcription factor could be an alternative target to PPAR‐γ in the control of severe malaria through parasite clearance 58. Nrf2 plays an essential role in the effective clean‐up process after ICH, perhaps via co‐ordinated efforts to enhance phagocytosis while concomitantly limiting oxidative stress 1. Sulforaphane was capable of enhancing RBC phagocytosis and improving haematoma resolution via activating Nrf2 and inducing CD36 expression in microglias 1. Monascin acts as a novel natural Nrf2 activator with PPAR‐γ agonist activity was confirmed by Nrf2 and PPAR‐γ reporter assays 59. Protective effects of ankaflavin against diabetes are mediated by the up‐regulation of the signalling pathway of Nrf2, which enhances antioxidant activity and serves as a PPAR‐γ agonist to enhance insulin sensitivity 54. Monascin and ankaflavin, the yellow pigments produced by Monascus species, have been proven to possess hypolipidaemic functions and less side‐effects 60. Dimethyl fumarate is an orally administered fumarate ester recently FDA approved for first‐line monotherapy of multiple sclerosis, which stimulates Nrf2 activity to attenuate hyperphosphataemia in vitro or vitamin D3‐induced in vivo vascular calcification 61. Carnosol increased the nuclear levels of Nrf2 and involved in the cytoprotective effects 62.
The agonists for the other scavenger receptors
CD163 agonists
To date, the Hb–haptoglobin (Hp) complex is the only known ligand of CD163, and neither Hp alone nor free Hb has been found to display high‐affinity binding to the receptor. Because the Hb–Hp complex binds to CD163 with high affinity and the receptor system has a high endocytotic capacity, CD163 is thought to mediate the clearance of Hb–Hp complexes from the blood 27. Glucocorticoid can induce CD163 expression in MMΦ which enhances their capacity to bind and internalize Hb–Hp complexes 63.
Haptoglobin(Hp) and haemopexin(Hx) agonists
Hp expression is induced by inflammatory cytokines, dexamethasone and adrenoceptor agonists. In contrast, Hp was inhibited by nicotinic acid and the PPARγ agonist, rosiglitazone 64. The transcription rate of Hx increased by the calcium ionophore A23187, ionomycin and phorbol 12‐myristate 13‐acetate (PMA) in serum‐starved H4IIE rat hepatoma cells 65. Activated Nrf2 binds to antioxidant response elements (ARE), which promotes the transcription of haptoglobin and haemopexin 66.
LRP1 agonists
LRP1/CD91 contributed to haem clearance and blood–brain barrier protection after ICH in mice. Our research showed that recombinant LRP1 as supplement provides a novel approach to ameliorate intracerebral haemorrhage brain injury via its pleiotropic neuroprotective effects 33. Intrathecal infusions of LRP1 agonists—RBD (the Receptor Binding Domain of alpha‐2‐macroglobulin) or PEX (the haemopexin domain of MMP‐9) result in axonal sprouting and regeneration after spinal cord injury via activating ERK and Akt pathways 67. Imatinib promotes LRP1‐dependent ERK activation and helps to the pro‐survival effects on β‐cells 68.
SRA agonists
Fucoidan, a SRA agonist, could promote macrophage apoptosis by repressing ER stressor triggered autophagy 69. Heptapeptide XD4 activates SRA on the glia by increasing the binding of Aβ to SRA, thereby promoting glial phagocytosis of Aβ oligomer in microglia and astrocytes 70.
CD47 agonists
CD47 stimulated by its ligands, thrombospondins (TSPs), the agonist sequences occur in all five isoforms of TSP, it is possible that any TSP isoform could be pro‐apoptotic 71. PKHB1, the serum‐stable CD47 agonist peptide, might overcome drug refractoriness of chronic lymphocytic leukaemia by the pro‐apoptotic potential of targeting cell 72.
Iron chelators
Our study showed that ferric iron chelators such as deferoxamine and deferiprone lowered iron deposition in brain following ICH 73. However, ferric iron chelation does not improve the outcome after ICH 33, 74. Clioquinol, a ferrous iron chelator, improved the neurological outcome and attenuated brain oedema and ROS production besides reducing iron levels 73. Another ferrous chelator, 2,2′‐bipyridine, is a potential means of ameliorating iron‐induced injury after ICH 75; unfortunately, another results failed to support the use of bipyridine against ICH 76, and the function of bipyridine on ICH is uncertain so far.
Conclusion
Hp–Hb–CD163 as well as Hx–haem–LRP1 is believed to be the most important endogenous garbage scavenging pathway which participates in haematoma/ blood components resolution following ICH. PPARγ–Nrf2 activates the aforementioned clearance pathway and then accelerates haematoma removal. So the above‐mentioned haematoma scavenger pathway as a novel targets for therapeutic interventions to treat ICH is prospective and valuable.
Conflict of interest
The authors confirm that there is no conflict of interests.
Acknowledgements
Funding source: This work was supported by a project from National Natural Science Foundation of China(Project number: 81771294).
References
- 1. Zhao X, Sun G, Ting SM, et al Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015; 133: 144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao X, Sun G, Zhang J, et al Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator‐activated receptor gamma in microglia/macrophages. Ann Neurol. 2007; 61: 352–62. [DOI] [PubMed] [Google Scholar]
- 3. Zhao X, Grotta J, Gonzales N, et al Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009; 40: S92–4. [DOI] [PubMed] [Google Scholar]
- 4. Fang H, Wang PF, Zhou Y, et al Toll‐like receptor 4 signaling in intracerebral hemorrhage‐induced inflammation and injury. J Neuroinflammation. 2013; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Husemann J, Loike JD, Anankov R, et al Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002; 40: 195–205. [DOI] [PubMed] [Google Scholar]
- 6. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012; 11: 720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xi G, Wagner KR, Keep RF, et al Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998; 29: 2580–6. [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Hua Y, Keep RF, et al Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002; 953: 45–52. [DOI] [PubMed] [Google Scholar]
- 9. Schilling M, Besselmann M, Muller M, et al Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005; 196: 290–7. [DOI] [PubMed] [Google Scholar]
- 10. Hu X, Li P, Guo Y, et al Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012; 43: 3063–70. [DOI] [PubMed] [Google Scholar]
- 11. Yu X, Guo C, Fisher PB, et al Scavenger receptors: emerging roles in cancer biology and immunology. Adv Cancer Res. 2015; 128: 309–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao D, Luo J, Chen D, et al CD36 regulates lipopolysaccharide‐induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Sci Rep. 2016; 6: 23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Schravendijk MR, Handunnetti SM, Barnwell JW, et al Normal human erythrocytes express CD36, an adhesion molecule of monocytes, platelets, and endothelial cells. Blood. 1992; 80: 2105–14. [PubMed] [Google Scholar]
- 14. Burger P, de Korte D, van den Berg TK, et al CD47 in Erythrocyte Ageing and Clearance – the Dutch Point of View. Transfus Med Hemother. 2012; 39: 348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olsson M, Nilsson A, Oldenborg PA. Target cell CD47 regulates macrophage activation and erythrophagocytosis. Transfus Clin Biol. 2006; 13: 39–43. [DOI] [PubMed] [Google Scholar]
- 16. Zhou X, Xie Q, Xi G, et al Brain CD47 expression in a swine model of intracerebral hemorrhage. Brain Res. 2014; 1574: 70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ni W, Mao S, Xi G, et al Role of erythrocyte CD47 in intracerebral hematoma clearance. Stroke. 2016; 47: 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR‐A) multifunctional? ‐ The mouse's tale. J Clin Invest. 2001; 108: 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley JL, Ozment TR, Li C, et al Scavenger receptor‐A (CD204): a two‐edged sword in health and disease. Crit Rev Immunol. 2014; 34: 241–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer's disease. Int J Alzheimers Dis. 2012; 2012: 489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Z, Zhong S, Liu Y, et al Scavenger receptor SRA attenuates microglia activation and protects neuroinflammatory injury in intracerebral hemorrhage. J Neuroimmunol. 2015; 278: 232–8. [DOI] [PubMed] [Google Scholar]
- 22. Yuan B, Shen H, Lin L, et al Scavenger receptor SRA attenuates TLR4‐induced microglia activation in intracerebral hemorrhage. J Neuroimmunol. 2015; 289: 87–92. [DOI] [PubMed] [Google Scholar]
- 23. Schaer DJ, Alayash AI, Buehler PW. Gating the radical hemoglobin to macrophages: the anti‐inflammatory role of CD163, a scavenger receptor. Antioxid Redox Signal. 2007; 9: 991–9. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Mori T, Sumii T, et al Hemoglobin‐induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002; 33: 1882–8. [DOI] [PubMed] [Google Scholar]
- 25. Qu Y, Chen J, Benvenisti‐Zarom L, et al Effect of targeted deletion of the heme oxygenase‐2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab. 2005; 25: 1466–75. [DOI] [PubMed] [Google Scholar]
- 26. Garton TP, He Y, Garton HJ, et al Hemoglobin‐induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Res. 2016; 1635: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti‐inflammatory response. Ann Med. 2004; 36: 347–54. [DOI] [PubMed] [Google Scholar]
- 28. Ma B, Day JP, Phillips H, et al Deletion of the hemopexin or heme oxygenase‐2 gene aggravates brain injury following stroma‐free hemoglobin‐induced intracerebral hemorrhage. J Neuroinflammation. 2016; 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011; 42: 1781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaer DJ, Vinchi F, Ingoglia G, et al Haptoglobin, hemopexin, and related defense pathways‐basic science, clinical perspectives, and drug development. Front Physiol. 2014; 5: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deuel JW, Vallelian F, Schaer CA, et al Different target specificities of haptoglobin and hemopexin define a sequential protection system against vascular hemoglobin toxicity. Free Radic Biol Med. 2015; 89: 931–43. [DOI] [PubMed] [Google Scholar]
- 32. Hvidberg V, Maniecki MB, Jacobsen C, et al Identification of the receptor scavenging hemopexin‐heme complexes. Blood. 2005; 106: 2572–9. [DOI] [PubMed] [Google Scholar]
- 33. Wang G, Manaenko A, Shao A, et al Low‐density lipoprotein receptor‐related protein‐1 facilitates heme scavenging after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2016; 37: 1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Storck SE, Meister S, Nahrath J, et al Endothelial LRP1 transports amyloid‐beta1‐42 across the blood‐brain barrier. J Clin Invest. 2016; 126: 123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garland P, Durnford AJ, Okemefuna AI, et al Heme‐hemopexin scavenging is active in the brain and associates with outcome after subarachnoid hemorrhage. Stroke. 2016; 47: 872–6. [DOI] [PubMed] [Google Scholar]
- 36. Zhao H, Hao S, Xu H, et al Protective role of nuclear factor erythroid 2‐related factor 2 in the hemorrhagic shock‐induced inflammatory response. Int J Mol Med. 2016; 37: 1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013; 53: 401–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jo C, Gundemir S, Pritchard S, et al Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014; 5: 3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim S, Choi KJ, Cho SJ, et al Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci Rep. 2016; 6: 24933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyle JJ, Johns M, Lo J, et al Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2011; 31: 2685–91. [DOI] [PubMed] [Google Scholar]
- 41. Ikeda Y, Sugawara A, Taniyama Y, et al Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator‐activated receptor gamma in macrophages via an interaction with NRF2. J Biol Chem. 2000; 275: 33142–50. [DOI] [PubMed] [Google Scholar]
- 42. Wu JS, Cheung WM, Tsai YS, et al Ligand‐activated peroxisome proliferator‐activated receptor‐gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14‐3‐3 epsilon upregulation. Circulation. 2009; 119: 1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reddy RC. Immunomodulatory role of PPAR‐gamma in alveolar macrophages. J Investig Med. 2008; 56: 522–7. [DOI] [PubMed] [Google Scholar]
- 44. Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF‐kappaB. CNS Neurosci Ther. 2015; 21: 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho HY, Gladwell W, Wang X, et al Nrf2‐regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010; 182: 170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luo Z, Aslam S, Welch WJ, et al Activation of nuclear factor erythroid 2‐related factor 2 coordinates dimethylarginine dimethylaminohydrolase/PPAR‐gamma/endothelial nitric oxide synthase pathways that enhance nitric oxide generation in human glomerular endothelial cells. Hypertension. 2015; 65: 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lehmann JM, Moore LB, Smith‐Oliver TA, et al An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator‐activated receptor gamma (PPAR gamma). J Biol Chem. 1995; 270: 12953–6. [DOI] [PubMed] [Google Scholar]
- 48. Liu J, Wang LN, Jia JP. Peroxisome proliferator‐activated receptor‐gamma agonists for Alzheimer's disease and amnestic mild cognitive impairment: a systematic review and meta‐analysis. Drugs Aging. 2015; 32: 57–65. [DOI] [PubMed] [Google Scholar]
- 49. Gonzales NR, Shah J, Sangha N, et al Design of a prospective, dose‐escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC). Int J Stroke. 2013; 8: 388–96. [DOI] [PubMed] [Google Scholar]
- 50. Zhao X, Zhang Y, Strong R, et al 15d‐Prostaglandin J2 activates peroxisome proliferator‐activated receptor‐gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006; 26: 811–20. [DOI] [PubMed] [Google Scholar]
- 51. Hsu WH, Lee BH, Pan TM. Monascin attenuates oxidative stress‐mediated lung inflammation via peroxisome proliferator‐activated receptor‐gamma (PPAR‐gamma) and nuclear factor‐erythroid 2 related factor 2 (Nrf‐2) modulation. J Agric Food Chem. 2014; 62: 5337–44. [DOI] [PubMed] [Google Scholar]
- 52. Kambayashi J, Liu Y, Sun B, et al Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003; 9: 2289–302. [DOI] [PubMed] [Google Scholar]
- 53. Park SY, Shin HK, Lee JH, et al Cilostazol ameliorates metabolic abnormalities with suppression of proinflammatory markers in a db/db mouse model of type 2 diabetes via activation of peroxisome proliferator‐activated receptor gamma transcription. J Pharmacol Exp Ther. 2009; 329: 571–9. [DOI] [PubMed] [Google Scholar]
- 54. Lee BH, Hsu WH, Chang YY, et al Ankaflavin: a natural novel PPARgamma agonist upregulates Nrf2 to attenuate methylglyoxal‐induced diabetes in vivo . Free Radic Biol Med. 2012; 53: 2008–16. [DOI] [PubMed] [Google Scholar]
- 55. Yagami T, Ueda K, Asakura K, et al Novel binding sites of 15‐deoxy‐Delta 12,14‐prostaglandin J2 in plasma membranes from primary rat cortical neurons. Exp Cell Res. 2003; 291: 212–27. [DOI] [PubMed] [Google Scholar]
- 56. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356: 2457–71. [DOI] [PubMed] [Google Scholar]
- 57. Ishii T, Itoh K, Ruiz E, et al Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4‐hydroxynonenal. Circ Res. 2004; 94: 609–16. [DOI] [PubMed] [Google Scholar]
- 58. Olagnier D, Lavergne RA, Meunier E, et al Nrf2, a PPARgamma alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog. 2011; 7: e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsu WH, Lee BH, Chang YY, et al A novel natural Nrf2 activator with PPARgamma‐agonist (monascin) attenuates the toxicity of methylglyoxal and hyperglycemia. Toxicol Appl Pharmacol. 2013; 272: 842–51. [DOI] [PubMed] [Google Scholar]
- 60. Lee CL, Hung YP, Hsu YW, et al Monascin and ankaflavin have more anti‐atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages. J Agric Food Chem. 2013; 61: 143–50. [DOI] [PubMed] [Google Scholar]
- 61. Ha CM, Park S, Choi YK, et al Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vascul Pharmacol. 2014; 63: 29–36. [DOI] [PubMed] [Google Scholar]
- 62. Chen CC, Chen HL, Hsieh CW, et al Upregulation of NF‐E2‐related factor‐2‐dependent glutathione by carnosol provokes a cytoprotective response and enhances cell survival. Acta Pharmacol Sin. 2011; 32: 62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schaer DJ, Boretti FS, Schoedon G, et al Induction of the CD163‐dependent haemoglobin uptake by macrophages as a novel anti‐inflammatory action of glucocorticoids. Br J Haematol. 2002; 119: 239–43. [DOI] [PubMed] [Google Scholar]
- 64. do Nascimento CO, Hunter L, Trayhurn P. Regulation of haptoglobin gene expression in 3T3‐L1 adipocytes by cytokines, catecholamines, and PPARgamma. Biochem Biophys Res Commun. 2004; 313: 702–8. [DOI] [PubMed] [Google Scholar]
- 65. Stred SE, Cote D, Weinstock RS, et al Regulation of hemopexin transcription by calcium ionophores and phorbol ester in hepatoma cells. Mol Cell Endocrinol. 2003; 204: 111–6. [DOI] [PubMed] [Google Scholar]
- 66. Belcher JD, Nath KA, Vercellotti GM. Vasculotoxic and Proinflammatory Effects of Plasma Heme: cell Signaling and Cytoprotective Responses. ISRN Oxidative Med. 2013; 2013: pii. 831596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoon C, Van Niekerk EA, Henry K, et al Low‐density lipoprotein receptor‐related protein 1 (LRP1)‐dependent cell signaling promotes axonal regeneration. J Biol Chem. 2013; 288: 26557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fred RG, Boddeti SK, Lundberg M, et al Imatinib mesylate stimulates low‐density lipoprotein receptor‐related protein 1‐mediated ERK phosphorylation in insulin‐producing cells. Clin Sci (Lond). 2015; 128: 17–28. [DOI] [PubMed] [Google Scholar]
- 69. Huang H, Li X, Zhuang Y, et al Class A scavenger receptor activation inhibits endoplasmic reticulum stress‐induced autophagy in macrophage. J Biomed Res. 2014; 28: 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang H, Su YJ, Zhou WW, et al Activated scavenger receptor A promotes glial internalization of abeta. PLoS ONE. 2014; 9: e94197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manna PP, Frazier WA. The mechanism of CD47‐dependent killing of T cells: heterotrimeric Gi‐dependent inhibition of protein kinase A. J Immunol. 2003; 170: 3544–53. [DOI] [PubMed] [Google Scholar]
- 72. Martinez‐Torres AC, Quiney C, Attout T, et al CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCgamma1 activation: evidence from mice and humans. PLoS Med. 2015; 12: e1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang G, Hu W, Tang Q, et al Effect comparison of both iron chelators on outcomes, iron deposit, and iron transporters after intracerebral hemorrhage in rats. Mol Neurobiol. 2016; 53: 3576–85. [DOI] [PubMed] [Google Scholar]
- 74. Auriat AM, Silasi G, Wei Z, et al Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome. Exp Neurol. 2012; 234: 136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu H, Wu T, Li M, et al Efficacy of the lipid‐soluble iron chelator 2,2'‐dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012; 45: 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Caliaperumal J, Wowk S, Jones S, et al Bipyridine, an iron chelator, does not lessen intracerebral iron‐induced damage or improve outcome after intracerebral hemorrhagic stroke in rats. Transl Stroke Res. 2013; 4: 719–28. [DOI] [PubMed] [Google Scholar]


