Abstract
The endocannabinoid system (ECS) is an endogenous signalling pathway involved in the control of several gastrointestinal (GI) functions at both peripheral and central levels. In recent years, it has become apparent that the ECS is pivotal in the regulation of GI motility, secretion and sensitivity, but endocannabinoids (ECs) are also involved in the regulation of intestinal inflammation and mucosal barrier permeability, suggesting their role in the pathophysiology of both functional and organic GI disorders. Genetic studies in patients with irritable bowel syndrome (IBS) or inflammatory bowel disease have indeed shown significant associations with polymorphisms or mutation in genes encoding for cannabinoid receptor or enzyme responsible for their catabolism, respectively. Furthermore, ongoing clinical trials are testing EC agonists/antagonists in the achievement of symptomatic relief from a number of GI symptoms. Despite this evidence, there is a lack of supportive RCTs and relevant data in human beings, and hence, the possible therapeutic application of these compounds is raising ethical, political and economic concerns. More recently, the identification of several EC‐like compounds able to modulate ECS function without the typical central side effects of cannabino‐mimetics has paved the way for emerging peripherally acting drugs. This review summarizes the possible mechanisms linking the ECS to GI disorders and describes the most recent advances in the manipulation of the ECS in the treatment of GI diseases.
Keywords: endocannabinoid system, gastrointestinal pathophysiology, functional gastrointestinal disorders, non‐alcoholic steatohepatitis, inflammatory bowel disease
| • Introduction |
| – Endocannabinoid‐related compounds |
| – The endocannabinoid system and the control of gastrointestinal motility |
| – The endocannabinoid system and the control of visceral sensitivity |
| – The endocannabinoid system and the control of intestinal inflammation |
| • The endocannabinoid system in gut pathophysiology |
| – The endocannabinoid system and functional dyspepsia |
| – The endocannabinoid system in irritable bowel syndrome |
| – The endocannabinoid system in inflammatory bowel disease |
| – The endocannabinoid system in liver disease |
| – Endocannabinoids in non‐alcoholic fatty liver disease |
| • Conclusions |
| • Conflict of interest |
Introduction
Cannabis sativa plant is the most commonly used illicit drug for recreational purposes worldwide, with estimated 16 million users in the United States 1, 2. At present, many patients use cannabis anecdotally to achieve symptomatic relief from a wide variety of symptoms, commonly of GI origin, particularly nausea and pain 3, 4, 5. The therapeutic efficacy of cannabis in the treatment of GI dysfunction relies on the fact that the GI tract is endowed with cannabinoid receptors and N‐arachidonoylethanolamine (anandamide, AEA) and 2‐arachidonoylglycerol (2‐AG), their best‐characterized endogenous ligands 6, 7. Together with their synthetizing and degrading enzymes, they embody the endocannabinoid system (ECS), a ubiquitous and complex system involved in the control of gut homoeostasis. Since first coined in 1995 8, the term ‘endocannabinoids’ (ECs) has been enlarged to a number of recently, yet only partially, identified endogenous ligands, such as 2‐arachidonoylglycerol ether (noladin ether), N‐arachidonoyl‐dopamine (NADA) and O‐arachidonoylethanolamine (virodhamine) 9. In recent years, several lipid‐derived mediators, closely resembling typical ECs, have been described, raising questions on the different pathophysiological role of these compounds 10, 11, 12, 13. These analogues [namely N‐linoleylethanolamine (LEA), N‐oleoylethanolamine (OEA), N‐palmitoylethanolamine (PEA) and N‐stearoylethanolamine (SEA)] are structurally related to classical ECs and have been shown to act synergistically, either enhancing the effects of prototypic ECs (the so‐called entourage effect) or displaying unique effects (seethe ‘Endocannabinoid‐related compounds’ section). An overview of the principal ECs and of the enzymes responsible for their metabolism is proposed in Figure 1. Different from other transmitters, the ECs and their congeners are not stored in intracytoplasmic vesicles, but synthetized from membrane precursors in an ‘on‐demand’ fashion 13. After their release into the extracellular space, these short‐lived compounds are rapidly removed from membrane transporters and degraded by specific enzymes (Fig. 1) 14. The ECs are able to exert their multifaceted activities by binding a large number of receptors that have not been fully identified, so far. The best‐characterized receptors are cannabinoid receptors 1 and 2 (CB1 and CB2), two G‐protein‐coupled receptors expressed in both peripheral and central nervous systems, as well as by a number of non‐neural cells 6, 15, 16. CB1 is responsible for the classical psychotropic effects of marijuana and is mainly expressed in the CNS 17. In the GI tract, CB1 is expressed in both myenteric and submucosal plexuses of the enteric nervous system (ENS), mostly by motoneurons, interneurons and primary afferent neurons but also by epithelial cells 18. Conversely, CB2 predominantly shows a peripheral distribution, with the highest rate of expression on immune cells 19, 20, but it is also found on enteric neurons 21. In rodent models, CB2 appears to be expressed by intestinal epithelial cells; however, this evidence has not been confirmed in both other animal models and human beings 22, 23, 24. As mentioned above, ECs and their related compounds exhibit several non‐CB1/CB2‐mediated effects by binding other receptors with different affinity. The orphan G‐protein‐coupled receptor 55 (GPR55), identified in 1999, has been proposed as the third CB receptor, and although it has been found in the jejunum, ileum and colon, its distribution has not been extensively studied 25. One of the best‐characterized non‐CB receptors for ECs is the transient receptor potential vanilloid type 1 (TRPV1), mainly located on the primary afferent nerve fibres 26. Originally identified as receptors for the capsaicin 27, TRPV1 receptors are known for being activated by NADA and AEA as effectively as capsaicin 28. AEA is a full agonist on TRPV1 receptors, but it also exerts indirect effects by binding CB1 29. Furthermore, a number of ECs have been shown to bind peroxisome proliferator‐activated receptors (PPARs). In vitro studies showed that AEA, noladin and virodhamine are receptor agonists to PPARα, while 2‐AG binds to PPARβ/δ 30. Taken together, the bewildering redundancy of the ECS and the different sites of action of the ECs account for the great variety of actions exhibited by these compounds in vivo.
Figure 1.
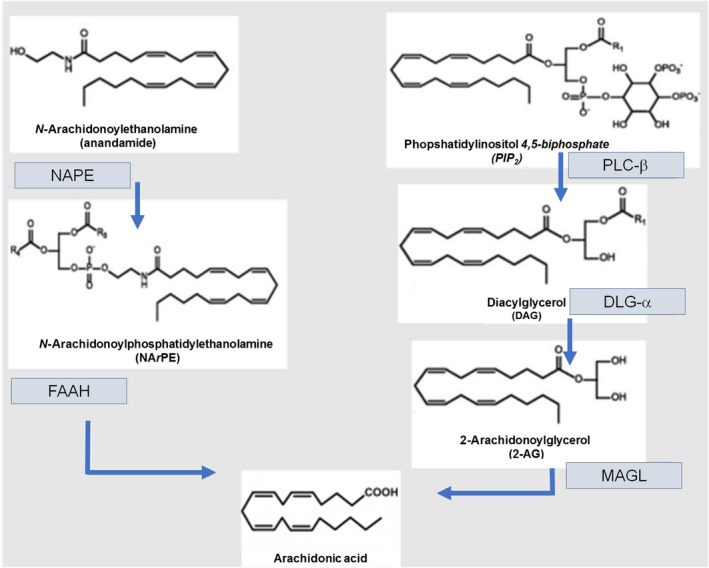
Schematic overview of the enzymes involved in EC metabolism. Anandamide (AEA) and 2‐acylglycerol (2‐AG) are the two best‐recognized stereotypical ECs. Both are synthetized by hydrolysis from membrane lipid precursors, namely N‐arachidonoyl‐phosphatidylethanolamine (NArPE) and phosphatidylinositol‐4,5‐bisphosphate (PIP2) for AEA and 2‐AG, respectively. Both AEA and 2‐AG, after the binding with CB receptors, are rapidly removed by membrane transporters and converted into arachidonic acid by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively.
Endocannabinoid‐related compounds
EC‐like compounds, such as N‐acylethanolamides (NAEs), have a close structural resemblance with classical ECs, but display no activity on CB receptors 10, 31, 32. However, these compounds share some biological activities and similar biosynthetic pathways of those of typical ECs, particularly AEA. AEA synthesis is, indeed, coupled with the formation of PEA, OEA and LEA 10, 33, 34. Although OEA and PEA do not directly activate cannabinoid receptors, they are thought to indirectly potentiate ECS signalling via the ‘entourage effect’ by either competing with stereotypical ECs for enzymatic degradation or increasing their receptor binding affinity 10 (Fig. 2). PEA and OEA are, indeed, both substrates of FAAH, the enzyme responsible for AEA degradation. By either competing with AEA for FAAH or inducing FAAH down‐regulation 35, 36, PEA and OEA could reduce AEA catabolism and ultimately increase AEA concentrations. Furthermore, independently of FAAH, PEA and OEA are able to enhance AEA effects at TRPV1 receptors 37, 38. OEA and PEA can activate, even if with different receptor affinity, PPARα, the G‐protein‐coupled receptor GPR119 and the TRPV1 39, 40, 41, 42. A growing body of evidence has shown that these compounds are involved in the control of a wide variety of functions, including the control of food intake 43, 44, neuroprotection 45 and inhibition of pain and inflammation 46, 47. PEA levels increase in inflamed tissues, possibly as a protective effect to exert its well‐recognized anti‐inflammatory and analgesic properties 46. In biopsies from patients with coeliac disease, levels of both PEA and AEA were increased 48. It has been shown that by selectively binding PPARα receptors, PEA is able to down‐regulate iNOS expression and nuclear factor‐κB (NFκB) activation, and in turn the inflammation in a number of chronic inflammatory conditions, including experimental and human models of inflammatory bowel disease (IBD) 49, 50, 51. PEA is indeed able to significantly inhibit the expression of S100B and Toll‐like receptor 4 on enteric glial cells, thus reducing inflammation induced by nuclear factor‐κB (NFκB) by selectively binding PPARα receptors 51. On the contrary, OEA was able to display antinociceptive properties in a PPAR‐a‐insensitive manner in mice 47.
Figure 2.
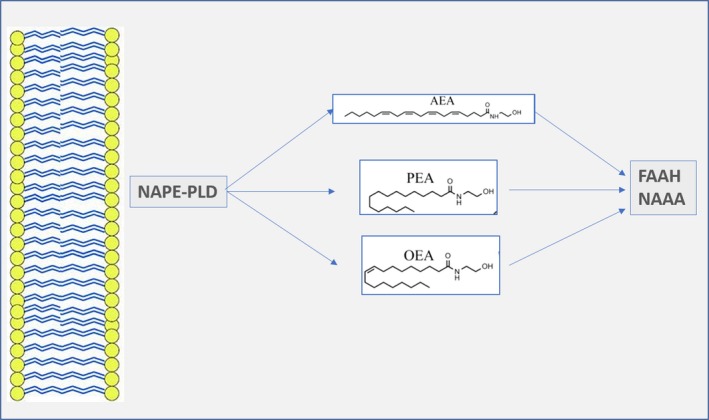
Biosynthesis and degradation of N‐acylethanolamides (NAEs) and possible points of interaction between AEA and its related compounds. Similar to AEA, N‐palmitoylethanolamine (PEA) and N‐oleoylethanolamine (OEA) are synthesized by N‐acylphosphatidylethanolamine‐specific phospholipase D (NAPE‐PLD) from membrane precursors. Unlike AEA, PEA and OEA exhibit no binding affinity on CB1/CB2 receptors, but they can enhance AEA activity at TRPV1 receptors. PEA and OEA are degraded by either fatty acid amide hydrolase (FAAH) or N‐acetylethanolamine‐hydrolysing acid amidase (NAAA). By competing with AEA for FAAH (mainly OEA) or by down‐regulating FAAH expression (predominantly PEA), they can increase AEA levels.
The endocannabinoid system and the control of gastrointestinal motility
In both animal and human GI tract, the ECs exert marked antipropulsive effects. This result is mainly mediated by the reduction in the release of acetylcholine via the activation of presynaptic CB1 18, 52, 53, 54. However, recent evidence suggests that along with the inhibition of acetylcholine release, the effects of the ECs on GI motility are likely to be related to the inhibition of all the components of the peristaltic reflex. In parallel with the inhibition of the release of acetylcholine, in rat models CB1 agonists were indeed able to significantly inhibit the release of both substance P and VIP, inhibiting, respectively, both the ascending contraction and the descending relaxation of the peristaltic reflex 55, 56, 57, 58. Furthermore, both the deletion of the CB1 gene 55, 56, 57 and the pharmacological blockade of these receptors 59, 60, 61 displayed prokinetic effects. Altogether, these lines of evidence seem to suggest that ECs are able to significantly reduce smooth muscle contractility, mainly by binding CB1. CB2 does not appear to play a major role in the control of intestinal motility under physiological conditions. However, studies on rodents have shown that intestinal hypermotility due to lipopolysaccharide (LPS) administration was abolished by CB2, but not by CB1 agonists 62. Hence, in animal models, CB2 agonism is more likely to inhibit intestinal motility in pathophysiological conditions associated with intestinal inflammation and immune activation.
The endocannabinoid system and the control of visceral sensitivity
Undoubtedly, the most documented effect of the ECS is the control of visceral sensitivity and, although empirically grounded, phytocannabinoid‐based treatments have been used for centuries in a number of conditions featured by chronic pain. In recent years, several studies have elucidated the molecular mechanism by which ECs are able to reduce visceral sensation and pain. The reduction in visceral sensitivity threshold to colorectal distension was found to be dependent on both CB1 activation and CB2 activation 63, 64, 65, 66, 67. Rousseaux et al. have shown that after colorectal distension, orally administered probiotics were able to reduce visceral sensation in rats in a CB2‐dependent fashion 68. Moreover, in pro‐inflammatory conditions, AM124 was able to reduce the bradykinin‐induced activation of primary afferents in wild‐type but not in CB2‐deficient mice 69, further supporting the evidence that CB2 is probably involved in the control of visceral hypersensitivity in inflammatory conditions. In rodents, visceral hypersensitivity due to water avoidance stress was significantly associated with a decreased expression and function of CB1, while a reciprocal increase in TRPV1 expression was found in dorsal root ganglion (DRG) neurons 70. CB1 and TRPV1 receptors are intimately connected, and CB1 is able to inhibit TRPV1 activity either directly or indirectly through the cyclic AMP–protein kinase A 71. The treatment of DRG neurons with anandamide, whose levels are increased in psychological stress, was able to reproduce the changes in TRPV1 and CB1 expressions, while administration of CB1 agonist and/or TRPV1 receptor antagonist was able to prevent these effects 70. Furthermore, injections of corticosteroids were able to increase anandamide expression and to reproduce the reciprocal changes in the expression of CB1 and TRPV1 receptors 70. Although not completely elucidated, the mechanism underlying the reduced expression of CB1 in chronic stress conditions might rely on increased methylation of the Cnr1 gene promoter by DNMT1, which results in epigenetic modifications of CB1 expression 72. Collectively, these findings indicate that the interplay between the cannabinoid and vanilloid signalling pathways may play an important role in stress‐induced visceral hyperalgesia 73, 74. In summary, both CB1 activation and CB2 activation have been linked to the control visceral sensitivity and stress‐induced hyperalgesia in animal models. The antinociceptive effects of CB1 are probably intimately connected to a reciprocal down‐regulation of TRPV1 receptors, while CB2 is likely able to counteract the sensitizing effects of inflammatory mediators, such as bradykinin, on peripheral endings of visceral afferents.
The endocannabinoid system and the control of intestinal inflammation
Over the past decade, many lines of evidence highlighting the role of the ECS in intestinal inflammation have been produced in both animal and pre‐clinical models 18, 75, 76. Although genetic studies failed to find any significant association between the polymorphisms in the gene encoding for FAAH and the risk of developing Crohn's disease (CD), homozygosis for the mutation Pro129Thr in FAAH gene was significantly associated with development of fistulas and extra‐intestinal manifestations in patients with CD 77. Also, in patients with ulcerative colitis (UC), the same FAAH genetic variant led to an earlier average onset of the inflammatory disease 77. Furthermore, in a recent case–control association analysis from a paediatric IBD population, the functional CB2‐R63 variant was significantly associated with the risk of developing IBDs and also linked to a more aggressive phenotype in both patients with CD and patients with UC 78. The pivotal role of the ECS in regulating intestinal inflammation has been confirmed by the evidence that both genetic ablation of FAAH and the pharmacological treatment with FAAH inhibitors prevented the development of colitis in rodents 79. In animal models, these effects are dependent on both CB1 and CB2. CB1 and CB2 agonists are indeed able to significantly reduce experimental colitis, while CB2 antagonists and CB1 knockout mice developed a more severe TNBS‐induced colitis 80, 81. Finally, it has been shown that an increase in AEA levels, induced by inhibitors of the catabolic or reuptake enzymes, significantly attenuates colitis in wild‐type mice, but not in CB1‐ and CB2‐deficient mice 82. In human beings, ex vivo studies have demonstrated a significantly increased expression of CB and EC levels in chronic inflammatory conditions, including IBDs, diverticulitis and coeliac disease 48, 74, 75. An overview of the reciprocal changes in CB receptors and EC level is reported in Table 1. However, both FAAH expression and levels of AEA have been reported to be decreased or increased in colitis from different studies, pointing towards the need for further studies to fully address the role of ECS in the modulation of intestinal inflammation.
Table 1.
Reported altered expression profile of endocannabinoid system (ECS) in intestinal disease
| Clinical condition | AEA | PEA | FAAH | CB1 | CB2 | Ref. | |
|---|---|---|---|---|---|---|---|
| Ileitis | Mouse | + | = | = | + | + | 54, 74 |
| Coeliac‐like atrophy | Rat | + | + | nd | nd | nd | 48 |
| Colitis | Mouse | +/− | nd | +/− | nd | nd | 82 |
| IBD | Human | +/− | * | +/= | +/− | + | 24, 62 |
| Diverticulitis | Human | + | = | nd | = | nd | 75 |
| FD | Human | nd | nd | nd | +/* | nd | 85 |
| IBS | Human | nd | * | nd | * | + | 105 |
| NAFLD/NASH | Human | + | nd | nd | + | * | 116, 117, 120, 121 |
+: increase; −: decrease; =: no significant change; nd: not determined; +/−: conflicting results; *: indirect evidence from administration of agonists/antagonists. IBD: inflammatory bowel disease; FD: functional dyspepsia; IBS: irritable bowel syndrome; NAFLD: non‐alcoholic fatty liver disease; NASH: non‐alcoholic steatohepatitis.
The endocannabinoid system in gut pathophysiology
The homoeostatic role of ECS, able to regulate GI functions peripherally and centrally, represents both a blessing and a curse, making it an appealing therapeutic target and, at the same time, a challenge in selectively modulating GI functions without altering the functionality of other organs. We will now discuss in detail the evidence produced on the role of the ECS in GI disorders, namely functional dyspepsia (FD) and irritable bowel syndrome (IBS), two of the main functional gastrointestinal disorders (FGIDs), IBDs and non‐alcoholic fatty liver disease (NAFLD). We will also review the most recent advances in the possible therapeutic exploitation of manipulating ECS in the treatment of these GI disorders.
The endocannabinoid system and functional dyspepsia
Although only few studies have investigated the potential effects of ECS in FD, there is evidence suggesting that the ECS might be an intriguing target in FD treatment, as it is involved in the modulation of some of the proposed mechanisms underlying FD pathophysiology 83, 84. In a recent study in patients with FD, Ly et al. have demonstrated a sustained increase in CB1 receptor availability in cerebral regions involved in the control of food intake and visceral sensitivity, suggesting for the first time a long‐term dysfunction in ECS signalling pathways in FD 85. However, whether this effect is a consequence of altered visceral sensitivity or of dysregulation in food intake still needs to be clarified. Impaired gastric accommodation, delayed gastric emptying and visceral hypersensitivity have been suggested as the underlying pathophysiological mechanisms of some FD symptoms, such as nausea, early satiety, post‐prandial fullness and pain 84, 86, 87. In experimental animals, CB receptor agonists have been shown to significantly reduce gastric emptying 88, 89. Similarly, oral administration of dronabinol (Δ9‐THC) was able to significantly reduce gastric emptying in human beings 90, 91. Furthermore, in healthy individuals, administration of a CB1 antagonist (rimonabant) was able to inhibit gastric accommodation, but not affecting gastric sensitivity, suggesting a role of ECS in the control of gastric accommodation 92. Although further studies are required to fully address the putative role of ECS in FD pathophysiology, the well‐recognized orexigenic and antiemetic effects of cannabino‐mimetics make the manipulation of ECS signalling pathway a promising strategy in FD treatment.
The endocannabinoid system in irritable bowel syndrome
Although the pathophysiology of IBS is still not completely understood, gut motility impairment, visceral hyperalgesia, low‐grade inflammation and gut–brain axis alterations have all been associated with symptoms onset 93; hence, the ECS may represent a new therapeutic target. As ECs are known to decrease GI motility 94, 95, dronabinol, a derivative of THC, has been tested in patients affected by diarrhoea‐predominant IBS (IBS‐D) showing variable results. It has been shown that this compound was effective in decreasing the colonic transit but not colonic sensitivity, and this effect was limited to those patients carrying CB1 receptor polymorphism rs806378 96, 97. Moreover, as the activation of CB1 may reduce GI transit, the use of its antagonist may be used to increase stool frequency in constipation‐predominant IBS (IBS‐C). Actually, a selective CB1 antagonist, namely rimonabant (SR141716A), was able to increase colonic motility in mice 61. Interestingly, also the inhibition of the 2‐AG synthesizer DAGL using orlistat was found to normalize stool frequency in a mouse model of chronic constipation, without affecting basal motility 98. In addition, several lines of evidence suggested that the increase in CB1 activity might lead to a reduction in visceral sensitivity 66, 99, 100, 101. Esfandyari et al. have tested the efficacy of dronabinol in visceral sensitivity in a randomized, double‐blind, placebo‐controlled trial showing its ability to increase colonic compliance and relaxation in vivo 90. However, a further study failed to find significant difference in terms of rectal compliance between dronabinol and placebo 97. This discrepancy may be due to a different expression of CB1 in colon and rectum. Finally, several studies revealed that the ECS also participates in immune response, mainly reducing the production of inflammatory cytokines. Given the evidence for a role of low‐grade inflammation in IBS, ECs may also improve IBS symptoms by decreasing the inflammatory response 102, 103, 104. All these lines of evidence confirm that the ECS may represent a new therapeutic target in IBS; however, the risk of adverse effect still limits the use of ECs in treating FGIDs. Therefore, EC‐like compounds able to modulate ECS signalling with a good safety profile and, more importantly, without central side effects appear as promising candidates in IBS treatment. Recently, a multi‐centre randomized, double‐blind, placebo‐controlled study has shown the efficacy of orally administered PEA in decreasing the pain severity in patients with IBS. The authors found a significantly increased expression of mast cells and CB2 in IBS, while the levels of OEA were significantly reduced. Furthermore, orally administered PEA significantly improved the pain severity in these patients; however, the authors concluded that it was less obvious whether this effect was dependent on the ECS‐induced modulation of visceral hyperalgesia or on mast cell stabilization; hence, further studies evaluating the relation between ECs, inflammation and IBS are needed 105.
The endocannabinoid system in inflammatory bowel disease
The lines of evidence showing the involvement of ECs in the regulation of inflammatory and immune response in the digestive tract inevitably promoted research on the role of ECs in IBD. The first evidence came from CB1 and CB2 knockout mice that showed a higher susceptibility to chemically induced colitis, suggesting that ECs play a key protective role against chronic inflammation 81, 106. Moreover, in vitro studies showed that AEA and other CB1 agonists promote wound closure in human colonic epithelium and hence might improve mucosal healing in patients with IBD 22. Furthermore, in vitro experiments showed that anandamide and 2‐AG increased intestinal permeability when apically administered on Caco‐2 cells, and an in vivo study in obese mice, a model of leaky gut, showed that the CB1 antagonist rimonabant was able to reduce plasmatic LPS level, confirming the role of ECs in regulating gut permeability 107, 108, 109. Interestingly, further studies have revealed that while CB1 mainly mediates the effects of ECs in a physiological setting, CB2 seems to assume a prevalent role during inflammatory process. Indeed, immunohistochemical studies showed that during inflammatory flares, the expression of CB2, but not of CB1, is modified and amplified 24, 62. This evidence is very intriguing as CB2 agonists may represent a new therapeutic strategy in IBD, acting directly and specifically on inflamed tissue, thus reducing central adverse effects. Finally, a protective effect of PEA has been demonstrated in human biopsies from patients with active UC, suggesting that exogenous administration of EC‐like amides may improve mucosal healing in patients with IBD 51. As NAEs are already available for treating neuropathic pain, showing a good efficacy and safety profile, further clinical trials to evaluate the therapeutic role of these compounds are clearly required.
The endocannabinoid system in liver disease
Liver plays a major role in human homoeostasis with numerous functions, including regulation of lipid and carbohydrate metabolism, plasma protein synthesis, hormone production and detoxification. The emerging role of ECs in homoeostasis and lipid metabolism led several authors to investigate the interactions between the ECS and liver functions in normal and pathological conditions. The cannabinoid receptors are widely distributed on both hepatocytes and cholangiocytes, as on Kupffer and stellate cells, and their expression is modified during liver injury 110, 111, 112, 113. In particular, it was found that the ECS is involved in hepatic haemodynamic, cellular regeneration, liver fibrosis and lipid metabolism. As known, liver haemodynamic dysregulation plays a central role in cirrhosis, indeed portal hypertension and systemic vasodilation are involved in all major cirrhotic complications, such as ascites, variceal bleeding, liver‐related cardiomyopathy and increased risk of cardiovascular events 114, 115. Remarkably, the hypotensive effects of ECs, mainly mediated via CB1 activation, have been associated with cirrhosis‐induced vasodilation, and increased levels of AEA have been found in peripheral blood of patients with cirrhosis 116, 117. In rodent models of cirrhosis, the administration of CB1 antagonist was found to decrease ascites and ameliorate sodium balance, and CB1 was shown to contribute to cardiac contractility alterations related to liver cardiomyopathy, suggesting that CB1 antagonists might be used to improve cardiovascular activity in cirrhosis 118, 119. ECs have also been associated with fibrosis progression in HCV‐infected patients, suggesting a profibrotic activity of ECs. Indeed, CB1 stimulation promotes the activity of myofibroblasts and stellate cells, likely via an increased TGF‐β production 120, 121. On the contrary, CB2 activation seems to play a protective role against fibrosis, promoting regeneration of liver cells after acute injury. Indeed, selective CB2 agonists have been found to slow fibrosis in a rat model of cirrhosis, and CB2−/− knockout mice are more sensitive to acute liver injury, showing a low regenerative response 122, 123. In summary, although ECs may worse cirrhosis progression and complications mainly via CB1 activation, specific CB2 agonists might slow liver fibrotic evolution.
Endocannabinoids in non‐alcoholic fatty liver disease
The emblematic role of ECS in metabolic syndrome and obesity is already known; indeed, the CB1 antagonist rimonabant has been proposed in obesity treatment due to its beneficial effects on both bodyweight and lipid profile. However, the neuropsychiatric adverse effects have limited the clinical use of this compound. Non‐alcoholic fatty liver disease (NAFLD) and non‐alcoholic steatohepatitis (NASH) are strongly associated with metabolic syndrome, representing the ‘liver response’ to obesity, dyslipidaemia and altered carbohydrate metabolism. As ECs play a key role in liver lipid metabolism, a great interest is raised on effects of ECs on fatty liver diseases 124. Cannabinoid receptors are involved in hepatic lipogenesis, inducing specific transcriptional factors, such as SREBPs (sterol regulatory element‐binding proteins). Indeed in a mouse model with a selective deletion of hepatic CB1, a significant reduction in lipid storage during high‐fat diet has been observed 125. Intriguingly, also lipid profile and insulin resistance were improved; however, no effects on BMI have been registered in this murine model, suggesting that other mechanisms are involved in bodyweight regulation 125. Altogether, these lines of evidence support the role of ECs in hepatic steatosis and fibrotic progression, opening the possibility of new therapeutic options in treatment of NAFLD and NASH; in particular, the efficacy and safety of the CB1 antagonist rimonabant are currently under investigation in a phase III clinical trial for treatment of NASH.
Conclusions
In the last years, accumulating lines of evidence have pointed out the homoeostatic role of the ECS in regulating intestinal motility, sensitivity and inflammation. An impairment of ECS signalling has been suggested to play a key role in several gastrointestinal disorders, such as FGIDs, IBDs and liver diseases. Even if conflicting results have been produced in vivo, convincing evidence suggests that pharmacological manipulation of this multifaceted system might provide new therapeutic options in treating GI diseases. The complexity and the redundancy of ECS make the manipulation of this complex system an appealing target for therapeutic purposes, although the possibility of central side effects strongly limited the current use of these compounds in clinical settings. Using peripherally acting drugs with no affinity on central cannabinoid receptors is an intriguing strategy, and as PEA formulations are already available for the treatment of chronic pain, further in vivo studies to test the clinical efficacy of these compounds are strongly warranted.
Conflict of interest
The authors have no conflicting interests to declare.
References
- 1. United Nations Office on Drugs and Crime . World Drug Report 2015 (United Nations publication, Sales No. E.15.XI.6). Available at http://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 2. Degenhardt L, Ferrari AJ, Calabria B, et al The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS ONE. 2013; 8: e76635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007; 4: 1614–48. [DOI] [PubMed] [Google Scholar]
- 4. Campbell FA, Tramèr MR, Carroll D, et al Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review BMJ. 2001; 323: 13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005; 59: 291–5. [DOI] [PubMed] [Google Scholar]
- 6. Devane WA, Hanus L, Breuer A, et al Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992; 258: 1946–9. [DOI] [PubMed] [Google Scholar]
- 7. Sugiura T, Kondo S, Sukagawa A, et al 2‐Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995; 215: 89–97. [DOI] [PubMed] [Google Scholar]
- 8. Di Marzo V, Fontana A. Anandamide, an endogenous cannabinomimetic eicosanoid: ‘killing two birds with one stone’. Prostaglandins Leukot Essent Fatty Acids. 1995; 53: 1–11. [DOI] [PubMed] [Google Scholar]
- 9. Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009; 60: 77–84. [DOI] [PubMed] [Google Scholar]
- 10. Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic? Curr Med Chem. 1999; 6: 757–73. [PubMed] [Google Scholar]
- 11. Lambert DM, Muccioli GG. Endocannabinoids and related N‐acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr Opin Clin Nutr Metab Care. 2007; 10: 735–44. [DOI] [PubMed] [Google Scholar]
- 12. Di Marzo V, Wang J. The Endocannabinoidome: The World of Endocannabinoids and Related Mediators. Amsterdam: Elsevier; 2015. ISBN 978‐0‐12‐420126‐2. [Google Scholar]
- 13. Piomelli D, Giuffrida A, Calignano A, et al The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000; 21: 218–24. [DOI] [PubMed] [Google Scholar]
- 14. Fezza F, Bari M, Florio R, et al Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014; 19: 17078–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda LA, Lolait SJ, Brownstein MJ, et al Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990; 346: 561–4. [DOI] [PubMed] [Google Scholar]
- 16. Munro S, Thomas KL, Abu‐Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993; 365: 61–5. [DOI] [PubMed] [Google Scholar]
- 17. Howlett AC, Barth F, Bonner TI, et al International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002; 54: 161–202. [DOI] [PubMed] [Google Scholar]
- 18. Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008; 57: 1140–55. [DOI] [PubMed] [Google Scholar]
- 19. Izzo AA. The cannabinoid CB(2) receptor: a good friend in the gut. Neurogastroenterol Motil. 2007; 19: 704–8. [DOI] [PubMed] [Google Scholar]
- 20. Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011; 50: 193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kano M, Ohno‐Shosaku T, Hashimotodani Y, et al Endocannabinoid‐mediated control of synaptic transmission. Physiol Rev. 2009; 89: 309–80. [DOI] [PubMed] [Google Scholar]
- 22. Wright KL, Rooney N, Feeney M, et al Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005; 129: 437–53. [DOI] [PubMed] [Google Scholar]
- 23. Buckley NE, Hansson S, Harta G, et al Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1998; 82: 1131–49. [DOI] [PubMed] [Google Scholar]
- 24. Marquéz L, Suárez J, Iglesias M, et al Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE. 2009; 4: e6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007; 152: 567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003; 140: 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caterina MJ, Schumacher MA, Tominaga M, et al The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature. 1997; 389: 816–24. [DOI] [PubMed] [Google Scholar]
- 28. Huang SM, Bisogno T, Trevisani M, et al An endogenous capsaicin‐like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002; 99: 8400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryskamp DA, Redmon S, Jo AO, et al TRPV1 and endocannabinoids: emerging molecular signals that modulate mammalian vision. Cells. 2014; 3: 914–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pertwee RG, Howlett AC, Abood ME, et al International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev. 2010; 62: 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheskin T, Hanus L, Slager J, et al Structural requirements for binding of anandamide‐type compounds to the brain cannabinoid receptor. J Med Chem. 1997; 40: 659–67. [DOI] [PubMed] [Google Scholar]
- 32. Griffin G, Tao Q, Abood ME. Cloning and Pharmacological Characterization of the Rat CB2 Cannabinoid Receptor. J Pharmacol Exp Ther. 2000; 292: 886–94. [PubMed] [Google Scholar]
- 33. Ben‐Shabat S, Fride E, Sheskin T, et al An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2‐arachidonoyl‐glycerol cannabinoid activity. Eur J Pharmacol. 1998; 353: 23–31. [DOI] [PubMed] [Google Scholar]
- 34. Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998; 359: 1–18. [DOI] [PubMed] [Google Scholar]
- 35. Fowler CJ, Jonsson KO, Tiger G. Fatty acid amide hydrolase, biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2‐arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem Pharmacol. 2001; 62: 517–26. [DOI] [PubMed] [Google Scholar]
- 36. Di Marzo V, Melck D, Orlando P. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti‐proliferative effect of anandamide in human breast cancer cells. Biochem J. 2001; 358: 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001; 506: 253–6. [DOI] [PubMed] [Google Scholar]
- 38. Borrelli F, Izzo AA. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract Res Clin Endocrinol Metab. 2009; 23: 33–49. [DOI] [PubMed] [Google Scholar]
- 39. Ho W‐SV, Barrett DA, Randall MD. ‘Entourage’ effects of N‐palmitoylethanolamide and N‐oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008; 155: 837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. LoVerme J, La Rana G, Russo R, et al The search for the palmitoylethanolamide receptor. Life Sci. 2005; 77: 1685–98. [DOI] [PubMed] [Google Scholar]
- 41. Capasso R, Matias I, Lutz B, et al Fatty acid amide hydrolase controls mouse intestinal motility in vivo . Gastroenterology. 2005; 129: 941–51. [DOI] [PubMed] [Google Scholar]
- 42. Matias I, Gonthier MP, Petrosino S, et al Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta‐cells. Br J Pharmacol. 2007; 152: 676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends in Endocrinol Metab. 2007; 18: 27–37. [DOI] [PubMed] [Google Scholar]
- 44. Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999; 143: 315–7. [DOI] [PubMed] [Google Scholar]
- 45. Ahn EH, Kim DW, Shin MJ, et al Pep‐1‐PEA‐15 protects against toxin‐induced neuronal damage in a mouse model of Parkinson's disease. Biochim Biophys Acta. 2014; 1840: 1686–700. [DOI] [PubMed] [Google Scholar]
- 46. Lo Verme J, Fu J, ‘Astarita G, et al The nuclear receptor peroxisome proliferator‐activated receptor‐alpha mediates the anti‐inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005; 67: 15–9. [DOI] [PubMed] [Google Scholar]
- 47. Suardı′az M, Estivill‐Torru′s G, Goicoechea C, et al Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007; 133: 99–110. [DOI] [PubMed] [Google Scholar]
- 48. D'Argenio G, Petrosino S, Gianfrani C, et al Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate‐treated rats. J Mol Med. 2007; 85: 523–30. [DOI] [PubMed] [Google Scholar]
- 49. Lowin T, Apitz M, Anders S, et al Anti‐inflammatory effects of N‐acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX‐2 dependent manner. Arthritis Res Ther. 2015; 17: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Impellizzeri D, Ahmad A, Bruschetta G, et al The anti‐inflammatory effects of palmitoylethanolamide (PEA) on endotoxin‐induced uveitis in rats. Eur J Pharmacol. 2015; 15: 28–35. [DOI] [PubMed] [Google Scholar]
- 51. Esposito G, Capoccia E, Turco F, et al Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4‐dependent PPAR‐α activation. Gut. 2014; 63: 1300–12. [DOI] [PubMed] [Google Scholar]
- 52. Mulè F, Amato A, Baldassano S, et al Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric reparations. Pharmacol Res. 2007; 56: 185–92. [DOI] [PubMed] [Google Scholar]
- 53. Croci T, Manara L, Aureggi G, et al In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br J Pharmacol. 1998; 125: 1393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Manara L, Croci T, Guagnini F, et al Functional assessment of neuronal cannabinoid receptors in the muscular layers of human ileum and colon. Dig Liver Dis. 2002; 34: 262–9. [DOI] [PubMed] [Google Scholar]
- 55. Yuece B, Sibaev A, Broedl U, et al Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007; 19: 744–53. [DOI] [PubMed] [Google Scholar]
- 56. Aviello G, Romano B, Izzo AA. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci. 2008; 1(Suppl): 81–93. [PubMed] [Google Scholar]
- 57. Sibaev A, Yuece B, Kemmer M, et al Cannabinoid‐1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2008; 296: G119–28. [DOI] [PubMed] [Google Scholar]
- 58. Grider JR, Mahavadi S, Li Y, et al Modulation of motor and sensory pathways of the peristaltic reflex by cannabinoids. Am J Physiol Gastrointest Liver Physiol. 2009; 297: G539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colombo G, Agabio R, Lobina C, et al Cannabinoid modulation of intestinal propulsion in mice. Eur J Pharmacol. 1998; 344: 67–9. [DOI] [PubMed] [Google Scholar]
- 60. Izzo AA, Mascolo N, Tonini M, et al Modulation of peristalsis by cannabinoid CB(1) ligands in the isolated guinea‐pig ileum. Br J Pharmacol. 2000; 129: 984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pinto L, Izzo AA, Cascio MG, et al Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002; 123: 227–34. [DOI] [PubMed] [Google Scholar]
- 62. Duncan M, Mouihate A, Mackie K, et al Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide treated rats. Am J Physiol Gastrointest Liver Physiol. 2008; 295: G78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006; 18: 949–56. [DOI] [PubMed] [Google Scholar]
- 64. Fioramonti J, Bueno L. Role of cannabinoid receptors in the control of gastrointestinal motility and perception. Expert Rev Gastroenterol Hepatol. 2008; 2: 385–97. [DOI] [PubMed] [Google Scholar]
- 65. Kikuchi A, Ohashi K, Sugie Y, et al Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF‐03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008; 106: 219–24. [DOI] [PubMed] [Google Scholar]
- 66. Brusberg M, Arvidsson S, Kang D, et al CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension‐induced visceral pain in rodents. J Neurosci. 2009; 29: 1554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ravnefjord A, Brusberg M, Kang D, et al Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur J Pharmacol. 2009; 611: 85–91. [DOI] [PubMed] [Google Scholar]
- 68. Rousseaux C, Thuru X, Gelot A, et al Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007; 13: 35–7. [DOI] [PubMed] [Google Scholar]
- 69. Hillsley K, McCaul C, Aerssens J, et al Activation of the cannabinoid 2 (CB2) receptor inhibits murine mesenteric afferent nerve activity. Neurogastroenterol Motil. 2007; 19: 769–77. [DOI] [PubMed] [Google Scholar]
- 70. Hong S, Zheng G, Wu X, et al Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011; 140: 627–37. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bhave G, Zhu W, Wang H, et al cAMP‐dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002; 35: 721–31. [DOI] [PubMed] [Google Scholar]
- 72. Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress‐induced visceral pain in the peripheral nervous system. Gastroenterology. 2015; 148: 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hong S, Fan J, Kemmerer ES, et al Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009; 58: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010; 126: 21–38. [DOI] [PubMed] [Google Scholar]
- 75. Guagnini F, Valenti M, Mukenge S, et al Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006; 55: 946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schicho R, Storr M. Targeting the endocannabinoid system for gastrointestinal diseases: future therapeutic strategies. Expert Rev Clin Pharmacol. 2010; 3: 193–207. [DOI] [PubMed] [Google Scholar]
- 77. Storr M, Emmerdinger D, Diegelmann J, et al The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment Pharmacol Ther. 2009; 29: 542–51. [DOI] [PubMed] [Google Scholar]
- 78. Strisciuglio C, Bellini G, Miele E, et al Cannabinoid Receptor 2 Functional Variant Contributes to the Risk for Pediatric Inflammatory Bowel Disease. J Clin Gastroenterol. 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 79. Sałaga M, Mokrowiecka A, Zakrzewski PK, et al Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH) . J Crohns Colitis. 2014; 8: 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Storr M, Emmerdinger D, Diegelmann J, et al The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn's disease. PLoS ONE. 2010; 5: e9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Massa F, Marsicano G, Hermann H, et al The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004; 113: 1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. D'Argenio G, Valenti M, Scaglione G, et al Up‐regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006; 20: 568–70. [DOI] [PubMed] [Google Scholar]
- 83. Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004; 127: 1239–55. [DOI] [PubMed] [Google Scholar]
- 84. Sarnelli G, Vandenberghe J, Tack J. Visceral hypersensitivity in functional disorders of the upper gastrointestinal tract. Dig Liver Dis. 2004; 36: 371–6. [DOI] [PubMed] [Google Scholar]
- 85. Ly HG, Ceccarini J, Weltens N, et al Increased Cerebral Cannabinoid‐1 Receptor Availability Is a Stable Feature of Functional Dyspepsia: A [18 F]MK‐9470 PET Study. Psychother Psychosom. 2015; 84: 149–58. [DOI] [PubMed] [Google Scholar]
- 86. Tack J, Piessevaux H, Coulie B, et al Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998; 115: 1346–1352. [DOI] [PubMed] [Google Scholar]
- 87. Tack J, Caenepeel P, Fischler B, et al Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001; 121: 526–35. [DOI] [PubMed] [Google Scholar]
- 88. Calignano A, La Rana G, Makriyannis A, et al Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur J Pharmacol. 1997; 340: R7–8. [PubMed] [Google Scholar]
- 89. Izzo AA, Capasso R, Pinto L, et al Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001; 132: 1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Esfandyari T, Camilleri M, Busciglio I, et al Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo‐controlled study. Am J Physiol Gastrointest Liver Physiol. 2007; 293: G137–45. [DOI] [PubMed] [Google Scholar]
- 91. Esfandyari T, Camilleri M, Ferber I, et al Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo‐controlled study. Neurogastroenterol Motil. 2006; 18: 831–8. [DOI] [PubMed] [Google Scholar]
- 92. Ameloot K, Janssen P, Scarpellini E, et al Endocannabinoid control of gastric sensorimotor function in man. Aliment Pharmacol Ther. 2010; 31: 1123–31. [DOI] [PubMed] [Google Scholar]
- 93. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016; doi: 10.1053/j.gastro.2016.02.032. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 94. Reichenbach ZW, Shey R. Cannabinoids and GI disorders: endogenous and exogenous. Curr Treat Options Gastroenterol. 2016; 14: 14461–77. [DOI] [PubMed] [Google Scholar]
- 95. Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol. 2004; 141: 1335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wong BD, Camilleri M, Busciglio I, et al Pharmacogenetics trial on a cannabinoid agonist shows reduced fasting colonic motility in patients with non‐constipated irritable bowel syndrome. Gastroenterology. 2011; 141: 1638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wong BD, Camilleri M, Eckert D, et al Randomized pharmacodynamics and pharmacogenetics trial on dronabinol effects on colon transit in irritable bowel syndrome‐diarrhea. Neurogastroenterol Motil. 2012; 24: 358–e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bashashati M, Nasser Y, Keenan C, et al Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation Br . J Pharmacol. 2015; 172: 3099–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fichna J, Wood JT, Papanastasiou M, et al Endocannabinoid and cannabinoid‐like fatty acid amine levels correlate with pain‐related symptom in patient with IBS‐D and IBS‐C: a pilot study. PLoS ONE. 2013; 8: e85073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Duncan M, Davison JS. Sharkey KA Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005; 22: 667–83. [DOI] [PubMed] [Google Scholar]
- 101. Booker L, Naidu PS, Razdan RK, et al Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend. 2009; 105: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barbara G, De Giorgio R, Stanghellini V, et al A role for inflammation in irritable bowel syndrome? Gut. 2002; 51: i41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hirotada A, Eikichi I, Kazuhiko N. Low‐grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. 2010; 1: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Greneisen WE, Turner H. Immunoreactive effects of cannabinoids: considerations for therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol. 2010; 10: 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barbara G, Cremon C, Bellacosa L, et al Randomized Placebo‐Controlled Multicenter Study on the effect of palmitoyl‐ethanolamide and polydatin on immune activation in patients with irritable bowel syndrome. Gastroenterology 2014. 146, Issue 5, Supp 1, Page S–124. [Google Scholar]
- 106. Engel MA, Kellermann CA, Burnat G, et al Mice lacking cannabinoid CB1‐CB2‐ receptors or both receptors show increased susceptibility to TNBS‐induced colitis. J Physiol Pharmacol. 2010; 61: 89–97. [PubMed] [Google Scholar]
- 107. Alhamoruni A, Lee AC, Wright KL, et al Pharmacological effects of cannabinoids on Caco2 cells culture model of intestinal permeability. J Phamacol Exp Ther. 2010; 335: 92–102. [DOI] [PubMed] [Google Scholar]
- 108. Muccioli GG, Naslain D, Bäckhed F, et al The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010; 6: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alhamoruni A, Wright KL, Larvin M, et al Cannabinoids mediate opposing effects on inflammation‐induced intestinal permeability Br . J Pharmacol. 2012; 165: 2598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu X, Liu Y, Hung S, et al Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006; 171: 31–8. [DOI] [PubMed] [Google Scholar]
- 111. Mukhopadhyay B, Liu J, Osei‐Hyiaman D, et al Transcriptional regulation of cannabinoid receptor‐1 expression in the liver by retinoic acid acting via retinoic acid receptor‐gamma. J Biol Chem. 2010; 285: 19002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Floreani A, Lazzari R, Macchi V, et al Hepatic expression of endocannabinoid receptors and their novel polymorphisms in primary biliary cirrhosis. J Gastroenterol. 2009; 45: 68–76. [DOI] [PubMed] [Google Scholar]
- 113. Tam J, Liu J, Mukhopadhyay B, et al Endocannabinoids and liver disease. Hepatology. 2011; 53: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002; 87: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997; 25: 1. [DOI] [PubMed] [Google Scholar]
- 116. Batkai S, Jarai Z, Wagner JA, et al Endocannabinoids acting at vascular CB1 receptors mediated the vasodilated state in advanced liver cirrhosis. Nat Med. 2001; 7: 827–32. [DOI] [PubMed] [Google Scholar]
- 117. Caraceni P, Viola A, Piscitelli Giannone F, et al Circulating and hepatic endocannabinoids and endocannabinoids‐related molecules in patients with cirrhosis. Liver Int. 2010; 30: 816–25. [DOI] [PubMed] [Google Scholar]
- 118. Domenicali M, Caraceni P, Giannone F, et al Cannabinoid type 1 receptor antagonism delays ascites formation in rats with cirrhosis. Gastroenterology. 2009; 137: 341–9. [DOI] [PubMed] [Google Scholar]
- 119. Gaskari SA, Homar H, Lee SS. Therapy insight: cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006; 3: 329–37. [DOI] [PubMed] [Google Scholar]
- 120. Teixeira‐Clerc F, Julien B, Grenard P, et al CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006; 12: 671–6. [DOI] [PubMed] [Google Scholar]
- 121. Hezod C, Roudot‐Thoraval F, Nguyen S, et al Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005; 42: 63–71. [DOI] [PubMed] [Google Scholar]
- 122. Munoz‐Luque J, Ros J, Fernandez‐Varo G, et al Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008; 324: 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lotersztajn S, Teixeira‐Clerc F, Julien B, et al CB2 receptors as a new therapeutic target for liver diseases. Br J Pharmacol. 2008; 153: 286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Caraceni P, Domenicali M, Giannone F, et al The role of the endocannabinoid system in liver diseases. Best Pract Res Clin Endocrinol Metab. 2009; 23: 65–77. [DOI] [PubMed] [Google Scholar]
- 125. Osei‐Hyiaman D, Liu J, Zhou L, et al Hepatic CB1 receptor is required for development of diet‐induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008; 118: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]


